Abstract
Bone marrow CD34+ cell apoptosis (annexin V), proliferation (Ki-67), and Bcl-2-related protein expression was evaluated by flow cytometry in 102 patients with myelodysplastic syndrome (MDS) and acute myeloid leukemia secondary to MDS (MDS-AML) and in 30 normal donors (NBM). Apoptosis was significantly increased in refractory anemia (RA)/RA with ringed sideroblasts (RARS) (56.9% [20.4%-93.6%]) and refractory anemia with excess blasts (RAEB) (51.2% [25.2%-76.6%]) compared with NBM (16.7% [3.4%-35.3%],P < .0001). In RA/RARS, apoptosis always exceeded proliferation (Ki-67-positivity, 26.1% [9.5%-47.8%]; apoptosis:proliferation ratio 2.08 [1.15-3.63]); whereas in RAEB, this ratio equalized (1.14 [0.93-2.08]) due to increased proliferation (40.4% [22%-69.5%]). Progression to RAEB in transformation (RAEB-t)/MDS-AML was associated with a significant reduction in apoptosis (22.3% [2.1%-53.2%];P < .0001) and proliferation (16.8% [1.9%-75.8%);P = .04; ratio 1.69 [0.16-12.21]). Pro-apoptotic (Bax/Bad) versus anti-apoptotic (Bcl-2/Bcl-X) Bcl-2-related protein ratios were increased in RA/RARS compared with NBM (2.57 [1.93-9.42] versus 1.89 [0.65-4.1]; P = .06), whereas disease progression was associated with significantly reduced ratios (1.16 [0.06-3.32]; P < .0001) due primarily to increased Bcl-2 expression. Apoptosis and Bax/Bad:Bcl-2/Bcl-X ratio were inversely correlated with both International Prognostic Scoring System score and cytogenetic risk group; highest levels observed in patients with low score and/or good risk cytogenetics. There was a trend toward an association between Bcl-2–related protein expression and apoptosis (P = .07). This study indicates that MDS progression arises through multiple hits that alter levels of CD34+cell apoptosis and proliferation. Early disease is associated with excessive apoptosis and elevated ratio of apoptosis to proliferation. Increased proliferative rates are observed in RAEB, whereas leukemic transformation arises through inhibition of apoptosis rather than excessive cell growth. Although disease progression is accompanied by a fall in pro-apoptotic versus anti-apoptotic Bcl-2–related protein ratios, heterogeneity in patterns of protein expression indicates that factors additional to Bcl-2 family members play a role in the deregulated apoptosis in MDS.
Introduction
The myelodysplastic syndromes are clonal stem cell disorders characterized by dysplastic hematopoiesis and an increased risk of leukemic transformation, especially in advanced subtypes: refractory anemia with excess blasts (RAEB) and RAEB in transformation (RAEB-t).1 Despite a normal or hypercellular marrow, most patients present with peripheral blood cytopenias. This paradox has recently been attributed to excessive programmed cell death or apoptosis of hematopoietic progenitors.2 3 Apoptosis in early disease may represent a pathophysiological mechanism whereby the hematopoietic system is able to abrogate defective and/or potentially harmful clones. Alternatively, an early “hit” in the multistep pathogenesis of myelodysplastic syndrome (MDS) could give rise to a clone with a proliferative advantage. Increased apoptosis may thus represent a homeostatic process to control cell numbers. In turn, leukemic progression could arise through the acquisition of genetic lesions that either block programmed cell death (PCD) or promote proliferation over and above apoptosis.
PCD can be triggered by a variety of extracellular stimuli and intracellular signals. Major regulators of these apoptotic pathways are members of the Bcl-2 family. These proteins function as homodimers or heterodimers, and it is the ratio of pro-apoptotic versus anti-apoptotic molecules that determines a cell's susceptibility to death signals.4 Deregulation of Bcl-2 family members has been demonstrated in a variety of human cancers, including hematological malignancies,5,6 and could feasibly underlie aberrant PCD observed in MDS. Certainly, increased expression of the anti-apoptotic protein Bcl-2 compared to pro-apoptotic Bax has been associated with disease progression in chronic lymphocytic leukemia.7 Moreover, in acute myeloid leukemia (AML), Bcl-2 overexpression is linked with CD34 positivity, resistance to chemotherapy, and short survival,6,8 features commonly attributed to disease arising from MDS. Studies demonstrating reduced Bcl-2 expression in CD34+ cells of patients with early MDS subtypes compared to advanced disease and normal controls lends further support for a role of the Bcl-2–related proteins in the pathogenesis of deregulated apoptosis and ineffective hematopoiesis in MDS.9,10 In the present study, the relationship between hematopoietic progenitor cell apoptosis and proliferation at different stages of MDS evolution was investigated by 2-color flow cytometry, using anti-CD34 monoclonal antibody (Mab) to identify progenitor cells, annexin V that binds to exposed phosphatidylserine on apoptotic cells11 and Ki-67 Mab that specifically binds to DNA of cycling cells.12 Permeabilized anti-CD34-labeled cells were also incubated with conjugated antibodies to 2 pro-apoptotic (Bax and Bad) and 2 anti-apoptotic (Bcl-2 and Bcl-X) Bcl-2 family members to evaluate whether deregulated apoptosis could be attributed to altered expression of the Bcl-2–related proteins.
Patients, materials, and methods
Patient characteristics
Fresh bone marrow (BM) aspirates were obtained from 102 patients with MDS or AML secondary to MDS (MDS-AML) and from 30 normal donors. A full explanation of the procedure was given prior to BM aspiration, and informed consent was obtained. The majority of aspirates were taken at the time of initial presentation. In remaining cases, patients treated with growth or differentiating agents or cytoreductive therapy within 3 months preceding analysis were excluded from the study. All samples were analyzed within 4 hours of aspiration.
The degree of apoptosis, proliferation, and Bcl-2–related protein expression was evaluated in 117, 56, and 44 cases, respectively. Data regarding Bcl-2–related protein expression has been previously published.13 According to the French, American, British (FAB) morphologic guidelines for MDS classification,14 48 patients had refractory anemia (RA), 4 had RA with ringed sideroblasts (RARS), 15 had RAEB, 7 had RAEB-t, and 28 had MDS-AML. Within this subset of patients, 6 had AML that had progressed from chronic myelomonocytic leukemia (CMML), and 5 had relapsed following chemotherapy. No patient had received any therapy other than supportive care for at least 1 month prior to BM examination. Median age was 67 years (range 17-92) with no significant differences between diagnostic categories. Hemoglobin (P = .002) as well as white cell (P = .004) and platelet counts (P = .0002), however, differed significantly between the disease subgroups. Of the 50 evaluable patients with early MDS, 38 (76%) had “good risk” cytogenetics according to the International Prognostic Scoring System (IPSS; normal karyotype or isolated deletions of the long arm of chromosome 5 or 20 or -Y).15 In contrast, 17 of 32 (53%) patients with RAEB-t/MDS-AML harbored a “poor risk” karyotype (> 2 chromosomal abnormalities and/or abnormalities of chromosome 7) with 14 (44%) demonstrating abnormalities of chromosome 7 (Table1).
Clinical and laboratory characteristics of myelodysplastic syndrome (MDS) and acute myeloid leukemia secondary to MDS patients
| Patient characteristics . | . | RA/RARS . | RAEB . | RAEB-t/MDS-AML . |
|---|---|---|---|---|
| No. | 52 | 15 | 35 | |
| Sex | ||||
| M | 31 (60%) | 11 (73%) | 17 (49%) | |
| F | 21 (40%) | 4 (27%) | 18 (51%) | |
| Age (y) | median | 62 | 72 | 67 |
| range | (19-92) | (37-86) | (17-81) | |
| Hemoglobin (1012/dL) | median | 11.6 | 10.6 | 9.4 |
| range | (5.0-15.8) | (6.1-14.0) | (3.8-12.5) | |
| WBC (109/L) | median | 4.7 | 2.5 | 10.9 |
| range | (0.5-8.0) | (0.9-22.2) | (0.6-230.1) | |
| Neutrophils (109/L) | median | 2.5 | 0.6 | 1.4 |
| range | (0.1-5.4) | (0.2-8.5) | (0.1-13.6) | |
| Platelets (109/L) | median | 120 | 47 | 40 |
| range | (9-358) | (16-319) | (4-431) | |
| Cytogenetics | ||||
| Good risk | 38/50 (76%) | 9/14 (64%) | 8/32 (25%) | |
| Intermediate risk | 8/50 (16%) | 3/14 (21%) | 7/32 (22%) | |
| Poor risk | 4/50 (8%) | 2/14 (15%) | 17/32 (53%) | |
| Chromosome 7 abnormalities | 3/50 (6%) | 2/14 (15%) | 14/32 (44%) | |
| IPSS score | ||||
| Low | 18 (37.5%) | 0 (0%) | 0 (0%) | |
| INT-1 | 27 (56%) | 8 (62%) | 0 (0%) | |
| INT-2 | 3 (6.5%) | 4 (31%) | 0 (0%) | |
| High | 0 (0%) | 1 (6%) | 7 (100%) |
| Patient characteristics . | . | RA/RARS . | RAEB . | RAEB-t/MDS-AML . |
|---|---|---|---|---|
| No. | 52 | 15 | 35 | |
| Sex | ||||
| M | 31 (60%) | 11 (73%) | 17 (49%) | |
| F | 21 (40%) | 4 (27%) | 18 (51%) | |
| Age (y) | median | 62 | 72 | 67 |
| range | (19-92) | (37-86) | (17-81) | |
| Hemoglobin (1012/dL) | median | 11.6 | 10.6 | 9.4 |
| range | (5.0-15.8) | (6.1-14.0) | (3.8-12.5) | |
| WBC (109/L) | median | 4.7 | 2.5 | 10.9 |
| range | (0.5-8.0) | (0.9-22.2) | (0.6-230.1) | |
| Neutrophils (109/L) | median | 2.5 | 0.6 | 1.4 |
| range | (0.1-5.4) | (0.2-8.5) | (0.1-13.6) | |
| Platelets (109/L) | median | 120 | 47 | 40 |
| range | (9-358) | (16-319) | (4-431) | |
| Cytogenetics | ||||
| Good risk | 38/50 (76%) | 9/14 (64%) | 8/32 (25%) | |
| Intermediate risk | 8/50 (16%) | 3/14 (21%) | 7/32 (22%) | |
| Poor risk | 4/50 (8%) | 2/14 (15%) | 17/32 (53%) | |
| Chromosome 7 abnormalities | 3/50 (6%) | 2/14 (15%) | 14/32 (44%) | |
| IPSS score | ||||
| Low | 18 (37.5%) | 0 (0%) | 0 (0%) | |
| INT-1 | 27 (56%) | 8 (62%) | 0 (0%) | |
| INT-2 | 3 (6.5%) | 4 (31%) | 0 (0%) | |
| High | 0 (0%) | 1 (6%) | 7 (100%) |
RA indicates refractory anemia; RARS, RA with ringed sideroblasts; RAEB, RA with excess blasts; RAEB-t, RAEB in transformation; MDS-AML, acute myeloid leukemia secondary to MDS; WBC, white blood count.
Apoptosis quantification
BM mononuclear cells were isolated by density gradient centrifugation over Ficoll-Paque (d = 1.077 g/mL; Pharmacia Biotech) and washed twice in phosphate-buffered saline (PBS) (Sigma). A total of 106 cells were used per sample. Cells were incubated with phycoerythrin (PE)-conjugated anti-CD34 Mab (Clone 8G12, Anti-HPCA-2, IgG1; Becton Dickinson) for 20 minutes at room temperature in the dark and then washed twice in PBS. Pelleted cells were resuspended in 100 μL binding buffer (10 mmol/L Hepes/NaOH, pH 7.4, 140 mmol/L NaCl, 2.5 mmol/L CaCl2; Bender Medsystems, Boehringer Ingelheim) and incubated with 2 μL fluorescein isothiocyanate (FITC)-conjugated annexin V (Bender Medsystems, Boehringer Ingelheim) for 10 minutes at room temperature in the dark. Cells were then resuspended in 400 μL binding buffer prior to flow cytometric analysis. Negative controls included MNCs incubated with neither CD34-PE Mab nor annexin V-FITC and cells incubated with CD34-PE Mab only.
Ki-67 expression
For cell surface and intracellular protein staining, fresh whole BM containing 106 white blood cells per sample was used. Cells were incubated with PE-conjugated anti-CD34 Mab (Clone 8G12, Anti-HPCA-2, IgG1; Becton Dickinson) for 20 minutes at room temperature in the dark and then washed twice in PBS. Samples were fixed in 100 μL “medium A” that contained formaldehyde (Fix & Perm, Caltag Laboratories, TCS Biologicals) for 15 minutes at room temperature in the dark and then washed in PBS to which 2.5% fetal calf serum (Sigma) and 0.1% sodium azide (Sigma) had been added (PBSF). MNCs were subsequently incubated with 100 μL permeabilization “medium B” (Fix & Perm, Caltag Laboratories, TCS Biologicals) and FITC-conjugated Ki-67 Mab (Clone MIB-1, IgG1; Immunotech, Coulter) for 20 minutes at 4°C in the dark and then washed in ice-cold PBSF. Cell pellets were resuspended in ice-cold PBSF prior to flow cytometric analysis. Cells incubated with FITC-conjugated isotype-specific antibodies (Serotec) were used as negative controls.
Bcl-2–related protein analysis
BM cells (106) were incubated with FITC-conjugated anti-CD34 Mab (Becton Dickinson) for 20 minutes at room temperature in the dark and then washed twice in PBS. Cells were fixed in 100 μL medium A for 15 minutes at room temperature in the dark and then washed in PBS. To prevent nonspecific binding of Mabs to Fc receptors, cells were pre-incubated with 10 μL rabbit-anti-mouse (RAM) immunoglobulins (Dako) for 10 minutes. Permeabilization medium B (100 μL) was subsequently added for a further 10 minutes prior to washing in ice-cold PBSF. To detect intracellular Bcl-2–related protein expression, cells were incubated with Mabs to Bax (Immunotech, Coulter), Bcl-2 (Dako), Bad, and Bcl-X (Transduction Laboratories, Affiniti Research Products) for 30 minutes on ice. After washing in ice-cold PBSF, cells were incubated with PE-conjugated RAM (Dako) for 30 minutes on ice, washed again, and resuspended in ice-cold PBSF prior to flow cytometric analysis. Negative controls were performed by incubating cells with isotype-specific antibodies (Sigma).
Flow cytometric analysis
Two-color analysis of annexin V, Ki-67 expression, and Bcl-2–related protein levels within CD34+ cell populations was performed, using an EPICS XL flow cytometer (Coulter). Data were acquired in list mode, acquiring at least 2 × 105 events per sample and/or at least 104 events within the CD34+ cell gate. Analysis was based on gating of subpopulations of CD34+ cells by forward scatter versus side scatter as well as side scatter versus fluorescence 2 for apoptosis and Ki-67 detection and fluorescence 1 for Bcl-2–related protein analysis. The percentage of positivity was determined by comparison of the fluorescence distribution histogram of positively stained cells to that of cells to which no annexin V was added for apoptosis, and to cells stained with appropriate isotype control for Ki-67 and Bcl-2–related protein analysis. The mean fluorescence intensity (MFI) value for each Bcl-2–related protein was calculated by dividing the MFI value of the positively stained cells by that of cells stained with an isotype control antibody. To determine individual Bcl-2–related protein levels, we calculated an Oncoprotein Index (OPI) as previously described,9 whereby OPI equals the product of the percentage of positive cells and MFI.
Statistical analysis
For the purposes of statistical evaluation, patients with RA and RARS were analyzed as one group. Comparison of CD34+ cell annexin V positivity and Ki-67 protein expression as well as apoptosis:proliferation (A:P) and Bax/Bad:Bcl-2/Bcl-X ratios between patients within clinically different disease subgroups was carried out, using the chi-square, Kruskal Wallis, and Mann Whitney tests. Correlations with clinical and laboratory parameters were calculated, using the Spearman rank test. In all statistical calculations, aP value of <.05 was considered significant.
Results
Degree of apoptosis
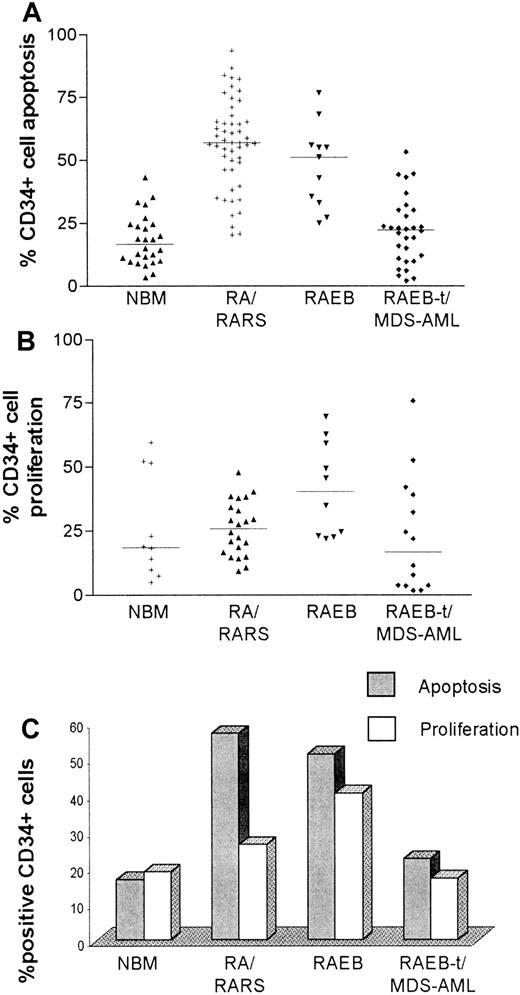
As shown in the representative set of experiments (Figure1), the degree of CD34+ cell apoptosis was significantly different between the diagnostic subgroups (chi-square 20.9; 2 degrees of freedom [df];P < .00005). CD34+ cell apoptosis was significantly increased in MDS FAB subtypes RA, RARS (median 56.9% [range 20.4%-93.6%], n = 50), and RAEB (51.2% [25.2%-76.6%], n = 11) compared to normal controls (median 16.7% [range 3.4%-35.3%], n = 26, P < .0001). Of the 50 patients with RA/RARS, 40 (80%) had more than 50% CD34+ cells undergoing PCD. Levels of apoptosis were significantly lower in RAEB-t/MDS-AML (median 22.3% [range 2.1%-53.2%], n = 30) compared to both RA/RARS (P < .0001) and RAEB (P = .0003) but did not differ from normal controls (Table2). Within this subgroup, patients with AML that had transformed from CMML (n = 5) had the lowest levels of PCD (median 6.2% [range 2.1%-27.8%], P = .03). Differences in CD34+ cell apoptosis between RA/RARS/RAEB and RAEB-t/MDS-AML remained significant even after removal of CMML-AML from this latter category. Comparison of early MDS with RAEB-t alone (P = .0003) and RAEB versus RAEB-t (P = .004) also demonstrated significant differences. When analyzing all patients, the percentage of apoptosis was positively correlated with hemoglobin concentration (Spearman r 0.32, confidence interval [CI] 0.19-0.5, P = .02) and platelet counts (Spearmanr 0.45,CI 0.26-0.6, P < .0001), whereas there was an inverse correlation with white cell count (Spearman r−0.26, CI −0.44 to −0.06, P = .01) and IPSS score (Spearman r −0.36, CI −0.56 to −0.12,P = .004). Within disease subgroups, however, levels of PCD were highly variable (Figure 2A) and could not be correlated with the aforementioned characteristics (data not shown).
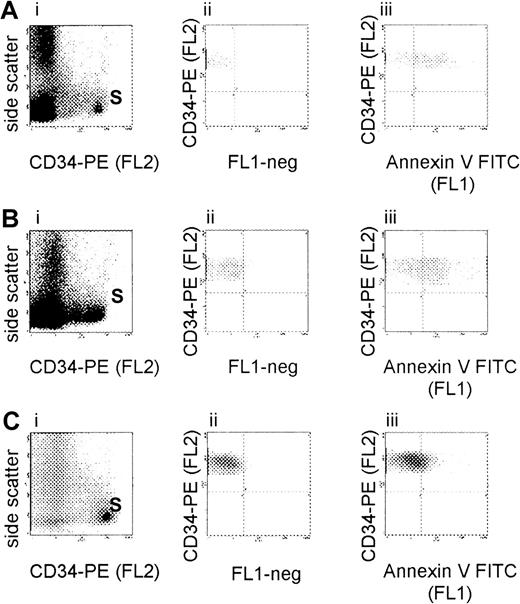
Flow cytometric evaluation of CD34+ cell apoptosis in MDS.
Bone marrow mononuclear cells from patients with MDS RA (A), RAEB (B), and RAEB-t (C) were isolated by density gradient centrifugation and incubated with phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody (Mab) and fluorescein isothiocyanate (FITC)-conjugated annexin V. Analysis was based on gating on CD34+ cell subpopulations (S) by forward scatter versus side scatter as well as side scatter versus fluorescence 2 (FL2) (i). Percentage of positivity was determined by comparison of the fluorescence distribution histogram (FL1) of positively stained cells (iii) to that of cells to which no annexin V was added (ii).
Flow cytometric evaluation of CD34+ cell apoptosis in MDS.
Bone marrow mononuclear cells from patients with MDS RA (A), RAEB (B), and RAEB-t (C) were isolated by density gradient centrifugation and incubated with phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody (Mab) and fluorescein isothiocyanate (FITC)-conjugated annexin V. Analysis was based on gating on CD34+ cell subpopulations (S) by forward scatter versus side scatter as well as side scatter versus fluorescence 2 (FL2) (i). Percentage of positivity was determined by comparison of the fluorescence distribution histogram (FL1) of positively stained cells (iii) to that of cells to which no annexin V was added (ii).
Median levels of apoptosis, proliferation, apoptosis:proliferation (A:P) ratio and Bax/Bad:Bcl2/BclX ratio in different diagnostic subgroups
| Disease subgroup . | Apoptosis (%) (range) . | Proliferation (%) (range) . | A:P ratio (range) . | Bax/Bad:Bcl-2/ Bcl-X ratio (range) . |
|---|---|---|---|---|
| NBM | 16.7 (3.4-35.3) | 18.7 (5.1-59.6) | 0.81 (0.48-1.6) | 1.89 (0.65-4.12) |
| RA/RARS | 56.9 (20.4-93.6) | 26.1 (9.5-47.8) | 2.08 (1.15-3.63) | 2.57 (1.93-9.42) |
| RAEB | 51.2 (25.2-76.6) | 40.4 (22-69.5) | 1.14 (0.93-2.06) | 1.19 (1.14-2.08) |
| RAEB-t/MDS-AML | 22.3 (2.1-53.2) | 16.85 (1.9-75.8) | 1.69 (0.16-12.2) | 0.85 (0.06-3.32) |
| Disease subgroup . | Apoptosis (%) (range) . | Proliferation (%) (range) . | A:P ratio (range) . | Bax/Bad:Bcl-2/ Bcl-X ratio (range) . |
|---|---|---|---|---|
| NBM | 16.7 (3.4-35.3) | 18.7 (5.1-59.6) | 0.81 (0.48-1.6) | 1.89 (0.65-4.12) |
| RA/RARS | 56.9 (20.4-93.6) | 26.1 (9.5-47.8) | 2.08 (1.15-3.63) | 2.57 (1.93-9.42) |
| RAEB | 51.2 (25.2-76.6) | 40.4 (22-69.5) | 1.14 (0.93-2.06) | 1.19 (1.14-2.08) |
| RAEB-t/MDS-AML | 22.3 (2.1-53.2) | 16.85 (1.9-75.8) | 1.69 (0.16-12.2) | 0.85 (0.06-3.32) |
NBM indicates normal donors; RA, refractory anemia; RARS, RA with ringed sideroblasts; RAEB, RA with excess blasts; RAEB-t, RAEB in transformation; MDS-AML, acute myeloid leukemia secondary to myelodysplastic syndrome.
Levels of CD34+ cell apoptosis and proliferation in different diagnostic subgroups.
To evaluate progenitor cell apoptosis, bone marrow mononuclear cells were isolated by density gradient centrifugation and incubated with phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody (Mab) and fluorescein isothiocyanate (FITC)-conjugated annexin V prior to 2-color flow cytometric analysis. Proliferating cells were identified by permeabilizing CD34-labeled cells and incubating with FITC-conjugated Ki-67 Mab. Percentage of positivity was determined by comparison of the fluorescence distribution histogram of positively stained cells to that of cells to which no annexin V was added for apoptosis, and to cells labeled with appropriate isotype control for Ki-67. As illustrated in the scatter plots, apoptosis (A) and proliferation (B) varied widely, even within diagnostic subgroups. (C) Bar chart that compares median levels of CD34+ cell apoptosis and proliferation in specific diagnostic categories.
Levels of CD34+ cell apoptosis and proliferation in different diagnostic subgroups.
To evaluate progenitor cell apoptosis, bone marrow mononuclear cells were isolated by density gradient centrifugation and incubated with phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody (Mab) and fluorescein isothiocyanate (FITC)-conjugated annexin V prior to 2-color flow cytometric analysis. Proliferating cells were identified by permeabilizing CD34-labeled cells and incubating with FITC-conjugated Ki-67 Mab. Percentage of positivity was determined by comparison of the fluorescence distribution histogram of positively stained cells to that of cells to which no annexin V was added for apoptosis, and to cells labeled with appropriate isotype control for Ki-67. As illustrated in the scatter plots, apoptosis (A) and proliferation (B) varied widely, even within diagnostic subgroups. (C) Bar chart that compares median levels of CD34+ cell apoptosis and proliferation in specific diagnostic categories.
Degree of proliferation
Maximal rates of proliferation were observed in patients with RAEB (median 40.35% [range 22%-69.5%], n = 10), which were higher than both normal controls (median 18.7% [range 5.1%-59.6%)], n = 10, P = .05) and RA/RARS (median 26.05% [range 9.5%-47.8%], n = 22, P = .02). Progression to RAEB-t/MDS-AML was associated with a significant reduction in Ki-67 positivity (median 16.85% [range 1.9%-75.8%], n = 14,P = .01) (Table 2). The degree of proliferation within diagnostic subgroups was highly variable, especially in advanced disease, (Figure 2B) and showed no correlation with clinical or laboratory characteristics or IPSS score (data not shown).
Apoptosis versus proliferation
CD34+ cell proliferation equaled or exceeded apoptosis in 8 of 10 (80%) normal controls (median A:P ratio 0.81 [range 0.48-1.6]), whereas in FAB subtypes RA/RARS, levels of CD34+ cell apoptosis always exceeded S-phase percentage (median A:P ratio 2.08 [range 1.15-3.63], P < .0001), with 13 of 22 (59.1%) patients having an A:P ratio greater than 2. Progression to RAEB was associated with an increase in Ki-67 positivity such that apoptosis mirrored proliferation in 8 of 10 (80%) cases (median A:P ratio 1.14 [range 0.94-2.06], P = .0004). The relationship between apoptosis and CD34+ cell division in RAEB-t/MDS-AML was highly variable (median A:P ratio 1.69 [range 0.16-12.21]); however in 10 of 14 (71.4%) cases, proliferation rates were lower than or matched levels of PCD (Figure 2C). The ratio of apoptosis to proliferation was significantly different between the 3 disease categories (chi-square 7.59, 2 df, P = .023) (Table 2). When considering the group as a whole, there was a significant inverse correlation between A:P ratio and IPSS score (Spearman r = −0.39 [CI −0.65 to −0.06)],P = .02). However, within disease subgroups, A:P ratio showed no correlation with laboratory parameters.
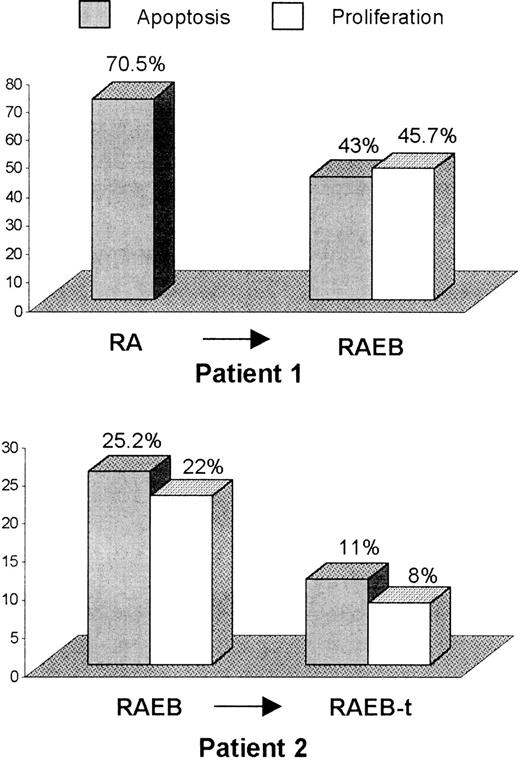
Disease progression and sequential patient samples
Two patients showed evidence of disease evolution during the period of study. In the first case, BM blast percentage increased from 3% to 9% non-erythroid cells, whereas in patient 2, transformation to RAEB-t was accompanied by karyotypic evolution 46, XX to 46, XX, del16 (q22-q24). MDS progression in both cases was associated with a fall in levels of CD34+ cell apoptosis. In the latter patient, in whom proliferation rates were additionally measured, evolution to RAEB-t was accompanied by a concomitant reduction in CD34+cell proliferation (Figure 3).
Sequential analysis of CD34+ cell apoptosis and proliferation during MDS progression.
Bone marrow CD34+ cell apoptosis and proliferation, using annexin V and Ki-67 labeling, respectively, was evaluated sequentially in 2 patients undergoing MDS evolution. BM blast percentage in patient 1 increased from 3% to 9% non-erythroid cells, whereas, in the second case, transformation from RAEB to RAEB-t was accompanied by karyotypic evolution.
Sequential analysis of CD34+ cell apoptosis and proliferation during MDS progression.
Bone marrow CD34+ cell apoptosis and proliferation, using annexin V and Ki-67 labeling, respectively, was evaluated sequentially in 2 patients undergoing MDS evolution. BM blast percentage in patient 1 increased from 3% to 9% non-erythroid cells, whereas, in the second case, transformation from RAEB to RAEB-t was accompanied by karyotypic evolution.
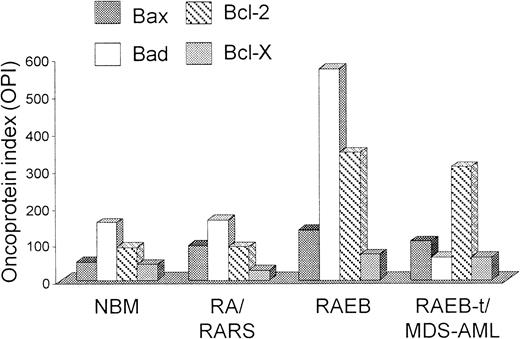
Bcl-2–related protein expression
As shown in Figure 4, patients with RA/RARS had a higher Bax OPI compared to normal controls (median 92.2 [range 12.2-913], n = 14 versus median 51.4 [range 9.2-170.7], n = 10), whereas progression to RAEB or RAEB-t/MDS-AML was associated with a significant increase in Bcl-2 expression (RA/RARS median OPI, 84.1 [range 9.1-681.7]; RAEB/RAEB-t/MDS-AML median OPI, 336.7 [range 1.1-6448], n = 20, P = .02) and a relative reduction in Bad. A higher pro-apoptotic (Bax/Bad) versus anti-apoptotic (Bcl-2/Bcl-X) apoptotic Bcl-2–related protein ratio was demonstrated in RA/RARS compared to normal controls, although this finding did not reach statistical significance (median 2.57 [range 1.93-9.42] versus median 1.89 [range 0.65-4.1], P = .06). Evolution to RAEB and RAEB-t/MDS-AML, however, was associated with a significant reduction in Bax/Bad versus Bcl-2/Bcl-X ratio (RAEB, median 1.19 [range 1.14-2.08], P = .04; RAEB-t/MDS-AML, median 0.85 [range 0.06-3.32], P = .0002) (Table 2). Of the 14 patients with RA/RARS, 12 (85.7%) had a pro-apoptotic versus anti-apoptotic Bcl-2–related protein ratio greater than 2; conversely, a ratio of 1 was observed in 12 of 20 (60%) patients with more advanced disease. In MDS patients, there was a significant positive correlation between Bax/Bad versus Bcl-2/Bcl-X ratio and platelet count (Spearman r = 0.46, 95% CI 0.11-0.71,P = .01) and a negative correlation with IPSS score (Spearman r = minus 0.67, 95% CI −0.87 to −0.26,P = .003). There was a trend toward an association between Bcl-2–related protein expression and apoptosis, although this finding failed to reach statistical significance (P = .07). Within disease subgroups however, patterns of Bcl-2-related protein expression were highly variable and showed no correlation with the aforementioned laboratory parameters.
Pro-apoptotic and anti-apoptotic Bcl-2–related protein expression in MDS/MDS-AML and normal controls.
BM samples were incubated with FITC-conjugated anti-CD34 Mab and fixed with formaldehyde. To prevent nonspecific binding of Mabs to Fc receptors, cells were pre-incubated with rabbit-anti-mouse (RAM) immunoglobulins, permeabilized, and then incubated with mouse-anti-human Mabs to Bax, Bad, Bcl-2, and Bcl-X on ice. Washed cells were subsequently incubated with PE-conjugated RAM on ice prior to flow cytometric analysis. The mean fluorescence intensity (MFI) value for each Bcl-2–related protein was calculated by dividing the MFI value of the positively labeled cells by that of cells stained with an isotype control antibody. To determine individual Bcl-2–related protein levels, we calculated an Oncoprotein Index (OPI) whereby OPI equals the product of the percentage of positive cells and MFI. Median Bax, Bad, Bcl-2, and Bcl-X OPI are plotted in specific diagnostic subgroups.
Pro-apoptotic and anti-apoptotic Bcl-2–related protein expression in MDS/MDS-AML and normal controls.
BM samples were incubated with FITC-conjugated anti-CD34 Mab and fixed with formaldehyde. To prevent nonspecific binding of Mabs to Fc receptors, cells were pre-incubated with rabbit-anti-mouse (RAM) immunoglobulins, permeabilized, and then incubated with mouse-anti-human Mabs to Bax, Bad, Bcl-2, and Bcl-X on ice. Washed cells were subsequently incubated with PE-conjugated RAM on ice prior to flow cytometric analysis. The mean fluorescence intensity (MFI) value for each Bcl-2–related protein was calculated by dividing the MFI value of the positively labeled cells by that of cells stained with an isotype control antibody. To determine individual Bcl-2–related protein levels, we calculated an Oncoprotein Index (OPI) whereby OPI equals the product of the percentage of positive cells and MFI. Median Bax, Bad, Bcl-2, and Bcl-X OPI are plotted in specific diagnostic subgroups.
Cytogenetics
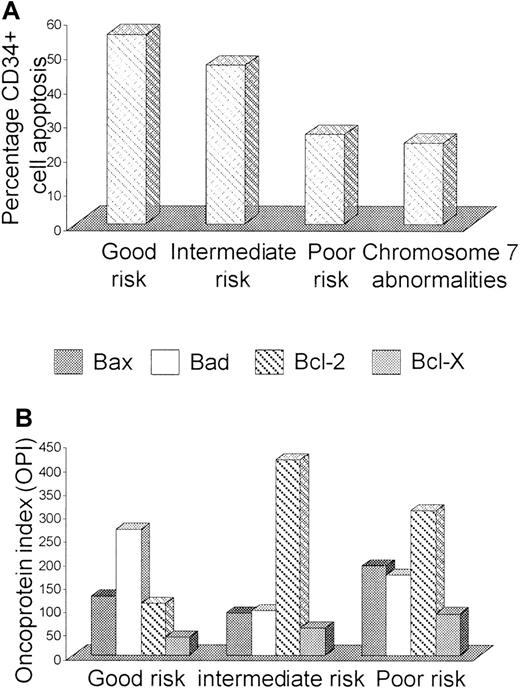
A significant relationship was observed between PCD and cytogenetic risk group; patients with good risk cytogenetics having the highest degrees of apoptosis (good risk, 55.1% [2.1%-93.6%]; intermediate risk, 46.1% [9.9%-83.8%]; poor risk, 25.8% [3%-86.5%], P = .02). The presence of chromosome 7 abnormalities was particularly associated with reduced CD34+ cell apoptosis (chromosome 7 abnormalities, 23.3% [3%-56.9%] verus other karyotype, 51.4% [9.9%-93.6%],P = .0007) (Figure 5A). Proliferation rates and A:P ratios showed no correlation with karyotype. Cytogenetic risk group also significantly influenced Bcl-2–related protein ratios, with the highest ratios observed in patients with a good risk karyotype (good risk, median 2.36 [range 1.17-9.42]; intermediate risk, median 1.14 [range 0.06-5.77]; poor risk, median 1.17 [range 0.4-2.89], P = .03). Low ratios in patients with intermediate or poor risk cytogenetics was primarily due to increased Bcl-2 expression (Figure 5B). There was also a trend for lower pro-apoptotic versus anti-apoptotic Bcl-2–related protein ratios in patients harboring chromosome 7 abnormalities (chromosome 7 abnormalities, median ratio, 1.16 [range 0.4-2.89] versus other karyotype, median ratio, 2.08 [range 0.05-9.42],P = .08) (Table 3).
Influence of cytogenetic risk group on CD34+cell apoptosis and Bcl-2–related protein expression.
The degree of CD34+ cell apoptosis (A) and Bax, Bad, Bcl-2, and Bcl-X expression (B) was compared in MDS/MDS-AML patients with good (normal karyotype or isolated deletions of 5q or 20q), intermediate (≤2 cytogenetic abnormalities, excluding isolated 5q or 20q deletions and chromosome 7 abnormalities) and poor risk (> 2 abnormalities and/or abnormalities of chromosome 7). Apoptosis was additionally evaluated in patients harboring aberrations of chromosome 7.
Influence of cytogenetic risk group on CD34+cell apoptosis and Bcl-2–related protein expression.
The degree of CD34+ cell apoptosis (A) and Bax, Bad, Bcl-2, and Bcl-X expression (B) was compared in MDS/MDS-AML patients with good (normal karyotype or isolated deletions of 5q or 20q), intermediate (≤2 cytogenetic abnormalities, excluding isolated 5q or 20q deletions and chromosome 7 abnormalities) and poor risk (> 2 abnormalities and/or abnormalities of chromosome 7). Apoptosis was additionally evaluated in patients harboring aberrations of chromosome 7.
Variations in median CD34+ cell apoptosis, proliferation, and Bcl-2–related protein ratios according to karyotype
| Characteristic . | Good risk (range) . | Intermediate risk (range) . | Poor risk (range) . | P . | Chromosome 7 abnormalities (range) . | P . |
|---|---|---|---|---|---|---|
| Apoptosis (%) | 55.1 (2.1-93.6) | 46.1 (9.9-83.8) | 25.9 (3-86.5) | .02 | 23.4 (3-56.9) | .0007 |
| Proliferation (%) | 27.6 (4-75.8) | 24.9 (8-38.5) | 28.6 (1.9-69.5) | N/S | 23.6 (1.9-52.6) | N/S |
| A:P ratio | 1.79 (0.16-5.7) | 1.91 (1.37-3.4) | 1.54 (0.8-12.2) | N/S | 1.79 (0.85-5.67) | N/S |
| Bax/Bad:Bcl-2/Bcl-X ratio | 2.36 (1.17-9.42) | 1.14 (0.06-5.77) | 1.17 (0.4-2.89) | .03 | 1.17 (0.4-2.89) | .08 |
| Characteristic . | Good risk (range) . | Intermediate risk (range) . | Poor risk (range) . | P . | Chromosome 7 abnormalities (range) . | P . |
|---|---|---|---|---|---|---|
| Apoptosis (%) | 55.1 (2.1-93.6) | 46.1 (9.9-83.8) | 25.9 (3-86.5) | .02 | 23.4 (3-56.9) | .0007 |
| Proliferation (%) | 27.6 (4-75.8) | 24.9 (8-38.5) | 28.6 (1.9-69.5) | N/S | 23.6 (1.9-52.6) | N/S |
| A:P ratio | 1.79 (0.16-5.7) | 1.91 (1.37-3.4) | 1.54 (0.8-12.2) | N/S | 1.79 (0.85-5.67) | N/S |
| Bax/Bad:Bcl-2/Bcl-X ratio | 2.36 (1.17-9.42) | 1.14 (0.06-5.77) | 1.17 (0.4-2.89) | .03 | 1.17 (0.4-2.89) | .08 |
A:P indicates apopotosis:proliferation ratio; N/S, not significant.
Discussion
Several authors3,9,13,16-21 have demonstrated that excessive intramedullary hematopoietic cell apoptosis may contribute toward the ineffective hematopoiesis characteristic of MDS. Studies demonstrating that early MDS is, in addition, a highly proliferative disorder, with the number of S-phase cells being significantly correlated with the degree of PCD,10,22 have prompted speculation that increased cell division in early disease is a compensatory mechanism to neutralize excessive apoptosis. Alternatively, increased PCD may represent a homeostatic process to counteract high proliferative rates. Data are conflicting, however, regarding the stage of MDS at which apoptosis and/or proliferation is most prominent or indeed, the nature of the primary cell involved.3,9 Moreover, alterations in the balance between cell growth and cell death with disease evolution and the nature of underlying lesions responsible for these changes have not been clearly elucidated. Inconsistencies between studies may be partly explained by the variable cellular composition of MDS BM as well as differences in methodology. Certainly, difficulties in discerning the precise cell type undergoing PCD and/or proliferation, using techniques that identify DNA strand breaks (in situ end labeling [ISEL], terminal dUTP nick-end labeling [TUNEL]) (apoptosis) and DNA incorporation of thymidine analogs (proliferation) in BM biopsy specimens, generally limits analysis to hematopoietic cells as a whole rather than specific cell subtypes.3,10,21 Moreover, TUNEL does not reliably discriminate between apoptosis and cell necrosis.23 In the present study, the degree of PCD and proliferation was evaluated by dual-labeling flow cytometric techniques. Although the precise phenotype of cells undergoing apoptosis in MDS has not yet been clearly defined,3,9 we limited assessment of PCD to CD34+ progenitors to minimize the influence of cellular heterogeneity of different MDS FAB subtypes on outcome. CD34+ cells were also selected for analysis as clonality studies indicate that MDS arises through neoplastic transformation of primitive hematopoietic stem cells.24
Apoptosis determination, using flow cytometric detection of annexin V binding, offers several advantages over older methods. First, the externalization of phosphatidylserine to which annexin V binds can be measured prior to in vitro detection of DNA damage,25enabling detection of cells in the earliest stages of apoptosis.11,26,27 Moreover, flow cytometric techniques are both rapid, minimizing in vitro induction of cell death, and permit examination of large numbers of cells. In accordance with previous studies, we found that CD34+ cell apoptosis was significantly increased in RA/RARS and RAEB compared to normal controls, whereas leukemic evolution was associated with a marked reduction in PCD.9,17,18 20 We also demonstrated a significant relationship between apoptosis, IPSS score, and cytogenetic risk group; patients with a low score and/or good risk cytogenetics have the highest degrees of annexin V positivity. Conversely, the presence of chromosome 7 abnormalities was associated with significantly reduced CD34+ cell apoptosis. The positive correlation between PCD, hemoglobin concentration, and platelet counts may be secondary to low levels of apoptosis observed in advanced MDS, a disease stage characteristically associated with worsening peripheral cytopenias. Similarly, the inverse correlation with white cell count is probably due to increased numbers of circulating peripheral blasts in RAEB-t/MDS-AML.
The degree of apoptosis in the present study is considerably higher than previously reported in a number of published series.9,17,21,28 This probably reflects differences in experimental design. Earlier studies predominantly focused on detection of DNA degradation or morphologic changes, which are late events in the process of apoptosis. Evaluation of BM mononuclear cells rather than stem cells alone may additionally lead to underestimation of the degree of PCD. Authors, who have quantified apoptosis through annexin V staining, have demonstrated similar levels of PCD to the present study. Indeed, Kliche and Andreef28 detected annexin V positivity in 53.4% (mean) BM cells in 28 MDS patients compared to 28% in 12 normal controls.29
In the majority of RA/RARS patients, levels of CD34+cell apoptosis greatly exceeded proliferation, with 13 of 22 cases having an A:P ratio greater than 2. This finding indicates that, in early disease, excessive apoptosis probably represents a pathophysiological mechanism rather than a compensatory process to counteract increased cell growth. Cell death might be triggered by the BM microenvironment, either through a relative deficiency of growth factors or mediated through stromal immunoregulatory cells in an attempt to abrogate potentially harmful clones. Certainly, studies demonstrating elevated tumor necrosis factor α protein or messenger RNA levels30-32 and the enhancement of in vitro colony formation through incubation of MDS marrow cells with cyclosporin or removal of T cells33 34 indicate that this may be the case. Alternatively, intrinsic genomic lesions that give rise to defective cell cycle machinery and/or DNA repair may induce PCD. The imbalance in favor of cell death in early MDS does not correspond with the belief that neoplastic disease arises through a clone(s) harboring a survival advantage. Moreover, an increased A:P ratio should logically give rise to stem cell exhaustion and a hypocellular marrow rather than hypercellularity frequently observed in MDS. Hypothetically, this paradox may be explained by the occurrence of phenotypically silent genomic events in early disease, which confer a survival or growth advantage on stem cells and their progeny, permitting clonal expansion. “Overt” MDS, characterized by progressive cytopenias, probably represents more advanced steps in the progression toward leukemia and may only arise at a stage at which the number and/or nature of genetic lesions are such that they are detected by DNA-damage surveillance mechanisms with resultant hematopoietic cell apoptosis.
In our study, progression to RAEB was associated with an increase in the number of Ki-67-positive cells such that the ratio between apoptosis and proliferation equalized. However, evolution to RAEB-t/MDS-AML was accompanied by a significant reduction in annexin V positivity with an accompanying fall in proliferation in most cases. Previous studies22,35 36 have also demonstrated a fall in the number of s-phase cells with disease progression, with patients with the lowest labeling index being at highest risk of leukemic transformation, indicating that evolution toward acute leukemia, in most cases, arises through genomic lesions that inhibit apoptotic control mechanisms rather than promoting cell growth.
Given the primary role of the Bcl-2–related proteins in the control of apoptotic pathways, we sought to determine whether altered expression of Bcl-2 family members was responsible for deregulated CD34+ cell apoptosis in MDS. Increased BM Bcl-2 expression with MDS progression has been previously documented.9 21However, because many of these studies have measured protein expression in all hematopoietic cells, it could be argued that observed alterations in Bcl-2 levels merely reflect the heterogeneity of cell type in different MDS FAB subgroups. With the use of dual-labeling flow cytometric techniques to restrict analysis to CD34+progenitors, we found higher Bax/Bad verus Bcl-2/Bcl-X ratios in early MDS compared to normal controls, which appeared to be due to a relative increase in Bax levels. Disease progression was accompanied by a significant fall in pro-apoptotic verus anti-apoptotic Bcl-2–related protein ratio due, in part, to a relative increase in Bcl-2 and a reduction in Bad expression. As with apoptosis, we found a significant negative correlation between Bax/Bad versus Bcl-2/Bcl-X ratios and both IPSS score and cytogenetic risk group. There was also a trend toward an association between Bcl-2–related protein expression and apoptosis, although this finding just failed to reach statistical significance. Patterns of Bcl-2–related protein expression, however, varied widely, even within disease subgroups, indicating that mechanisms additional to Bcl-2–related protein expression are involved in the regulation of apoptosis in MDS.
Our study indicates that MDS progression arises through multiple genomic hits that alter both the balance between apoptosis and proliferation and the ratio of pro-apoptotic versus anti-apoptotic Bcl-2–related proteins. As well as providing a model for the multistep pathogenesis of cancer in general, a clearer understanding of the molecular events leading to deregulation of the balance between cell growth and cell death in MDS should permit the identification of therapeutic targets, diagnostic markers, and useful indicators of prognosis.
Acknowledgments
We are indebted to Keith L. Fishlock for his assistance in flow cytometric analysis; Barbara Czepulkowski for cytogenetic analysis; Dr F. Al Refaie, Princess Alexandra Hospital, Harlow; Dr R. Carr, St Thomas' Hospital, London; Dr J. Duncan, Royal Sussex County Hospital, Brighton; Drs C. G. Taylor and D. S. G. Gillett, Pembury Hospital; Dr J. P. L. A. Hayes, All Saint's Hospital, Chatham; Drs R. M. Ireland and P. Black, Greenwich Hospital; Drs R. Jan-Mohamed and R. Kaczmarski, The Hillingdon Hospital; Dr Mir, Lewisham Hospital; Drs S. M. B. Rassam and S. Ward, Queen Mary's Hospital, Sidcup; Dr V. Ratnayake, The William Harvey Hospital, Ashord; Dr I. R. Samaratunga, Farnborough Hospital; Dr B. Wells, The James Paget Hospital, Great Yarmouth; and Dr Y. F. Williams, The Kent and Canterbury Hospital for donation of patient samples; and to Dr R. Hooper for his help in statistical analysis.
Supported by a grant from the Leukaemia Research Fund, United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ghulam J. Mufti, The Department of Haematological Medicine, Guy's, King's, Thomas' School of Medicine, Denmark Hill Campus, Bessemer Road, London, SE5 8RX, UK; e-mail:ghulam.mufti@kcl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal