Abstract
Cell-mediated immunity and T-lymphocyte maturation are impaired in HIV-infected children. These abnormalities would be detected in HIV-uninfected offspring of HIV women (seroreverters [SR]) if HIV or its soluble proteins could cross the placental barrier. Immunophenotypic analyses were performed in 20 healthy HIV-uninfected newborns of HIV-infected mothers (SR), and in 14 healthy newborns of HIV-negative women (UC). The same analyses were performed in 3 groups of older children: SR (n = 41); UC (n = 15); and HIV-infected children (n = 25). Antigen-specific cells were evaluated with ELISpot and fluorimetric analyses; IL-7 serum concentration was measured by enzyme-linked immunosorbent assay (ELISA). Results showed that in SR newborns: (1) the CD4/CD8 ratio was reduced, (2) CD4+ and CD8+ naive T-cell percentages were decreased, (3) percentage of activated CD8+ T cells was increased, and (4) percentages of CD3+/4−/8− (DN) and DN/25−/44+ were augmented. These abnormalities were partially retained in older SR children. CD4+ and CD8+ HIV-specific cells were detected in a portion of newborn SRs but not in older SRs. Serum IL-7 was augmented both in newborn and older SRs. Cell-mediated immunity and T-cell maturation are altered even in HIV-uninfected newborns of HIV-infected mothers; these abnormalities persist over time. The biologic significance of these observations and potential subsequent clinical events should be investigated in larger cohorts of seroreverters.
Introduction
Mother-to-child transmission of HIV is the primary route of infection in infants.1-3 Vertical HIV infection occurs in 25% to 30% of pregnancies. Prepartum and intrapartum prophylaxis with antiretroviral agents, along with elective cesarean section and avoidance of breastfeeding, have nevertheless reduced appreciably the incidence of this modality of infection.4-7
Vertical HIV transmission can be the result of in utero or intrapartum infection.1-3 That at least part of these infections occurs in utero confirms that the placental barrier is not impermeable to maternal pathogens. Transplacental passage of pathogens is a well-known phenomenon, hence the observation that maternal infection with Toxoplasma gondii or cytomegalovirus can result in fetal pathologies. Vertical transmission of pathogens is facilitated by: (1) lack of HLA Class I expression by the syncytiotrophoblast, (2) multiple vesicular and immunoglobulin transport pathways; and (3) the presence of physical defects in the trophoblast layers throughout gestation.8 T-placental barrier can thus be permeable to microorganisms and proteins.
In the case of HIV infection, in utero exposure to the virus can be associated with 2 possible outcomes: overt HIV infection and lack thereof.9-16 Pediatric HIV infection results in a complex pattern of quantitative and qualitative defects involving the immune system that has been described in depth.17 In utero exposure not resulting in infection, and the possibility that HIV and/or viral particles penetrate across the placental barrier even in the absence of fetal infection, is supported by experimental results. Thus, exposure to HIV in HIV-uninfected newborns of HIV-seropositive mothers is shown by the observation that HIV-specific T helper9,10 and cytotoxic T cells11-15 can be detected in these newborns. Additionally, a recent report showed that susceptibility to apoptosis is augmented in cord blood T lymphocytes from infants of HIV-infected mothers.16 Because in HIV-infected women the fetus would develop in a microenvironment enriched with viral particles and/or whole virus, immune abnormalities could be observed even in HIV-uninfected newborns of HIV-seropositive mothers. In particular, because HIV recognizes CD4 as its main receptor,18 19 if immune abnormalities occur in these newborns, an impairment of T- but not of B-lymphocyte function and/or development, would be expected even in HIV-uninfected newborns of HIV-infected mothers.
To verify this hypothesis, we performed in-depth immunologic analyses in healthy HIV-uninfected newborns of HIV-infected mothers and in healthy newborns of HIV-negative women. The same analyses were also performed in older HIV-exposed and -unexposed healthy children as well as in a group of HIV-infected children.
Results confirm the hypothesis that abnormalities in cell-mediated immunity and T-cell development are present in HIV-uninfected newborns of HIV-infected mothers. Surprisingly, some of these immune abnormalities persist during childhood.
Patients, materials, and methods
Patients and controls
Twenty HIV-uninfected newborns of HIV-infected mothers (mean age 30 days) and 14 HIV-uninfected newborns of healthy mothers (mean age 30.5 days) were consecutively enrolled in the study. Infants born of HIV-infected mothers were shown to be HIV-uninfected and defined as seroreverters if they: (1) had negative HIV-polymerase chain reaction (PCR) tests at birth and at 1 and 3 months of age or (2) became HIV-seronegative after 6 months of age. The demographic characterization of these newborns is presented in Table1. Forty-two older HIV-uninfected children (mean age 7.2 years) of HIV-infected mothers, 15 HIV-uninfected children (mean age 6.2 years) of healthy mothers, and 25 HIV-infected children (mean age 5.8 years) were also enrolled in the protocol. The demographics of these older children are presented in Table 2. Written informed consent was obtained from the relatives of or the person legally responsible for the children included in the study, and the study was approved by the institutional review board of Ospedale L. Sacco.
Demographic characterization of 34 healthy, HIV-uninfected newborns of either HIV-infected or HIV-uninfected mothers (demographic data from the mothers are shown as well)
| . | Newborn SR (n = 20) . | Newborn UC (n = 14) . |
|---|---|---|
| Age (d) | 30.8 ± 22.5 | 30.5 ± 24.1 |
| Gestational age (wk) | 37.2 ± 1.1 | 38.1 ± 1.9 |
| Gestational age < 37 wk | 6/20 (30%) | 3/14 (21%) |
| Birth weight (g) | 2680 ± 536 | 3071 ± 435 |
| Birth weight < 2500 g | 6/20 (30%) | 3/14 (21%) |
| Cesarean section | 16/20 | 1/14 |
| ZDV prophylaxis | 20/20 | 0/14 |
| HCV infection | 2/20 | 0/14 |
| CMV infection | 1/20 | 0/14 |
| HIV-infected mothers | HIV-uninfected mothers | |
| Maternal age (y) | 29.0 ± 9.2 | 30.4 ± 8.3 |
| Primiparity | 11/20 (55%) | 7/14 (50%) |
| Previous drug use* | 8/20 (40%) | 0/14 |
| STD† | 2/20 (10%) | 1/14 (7%) |
| . | Newborn SR (n = 20) . | Newborn UC (n = 14) . |
|---|---|---|
| Age (d) | 30.8 ± 22.5 | 30.5 ± 24.1 |
| Gestational age (wk) | 37.2 ± 1.1 | 38.1 ± 1.9 |
| Gestational age < 37 wk | 6/20 (30%) | 3/14 (21%) |
| Birth weight (g) | 2680 ± 536 | 3071 ± 435 |
| Birth weight < 2500 g | 6/20 (30%) | 3/14 (21%) |
| Cesarean section | 16/20 | 1/14 |
| ZDV prophylaxis | 20/20 | 0/14 |
| HCV infection | 2/20 | 0/14 |
| CMV infection | 1/20 | 0/14 |
| HIV-infected mothers | HIV-uninfected mothers | |
| Maternal age (y) | 29.0 ± 9.2 | 30.4 ± 8.3 |
| Primiparity | 11/20 (55%) | 7/14 (50%) |
| Previous drug use* | 8/20 (40%) | 0/14 |
| STD† | 2/20 (10%) | 1/14 (7%) |
SR = healthy HIV-uninfected children of HIV-infected mothers (seroreverters); UC = healthy children of HIV-negative women (uninfected controls); ZDV = zidovudine; HCV = hepatitis C virus; CMV = cytomegalovirus; STD = sexually transmitted disease. Mean ± standard deviation.
In all cases, drug use stopped at least 2 years before pregnancy.
Two chlamydial infections; 2 Candida albicans infections.
Demographic characterization of 57 healthy, HIV-uninfected children of either HIV-infected or HIV-uninfected mothers (data from 25 vertically HIV-infected children included in the study are shown as well)
| . | SR children (n = 42) . | UC children (n = 15) . | HIV+ children (n = 25) . |
|---|---|---|---|
| Age (y) | 7.2 ± 2.8 | 6.2 ± 4.0 | 5.8 ± 3.7 |
| Age < 5 y | 6/42 (14%) | 2/15 (13%) | 5/25 (20%) |
| CD4 (cells/μL) | 1278 ± 408 | 1551 ± 534 | 1158 ± 797 |
| Cesarean section | 4/42 | 2/15 | 4/25 |
| ZDV prophylaxis | 0/42 | 0/15 | 0/25 |
| HCV infection | 3/42 | 0/15 | 1/25 |
| CMV infection | 1/42 | 0/15 | 2/25 |
| . | SR children (n = 42) . | UC children (n = 15) . | HIV+ children (n = 25) . |
|---|---|---|---|
| Age (y) | 7.2 ± 2.8 | 6.2 ± 4.0 | 5.8 ± 3.7 |
| Age < 5 y | 6/42 (14%) | 2/15 (13%) | 5/25 (20%) |
| CD4 (cells/μL) | 1278 ± 408 | 1551 ± 534 | 1158 ± 797 |
| Cesarean section | 4/42 | 2/15 | 4/25 |
| ZDV prophylaxis | 0/42 | 0/15 | 0/25 |
| HCV infection | 3/42 | 0/15 | 1/25 |
| CMV infection | 1/42 | 0/15 | 2/25 |
SR = healthy HIV-uninfected children of HIV-infected mothers (seroreverters); UC = healthy children of HIV-negative women (uninfected controls); HIV+ = HIV-infected children; ZDV = zidovudine; HCV = hepatitis C virus; CMV = cytomegalovirus.
Mean values ± standard deviations are shown.
Blood drawing and processing
Whole blood was collected by venopuncture in EDTA-containing vacutainer tubes (Becton Dickinson, Rutherford, NJ). Peripheral blood mononuclear cells (PBMCs) were separated on lymphocyte separation medium (Organon Teknika, Durham, NC), washed in phosphate-buffered saline (PBS), and the number of viable leukocytes was determined by trypan blue exclusion.
Immunophenotypic analyses
Lymphocyte subsets were evaluated using an Epics XL flow-cytometer (Coulter Electronics, Miami Lakes, FL) using 100 μL of EDTA peripheral blood incubated 30 minutes at 4°C with fluorochrome-labeled monoclonal antibodies. Erytrocyte lysis was obtained, after incubation with the Immuno-Prep Epics Kit (Coulter Electronics) and Q-prep Work Station (Coulter Electronics). Lymphocytes were analyzed using forward- and side- scatter properties. For each sample, multiparametric data were acquired for 5000 events.
Determination of intracellular cytokine in antigen- and mitogen-stimulated T lymphocytes
PBMCs were resuspended in 200 μL of RPMI1640 media (Sigma, St Louis, MO) supplemented with 2% AB+ serum (Sigma). PBMCs were incubated for 18 hours with: (1) a pool of previously described 5 synthetic peptides from the glycoprotein (gp)160 envelope of HIV-120 (2.5 μmol/L final concentration) (env); (2) influenza virus vaccine (A/Taiwan, A/Shanghai, and B/Victoria), 24 μg/L (final dilution 1:1000)(negative control); and (3) PMA (50 ng/mL)+ ionomycin (1 μmol/L)(positive control). Antibody to CD28 (R&D Systems, Minneapolis, MN) was added during incubation at a dose of 1 μg per well to facilitate costimulation.
Interferon gamma ELISpot assays
The 96-well nitrocellulose plates were precoated with a first layer IFNγ monoclonal antibodies (Mabtech, Nacka, Sweden). 2 × 105 cells per well PBMCs were then added in duplicate wells either with env (20 μmol/L final concentration) in the presence or the absence of neutralizing anti-CD4 monoclonal antibody (see below), with no peptide (negative control), or in 1:100 PHA (M form; Sigma, St Louis, MO) (positive control). The 5 peptides used in the stimulation are promiscuous as they are recognized by multiple HLA Class I molecules (including HLA A1, A2, A3, A9, A25, A26, A29).21 Because these epitopes can also be recognized by HLA Class II molecules,26 IFNγ production by CD4+ was blocked by preincubating PBMCs with 100 ng/mL of neutralizing recombinant human CD4 monoclonal antibody (R&D). Plates were incubated overnight at 37°C in 7% CO2, then the cells were discarded and the plates incubated at room temperature for 3 hours. A second biotinylated anti-IFNγ monoclonal antibody (7-B6-1 biotin; Mabtech), followed by streptavidin-conjugated alkaline phosphatase (Mabtech) for 2 hours were subsequently used. IFNγ-producing cells were detected using an alkaline phosphatase-conjugate substrate kit (Bio-Rad laboratories, Hercules, CA). The spots were counted by eye, and the numbers were confirmed using a dissecting microscope (× 40). HIV-specific responses were reported as number of spot-forming units (SFU) per 106mononuclear cells after subtraction of background IFNγ secretion. Only responses observed in the wells in which IFNγ production by CD4+ was blocked by preincubation of PBMCs with the anti-CD4 antibody are reported. A positive response was defined as follows: (1) more than 20 HIV-specific SFU/106 cells and (2) HIV-stimulated SFU exceed background by a factor of at least 2.
Measurement of serum IL-7
IL-7 was measured in serum samples using an enzyme-linking immunosorbent assay (ELISA) kit (Quantikine HS human IL-7; R&D) according to the instructions of the manufacturer.
Statistical analysis
The differences between the mean values of different groups were calculated for each variable (possibly transformed to approximate normality or square root transformation) by using multiple linear regression with dummy variables adjusted for CD4 and CD8 numbers.
Results
Immune abnormalities in healthy newborns of HIV-infected and HIV-uninfected mothers
Twenty healthy newborns of HIV-infected mothers (SRs) and 14 healthy newborns of HIV-uninfected women (unexposed controls, UCs) were included in the study. The mean age was comparable between the 2 groups (SR = 30 days; UC = 30.5 days) (Table 1). A panel of immunophenotypic markers was evaluated both in SRs and UCs. No differences were observed between the 2 groups in the absolute number or the percentages of natural killer (CD16+) and B lymphocytes (CD19+) (data not shown). A complex pattern of alteration was nevertheless detected when T-lymphocyte subsets were analyzed. Thus, in SRs compared with UCs, the percentage and absolute number of: (1) CD4+ T cells was reduced (P = .069), CD8+ T cells augmented (P < .0001), and consequently the CD4/CD8 ratio was diminished (P = .00015); (2) CD4+/45RA/62+ (naive) lymphocytes were reduced (P < .0001) and CD4+/45RO (memory) cells augmented (P < .001); (3) CD8+/RA+LFAlow (naive) lymphocytes were reduced (P = .0033) and CD8+/RA+LFAhigh (“revertant” CD8+ T cells suggested to be highly enriched in memory T lymphocytes) augmented (P = .0022); (4) CD8+/38dim lymphocytes were reduced (P < .001) and CD8+/CD38bright(activated) cells augmented (P < .0001); and finally, (5) CD3+/4−/8− (double negative [DN]) and DN/25−/44+ T lymphocytes were augmented (P < .0001 in both cases)(Table3).
Immunophenotipic analyses (percentages of cells) in 2 groups of newborns
| Marker . | SR (n = 20) . | UC (n = 14) . | Statistical significance (P value) . |
|---|---|---|---|
| CD4 | 52.1 ± 4.9 | 59 ± 4.6 | .069 |
| CD8 | 22.1 ± 5.0 | 16.1 ± 3.4 | < .0001 |
| CD4/CD8 ratio | 2.7 ± 1.1 | 3.8 ± 0.9 | .00015 |
| CD4/RA/62 | 74.7 ± 8.9 | 89.7 ± 3.7 | < .0001 |
| CD4/RO | 24.9 ± 9.6 | 10.3 ± 3.3 | < .0001 |
| CD8/RA/LFAlow | 58.9 ± 15.3 | 77.7 ± 3.8 | .0033 |
| CD8/RA/LFAhigh | 22.1 ± 4.3 | 9.6 ± 4.1 | .0027 |
| CD8/38dim | 49.3 ± 13.9 | 79.7 ± 3.1 | < .0001 |
| CD8/38bright | 24.2 ± 11.1 | 1.8 ± 1.3 | <.0001 |
| CD3 DN | 9.3 ± 2.6 | 3.3 ± 1.1 | < .0001 |
| CD3 DN/44 | 8.8 ± 2.7 | 3.1 ± 0.9 | <.0001 |
| Marker . | SR (n = 20) . | UC (n = 14) . | Statistical significance (P value) . |
|---|---|---|---|
| CD4 | 52.1 ± 4.9 | 59 ± 4.6 | .069 |
| CD8 | 22.1 ± 5.0 | 16.1 ± 3.4 | < .0001 |
| CD4/CD8 ratio | 2.7 ± 1.1 | 3.8 ± 0.9 | .00015 |
| CD4/RA/62 | 74.7 ± 8.9 | 89.7 ± 3.7 | < .0001 |
| CD4/RO | 24.9 ± 9.6 | 10.3 ± 3.3 | < .0001 |
| CD8/RA/LFAlow | 58.9 ± 15.3 | 77.7 ± 3.8 | .0033 |
| CD8/RA/LFAhigh | 22.1 ± 4.3 | 9.6 ± 4.1 | .0027 |
| CD8/38dim | 49.3 ± 13.9 | 79.7 ± 3.1 | < .0001 |
| CD8/38bright | 24.2 ± 11.1 | 1.8 ± 1.3 | <.0001 |
| CD3 DN | 9.3 ± 2.6 | 3.3 ± 1.1 | < .0001 |
| CD3 DN/44 | 8.8 ± 2.7 | 3.1 ± 0.9 | <.0001 |
SR = healthy HIV-uninfected children of HIV-infected mothers (seroreverters); UC = healthy children of HIV-negative women (uninfected controls); DN = double negatives; LFA = leukocyte function-associated antigen.
Mean values ± standard deviations are shown.
CD4+/− antigen- and mitogen-specific lymphocyte in newborns
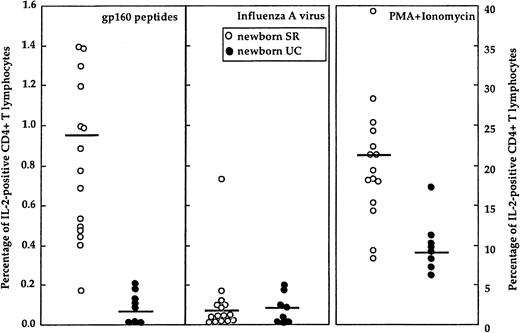
Memory cells (CD45 R0) are augmented in SR newborns compared with UC newborns. To verify whether HIV-specific lymphocyte responses could be detected in SRs, we quantified IL-2 producing CD4+ cells on stimulation with env-peptides. Influenza A virus (FLU)–stimulated and PMA+ionomycin-stimulated CD4+ T cells were used as negative and positive controls, respectively. Results are summarized as follows: (1) HIV-specific CD4+ T cells were undetectable in UCs but present and strong in SRs (P = .001), (2) FLU-specific CD4+ T cells were undetectable in both UCs and SRs, and (3) the frequency of mitogen-responsive CD4+ T lymphocytes was augmented in SRs compared with UCs (P = .001). Results are shown in Figure1.
Results of CD4+ T cells in SRs and UCs.
Percentage of antigen- and mitogen-stimulated IL-2 positive CD4+ T lymphocytes in healthy newborns of HIV-infected (newborn SR, ○) or HIV-uninfected (newborn UC, ●) mothers. The horizontal bar indicates mean values.
Results of CD4+ T cells in SRs and UCs.
Percentage of antigen- and mitogen-stimulated IL-2 positive CD4+ T lymphocytes in healthy newborns of HIV-infected (newborn SR, ○) or HIV-uninfected (newborn UC, ●) mothers. The horizontal bar indicates mean values.
Quantitation of IFNγ-secreting CD8+/− T cells on stimulation with HIV envelope peptides in newborns
The number of CD8+ HIV-specific IFNγ-secreting cells was compared in SRs (n = 8) and UCs (n = 7). The mean number of IFNγ-secreting cells was similar in SRs (11 ± 4 per 106 cells) and in UCs (7 ± 5 per 106 cells). Nevertheless, an augmented number of HIV-specific IFNγ-secreting cells (greater than 2-fold the background) was detected in 3 of 8 SRs (39, 64, 72 per 106 cells)(the same newborns in whom the highest frequencies of HIV-specific, IL-2-secreting CD4+ T cells was detected) but in none of the UCs (data not shown).
Immune abnormalities in older seroreverters, unexposed controls, and HIV-infected children
The results obtained in newborns were verified in older children, who were divided in 3 categories: SR (n = 41), UC (n = 15), and HIV-infected children (n = 25), a group that, because of the success of pharmacologic prophylaxis in reducing mother-to-infant transmission of HIV was thankfully not available in the newborn population. None of the SRs underwent zidovudine prophylaxis at the time of delivery (Table2). CD4 counts of HIV-infected children were reduced compared with both groups of uninfected individuals; this variable was thus included in the statistical analyses. Results confirmed that, even in older SRs compared with UCs, the percentage of: (1) CD4+/45RA/62+ (naive) lymphocytes was reduced (P = .03) and that of CD4+/45RO (memory) cells augmented (P = .001), (2) CD8+/CD38dim cells was reduced (P = .004) and that of CD8/38 bright cells augmented (P = .01), and (3) DN and DN/CD25−/CD44+ cells was augmented (P = .02 and .001, respectively). In all these cases, the immunophenotype of SR was similar to the one observed in HIV-infected children (Table 4).
Immunophenotipic analyses (percentages of cells) in 3 groups of older children (mean age = 7 years)
| Marker . | SR (n = 41) . | UC (n = 15) . | HIV+ (n = 25) . | Statistical significance (P value) . | ||
|---|---|---|---|---|---|---|
| SR vs UC . | SR vs HIV+ . | UC vs HIV+ . | ||||
| CD4 | 38.4 ± 6.8 | 40.4 ± 4.6 | 28.2 ± 9.9 | ns | .0004 | < .0001 |
| CD8 | 24.2 ± 5.3 | 25.6 ± 3.7 | 42.9 ± 11.1 | ns | < .0001 | .0001 |
| CD4/CD8 ratio | 1.7 ± 0.7 | 1.6 ± 0.3 | 0.8 ± 0.5 | ns | < .0001 | < .0001 |
| CD4/RA/62 | 62.9 ± 9.8 | 71.8 ± 8.6 | 57.9 ± 9.6 | .03 | ns | .001 |
| CD4/RO | 37.1 ± 10.1 | 29.1 ± 8.6 | 42.3 ± 14.2 | .001 | ns | .001 |
| CD8/RA/LFAlow | 40.7 ± 8.5 | 48.9 ± 4.2 | 22.7 ± 4.1 | ns | .0001 | < .0001 |
| CD8/RA/LFAhigh | 25.3 ± 2.2 | 26.6 ± 4.7 | 28.2 ± 3.5 | ns | ns | ns |
| CD8/38dim | 34 ± 13.7 | 48.7 ± 6.2 | 47.5 ± 13.6 | .004 | .003 | ns |
| CD8/38bright | 15.5 ± 5.6 | 5.6 ± 1.4 | 25.4 ± 8.1 | .01 | .009 | .001 |
| CD3 DN | 9.3 ± 2.2 | 6.5 ± 2.3 | 11.9 ± 4.9 | .02 | ns | .002 |
| CD3 DN/44 | 8.4 ± 2.7 | 5.1 ± 3.7 | 11.5 ± 5.1 | .001 | ns | .001 |
| Marker . | SR (n = 41) . | UC (n = 15) . | HIV+ (n = 25) . | Statistical significance (P value) . | ||
|---|---|---|---|---|---|---|
| SR vs UC . | SR vs HIV+ . | UC vs HIV+ . | ||||
| CD4 | 38.4 ± 6.8 | 40.4 ± 4.6 | 28.2 ± 9.9 | ns | .0004 | < .0001 |
| CD8 | 24.2 ± 5.3 | 25.6 ± 3.7 | 42.9 ± 11.1 | ns | < .0001 | .0001 |
| CD4/CD8 ratio | 1.7 ± 0.7 | 1.6 ± 0.3 | 0.8 ± 0.5 | ns | < .0001 | < .0001 |
| CD4/RA/62 | 62.9 ± 9.8 | 71.8 ± 8.6 | 57.9 ± 9.6 | .03 | ns | .001 |
| CD4/RO | 37.1 ± 10.1 | 29.1 ± 8.6 | 42.3 ± 14.2 | .001 | ns | .001 |
| CD8/RA/LFAlow | 40.7 ± 8.5 | 48.9 ± 4.2 | 22.7 ± 4.1 | ns | .0001 | < .0001 |
| CD8/RA/LFAhigh | 25.3 ± 2.2 | 26.6 ± 4.7 | 28.2 ± 3.5 | ns | ns | ns |
| CD8/38dim | 34 ± 13.7 | 48.7 ± 6.2 | 47.5 ± 13.6 | .004 | .003 | ns |
| CD8/38bright | 15.5 ± 5.6 | 5.6 ± 1.4 | 25.4 ± 8.1 | .01 | .009 | .001 |
| CD3 DN | 9.3 ± 2.2 | 6.5 ± 2.3 | 11.9 ± 4.9 | .02 | ns | .002 |
| CD3 DN/44 | 8.4 ± 2.7 | 5.1 ± 3.7 | 11.5 ± 5.1 | .001 | ns | .001 |
SR = healthy HIV-uninfected children of HIV-infected mothers (seroreverters); UC = healthy children of HIV-negative women (uninfected controls); HIV+ = HIV-infected children; ns = statistically not significant; DN = double negatives; LFA = leukocyte function-associated antigen.
Mean values ± standard deviations are shown.
CD4+/− antigen- and mitogen-specific lymphocyte in older children
IL-2–producing CD4+ cells were measured in older SRs (n = 8), UCs (n = 8), and HIV-infected children (n = 6). Results showed that: (1) HIV-specific CD4+ T cells were detected only in HIV-infected children; (2) FLU-specific CD4+ T cells were present in SR, UC, and HIV-infected children; and (3) the frequency of mitogen-responsive CD4+ T lymphocytes was comparable in the 3 groups (Table 5).
Percentage of CD4+ T lymphocytes of children in which intracellular interleukin 2 was detected on antigen- or mitogen-stimulation
| Category . | Stimulation . | ||
|---|---|---|---|
| Gp160 peptides . | Influenza A virus . | PMA + ionomycin . | |
| SR (n = 8) | 0.01 (0-0.02) | 0.95 (0.42-1.11) | 15.4 (10.2-32.4) |
| UC (n = 8) | 0.01 (0-0.03) | 1.12 (0.51-1.17) | 15.7 (11.9-30.6) |
| HIV+ (n = 6) | 0.81 (0.41-1.2) | 0.77 (0.44-0.79) | 17.6 (9.22-29.8) |
| Category . | Stimulation . | ||
|---|---|---|---|
| Gp160 peptides . | Influenza A virus . | PMA + ionomycin . | |
| SR (n = 8) | 0.01 (0-0.02) | 0.95 (0.42-1.11) | 15.4 (10.2-32.4) |
| UC (n = 8) | 0.01 (0-0.03) | 1.12 (0.51-1.17) | 15.7 (11.9-30.6) |
| HIV+ (n = 6) | 0.81 (0.41-1.2) | 0.77 (0.44-0.79) | 17.6 (9.22-29.8) |
SR = healthy children of HIV-infected mothers; UC = healthy children of HIV-uninfected mothers; HIV+ = HIV-infected children.
Mean values and range are shown.
Quantitation of CD8+/−IFNγ-secreting T cells on stimulation with HIV envelope peptides in older children
CD8+ HIV-specific IFNγ-secreting cells were analyzed in SRs (n = 8), UCs (n = 8), and HIV-infected children (n = 6) and were detected in HIV-infected children (3 of 6 children were positive) but not in SRs or UCs (data not shown).
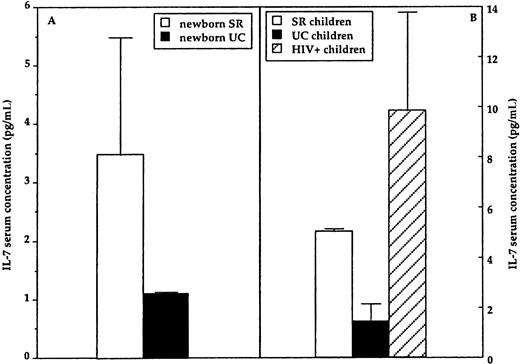
Serum IL-7 in newborns and older children
Serum IL-7 concentration was measured in newborns as well as in older SRs and UCs, and in HIV-infected children. Results showed serum IL-7 concentration to be significantly increased both in newborns and older SRs compared with newborn and older UCs (P = .02 and .01, respectively), with the highest levels present in HIV+ children (Figure 2). A trend, which, nevertheless, did not reach statistical significance, was noticed between higher serum IL-7 concentrations and lower CD4+ and CD4+/45RA/62+ T-lymphocyte counts in SR newborns as well as in SR and HIV+children.
Results shown for serum IL-7 concentration.
(A) IL-7 serum concentration in healthy newborn of HIV-infected (newborn SR, ■) or HIV-uninfected (newborn UC, ▪) mothers. (B) IL-7 serum concentration in older healthy children of HIV-uninfected (UC children, ▪) or HIV-infected (SR children, ■) mothers. IL-7 serum concentration in vertically HIV-infected children (▨) is shown as well. Mean and SD are shown.
Results shown for serum IL-7 concentration.
(A) IL-7 serum concentration in healthy newborn of HIV-infected (newborn SR, ■) or HIV-uninfected (newborn UC, ▪) mothers. (B) IL-7 serum concentration in older healthy children of HIV-uninfected (UC children, ▪) or HIV-infected (SR children, ■) mothers. IL-7 serum concentration in vertically HIV-infected children (▨) is shown as well. Mean and SD are shown.
Discussion
Vertical HIV infection occurs in 30% of cases in the absence of effective interventions.1-3 It is known that exposure to HIV or its soluble products can be detected in healthy, uninfected newborns of HIV-infected mothers.9-16 Thus, different studies have shown the presence of HIV-specific T helper9,10 and cytotoxic11-15 T cells in these uninfected newborns and have suggested that the activation of HIV-specific cell-mediated immunity could be associated with protection from infection.10 The results of this study show that intrauterine exposure to HIV or its soluble factors may occur during the pregnancies of HIV-infected mothers and appears to affect fetal immune maturation.
Because the immune system develops during intrauterine life, and intrathymic maturation and selection of T lymphocytes are known to occur in this period,22-24 we investigated whether these processes could be influenced by the presence of HIV and/or its soluble proteins. This working hypothesis was further reinforced by the observation that thymic abnormalities are described in fetuses aborted from HIV-seropositive women, even in the absence of thymic HIV infection.25 In particular, because HIV infection disrupts cell-mediated immunity,26-28 and the envelope glycoproteins of HIV bind to CD4,18 19 we would have expected immune abnormalities to be prevalent in the T-lymphocyte compartment. Results showed that, whereas no alterations are detected in either B lymphocytes or natural killer cells, a complex pattern of defects in CD4+ and CD8+ T-lymphocyte subpopulations and a peripheral increase in immature T lymphocytes are detected in HIV-uninfected newborns of HIV-infected mothers; these abnormalities tend to persist over time and are still present in older healthy children of HIV-infected mothers. Thus, we report that the physiologic development of the immune system is impaired in HIV-uninfected newborns of HIV-infected mothers.
All the pregnant mothers and newborns included in the study received zidovudine (according to PACTG 076), a protocol that, together with cesarean section,29-31 reduces the incidence of vertical infection.4-7 The immune abnormalities detected in uninfected newborns of HIV-seropositive mothers are not likely to be secondary to drug-related effects because the same abnormalities were observed in older children of HIV-infected mothers who did not undergo prophylaxis with zidovudine or any other antiviral drug.
Memory T cells were augmented and naive T lymphocytes were diminished in HIV-uninfected newborns of HIV-infected mothers suggesting that massive antigenic exposure is occurring in these individuals. Analysis of antigen-stimulated IL-2 production showed the presence of a significant number of HIV-specific CD4+ T helper cells in HIV-uninfected newborns. The observation that the frequency of influenza-specific T helper cells was negligible in the same individuals strongly suggests that the alterations in the memory or naive cells detected in these newborns are associated with exposure to HIV and/or its soluble products. Detection of recall antigen-specific CD4+ T cells at birth is indeed a rare event and, in particular, influenza-specific T-helper responses are not present at birth in either HIV-infected or healthy children.32-35 The immune abnormalities observed in HIV-uninfected newborns of HIV-infected mothers could therefore be secondary to exposure to HIV and/or viral soluble products. That immune abnormalities are present in HIV-uninfected newborns of HIV-infected mothers was recently suggested by the observation that IL-12 production is impaired in cord blood lymphocytes of these newborns36; cord blood T lymphocytes of HIV-exposed but uninfected newborns were also shown to be more susceptible to apoptosis.16
Although high frequencies of HIV-specific CD4+ T helper cells were detected in most HIV-uninfected newborns of HIV-infected mothers, HIV-specific, IFNγ-secreting CD8 T lymphocytes were present only in a minority of individuals. This observation suggests that intrauterine exposure in HIV-uninfected newborns of HIV-infected mothers is mostly subsequent to transplacental diffusion of HIV-soluble proteins and not to live and replication-competent HIV. Infection with the virus would, in fact, result in presentation of viral antigens in association with HLA Class I molecules, and elicitation of a CD8-mediated immune response. Transplacentar diffusion of massive quantities of HIV-soluble products, and possibly of proinflammatory cytokines, may also justify the observation that CD4+ T cells of healthy newborns of HIV-infected mothers are hyperresponsive to mitogens.
An elevated number of DN/CD25−/CD44+ T lymphocytes was detected in both healthy newborns and older children of HIV-infected mothers. These lymphocytes are immature cells that eventually mature into T-cell receptor- and CD4- or CD8-expressing T lymphocytes.37-39 The augmentation of these cells suggests that the physiologic thymic maturation pathway is impaired in HIV-uninfected children of HIV-infected mothers. In particular, because (1) gp120 binds to CD4 on the surface of lymphocytes,18,19and (2) intrathymic lymphocyte development is contingent on the possibility for thymocytes to recognize HLA Class II molecules expressed on nurse cells,40,41 immune abnormalities affecting T-lymphocyte development could be expected if gp120 would prevent the physiologic CD4-HLA Class II interactions. CD4+T cells are indeed reduced, CD8+ T cells augmented, and the CD4/CD8 ratio altered in HIV-uninfected newborns of HIV-infected mothers. The hypothesis that thymic development might be impaired in these newborns is further reinforced by results showing that Vβ families are perturbed in CD4 and CD8+ T lymphocytes of HIV-uninfected newborns of HIV-infected mothers.42
In this context, it is important to underline that serum IL-7 concentrations were found to be elevated in newborn and older healthy children of HIV-infected mothers. IL-7 is required for thymopoiesis, enhances the survival of developing thymocytes and mature T cells by up-regulating bcl-2, and functions as a maturation factor for postthymic naive T cells. Additionally, the IL-7 receptor is crucial in thymic development, as its expression is down-regulated in thymocytes that fail the positive selection process and up-regulated in positively selected thymocytes.43,44 IL-7 is augmented during T-cell reconstitution43 and in HIV-infected individuals. In particular, recent results showed the presence of an inverse correlation between serum IL-7 concentrations and CD4 as well as CD4+/45RA+/62L+ T cells in HIV-infected children.45 The augmented levels of serum IL-7 observed in newborn and older healthy children of HIV-infected mothers thus are consistent with the observation that T-cell homeostasis is impaired in these individuals.
As suggested by previously published data,10 the frequency of HIV-specific CD4+ T cells diminishes after birth and is comparable in older children of HIV-infected and uninfected mothers. These results show that antigen-specific immune activation is decreased once exposure to the antigen ceases and, in this case, suggest that exposure to HIV is indeed an intrauterine event and is interrupted after birth. It is nevertheless interesting that, even years after the exposure to HIV is ended, impairments in CD4+ and CD8+ T lymphocytes persist. These results strongly suggest that the maturation/developmental abnormalities associated with intrauterine exposure to HIV are long-lasting and persist after the exposure to the virus has ceased. These observations raise the concern that these children might be more susceptible to diseases such as autoimmune conditions and certain infections. Longitudinal studies on multiple cohorts of HIV-exposed and uninfected children will be needed to clarify this issue.
Acknowledgments
We are grateful to Drs Gene Shearer, Cristal Mackall, and Claire Cougnet, EIB, NCI, NIH, Bethesda, MD, for critically reading this manuscript and offering useful suggestions. We are also grateful to Drs Daria Trabattoni, Mara Biasin, Marta Cogliati, and Maria Luisa Fusi.
Supported by grants from Istituto Superiore di Sanita I and II Progetto AIDS 1998-99.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mario Clerici, Chair of Immunology, DISP LITA Vialba, Via GB Grassi, 74, 20157 Milano, Italy; e-mail:mago@mailserver.unimi.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal