Abstract
The ability of human γδ-T cells to mediate a number of in vitro functions, including innate antitumor and antiviral activity, suggests these cells can be exploited in selected examples of adoptive immunotherapy. To date, however, studies to examine such issues on a clinical scale have not been possible, owing in large measure to the difficulty of obtaining sufficient numbers of viable human γδ-T cells given their relative infrequency in readily available tissues. Standard methods used to expand human T cells often use a combination of mitogens, such as anti–T-cell receptor antibody OKT3 and interleukin (IL)-2. These stimuli, though promoting the expansion of αβ-T cells, usually do not promote the efficient expansion of γδ-T cells. CD2-mediated, IL-12–dependent signals that result in the selective expansion of human γδ-T cells from cultures of mitogen-stimulated human peripheral blood mononuclear cells are identified. It is first established that human γδ-T cells are exquisitely sensitive to apoptosis induced by T-cell mitogens OKT3 and IL-2. Next it is shown that the CD2-mediated IL-12–dependent signals, which lead to the expansion of γδ-T cells, do so by selectively protecting subsets of human γδ-T cells from mitogen-induced apoptosis. Finally, it is demonstrated that apoptosis-resistant γδ-T cells are capable of mediating significant antitumor cytotoxicity against a panel of human-derived tumor cell lines in vitro. Both the biologic and the practical implications of induced resistance to apoptosis in γδ-T cells are considered and discussed because these findings may play a role in the development of new forms of adoptive cellular immunotherapy.
Introduction
Human T lymphocytes recognize and respond to antigens via a clonally expressed T-cell receptor (TCR). Whereas most mature T cells express an αβ-TCR heterodimer, a few express an alternative γδ-TCR heterodimer.1-5 Although the physiologic role of human γδ-T cells remains unclear, evidence continues to accumulate to suggest that γδ-T cells are involved in a number of important physiologic and disease-related processes. For example, both murine and human γδ-T cells have been shown6-10 to exhibit major histocompatibility complex (MHC)-unrestricted cytotoxicity against some tumors, in vitro and in vivo. In addition, γδ-T cells have been shown11-13 to exert antiviral activity against a number of human pathogens, including the human immunodeficiency virus. It has also been proposed14 that γδ-T cells may play a role in wound healing or tissue repair through the elaboration of a number of growth factors, including keratinocyte growth factor. Recently, in both human clinical studies and experimental animal models of allogeneic bone marrow transplantation, it has been recognized that donor-derived γδ-T cells may serve as facilitating cells, promoting the engraftment of donor hematopoietic stem cells across varying degrees of MHC disparity.15 16
However, the exploitation of γδ-T cells for specific therapeutic ends remains largely unrealized, largely because of the extreme difficulty of obtaining sufficient numbers of viable γδ-T cells given their relative infrequency in peripheral blood (PB) or other readily available tissues. Simply isolating γδ-T cells from fresh PB or bone marrow is likely to prove impractical. Expanding γδ-T cells ex vivo using a variety of mitogenic stimuli, including anti-CD3 or anti-TCRγδ antibodies, is an attractive alternative means by which to obtain sufficient numbers of these cells. However, for reasons that are not entirely clear, human γδ-T cells, when compared to αβ-T cells, appear to undergo apoptosis or activation-induced cell death more readily upon TCR/CD3 engagement, especially in the presence of IL-2.17 Human γδ–T-cell clones have also been shown18 19 to readily undergo apoptosis when stimulated simultaneously by anti-TCR monoclonal antibody (mAb) plus exogenous IL-2, leading some to propose that the induction of programmed cell death upon repeated mitogenic stimulation might serve as a regulatory mechanism whereby excessive in vivo γδ–T-cell proliferation is prevented. In any event, the fact that γδ-T cells may simply die upon ex vivo expansion may represent a serious obstacle to developing approaches to incorporate γδ-T cells into any form of adoptive cellular immunotherapy.
Here, we identify a CD2-mediated, IL-12–dependent signal that results in the selective expansion of human γδ-T cells in mitogen-stimulated human peripheral blood mononuclear cell (PBMC) cultures. Using 4-color flow cytometry integrating surface staining with annexin V and propidium iodide (PI) uptake, we first confirm in PBMC cultures the findings of others that human γδ-T cells are indeed exquisitely sensitive to apoptosis induced by T-cell mitogens. Using these same methods, we then establish that the CD2-mediated, IL-12–dependent signals that lead to the observed expansion of γδ-T cells do so by selectively protecting a subset of human γδ-T cells from programmed cell death induced by mitogenic stimulation, in particular IL-2. Finally, we demonstrate that highly purified apoptosis-resistant human γδ-T cells can mediate antitumor activity against a variety of human tumor cell lines in vitro. Both the biologic and the practical implications of these findings are considered and discussed.
Materials and methods
PBMC and adherent cell-depleted PBMC
PBMC were isolated by Ficoll gradient centrifugation of whole blood obtained from healthy human volunteers. Where indicated, PBMC were depleted of monocytes by the removal of plastic-adherent cells, as described.20
Generation and maintenance of cell cultures
Cultures were initiated at a cell density of 1 × 106 cells/mL in 24-well flat-bottom tissue culture trays (Costar, Cambridge, MA) and maintained in 5% CO2 at 37°C in RPMI-1640 with 10% fetal bovine serum (HyClone, Logan, UT), 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, and 50 μmol/L 2-ME. On the day of culture initiation (day 0), human recombinant interferon (rIFN)-γ (1000 U/mL; Boehringer Mannheim, Indianapolis, IN); human rIL-12 (10 U/mL; R&D Systems, Minneapolis, MN), and mouse antihuman CD2 mAb clone S5.2 (1-10 μg/mL, mouse IgG2a; Becton Dickinson, San Jose, CA) were added. Twenty-four hours later (day 1), cultures were stimulated with 10 ng/mL anti-CD3 mAb OKT3 (mouse IgG2a; Orthobiotec, Raritan, NJ) and 300 U/mL human rIL-2 (Boehringer Mannheim). Where indicated, neutralizing monoclonal antihuman IL-12 antibody or an irrelevant isotype control antibody (R&D Systems) was added as a single dose at a final concentration of 25 μg/mL. Neutralizing monoclonal antihuman CD58 mAb clone L306 (mouse IgG2a; Becton Dickinson) or IgG2aisotype control antibody were added to cultures where indicated (5 μg/mL). Fresh medium with 10 U/mL IL-2 was added every 7 days. Where indicated, mAb OKT3 and mAb S5.2 were bound to plastic tissue culture plates as described.21
[3H]-thymidine proliferation assay
Proliferation assays were performed using standard methods as described in figure text. Assays were performed in triplicate with data presented as mean counts per minute (cpm) (± SD).
Surface staining and purification of cells by FACS
Cells were stained using fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or allophycocyanin (APC)-directly conjugated mAbs recognizing CD3, CD5, TCR-γδ, or TCR-αβ or directly conjugated isotype-matched irrelevant control antibodies (Becton Dickinson). Cells were stained for 30 minutes at 4°C in Hank's buffered saline solution (Mediatech, Herndon, VA) containing 2% fetal bovine serum (FBS) and immediately analyzed using a FACScalibur flow cytometer or sorted using a FACS Vantage cell sorter (Becton Dickinson). PI uptake was used to exclude nonviable cells. Data analysis was performed using CellQuest software (Becton Dickinson).
Four-color flow cytometry using annexin V-FITC and PI to measure apoptosis in αβ- and γδ-T cells
Cells were first surface stained (1 × 105 total cells in 100 μL) using anti-CD3–APC and anti-TCR-γδ–PE mAbs. Cells were washed twice with cold phosphate-buffered saline (PBS), washed twice again with annexin binding buffer (Apoptosis Detection Kit; R&D Systems), and then resuspended in 100 μL binding buffer. After the addition of annexin V-FITC and PI, cells were incubated for 15 minutes at room temperature in the dark. At this point, cells were kept on ice to prevent the capping and internalization of surface-bound mAbs. Calibration and compensation of all fluorescence detectors was performed using cells stained with individual positive and negative control reagents in the presence or absence of annexin V-FITC, PI, or both.
Induction of apoptosis in tumor target cells by cocultured human lymphocytes
Tumor target cells (2.5 × 104 in 500 μL complete RPMI) were cultured alone or cocultured with either human αβ- or γδ-T cells at varying effector–target (E:T) ratios (1:1 to 20:1) in sterile 5-mL round-bottom polystyrene tubes (Falcon, BD Labware, Franklin Lakes, NJ). Cells were incubated for 4 hours at 37°C in 5% CO2, after which they were washed with PBS and resuspended in 100 μL annexin binding buffer to which annexin V-FITC was added. After 15 minutes at room temperature in the dark, cocultured cells were gently vortexed to disrupt any tumor–lymphocyte aggregates, and they immediately were analyzed by flow cytometry. Voltages in the forward-scatter (FSC) and side-scatter (SSC) detectors were set to permit the discrimination of tumor cells (high FSC and high SSC) from lymphocytes (low FSC and low SSC) on the basis of light scatter. Electronically gated tumor cells were subsequently analyzed for the binding of annexin V-FITC using the FL1 detector.
Measurement of target cell viability on coculture with human lymphocyte for longer periods at lower E:T ratios
We modified a previously described method to distinguish living from dead cells.22 Briefly, target cells (2 × 103 in 100 μL complete RPMI) were cultured alone or cocultured with either human αβ-or γδ-T cells at various E:T ratios in 96-well flat-bottom tissue culture trays (Corning Glassware, Corning, NY). Cells were incubated for 18 hours at 37°C in 5% CO2, after which 100 μL of a solution containing 10 μg/mL ethidium bromide and 3 μg/mL acridine orange (Sigma, St Louis, MO) was added. Cells were immediately viewed using an inverted fluorescence microscope illuminated with a 300-W xenon light source (Intracellular Imaging, Cincinnati, OH). By using a blue filter set configured to excite for fluorescein (470 ± 20 nm excitation filter, 500 nm dichroic/beamsplitter filter, 515 nm emitter filter; Chroma Technology, Brattleboro, VT), live cells were observed to fluoresce green (acridine orange) whereas dead cells fluoresced orange (ethidium bromide).
Chromium Cr 51 release cytotoxicity assay
Human cervical carcinoma cell line HeLa (ATCC); human melanoma cell lines SK-MEL-3, SK-MEL-5, and SK-MEL-28 (ATCC and kindly provided by Dr B. McAlpine, Emory University, Atlanta, GA); human T-lymphoblastoid cell line CCRF-HSB-2 (ATCC); human peripheral blood lymphoblast cell line NC-37 (ATCC); human myeloid leukemia cell line K-562 (ATCC), human ovarian cancer cell line SK-OV-3 (ATCC), and human B-cell lymphoma line OCI-Ly823 were used as targets for chromium release assays. Target cells were labeled with 100 μCi Na251CrO4 (Amersham Pharmacia Biotech, Piscataway, NJ) from 2 hours to overnight at 37° C, after which cells were washed, trypsinized, and resuspended in RPMI containing 10% FBS. Cells were then plated (2 × 103/well) in 96-well V-bottom microtiter trays. Purified αβ- or γδ-T cells in varying numbers were added to target cells in a final volume of 150 μL. Trays were briefly centrifuged and then incubated for 4 hours at 37° C, after which 50 μL supernatant was removed to determine 51Cr release in cpm. Percentage specific target cell lysis was calculated as [(experimental release−spontaneous release)/(maximum release−spontaneous release)]×100. Maximum and spontaneous release were respectively determined by adding either 0.1% Triton X-100 or culture medium alone to labeled target cells in the absence of effector cells. Data are presented as the mean (± SD) of triplicate samples.
Results
Mitogenic stimulation of PBMC in the presence of anti-CD2 mAb S5.2 results in a large expansion of γδ-T cells
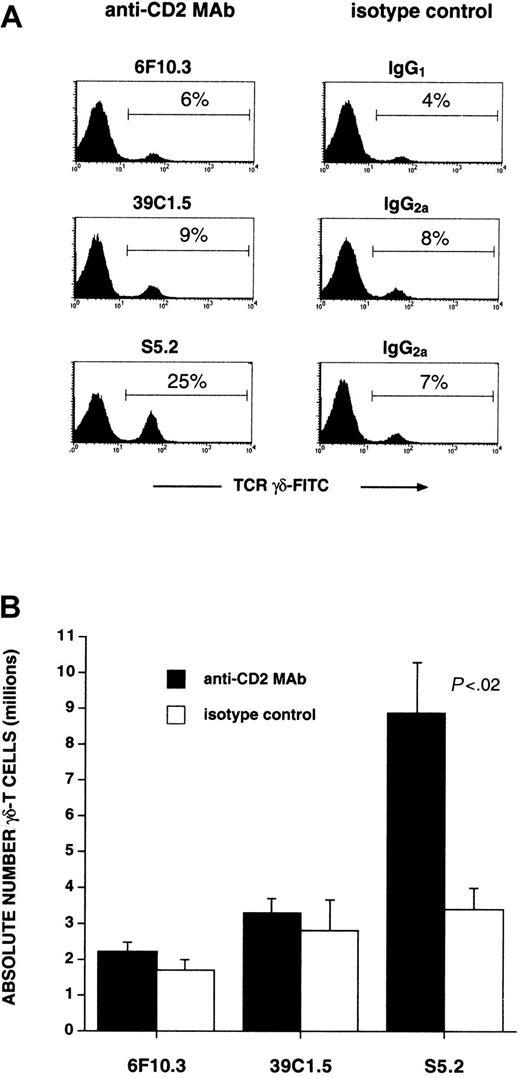
Previously, we described the expansion of human CD56+αβ-T cells arising in OKT3/IL-2-stimulated PBMC cultures, particularly if these cultures were first primed with IFN-γ 24 hours before stimulation with mitogens.24 25 In the process of examining the role of various surface antigens involved in CD56+ αβ–T-cell expansion, the inclusion of one particular mouse antihuman CD2 mAb (S5.2, IgG2a), but not its isotype control, resulted in a large increase in the percentage (Figure 1A) and absolute number of γδ-T cells (Figure 1B). Table 1 shows the results of experiments performed using PBMC obtained from several additional healthy donors. Data are presented as the mean fold expansion of these cultures (± SD, n = 5) determined after equivalent-length short-term cultures.
Anti-CD2 mAb S5.2 induces γδ–T-cell expansion from mitogen-stimulated PBMC cultures.
Cultures of human PBMC were initiated (day 0) by pre-incubating with IFN-γ (1000 U/mL) and then 24 hours later (day 1) with the addition of both OKT3 (10 ng/mL) and IL-2 (300 U/mL). The indicated anti-CD2 mAb or its corresponding isotype control antibody (5 μg/mL) was added on day 0. After 7 to 10 days, cultures were analyzed by FACS. Viable T-lymphocytes were first identified by gating on the CD3-PE+ and PI− populations. (A) Percentage of T-lymphocytes staining with an anti-γδ-TCR–FITC mAb is shown in each histogram. Results are representative of experiments performed using materials obtained from at least 5 different persons. (B) Numbers of γδ-T cells found in cultures initiated in the presence of the indicated anti-CD2 mAbs or isotype controls (P < .02 between mAb S5.2 and IgG2a control). Other antihuman CD2 mAbs tested—6F10.3 (mouse IgG1), 39C1.5 (rat IgG2a), and LT-2 (mouse IgG2b, not shown)—do not induce γδ–T-cell expansion. Data represent absolute numbers of γδ-T cells (mean ± SD) determined in quadruplicate; experiments were performed at least 3 separate times from samples obtained from different persons.
Anti-CD2 mAb S5.2 induces γδ–T-cell expansion from mitogen-stimulated PBMC cultures.
Cultures of human PBMC were initiated (day 0) by pre-incubating with IFN-γ (1000 U/mL) and then 24 hours later (day 1) with the addition of both OKT3 (10 ng/mL) and IL-2 (300 U/mL). The indicated anti-CD2 mAb or its corresponding isotype control antibody (5 μg/mL) was added on day 0. After 7 to 10 days, cultures were analyzed by FACS. Viable T-lymphocytes were first identified by gating on the CD3-PE+ and PI− populations. (A) Percentage of T-lymphocytes staining with an anti-γδ-TCR–FITC mAb is shown in each histogram. Results are representative of experiments performed using materials obtained from at least 5 different persons. (B) Numbers of γδ-T cells found in cultures initiated in the presence of the indicated anti-CD2 mAbs or isotype controls (P < .02 between mAb S5.2 and IgG2a control). Other antihuman CD2 mAbs tested—6F10.3 (mouse IgG1), 39C1.5 (rat IgG2a), and LT-2 (mouse IgG2b, not shown)—do not induce γδ–T-cell expansion. Data represent absolute numbers of γδ-T cells (mean ± SD) determined in quadruplicate; experiments were performed at least 3 separate times from samples obtained from different persons.
Expansion of mitogen-stimulated human γδ-T cells is augmented by anti-CD2 mAb S5.2 in the presence of recombinant human IL-12
| Culture condition* . | Fold expansion γδ-T cells† . | |||
|---|---|---|---|---|
| IFN-γ . | Anti-CD2 . | IL-12 . | Mean ± SD (n = 5) . | P . |
| + | IgG2a | PBS | 17.7 ± 11.4 | |
| + | IgG2a | + | 20.9 ± 10.5 | NS |
| + | PBS | + | 23.0 ± 13.1 | NS |
| + | 0.1 μg/mL | + | 41.9 ± 20.3 | .08 |
| + | 1 μg/mL | + | 72.2 ± 60.8 | .10 |
| + | 5 μg/mL | + | 67.1 ± 33.7 | <.02 |
| Culture condition* . | Fold expansion γδ-T cells† . | |||
|---|---|---|---|---|
| IFN-γ . | Anti-CD2 . | IL-12 . | Mean ± SD (n = 5) . | P . |
| + | IgG2a | PBS | 17.7 ± 11.4 | |
| + | IgG2a | + | 20.9 ± 10.5 | NS |
| + | PBS | + | 23.0 ± 13.1 | NS |
| + | 0.1 μg/mL | + | 41.9 ± 20.3 | .08 |
| + | 1 μg/mL | + | 72.2 ± 60.8 | .10 |
| + | 5 μg/mL | + | 67.1 ± 33.7 | <.02 |
PBMC were obtained from 5 healthy donors; short-term cultures of PBMC from each donor were initiated, maintained, and analyzed for the fold expansion of γδ-T cells after equivalent culture durations.
On day 0, IFN-γ (1000 U/mL), anti-CD2 mAb S5.2 (indicated concentration, isotype control IgG2a, or PBS) and IL-12 (10 U/mL or PBS) were added to PBMC cultures (1 × 106cells/mL). Twenty-four hours later (day 1), all cultures were stimulated with OKT3 (10 ng/mL) and IL-2 (300 U/mL).
Data are means (±SD) from separate experiments performed using PBMC derived from 5 persons. P values are between indicated condition and control (IFN-γ + IgG2a + PBS), derived using a standard Student t test.
Expansion of γδ-T cell induced by anti-CD2 mAb S5.2 requires the presence of IL-12 and occurs as a consequence of an increase in γδ–T-cell absolute numbers
The importance of IL-12 in the mAb S5.2-mediated expansion of γδ-T cell is shown in Figure 2, where the greatest percentage and absolute numbers of γδ-T cells are found in cultures initiated in the presence of anti-CD2 mAb S5.2 and exogenous IL-12. Importantly, if a neutralizing mAb to human IL-12 (but not its isotype control, not shown) is added to cultures initiated in the presence of mAb S5.2, both the percentage of γδ-T cells (Figure2A, lower histogram) and the absolute number of γδ-T cells (Figure2B, right column, anti–IL-12) are significantly diminished. Furthermore, as indicated in Figure 2, panel C, γδ–T-cell expansion induced by the addition of S5.2 and exogenous IL-12 does not occur as a consequence of the inhibition of αβ–T-cell growth or expansion. In addition, from these data we conclude that 5 μg/mL is the optimum mAb S5.2 concentration to promote γδ–T-cell expansion because in most instances, at higher concentrations (>10 μg/mL), culture growth is often globally inhibited in the presence ofeither the anti-CD2 mAb S5.2 or the corresponding isotype control IgG2a antibody (not shown). We attribute this finding to possibly the nonspecific inhibitory effects of the low concentration of sodium azide present in the mAb S5.2 preparation.
Expansion of γδ-T cell induced by anti-CD2 mAb S5.2 requires the presence of IL-12 and occurs as a consequence of an increase in γδ–T-cell absolute numbers.
Mitogen-stimulated PBMC cultures were initiated as described above. All cultures were primed with IFN-γ on the day of culture initiation (day 0) in the presence of anti-CD2 mAb S5.2 (or IgG2a isotype control, not shown). In addition, IL-12, PBS control, or antihuman IL-12 mAb (or isotype control for anti–IL-12 mAb, not shown) was included in these cultures. Twenty-four hours later (day 1), all cultures were stimulated with mitogenic OKT3 and IL-2. After 7 days, both the percentage and the absolute number of γδ-T cells were determined in cultures. Viable T cells were first identified by gating on the CD3-PE+ and PI− populations. (A) Percentage of T cells staining with an anti-γδ-TCR–FITC mAb is shown in each histogram. (B) Absolute number of γδ-T cells found in indicated culture conditions (mean ± SD) determined in quadruplicate. These results are representative of experiments performed using materials obtained from at least 8 different persons. (C) Mitogen-stimulated PBMC cultures were initiated as described above (day 0, IFN-γ; day 1, OKT3 and IL-2). On day 0, either IL-12 (10 U/mL) or PBS (−) was added to cultures. Likewise, anti-CD2 mAb S5.2 (or IgG2a isotype control, not shown) was added at the indicated concentration (μg/mL). After 14 days, absolute numbers of both αβ-T cells and γδ-T cells in cultures were determined by multiplying the total cell number in culture by the percentage of αβ- and γδ-T cells, as measured by FACS. Data are presented as fold expansion (mean ± SD) over starting numbers of αβ-T cells (open bars) and γδ-T cells (solid bars), determined in triplicate. Results are representative of experiments performed using materials obtained from at least 8 different persons.
Expansion of γδ-T cell induced by anti-CD2 mAb S5.2 requires the presence of IL-12 and occurs as a consequence of an increase in γδ–T-cell absolute numbers.
Mitogen-stimulated PBMC cultures were initiated as described above. All cultures were primed with IFN-γ on the day of culture initiation (day 0) in the presence of anti-CD2 mAb S5.2 (or IgG2a isotype control, not shown). In addition, IL-12, PBS control, or antihuman IL-12 mAb (or isotype control for anti–IL-12 mAb, not shown) was included in these cultures. Twenty-four hours later (day 1), all cultures were stimulated with mitogenic OKT3 and IL-2. After 7 days, both the percentage and the absolute number of γδ-T cells were determined in cultures. Viable T cells were first identified by gating on the CD3-PE+ and PI− populations. (A) Percentage of T cells staining with an anti-γδ-TCR–FITC mAb is shown in each histogram. (B) Absolute number of γδ-T cells found in indicated culture conditions (mean ± SD) determined in quadruplicate. These results are representative of experiments performed using materials obtained from at least 8 different persons. (C) Mitogen-stimulated PBMC cultures were initiated as described above (day 0, IFN-γ; day 1, OKT3 and IL-2). On day 0, either IL-12 (10 U/mL) or PBS (−) was added to cultures. Likewise, anti-CD2 mAb S5.2 (or IgG2a isotype control, not shown) was added at the indicated concentration (μg/mL). After 14 days, absolute numbers of both αβ-T cells and γδ-T cells in cultures were determined by multiplying the total cell number in culture by the percentage of αβ- and γδ-T cells, as measured by FACS. Data are presented as fold expansion (mean ± SD) over starting numbers of αβ-T cells (open bars) and γδ-T cells (solid bars), determined in triplicate. Results are representative of experiments performed using materials obtained from at least 8 different persons.
Anti-CD2 mAb S5.2 induces γδ–T-cell expansion by an agonistic and not a blocking interaction with CD2
The existence of accessory or alternative CD2 signaling pathways triggered by mAbs to CD2, which function exclusively in γδ-T cells, has previously been suggested by several investigators.18,26 Although most anti-CD2 mAbs capable of delivering proliferative signals to either αβ- or γδ-T cells appear to do so only if combined with a second anti-CD2 mAb recognizing a separate CD2 epitope, single epitope-binding anti-CD2 mAbs have been reported that appear to preferentially stimulate γδ-T cells.18,26 27 We performed the following experiments to show that mAb S5.2 functions in an agonistic and not a blocking capacity, thereby initiating rather than inhibiting CD2 signaling events that contribute to IL-12–dependent γδ–T-cell expansion.
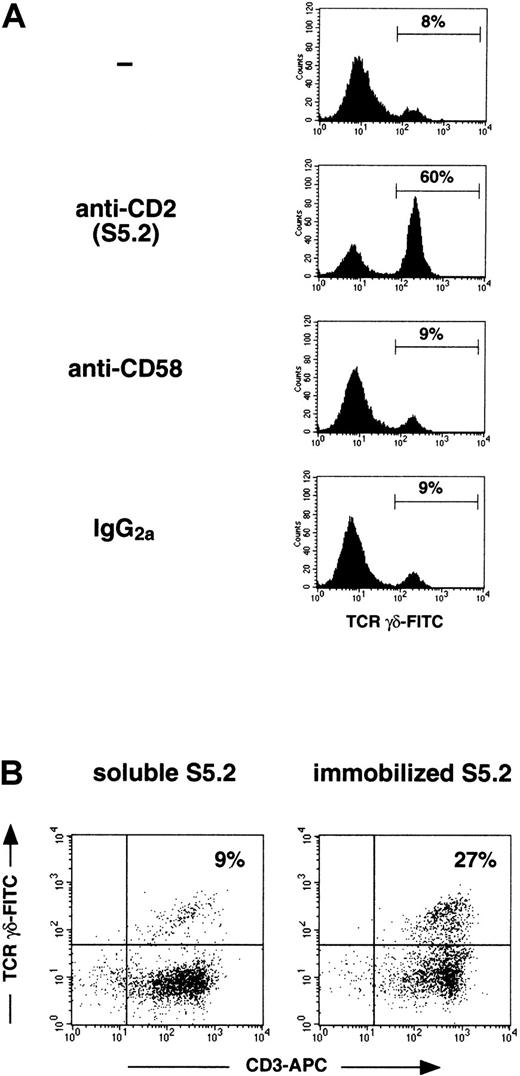
In mice and humans, both CD58 (LFA-3) and CD48 have been shown to serve as ligands for CD2; in humans, however, only CD58 has been shown to interact with CD2 on T cells in a functionally significant manner.28-31 We reasoned, therefore, that if anti-CD2 mAb S5.2 were inducing γδ–T-cell expansion by blockinginteractions between CD2 on γδ-T cells and CD58 expressed on other cells in culture, then the effect of a neutralizing anti-CD58 mAb would be the same—the enhancement of γδ–T-cell expansion. This is clearly not the case, as is shown in Figure3, panel A. To further demonstrate that mAb S5.2 is acting in an agonistic rather than an inhibitory manner, we next compared the capacity of both soluble and immobilized mAb S5.2 to induce γδ–T-cell expansion. Antibodies that bind to specific cell surface receptors usually cannot trigger signaling through these receptors unless immobilized or cross-linked. Consistent with this, previous reports18 26 have shown that the single anti-CD2 mAbs known to induce proliferative responses in γδ–T-cell clones appear to do so only if immobilized or if accessory cells that can cross-link the mAb through Fc receptors (FcR) are present. Because CD14+ cells (monocytes) present in our mitogen-stimulated cultures express FcR capable of cross-linking mAb S5.2 (mouse IgG2a), we performed the following experiments using PBMC first depleted of monocytes (<0.1% CD14+ cells; not shown). Figure 3, panel B shows that in mitogen-stimulated cultures, γδ-T cells can be induced to expand significantly by immobilized, but not soluble, mAb S5.2.
Anti-CD2 mAb S5.2 induces γδ–T-cell expansion through an agonistic and not a blocking interaction with CD2.
(A) Anti-CD2 mAb S5.2 does not induce γδ–T-cell expansion by disrupting a CD2–CD58 interaction. Mitogen-stimulated PBMC cultures were initiated as described above, now with the inclusion of IL-12 (day 0, IFN-γ, IL-12; day 1, OKT3 and IL-2). On day 0, either PBS (−), mAb S5.2 (mouse IgG2a), antihuman-CD58 mAb L066.4 (mouse IgG2a), or mouse IgG2a isotype control was added separately to identical cultures. After 14 days, cultures were analyzed using FACS. Viable T cells were first identified by gating on the CD3-PE+ and PI− populations. Percentage of T cells staining with an anti-γδ-TCR–FITC mAb is shown in each histogram. Results are representative of experiments performed using materials obtained from at least 3 different persons. (B) Immobilized, but not soluble, anti-CD2 mAb S5.2 can induce γδ–T-cell expansion in mitogen-stimulated, monocyte-depleted PBMC cultures. Monocyte-depleted PBMC cultures were initiated as described above, stimulated on day 0 with IFN-γ, IL-12, and either soluble or plastic-immobilized mAb S5.2. Twenty-four hours later (day 1), cultures were mitogenically stimulated with IL-2 and plastic-immobilized OKT3. After 21 days, cultures were analyzed using FACS; the percentage CD3-APC+/γδ-TCR-FITC+ cells in each dot plot was indicated. Immobilized or soluble IgG2a (isotype control for mAb S5.2) had a minimal effect on γδ–T-cell expansion (not shown). Results are representative of experiments performed using materials obtained from at least 3 different persons.
Anti-CD2 mAb S5.2 induces γδ–T-cell expansion through an agonistic and not a blocking interaction with CD2.
(A) Anti-CD2 mAb S5.2 does not induce γδ–T-cell expansion by disrupting a CD2–CD58 interaction. Mitogen-stimulated PBMC cultures were initiated as described above, now with the inclusion of IL-12 (day 0, IFN-γ, IL-12; day 1, OKT3 and IL-2). On day 0, either PBS (−), mAb S5.2 (mouse IgG2a), antihuman-CD58 mAb L066.4 (mouse IgG2a), or mouse IgG2a isotype control was added separately to identical cultures. After 14 days, cultures were analyzed using FACS. Viable T cells were first identified by gating on the CD3-PE+ and PI− populations. Percentage of T cells staining with an anti-γδ-TCR–FITC mAb is shown in each histogram. Results are representative of experiments performed using materials obtained from at least 3 different persons. (B) Immobilized, but not soluble, anti-CD2 mAb S5.2 can induce γδ–T-cell expansion in mitogen-stimulated, monocyte-depleted PBMC cultures. Monocyte-depleted PBMC cultures were initiated as described above, stimulated on day 0 with IFN-γ, IL-12, and either soluble or plastic-immobilized mAb S5.2. Twenty-four hours later (day 1), cultures were mitogenically stimulated with IL-2 and plastic-immobilized OKT3. After 21 days, cultures were analyzed using FACS; the percentage CD3-APC+/γδ-TCR-FITC+ cells in each dot plot was indicated. Immobilized or soluble IgG2a (isotype control for mAb S5.2) had a minimal effect on γδ–T-cell expansion (not shown). Results are representative of experiments performed using materials obtained from at least 3 different persons.
IL-12–dependent mAb S5.2-mediated signaling through CD2 protects γδ-T cells from activation-induced cell death
Especially in the presence of IL-2, γδ-T cells rapidly undergo apoptosis after receiving mitogenic stimuli through the TCR.17 Thus, one possible interpretation of our findings is that CD2 engagement by mAb S5.2 in the presence of IL-12 provides a signal to a subset of γδ-T cells that renders them resistant to activation-induced cell death caused by mitogenic OKT3 and IL-2.
Annexin V binds with high affinity to phosphatidylserine (PS), which is normally confined to the inner plasma membrane leaflet of nonapoptotic cells; the appearance of PS on the outer plasma membrane leaflet is an early event associated with apoptosis. These findings have been exploited to allow the examination of apoptosis by flow cytometric means.32 33 Thus, annexin V-FITC, in combination with directly conjugated antibodies, can be used to detect apoptosis occurring in phenotypically defined subpopulations of cells within heterogeneous cell cultures.
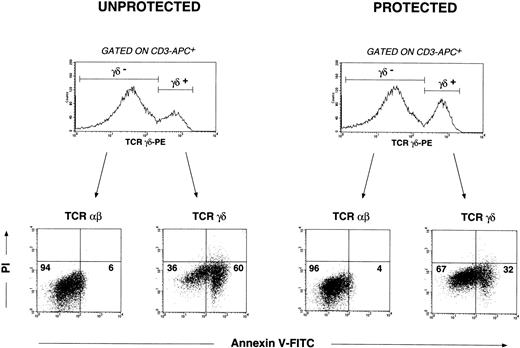
To demonstrate that CD2 engagement by mAb S5.2 in the presence of IL-12 protects γδ-T cells from activation-induced cell death, we performed the following experiment. By convention, we designated day 0 stimuli (IFN-γ, IL-12, and anti-CD2 mAb S5.2) as protective signals. PBMC cultures were initiated as described above. Those receiving day 0 stimuli were defined as protected; those not receiving day 0 stimuli (PBS only) were defined as unprotected. All cultures received OKT3 and IL-2 on day 1. After receiving the day 1 mitogenic signals, γδ- and αβ–T-cell populations within protected and unprotected cultures were assessed for apoptosis using 4-color flow cytometry 3 days after mitogen stimulation. As shown in Figure4, in the absence of protective day 0 signals, mitogen stimulation induces apoptosis in most γδ-T cells. In contrast, apoptosis occurs to a far lesser extent in γδ-T cells receiving day 0 protective signals. These results also show that apoptosis occurring in αβ-T cells in response to mitogenic stimulation is negligible under either of these conditions. In this regard, αβ-T cells serve as a control and support in part our argument that it is the γδ–T-cell compartment in cultures that is most affected by our manipulations.
CD2-mediated IL-12–dependent signals render human γδ-T cells resistant to mitogen-induced apoptosis: analysis by 4-color flow cytometry.
PBMC cultures were initiated as described above. Those receiving day 0 signals (IFN-γ, IL-12, and anti-CD2 mAb S5.2) by convention were defined as protected. Those receiving no anti-CD2 mAb S5.2 or IL-12 on day 0 (PBS only) were defined as unprotected. All cultures received OKT3 and IL-2 24 hours later (day 1 mitogenic signals). Both αβ- and γδ–T-cell populations were first delineated by electronic gating on the corresponding αβ- and γδ-T cells defined by anti-CD3-APC and anti-TCR-γδ–PE mAbs. Apoptosis occurring in αβ- and γδ–T-cell populations was then determined examining the uptake of annexin V-FITC and PI in the respective gated events. Cells incubated with anti-human CD95/Fas mAb CH11 (mouse IgM,) or mouse IgM isotype control antibody were used as positive and negative controls, respectively, to define apoptotic, viable, and necrotic quadrants within dot plots. Percentages of αβ- or γδ-T cells appearing in the corresponding dot plot quadrants are indicated: viable (annexin−/PI−), apoptotic (annexin+/PI−), and necrotic (annexin+/PI+). Results shown are representative of experiments performed using materials obtained from at least 4 different persons.
CD2-mediated IL-12–dependent signals render human γδ-T cells resistant to mitogen-induced apoptosis: analysis by 4-color flow cytometry.
PBMC cultures were initiated as described above. Those receiving day 0 signals (IFN-γ, IL-12, and anti-CD2 mAb S5.2) by convention were defined as protected. Those receiving no anti-CD2 mAb S5.2 or IL-12 on day 0 (PBS only) were defined as unprotected. All cultures received OKT3 and IL-2 24 hours later (day 1 mitogenic signals). Both αβ- and γδ–T-cell populations were first delineated by electronic gating on the corresponding αβ- and γδ-T cells defined by anti-CD3-APC and anti-TCR-γδ–PE mAbs. Apoptosis occurring in αβ- and γδ–T-cell populations was then determined examining the uptake of annexin V-FITC and PI in the respective gated events. Cells incubated with anti-human CD95/Fas mAb CH11 (mouse IgM,) or mouse IgM isotype control antibody were used as positive and negative controls, respectively, to define apoptotic, viable, and necrotic quadrants within dot plots. Percentages of αβ- or γδ-T cells appearing in the corresponding dot plot quadrants are indicated: viable (annexin−/PI−), apoptotic (annexin+/PI−), and necrotic (annexin+/PI+). Results shown are representative of experiments performed using materials obtained from at least 4 different persons.
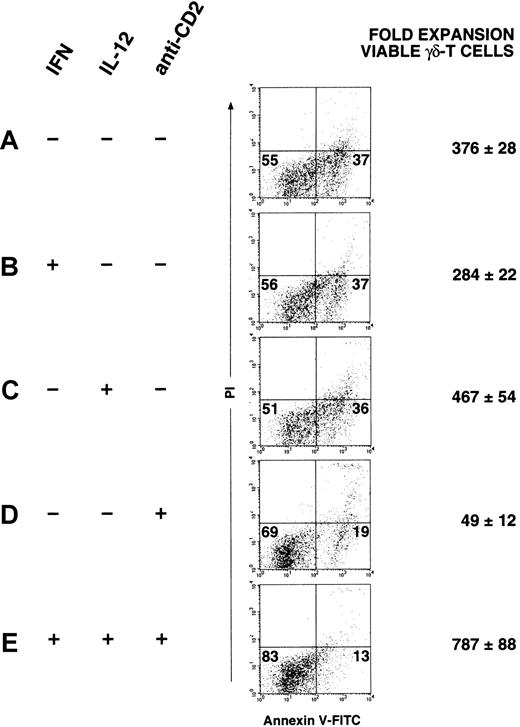
To demonstrate that the combination of CD2-mediated signals and IL-12 signaling promotes the expansion of apoptosis-resistant γδ-T cells, the following experiment was performed. Separate PBMC cultures were prepared (Figure 5A-E) receiving on day 0 as indicated, IFN-γ, IL-12, or anti-CD2 mAb S5.2. After 24 hours (day 1), all cultures received mitogenic stimulation with OKT3 and IL-2; after 21 days, γδ-T cells in each culture were analyzed for apoptosis. The percentages of viable and apoptotic cells in each dot plot are indicated in the corresponding quadrants and in the mean fold expansion (±SD) of apoptosis-resistant γδ-T cells in these cultures. As shown in Figure 5, panel E, the smallest percentage of apoptotic γδ-T cells and the greatest fold expansion of nonapoptotic γδ-T cells is found in cultures that received protective signals, including both exogenous IL-12 and anti-CD2 mAb S5.2. In contrast, a greater percentage of apoptotic γδ-T cells (annexin+/PI−) and a significantly lower expansion of viable γδ-T cells are noted in cultures to which no mAb S5.2 was added (Figure 5A-C). Interestingly, as shown in Figure 5, panel D, in cultures to which only mAb S5.2 has been added (no exogenous IL-12 or IFN-γ), it appears that though a significant proportion of γδ-T cells in these cultures remains viable, a significantly reduced expansion of viable γδ-T cell occurs. We interpret this to indicate that whereas engagement of CD2 with mAb S5.2 may generate a critical signal that induces resistance to apoptosis in mitogen-stimulated γδ-T cells, these signals in the absence of IL-12 are not sufficient to induce a significant expansion of apoptosis-resistant γδ-T cells. Thus, in conjunction with CD2-mediated signals, IL-12 appears to act synergistically to induce the greatest degree of expansion of apoptosis-resistant γδ-T cells. It is especially important to emphasize that day 0 signals alone (IFN-γ, IL-12, and anti-CD2 mAb S5.2), without day 1 signals (OKT3 and IL-2), cause no significant αβ- or γδ–T-cell proliferation (not shown).
Both CD2-mediated signals and IL-12 signaling contribute to the expansion of apoptosis-resistant γδ-T cells.
On day 0, separate PBMC cultures were initiated. Where indicated (+), IFN-γ (1000 U/mL), IL-12 (10 U/mL), or anti-CD2 mAb S5.2 (5 μg/mL) was added to cultures with PBS (−) added as a control. After 24 hours, all cultures received mitogenic stimulation with OKT3 and IL-2 (day 1). Cultures were maintained and expanded as described, and, after 21 days, γδ-T cells in each culture (first gated as CD3-APC+, TCR-γδ-PE+) were analyzed for apoptosis using 4-color FACS, as described above. The percentages of viable (annexin−/PI−) and apoptotic (annexin+/PI−) γδ-T cells in each dot plot are indicated in the corresponding quadrants. The absolute number of viable γδ-T cells (annexin−/PI−) found in each culture was determined with data expressed as the mean fold expansion of viable γδ-T cells (± SD), determined in triplicate. Results shown are representative of experiments performed using materials obtained from at least 3 different persons.
Both CD2-mediated signals and IL-12 signaling contribute to the expansion of apoptosis-resistant γδ-T cells.
On day 0, separate PBMC cultures were initiated. Where indicated (+), IFN-γ (1000 U/mL), IL-12 (10 U/mL), or anti-CD2 mAb S5.2 (5 μg/mL) was added to cultures with PBS (−) added as a control. After 24 hours, all cultures received mitogenic stimulation with OKT3 and IL-2 (day 1). Cultures were maintained and expanded as described, and, after 21 days, γδ-T cells in each culture (first gated as CD3-APC+, TCR-γδ-PE+) were analyzed for apoptosis using 4-color FACS, as described above. The percentages of viable (annexin−/PI−) and apoptotic (annexin+/PI−) γδ-T cells in each dot plot are indicated in the corresponding quadrants. The absolute number of viable γδ-T cells (annexin−/PI−) found in each culture was determined with data expressed as the mean fold expansion of viable γδ-T cells (± SD), determined in triplicate. Results shown are representative of experiments performed using materials obtained from at least 3 different persons.
Late, but not early, enhanced γδ–T-cell proliferation characterizes mAb S5.2 and IL-12–induced γδ–T-cell expansion
We have postulated that signaling through CD2 in the presence of IL-12 can protect γδ-T cells from mitogen-induced apoptosis. Alternatively, these signals might be leading to enhanced γδ–T-cell expansion by simply providing an early proliferative advantage to γδ-T cells compared with αβ-T cells. The following experiments were performed to show that this is not the case. By measuring [3H]-thymidine incorporation, we compared proliferative capacities of γδ- and αβ-T cells at bothearly time points (culture initiation, Figure6A) and late time points (3 week cultures, Figure 6B). These data show that γδ-T cells isolated early from protected cultures do not proliferate to a greater degree than αβ-T cells isolated from identical cultures. This is in contrast to γδ-T cells isolated later from protected cultures, which clearly manifest enhanced proliferative capacities compared to αβ-T cells. These data do not support a model where overrepresentation of γδ-T cells in longer-term S5.2-treated cultures occurs as a consequence of an early γδ–T-cell proliferative advantage.
[3H]-thymidine incorporation in sorted, highly purified αβ- and γδ-T cells: late, but not early, enhanced γδ–T-cell proliferation induced by mAb S5.2.
Protected PBMC cultures were initiated as described with all cultures receiving IFN-γ, IL-12, and mAb S5.2 on day 0 and OKT3 and IL-2 on day 1. (A) Early time points. After 24 hours (day 2), αβ- and γδ-T cells were sorted to high purity using FACS (greater than 98% pure and greater than 96% viable; not shown). Then they were plated at equivalent densities (5000 cells/well) in 96-well microtiter trays and were either stimulated with IL-2 at 100 U/mL or left unstimulated (PBS; indicated as no IL-2). After an additional 24 hours, [3H]-thymidine was added to cultures; 18 hours later, cells were harvested onto glass fiber filters. (B) Late time points. As above, but after 3 weeks, αβ- and γδ-T cells were sorted to high purity from cultures initiated in parallel; these cells were then assessed for proliferative capacity as described. Data are presented as mean cpm (± SD) of triplicate determinations. Results are representative of experiments performed using materials obtained from at least 2 different persons.
[3H]-thymidine incorporation in sorted, highly purified αβ- and γδ-T cells: late, but not early, enhanced γδ–T-cell proliferation induced by mAb S5.2.
Protected PBMC cultures were initiated as described with all cultures receiving IFN-γ, IL-12, and mAb S5.2 on day 0 and OKT3 and IL-2 on day 1. (A) Early time points. After 24 hours (day 2), αβ- and γδ-T cells were sorted to high purity using FACS (greater than 98% pure and greater than 96% viable; not shown). Then they were plated at equivalent densities (5000 cells/well) in 96-well microtiter trays and were either stimulated with IL-2 at 100 U/mL or left unstimulated (PBS; indicated as no IL-2). After an additional 24 hours, [3H]-thymidine was added to cultures; 18 hours later, cells were harvested onto glass fiber filters. (B) Late time points. As above, but after 3 weeks, αβ- and γδ-T cells were sorted to high purity from cultures initiated in parallel; these cells were then assessed for proliferative capacity as described. Data are presented as mean cpm (± SD) of triplicate determinations. Results are representative of experiments performed using materials obtained from at least 2 different persons.
IL-2 is a potent inducer of apoptosis in unprotected but not protected γδ-T cells
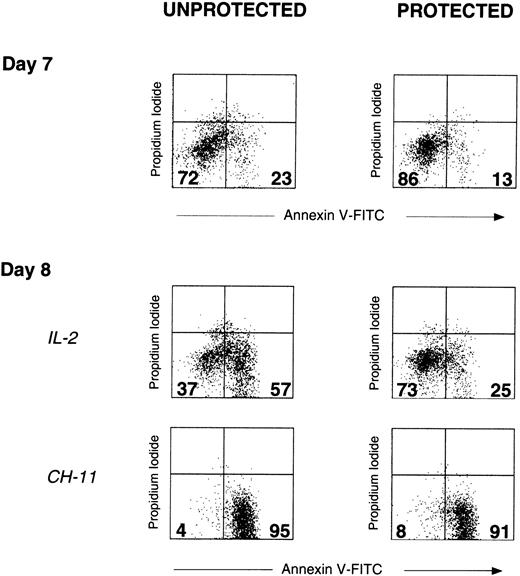
Despite the significant early mitogen-induced apoptosis occurring in unprotected γδ-T cells, after 7 days, surviving γδ-T cells are present in these cultures, though to a lesser extent than in protected cultures (Figure 7). Nonetheless, 1 day after the subsequent addition of IL-2 (day 8), a significantly greater percentage of unprotected but not protected γδ-T cells are induced to undergo apoptosis. This indicates that compared to unprotected γδ-T cells, protected γδ-T cells remain relatively resistant to IL-2–induced apoptosis. Furthermore, the ability of agonistic mouse antihuman CD95/Fas mAb CH11 to induce apoptosis in both protected and unprotected γδ-T cells suggests that the greater resistance to apoptosis of unprotected γδ-T cells does not result from a simple loss of CD95/Fas expression and is supported by the findings that CD95/Fas expression determined by FACS does not differ between protected and unprotected γδ-T cells (data not shown).
IL-2 is a potent inducer of apoptosis in unprotected but not protected γδ-T cells.
Unprotected and protected PBMC cultures were initiated on day 0, as described above. All cultures received OKT3 and IL-2 after 24 hours (day 1 mitogenic signals). On day 7, γδ-T cells in both unprotected and protected cultures were analyzed for apoptosis, as measured by the uptake of annexin V-FITC and PI (upper dot plots, day 7). The percentages of viable γδ-T cells (annexin−/PI−) and apoptotic γδ-T cells (annexin+/PI−) in each dot plot are indicated in the corresponding quadrants. Subsequently, IL-2 (100 U/mL) was added to equivalent numbers of cells from both protected and unprotected PBMC cultures. After overnight incubation, apoptosis in γδ-T cells was once again determined (middle dot plots, day 8, IL-2). Agonistic mouse antihuman CD95/Fas mAb CH11 (mouse IgM) was included in identical cultures as a positive control (lower dot plots, day 8, CH-11). Day 8 cultures (protected and unprotected) to which PBS alone or to which mouse IgM isotype control for CH11 was added were essentially unchanged with respect to apoptosis when compared to day 7 cultures (not shown). Addition of IL-2 had a minimal effect on apoptosis detected in αβ-T cells within either protected or unprotected cultures (not shown). Results are representative of experiments performed using materials obtained from at least 2 different persons.
IL-2 is a potent inducer of apoptosis in unprotected but not protected γδ-T cells.
Unprotected and protected PBMC cultures were initiated on day 0, as described above. All cultures received OKT3 and IL-2 after 24 hours (day 1 mitogenic signals). On day 7, γδ-T cells in both unprotected and protected cultures were analyzed for apoptosis, as measured by the uptake of annexin V-FITC and PI (upper dot plots, day 7). The percentages of viable γδ-T cells (annexin−/PI−) and apoptotic γδ-T cells (annexin+/PI−) in each dot plot are indicated in the corresponding quadrants. Subsequently, IL-2 (100 U/mL) was added to equivalent numbers of cells from both protected and unprotected PBMC cultures. After overnight incubation, apoptosis in γδ-T cells was once again determined (middle dot plots, day 8, IL-2). Agonistic mouse antihuman CD95/Fas mAb CH11 (mouse IgM) was included in identical cultures as a positive control (lower dot plots, day 8, CH-11). Day 8 cultures (protected and unprotected) to which PBS alone or to which mouse IgM isotype control for CH11 was added were essentially unchanged with respect to apoptosis when compared to day 7 cultures (not shown). Addition of IL-2 had a minimal effect on apoptosis detected in αβ-T cells within either protected or unprotected cultures (not shown). Results are representative of experiments performed using materials obtained from at least 2 different persons.
In vitro antitumor activity of apoptosis-resistant γδ-T cells measured against tumor cell lines
We next examined whether apoptosis-resistant γδ-T cells exert measurable antitumor activity against human tumor cells in vitro. We explored this question using 3 distinct methods. In all experiments, apoptosis-resistant γδ-T cells and control αβ-T cells were expanded and isolated simultaneously from a given individual. In virtually all instances, control αβ-T cells derived fromeither protected (day 0 plus day 1 signals) or unprotected cultures (day 1 mitogenic stimulation alone) were indistinguishable with regard to antitumor activity (not shown). Thus, αβ-T cells derived from protected or unprotected cultures were used interchangeably as controls for MHC-restricted alloreactivity.
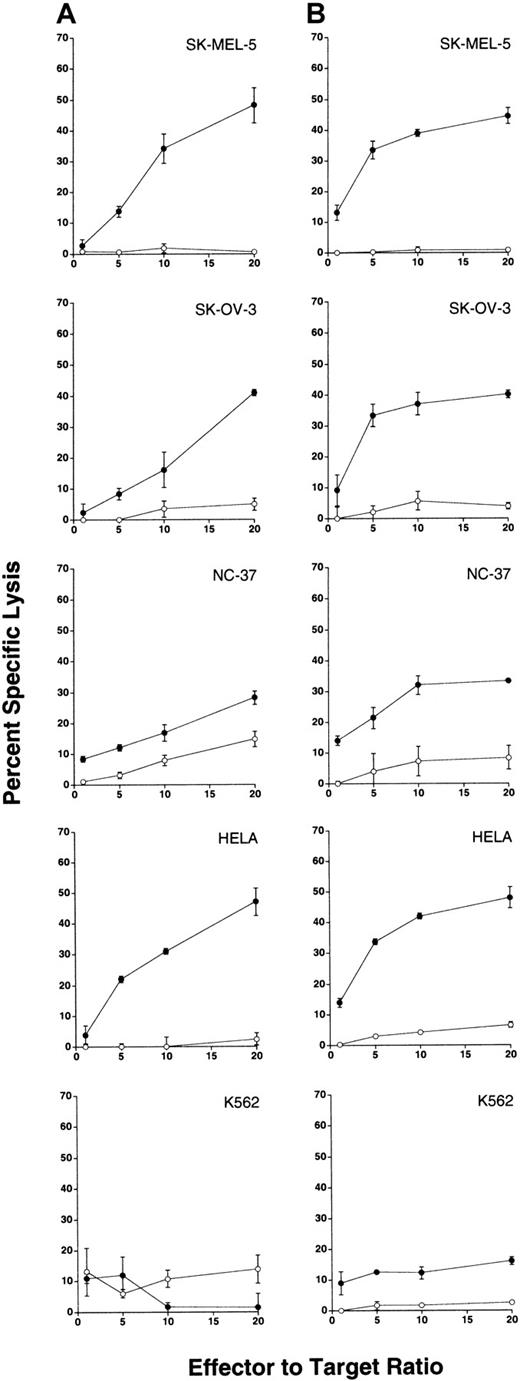
We first examined the antitumor activity of apoptosis-resistant γδ-T cells against a number of human tumor cell lines using a standard 4-hour 51Cr-release assay. Labeled tumor cells were incubated with apoptosis-resistant γδ-T cells or control αβ-T cells derived from a given person. Specific tumor lysis was measured, and results obtained from 2 separate persons are shown in Figure 8, panel A and panel B, respectively. Table 2 compiles the results of additional experiments performed using apoptosis-resistant γδ-T cells and control αβ-T cells derived from other healthy donors and tested against the indicated tumor cell lines. To allow comparison of multiple experiments, these data are presented as percentage- specific tumor lysis at an E:T ratio of 20:1.
Antitumor activity of apoptosis-resistant γδ-T cells demonstrated against human tumor cell lines.
Purified γδ- and αβ-T cells used as effector cells were sorted from 21-day cultures and were routinely enriched from cultures to 97% or greater pure and 98% or greater viable (not shown). To avoid the activation of T cells by the engagement of TCR, γδ-T cells were sorted as αβ-TCR−, CD5+ cells. Similarly, αβ-T cells were sorted as γδ-TCR−, CD5+ cells. After sorting, all lymphocytes used as effector cells were cultured overnight in complete RPMI containing 10 U/mL IL-2 and were routinely found to be 95% or greater viable (not shown).51Cr-labeled tumor cell targets (SK-MEL-5, SK-OV-3, NC-37, HeLa, and K-562) were incubated at the indicated E:T ratios with apoptosis-resistant γδ-T cells (filled circles) or control αβ-T cells (open circles) derived from 2 separate persons (column A and column B, respectively). After a 4-hour incubation at 37°C, supernatants were removed to determine 51Cr release in cpm. Data are presented as the mean percentage specific target lysis (± SD) of triplicate determinations.
Antitumor activity of apoptosis-resistant γδ-T cells demonstrated against human tumor cell lines.
Purified γδ- and αβ-T cells used as effector cells were sorted from 21-day cultures and were routinely enriched from cultures to 97% or greater pure and 98% or greater viable (not shown). To avoid the activation of T cells by the engagement of TCR, γδ-T cells were sorted as αβ-TCR−, CD5+ cells. Similarly, αβ-T cells were sorted as γδ-TCR−, CD5+ cells. After sorting, all lymphocytes used as effector cells were cultured overnight in complete RPMI containing 10 U/mL IL-2 and were routinely found to be 95% or greater viable (not shown).51Cr-labeled tumor cell targets (SK-MEL-5, SK-OV-3, NC-37, HeLa, and K-562) were incubated at the indicated E:T ratios with apoptosis-resistant γδ-T cells (filled circles) or control αβ-T cells (open circles) derived from 2 separate persons (column A and column B, respectively). After a 4-hour incubation at 37°C, supernatants were removed to determine 51Cr release in cpm. Data are presented as the mean percentage specific target lysis (± SD) of triplicate determinations.
Percentage specific lysis (51Cr release) of human tumor cell targets by apoptosis-resistant γδ-T cells and control αβ-T cells derived from healthy donors
| Tumor target* . | Lymphocyte source, age (y)/gender† . | Effector lymphocytes added and resultant specific lysis‡ . | |
|---|---|---|---|
| γδ-T cells . | αβ-T cells . | ||
| SK-MEL-3 | 38/M | 19.8 ± 0.9 | 3.5 ± 0.8 |
| 20/F | 16.2 ± 2.9 | 4.8 ± 1.9 | |
| 28/F | 16.6 ± 1.9 | 4.1 ± 2.0 | |
| SK-MEL-5 | 38/M | 14.1 ± 0.6 | 2.9 ± 0.9 |
| 28/F | 26.2 ± 5.4 | 5.3 ± 2.2 | |
| SK-MEL-28 | 38/M | 2.8 ± 0.7 | 0.5 ± 1.3 |
| 20/F | 1.4 ± 1.2 | 2.1 ± 0.4 | |
| HeLa | 41/F | 75.3 ± 2.0 | 9.1 ± 0.4 |
| 47/F | 65.7 ± 1.0 | 8.5 ± 1.2 | |
| OCI-Ly8 | 35/M | 60.1 ± 2.0 | 45.2 ± 4.7 |
| HSB-2 | 30/M | 23.0 ± 0.4 | ND |
| K562 | 47/F | 3.5 ± 0.2 | 1.6 ± 0.2 |
| Tumor target* . | Lymphocyte source, age (y)/gender† . | Effector lymphocytes added and resultant specific lysis‡ . | |
|---|---|---|---|
| γδ-T cells . | αβ-T cells . | ||
| SK-MEL-3 | 38/M | 19.8 ± 0.9 | 3.5 ± 0.8 |
| 20/F | 16.2 ± 2.9 | 4.8 ± 1.9 | |
| 28/F | 16.6 ± 1.9 | 4.1 ± 2.0 | |
| SK-MEL-5 | 38/M | 14.1 ± 0.6 | 2.9 ± 0.9 |
| 28/F | 26.2 ± 5.4 | 5.3 ± 2.2 | |
| SK-MEL-28 | 38/M | 2.8 ± 0.7 | 0.5 ± 1.3 |
| 20/F | 1.4 ± 1.2 | 2.1 ± 0.4 | |
| HeLa | 41/F | 75.3 ± 2.0 | 9.1 ± 0.4 |
| 47/F | 65.7 ± 1.0 | 8.5 ± 1.2 | |
| OCI-Ly8 | 35/M | 60.1 ± 2.0 | 45.2 ± 4.7 |
| HSB-2 | 30/M | 23.0 ± 0.4 | ND |
| K562 | 47/F | 3.5 ± 0.2 | 1.6 ± 0.2 |
Comparison of cytotoxicity at E:T ratios of 20:1.
Human tumor cell lines are described in “Materials and methods.”
Highly pure (>97%, not shown) apoptosis-resistant γδ- and control αβ-T lymphocytes were isolated by FACS from 21-day cultures of PBMC derived from healthy donors, as described in “Materials and methods.” Age and sex of PBMC donors are indicated.
Tumor target cells were labeled with 51Cr and cocultured with effector lymphocytes, as described in “Materials and methods.” Either apoptosis-resistant γδ-T cells or control αβ-T cells derived from the indicated donors were added at various E:T ratios (0.5:1 to 40:1). Percentage specific tumor lysis (mean ± SD of triplicate determinations) at an E:T ratio of 20:1 shown for comparison.
ND, not done.
Although 51Cr-release assays remain an established means by which to measure the in vitro cytotoxic activity of effector lymphocytes, we performed the following experiment to demonstrate that we could also measure the specific induction of apoptosis in sensitive target cells on coculture with effector γδ-T cells. HeLa cells were initially chosen for further experimentation. HeLa cells were cultured alone or cocultured for 4 hours with apoptosis-resistant γδ-T cells or control αβ-T cells at varying E:T ratios (1:1 to 20:1). As shown in Figure 9, panel A, T-lymphocytes alone (left plot) and HeLa target cells alone (center plot) have characteristic light scatter properties that allow both cell populations to be distinguished, even when mixed together in coculture (right plot). By gating only on HeLa cells, it was then possible to analyze HeLa target cells for their uptake of annexin V-FITC; this served as a measure of the ability of cocultured γδ- or αβ-T cells to induce apoptosis (Figure 9B). These representative data demonstrate that apoptosis-resistant γδ-T cells can induce a significantly greater degree of apoptosis in HeLa cells when compared to control αβ-T cells. Similar results were obtained using other target cells such as human melanoma cell lines (not shown).
Co-culture of tumor cells with apoptosis-resistant γδ-T cells: detection of tumor cell death.
(A) T lymphocytes alone (left plot) and HeLa target cells alone (center plot) have characteristic light scatter properties that allow each cell population to be distinguished, even when mixed together in coculture (right plot). (B) HeLa cells were cocultured for 4 hours with apoptosis-resistant γδ-T cells (TCR-γδ) or control αβ-T cells (TCR-αβ) at the indicated E:T ratios (0:1 to 20:1). Cocultured cells were then analyzed using FACS. Gating on the appropriate cell population (high forward scatter and high side scatter), uptake of annexin V-FITC by HeLa cells was determined and was taken as a measure of the ability of cocultured αβ- or γδ-T cells to induce apoptosis. Light microscopy and FACS using anti-CD3 mAbs were used to confirm that no tumor–lymphocyte aggregates remained after vortexing samples (not shown). Data are presented as histograms, and the percentage of HeLa cells staining with annexin V-FITC (and thus apoptotic) is indicated. These data are representative of experiments performed at least 3 times on materials obtained from 3 separate persons. (C) Measurement of HeLa target cell viability after coculture with apoptosis-resistant γδ-T cells or control αβ-T cells for longer periods at lower E:T ratios. Target HeLa cells were cultured alone or were cocultured with either human αβ- or γδ-T cells at a 1:1 E:T ratio for 18 hours. On the addition of ethidium bromide and acridine orange, cells were immediately viewed under fluorescence. As viewed using a 20× objective lens, tumor cells were readily distinguished from effector lymphocytes by size alone, permitting the enumeration of live (green) and dead (orange) tumor cells in each well. The percentage of tumor cells remaining viable was thus derived by dividing the number of green tumor cells by the number of green plus orange tumor cells ([green]/[green + orange]) in each well. Quantitations were performed in quadruplicate, with data presented as the mean viable tumor cells remaining per high-power field ± SD. Parallel determinations using trypan blue and a standard inverted microscope were also made and were in agreement with the results of these studies (not shown). These data are representative of experiments performed at least 3 times on materials obtained from 4 separate persons.
Co-culture of tumor cells with apoptosis-resistant γδ-T cells: detection of tumor cell death.
(A) T lymphocytes alone (left plot) and HeLa target cells alone (center plot) have characteristic light scatter properties that allow each cell population to be distinguished, even when mixed together in coculture (right plot). (B) HeLa cells were cocultured for 4 hours with apoptosis-resistant γδ-T cells (TCR-γδ) or control αβ-T cells (TCR-αβ) at the indicated E:T ratios (0:1 to 20:1). Cocultured cells were then analyzed using FACS. Gating on the appropriate cell population (high forward scatter and high side scatter), uptake of annexin V-FITC by HeLa cells was determined and was taken as a measure of the ability of cocultured αβ- or γδ-T cells to induce apoptosis. Light microscopy and FACS using anti-CD3 mAbs were used to confirm that no tumor–lymphocyte aggregates remained after vortexing samples (not shown). Data are presented as histograms, and the percentage of HeLa cells staining with annexin V-FITC (and thus apoptotic) is indicated. These data are representative of experiments performed at least 3 times on materials obtained from 3 separate persons. (C) Measurement of HeLa target cell viability after coculture with apoptosis-resistant γδ-T cells or control αβ-T cells for longer periods at lower E:T ratios. Target HeLa cells were cultured alone or were cocultured with either human αβ- or γδ-T cells at a 1:1 E:T ratio for 18 hours. On the addition of ethidium bromide and acridine orange, cells were immediately viewed under fluorescence. As viewed using a 20× objective lens, tumor cells were readily distinguished from effector lymphocytes by size alone, permitting the enumeration of live (green) and dead (orange) tumor cells in each well. The percentage of tumor cells remaining viable was thus derived by dividing the number of green tumor cells by the number of green plus orange tumor cells ([green]/[green + orange]) in each well. Quantitations were performed in quadruplicate, with data presented as the mean viable tumor cells remaining per high-power field ± SD. Parallel determinations using trypan blue and a standard inverted microscope were also made and were in agreement with the results of these studies (not shown). These data are representative of experiments performed at least 3 times on materials obtained from 4 separate persons.
Finally, we examined the ability of apoptosis-resistant γδ-T cells to kill tumor targets using acridine orange and ethidium bromide uptake as a means to distinguish live from dead target cells. As shown in Figure 9, panel C, at an E:T ratio as low as 1:1, HeLa tumor cell viability was significantly decreased after 18 hours of coculture with apoptosis-resistant γδ-T cells but not with control αβ-T cells. Although in agreement with the 51Cr-release and annexin data above, these experiments may be of additional biologic and even clinical significance given that this degree of tumor cell killing was induced at a significantly lower E:T ratio (1:1). Higher E:T ratios (ranging from 5:1 to 20:1, not shown) resulted in a similar induction of target cell death with the addition of γδ-T cells but not control αβ-T cells.
Discussion
The existence of alternative CD2 signaling pathways that function predominantly, if not exclusively, in γδ- but not αβ-T cells has been established by the important work of others.18,19 34 These pathways were largely revealed by the recognition that certain anti-CD2 mAbs could generate signals in cloned T cells, resulting in proliferation as measured by standard means, such as [3H]-thymidine incorporation. Although our current work is in agreement with these fundamental findings, we are able to extend these findings in several important biologic and possibly clinically relevant ways: our recognition that anti-CD2 mAb S5.2 can generate IL-12–dependent signals that protect human γδ-T cells from mitogen-induced apoptosis now provides us with the biologic basis for obtaining large numbers of viable and, as we show,functional human γδ-T cells.
The relation between CD2 signaling, IL-12, and acquired resistance to apoptosis in γδ-T cells is likely complex, but several observations may help elucidate the mechanisms underlying our observation, beginning with signaling through CD2 itself. In most studies, the capacity of anti-CD2 mAbs to signal through CD2 is almost entirely assessed in terms of proliferation.26,27,35,36 However, engagement of CD2 is not always associated with transduction of proliferative signals. Breitmeyer and Faustman37 have shown that the rosetting of human T cells by sheep red blood cells (which express a functional homologue of human CD58/LFA3, the natural ligand for CD2) results in the paralysis of TCR/CD3-mediated signal transduction and activation. These studies demonstrated that both calcium mobilization and proliferative responses to subsequent mitogenic anti-TCR antibodies were blocked for up to 48 hours after CD2 engagement. Similarly, in a more recent report, Miller et al38 showed that interaction between CD58/LFA-3 and CD2 can lead to T-cell unresponsiveness to antigenic or mitogenic stimuli in vitro. Conversely, it has been reported that the ability of certain anti-CD2 mAbs to stimulate γδ–T-cell clones was significantly diminished by the co-engagement of CD3, suggesting a possible antagonistic interaction between γδ-T cell CD2 and CD3 signaling pathways.26
Reasoning along these lines, we propose that γδ–T-cell expansion may occur in our system, not simply as a consequence of CD2-mediated proliferative signals per se but rather as a consequence of postreceptor CD2-mediated disruption or moderation of signals that could otherwise induce apoptosis in already apoptosis-prone γδ-T cells. In such a model, it is particularly important to emphasize that mAb S5.2 functions in an agonistic rather than in a blocking capacity,initiating rather than inhibiting CD2 signaling events (Figures 3).
In any event, it is important to note that CD2 signaling in the absence of IL-12 is insufficient to lead to the expansion of apoptosis-resistant γδ-T cells in mitogen-stimulated PBMC cultures, as is established by the neutralization experiments shown in Figure 2, panels A and B. This suggests that CD2 signaling is necessary, but not sufficient, for the expansion of apoptosis-resistant γδ-T cells. Conversely, it is evident that in the absence of CD2 signaling, IL-12 can neither enhance γδ–T-cell expansion (Figure 2C, Table 1) nor inhibit apoptosis (Figure 5C) in mitogen-stimulated γδ-T cells. Taken together, these data suggest that, in our system, γδ-T cells do not optimally respond to IL-12 without first receiving signals through CD2. Such an interpretation would be in keeping with the findings of Gollub et al,29 30 who have previously shown that responsiveness to IL-12 in activated T cells was indeed regulated and dependent on signals delivered through CD2. Thus, the requirement for CD2 signaling in our system might be explained on the basis that these signals simply enhance responsiveness to IL-12 in γδ-T cells, leading to apoptosis resistance. This would be in part supported by data that show IL-12–receptor (IL-12R) is found to be up-regulated on protected but not on unprotected γδ-T cells (unpublished data, manuscript in preparation).
It is unclear how IL-12 might inhibit apoptosis in mitogen-stimulated γδ-T cells, though the observations of Perussia et al39may provide an important clue. In one study, it was shown that whereas IL-12 always acts synergistically with IL-2 in inducing αβ–T-cell proliferation, in contrast, IL-12 could significantly inhibit IL-2–induced proliferation in resting γδ-T cells.39 Thus, it is conceivable that during the first critical 24 hours (day 0) of our protected cultures, responsiveness to IL-12 is first established through CD2-mediated signals delivered by mAb S5.2. Subsequent strong mitogenic signals delivered on day 1,in particular, IL-2, would then be less able to induce apoptosis in γδ-T cells. Thus, γδ-T cells spared this initial IL-2–mediated apoptosis would be those observed to expand in our cultures. That resistance to apoptosis in γδ-T cells might be a consequence of blunted responsiveness to IL-2 is supported by the findings shown in Figure 7, where clearly a differential susceptibility to IL-2–induced apoptosis is noted between protected and unprotected γδ-T cells. This is consistent with our observation that protected γδ-T cells fail to up-regulate the expression of CD25/IL-2Rα when compared to unprotected γδ-T cells (unpublished data, manuscript in preparation) and is further supported by our findings that γδ-T cells isolated early from protected cultures appear to proliferate to a lesser extent in response to IL-2 (Figure 6).
Although the principal tenet of our model is that γδ-T cells in protected cultures preferentially expand as a consequence of acquired resistance to apoptosis, it is important to appreciate that γδ-T cells can and do expand in unprotected cultures as well, albeit to a lesser extent (Figures 1B, 2C, 5, Table 1). This must be taken into account, particularly when the magnitude of γδ–T-cell expansion is compared between various culture conditions, especially after longer periods of expansion (Figure 5). In view of this, it must be emphasized that in protected cultures, not all γδ-T cells are resistant to apoptosis; conversely, in unprotected cultures, not all γδ-T cells are apoptotic (Figures 4, 5, 7). This suggests that protective signals might simply alter the proportion of apoptosis-resistant and -sensitive γδ-T cells present at a given time in culture. Hence, if a significant fraction of γδ-T cells in protected cultures acquire resistance to apoptosis early, even if only transiently, this in itself could account for the greater γδ–T-cell expansion observed at later time points. This is supported by the observation that in both protected and unprotected cultures, the proportions of viable and apoptotic γδ-T cells change in a predictable manner over time: at very early time points (up to culture day 1), protected and unprotected cultures contain comparable total numbers and similar proportions of viable and apoptotic γδ-T cells (not shown). However, by culture day 2 to day 4, though total γδ–T-cell numbers still remain comparable (not shown), protected cultures are routinely found to contain a greater proportion of apoptosis-resistant γδ-T cells (Figure 4). Eventually, as protected and unprotected cultures enter exponential growth phases (usually simultaneously between day 6 and day 10; not shown), protected cultures—already containing a larger proportion of viable γδ-T cells (Figure 7)—invariably proceed to surpass unprotected cultures with regard to expansion of viable γδ-T cells (Figure 1). This survival advantage of γδ-T cells in protected cultures is further accentuated after restimulation with IL-2 (Figure 7) and is ultimately reflected in the larger total numbers of viable γδ-T cells found in protected cultures at even later time points up to 45 days and beyond (not shown). Although we do not propose that protective signals render γδ-T cells permanently resistant to apoptosis, it is interesting to note that by the third week in culture, compared to unprotected cultures, protected cultures still contain a larger proportion of apoptosis-resistant γδ-T cells, though the differences in the magnitude of γδ-T cell–expansion is often no longer as great (Figure 5).
With regard to antitumor activity, the data provided in Figures 8 and 9and in Table 2 establish that apoptosis-resistant γδ-T cells, expanded and isolated from a number of persons, do indeed possess the ability to kill a variety of human tumors in vitro as measured by several methods. Although the mechanism of γδ–T-cell tumor recognition is not addressed here, several points are noteworthy. First, in virtually all instances in which killing is observed, apoptosis-resistant γδ-T cells were found to kill tumor targets to a significantly greater degree than αβ-T cells on a cell-for-cell basis. These data suggest that γδ-T cells recognize target cells by mechanisms distinct from those used by αβ-T cells, as is known.1,2,4 Second, it is interesting to note that a number of tumor cell lines of epithelial origin were found to be relatively sensitive to killing by γδ-T cells. This is especially intriguing given the recent findings that γδ-T cells expressing particular Vδ TCR can recognize an MHC class I-related molecule frequently expressed on tumor cells of epithelial origin.40-42 Third, that the prototypic natural killer (NK)–sensitive target K562 is found to be relatively resistant to γδ-T-cell–mediated killing might also suggest the involvement of mechanisms distinct from NK or lymphokine-activated killer cell–mediated killing. These cytotoxicity data, though not exhaustive—especially with respect to the number of donors tested or the number of tumor targets assessed—nevertheless serve to underscore the practical significance of our findings, namely that an otherwise rare subset of human lymphocytes can now readily be expanded, isolated, and subjected to more rigorous study. This is particularly relevant as one considers the potential for using such cells in various forms of adoptive immunotherapy.
Whether γδ-T cells have therapeutically exploitable biologic properties such as antiviral, antitumor, or hematopoietic stem cell graft-facilitating effects, remains to be determined. Although far larger numbers of apoptosis-resistant γδ-T cells would be required to design adoptive cellular therapy clinical experiments, it should be emphasized that usually only 2 mL PBMC (1 × 106cells/mL), derived from 3 to 5 mL fresh blood, is used as starting material to generate cultures larger than 50 to 100 × 106 T cells containing 40% to 60% γδ-T cells after 2 to 3 weeks or longer. Thus, with proper culture optimization, many more than 1 × 109 viable γδ-T cells could readily be obtained after ex vivo expansion using, as starting materials, safely procurable volumes of fresh autologous or allogeneic peripheral blood. Such γδ-T-cell–enriched products could readily be subjected to positive or negative selection techniques (immunomagnetic columns, high-speed FACS, panning, and so on) to obtain a product essentially “pure” in terms of γδ–T-cell content and, thus, ideal for examining clinical questions, something that was until now not practically possible.
Acknowledgment
We thank Christopher Ferrigno for his technical contributions to this work.
Supported by a grant from the Robert Wood Johnson Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard D. Lopez, BMT Program, THT-541, University of Alabama at Birmingham, 1900 University Boulevard, Birmingham AL 35294; e-mail: richard.lopez@ccc.uab.edu.


![Fig. 6. [3H]-thymidine incorporation in sorted, highly purified αβ- and γδ-T cells: late, but not early, enhanced γδ–T-cell proliferation induced by mAb S5.2. / Protected PBMC cultures were initiated as described with all cultures receiving IFN-γ, IL-12, and mAb S5.2 on day 0 and OKT3 and IL-2 on day 1. (A) Early time points. After 24 hours (day 2), αβ- and γδ-T cells were sorted to high purity using FACS (greater than 98% pure and greater than 96% viable; not shown). Then they were plated at equivalent densities (5000 cells/well) in 96-well microtiter trays and were either stimulated with IL-2 at 100 U/mL or left unstimulated (PBS; indicated as no IL-2). After an additional 24 hours, [3H]-thymidine was added to cultures; 18 hours later, cells were harvested onto glass fiber filters. (B) Late time points. As above, but after 3 weeks, αβ- and γδ-T cells were sorted to high purity from cultures initiated in parallel; these cells were then assessed for proliferative capacity as described. Data are presented as mean cpm (± SD) of triplicate determinations. Results are representative of experiments performed using materials obtained from at least 2 different persons.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3827/5/m_h82300427006.jpeg?Expires=1767766187&Signature=Jy5-yoNw3V7Xo9n2PqeodnxzcFyTXG4HoSZu81meoMl-Y0l1NOFNX3MfQK-xd2UF5H~T7R0kzYkCBF8QwFZ8fzqMWCLM36TAgJvDwe~JClObnvb5Il6l-5jOdDR3kl7BrhoGJyPLwj1fOqT4ifwGsieiJGqKoklC-k94gf2dEm0ujB3h6VIYz8hERk161K1KJyKL7aEckIhy1LtN8j-C0T6nn3yAlUeWc7yRTFgYN71NqTHC5jILGxRQ3kViAPtNBYMe3wIJMszME5IidAg05xJdNnRemSbvcE4wn~X51OH4lDpPkE1E8XLjw6uGFpY2GfIiAfi8qwsmyoTu5Sw9LQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 9. Co-culture of tumor cells with apoptosis-resistant γδ-T cells: detection of tumor cell death. / (A) T lymphocytes alone (left plot) and HeLa target cells alone (center plot) have characteristic light scatter properties that allow each cell population to be distinguished, even when mixed together in coculture (right plot). (B) HeLa cells were cocultured for 4 hours with apoptosis-resistant γδ-T cells (TCR-γδ) or control αβ-T cells (TCR-αβ) at the indicated E:T ratios (0:1 to 20:1). Cocultured cells were then analyzed using FACS. Gating on the appropriate cell population (high forward scatter and high side scatter), uptake of annexin V-FITC by HeLa cells was determined and was taken as a measure of the ability of cocultured αβ- or γδ-T cells to induce apoptosis. Light microscopy and FACS using anti-CD3 mAbs were used to confirm that no tumor–lymphocyte aggregates remained after vortexing samples (not shown). Data are presented as histograms, and the percentage of HeLa cells staining with annexin V-FITC (and thus apoptotic) is indicated. These data are representative of experiments performed at least 3 times on materials obtained from 3 separate persons. (C) Measurement of HeLa target cell viability after coculture with apoptosis-resistant γδ-T cells or control αβ-T cells for longer periods at lower E:T ratios. Target HeLa cells were cultured alone or were cocultured with either human αβ- or γδ-T cells at a 1:1 E:T ratio for 18 hours. On the addition of ethidium bromide and acridine orange, cells were immediately viewed under fluorescence. As viewed using a 20× objective lens, tumor cells were readily distinguished from effector lymphocytes by size alone, permitting the enumeration of live (green) and dead (orange) tumor cells in each well. The percentage of tumor cells remaining viable was thus derived by dividing the number of green tumor cells by the number of green plus orange tumor cells ([green]/[green + orange]) in each well. Quantitations were performed in quadruplicate, with data presented as the mean viable tumor cells remaining per high-power field ± SD. Parallel determinations using trypan blue and a standard inverted microscope were also made and were in agreement with the results of these studies (not shown). These data are representative of experiments performed at least 3 times on materials obtained from 4 separate persons.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3827/5/m_h82300427009.jpeg?Expires=1767766187&Signature=EZKvcTLwBtkndJODzRv4Qut~XKEdDI17Its9qPfRELHi~uFV9dcpYzRexzw3yDixVoLkDoxTMtjPhL9C6vB8sh-pPA1rWHqIzyD7IV5NMvTpgDbLF8WAD6b8Dw~ppnMiyWaok~1khA5zpzlpxYj3DftR3rw65B~ezBZuTjk-gjQMVCaYK7vRXARIyICLFV-9SoJUiBvioztpDgtPHB6fVauHOYfDXiL-04RIsmp4j~sopy70FJDJK0rmakIZlFf7w1uCBuEHiB6QLTHzXNDwe8O-hCFXDNB33KPsNxK-UG1OnLiXSpT2fk84ADDGY8515WmbJe7lBLlKD1HlJxi81A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal