Abstract
How platelet shape change initiated by a collagen-related peptide (CRP) specific for the GPVI/FcRγ-chain complex (GPVI/FcRγ-chain) is coupled to SLP-76, phosphoinositide (PI) 3-kinase, and gelsolin is reported. As shown by video microscopy, platelets rapidly round and grow dynamic filopodial projections that rotate around the periphery of the cell after they contact a CRP-coated surface. Lamellae subsequently spread between the projections. All the actin-driven shape changes require SLP-76 expression. SLP-76 is essential for the Ca++mobilization induced by CRP, whereas PI 3-kinase only modulates it. The extension of lamellae requires net actin assembly and an exposure of actin filament barbed ends downstream of PI 3-kinase. Gelsolin expression is also required for the extension of lamellae, but not for the formation of filopodia. Altogether, the data describe the role of SLP-76 in the platelet activation initiated by GPVI/FcRγ-chain and the roles of PI 3-kinase and gelsolin in lamellae spreading.
Introduction
Blood platelets play a critical role in hemostasis. After blood vessel injury and disruption of the endothelial layer, platelets adhere to collagen in the basement membrane through glycoprotein (GP) Ia-IIa (integrin α2β1) and are activated by ligation of the GPVI/Fc receptor γ-chain complex (GPVI/FcRγ-chain).1 They rapidly change from discoid shapes to their activated forms. The process of platelet shape change has been well studied in vitro with soluble agonists such as thrombin. Both the assembly of actin into filaments and the incorporation of many actin-associated and signaling molecules into the platelet actin-based cytoskeleton are required.2
The intracellular signaling pathway leading to activation of platelets by thrombin begins with the activation of phospholipase C-β (PLC-β) through a trimeric G-protein–coupled receptor.3 4 Active PLC-β hydrolyzes plasma membrane polyphosphoinositides (ppIs), notably phosphatidylinositol 4,5-bisphosphate, to form inositol 1,4,5-trisphosphate and 1,2-diacylglycerol, which, respectively, mobilizes Ca++ from the internal stores and activates protein kinase C.
Activation of platelets by thrombin results in the formation of filopodia and in cell spreading by the extension of lamellae.5 Filopodia and lamellae are composed of bundles of long filaments and orthogonal arrays of short filament networks, respectively. The extension of lamellae observed in platelets activated by thrombin requires the severing of actin filaments present in the resting cell, the formation and activation of barbed-end nucleation sites, and the addition of actin monomers onto these nucleation sites to double the F-actin content.5 We have argued, based on the structural changes that normally occur in platelets and the lack thereof in the platelets of gelsolin-deficient mice, that 75% of the actin nucleation activity derives from Ca++-activated, gelsolin-based filament fragmentation and the subsequent uncapping of these filaments by membrane ppIs.6,7 Production of ppIs requires the activation of the small GTPase Rac,8 and a rapid and robust activation of Rac follows ligation of the thrombin receptor.9 Phosphoinositide (PI) 3-kinase is not required for the platelet spreading mediated by thrombin,10 but it is involved in platelet spreading over fibrinogen-coated surfaces mediated by adenosine diphosphate (ADP)11 and in platelet actin assembly initiated by the fibrinogen receptor, the integrin αIIbβ3.10
In contrast, less is known about platelet shape change induced by GPVI/FcRγ-chain. GPVI is a member of the immunoglobulin superfamily,12 and FcRγ-chain belongs to the family of the immunoreceptor tyrosine-based activation motif (ITAM)-containing receptors. Human platelets express 2 ITAM-containing receptors, the receptor for the Fc domain of IgGs, FcγRIIA, and GPVI/FcRγ-chain.1 FcγRIIA is expressed in human, but not in mouse, platelets, whereas GPVI/FcRγ-chain is expressed in both. Part of the intracellular signaling pathway from GPVI/FcRγ-chain has been identified.13 Platelet stimulation with collagen or a cross-linked collagen-related peptide (CRP) selective for GPVI/FcRγ-chain significantly increases tyrosine phosphorylation of multiple proteins, including FcRγ-chain, the protein tyrosine kinase Syk, SLP-76, and phospholipase C-γ2 (PLC-γ2).13,14 A model has been proposed in which Syk binds to the phosphorylated ITAM of FcRγ-chain through its tandem Src homology (SH2) domains. Phosphorylated SLP-76 recruits PLC-γ2 to the plasma membrane, where Syk phosphorylates and activates it.14,15 In support of this model, tyrosine phosphorylation of PLC-γ2 and platelet activation induced by collagen and CRP are abolished in FcRγ-chain–, Syk-, and SLP-76–deficient mice.13-15
SLP-76 is an adaptor protein predominantly expressed in hematopoietic cells, notably in T cells and in myeloid cells.16-18 It contains multiple N-terminal tyrosine phosphorylation sites, a central proline-rich region that constitutively associates with the SH3 domains of the adaptor protein Grb2,16 and aC-terminal SH2 domain. After ligation of the T-cell antigen receptor, SLP-76 is rapidly tyrosine phosphorylated by ZAP-7019-21 and associates with Vav,22 a guanine nucleotide exchange factor for Rac. In addition, SLP-76 interacts through its SH2 domain with Fyb/SLAP-130.23,24SLP-76–deficient mice have severe impairment of T-cell development.18,25 They also manifest a bleeding diathesis resulting in significant perinatal mortality,15,25 similar to Syk-deficient mice.13 Surviving adults show massive bleeding of intraperitoneal fluid and diffuse edema of the neck, thorax, and intestinal regions.
PI 3-kinase also plays a role in GPVI/FcRγ-chain signaling.26 PLC-γ2 moves to the plasma membrane by binding ppIs phosphorylated in the D3 position of the inositol ring by its pleckstrin homology and/or SH2 domains,27,28 where it now hydrolyzes phosphatidylinositol 4,5-bisphosphate. PI 3-kinase binds to the phosphorylated ITAM of FcRγ-chain after platelet activation induced by collagen or CRP,29 and its inhibition by wortmannin or LY294002 affects the platelet responses downstream of GPVI/ FcRγ-chain.26 30
The aim of the current study was to investigate the mechanisms of platelet shape changes induced by GPVI/FcRγ-chain and to connect signaling to actin with known players of intracellular signaling pathways induced by GPVI/FcRγ-chain and the thrombin receptor. We report that the activation of platelets on CRP-coated surfaces leads to the formation of actin-driven motile protrusions and to the extension of lamellae. Our results confirm that the adaptor protein SLP-76 is critical for platelet activation by CRP, acting downstream of Syk for the tyrosine phosphorylation and activation of PLC-γ2. PI 3-kinase is required for the exposure of filament barbed ends and for actin assembly, both of which lead to the spreading of lamellae mediated by GPVI/FcRγ-chain. Finally, as we have reported for the thrombin receptor,6 gelsolin is required for the extension of lamellae that occurs downstream of GPVI/FcRγ-chain, but not for filopodia formation.
Materials and methods
Human and mouse platelet preparation and stimulation
Blood from healthy human volunteers was collected into 0.1 vol Aster-Jandl anticoagulant. Platelet-rich plasma (PRP) was prepared by centrifugation at 100g for 20 minutes, and platelets were separated from PRP by gel-filtration. Blood was collected from normal (wild-type [WT]), SLP-76–deficient,25 and gelsolin-deficient6 mice by retro-orbital plexus bleeding and was anticoagulated in 0.1 vol Aster-Jandl anticoagulant. PRP was obtained by centrifugation of the blood at 100g for 6 minutes, followed by centrifugation of the supernatant and the buffy coat at 100g for 6 minutes. Mouse platelets were isolated from PRP using a metrizamide gradient.31 Briefly, platelets were concentrated between 25% and 10% metrizamide phases in 140 mmol/L NaCl, 5 mmol/L KCl, 12 mmol/L trisodium citrate, 10 mmol/L glucose, 12.5 mmol/L sucrose, pH 6, by centrifugation at 1100g for 12 minutes. This washing procedure was duplicated, and finally platelets were resuspended in 140 mmol/L NaCl, 3 mmol/L KCl, 0.5 mmol/L MgCl2, 5 mmol/L NaHCO3, 10 mmol/L glucose, 10 mmol/L HEPES, pH 7.4. The concentration of human and mouse platelets was adjusted to 3 × 108/mL, and platelets were allowed to rest for 30 minutes at 37°C before use. Platelets were stimulated with 3 μg/mL CRP prepared and cross-linked as previously described.32
Video microscopy
Twenty-five millimeter round coverslips were attached with a petroleum jelly–paraffin wax mixture to the bottom of 35-mm plastic Petri dishes, with a 10-mm hole punched in the bottom. Coverslips were coated with 6 μg/mL CRP in phosphate-buffered saline (PBS) for 2 hours, followed by extensive blocking with fatty acid-free 3% (wt/vol) bovine serum albumin (BSA) in PBS. Petri dishes were maintained at 37°C with a Harvard Apparatus (Holliston, MA) temperature controller TC-202. Platelets were imaged on a Zeiss inverted microscope with differential interference contrast optics and 100× oil immersion objective. Images were captured with a Hamamatsu C2400 CCD camera (Japan), processed for background subtraction and frame averaging with a Hamamatsu ARGUS image processor, and digitally recorded on a Macintosh computer equipped with a SCION frame grabber LG-3 (Frederick, MD).
Electron microscopy
Platelets were attached to the surfaces of 5-mm round CRP-coated (6 μg/mL) coverslips by centrifugation at 330g for 5 minutes at 37°C. Platelets either were fixed by the addition of 1% (vol/vol) glutaraldehyde for 10 minutes to view the topology of the cells or they were extracted with 0.75% Triton X-100 in 60 mmol/L Pipes, 25 mmol/L HEPES, 10 mmol/L EGTA, and 2 mmol/L MgCl2(PHEM buffer) containing protease inhibitors and 2 μmol/L phallacidin for 2 minutes. Cytoskeletons were fixed with 1% glutaraldehyde in PHEM buffer as previously described.5The coverslips were washed in water, rapidly frozen, freeze-dried, and coated with 1.4 nm tantalum–tungsten and 4 nm carbon. Replicas were picked up on carbon–formvar-coated copper grids and photographed at 100 kV in a JEOL 1200-EX electron microscope.
Tyrosine phosphorylation of PLC-γ2
Cell lysates, prepared in 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS) in PBS with 1 mmol/L sodium orthovanadate, 100 μg/mL phenylmethylsulfonyl fluoride, and 1× Complete proteinase inhibitors (Boehringer-Mannheim), were precleared for 2 hours with normal rabbit serum coupled to protein G–Sepharose beads, then immunoprecipitated overnight with a polyclonal anti-PLC-γ2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) bound to protein G–Sepharose beads. The immune complexes were solubilized in SDS–polyacrylamide gel electrophoresis (PAGE) loading buffer33 containing 5% β-mercaptoethanol. After they were boiled for 5 minutes, proteins were separated by SDS-PAGE on an 8% polyacrylamide gel and transferred to an Immobilon-P membrane (Millipore). The membrane was incubated in a blocking solution (100 mmol/L NaCl, 20 mmol/L Tris/HCl, pH 7.4) containing 1% BSA, then probed with a 1:1 mixture of 4G10 (Upstate Biotechnology) and PY20 (Transduction Laboratories) anti-phosphotyrosine monoclonal antibodies. Detection was performed with an enhanced chemiluminescence system (Pierce).
Intracellular free calcium concentration ([Ca++]i) measurements
[Ca++]i measurements were performed on indo1–am-labeled platelets, as previously described.34 Briefly, indo1 fluorescence was recorded using a spectrofluorometer (LS50; Perkin-Elmer Cetus Instruments, Norwalk, CT). Excitation and emission wavelengths were 331 and 410 nm, respectively. [Ca++]i was calibrated according to Grynkiewicz et al35: [Ca++]i = Kd × (F − Fmin)/(Fmax − F), where F is the measured fluorescence intensity, and Fminand Fmax are the fluorescence intensities obtained without external Ca++ and at saturating Ca++, respectively. Kd for indo1 was taken to be 250 nmol/L.
Measurement of F-actin content
Resting or activated platelets in suspension were fixed in 3.4% (vol/vol) formaldehyde and permeabilized with 0.1% Triton X-100 in the presence of 10 μmol/L fluorescein isothiocyanate (FITC)–phalloidin (Sigma). Bound FITC–phalloidin was quantitated by FACS analysis using a Becton Dickinson flow cytometer. In total, 10 000 events were analyzed for each sample.
Measurement of filament ends
Resting or CRP-activated platelets in suspension were extracted with 0.1% Triton in PHEM buffer containing protease inhibitors and 2 μmol/L phallacidin. Then, 185 μL 100 mmol/L KCl, 2 mmol/L MgCl2, 0.5 mmol/L adenosine triphosphate, 0.1 mmol/L EGTA, 0.5 mmol/L dithiothreitol, and 10 mmol/L Tris, pH 7.0 were added to 100 μL platelet lysate, and the polymerization rate assay was started by the addition of 15 μL monomeric pyrene-labeled rabbit skeletal muscle actin to a final concentration of 1 μmol/L. Pyrene-actin fluorescence was recorded using a spectrofluorometer (LS50; Perkin-Elmer Cetus Instruments). Excitation and emission wavelengths were 366 and 386 nm, respectively. Activity inhibited by 2 μmol/L cytochalasin B is defined as barbed-end actin assembly. Activity not inhibited by cytochalasin B is defined as pointed-end actin assembly. The number of actin filament ends was determined as previously described.5 There were 1.5 × 107 platelets per assay. Initial barbed- and pointed-end addition rates in 1 μmol/L actin solution are 10 and 1 monomers s−1, respectively.
Results
Morphology of platelets activated by CRP
A reproducible series of morphologic changes occurs when human platelets contact a CRP-coated surface (Figure1). As initial contact with the surface is made, platelets convert from discs to round or spherical forms. This event is rapidly followed by the extension of filopodia from the rounded cell. Filopodial growth is a dynamic process, detectable first as a rippling and/or a bulging at the surface of platelets followed by the extension of filopodia. Filopodia continue to extend and withdraw as the cells flatten onto the surface. The central bodies of platelets activated on CRP remain 3-dimensional and display dynamic membrane activity, including the formation of unique blunt motile filopodia that rotate around the cell. Simultaneous with filopodia formation and movement, there is a partial filling of the spaces between filopodia with small lamellae-like extensions of membrane.
Morphologic changes occurring in human platelets activated on CRP-coated surfaces.
Representative morphology of a single human platelet activated on a CRP-coated surface. Platelets were allowed to adhere to CRP-coated glass coverslips. Attachment and activation were followed by video microscopy. Images shown are captured at 2-minute intervals after the initial adhesion and activation reaction. Major morphologic changes occur in CRP-activated platelets: adherence effects a rounding of the discoid cell; filopodia are protruded and retracted; filopodia form and rotate; and spaces between filopodia are filled by lamellipodia protrusion. Time is indicated in minutes.
Morphologic changes occurring in human platelets activated on CRP-coated surfaces.
Representative morphology of a single human platelet activated on a CRP-coated surface. Platelets were allowed to adhere to CRP-coated glass coverslips. Attachment and activation were followed by video microscopy. Images shown are captured at 2-minute intervals after the initial adhesion and activation reaction. Major morphologic changes occur in CRP-activated platelets: adherence effects a rounding of the discoid cell; filopodia are protruded and retracted; filopodia form and rotate; and spaces between filopodia are filled by lamellipodia protrusion. Time is indicated in minutes.
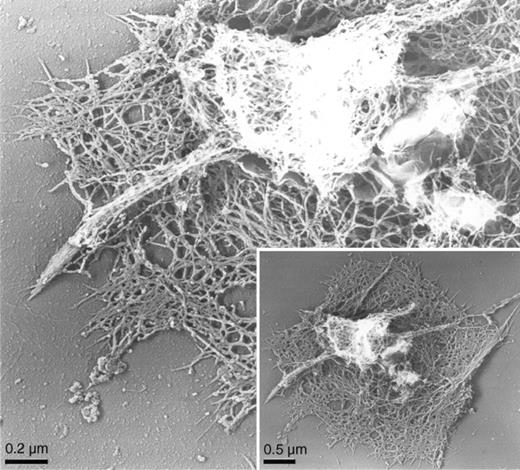
High-resolution electron microscopy reveals the underlying structure of the activated human platelet cytoskeleton and the arrangement of actin filaments within the specific cellular processes described (Figure2). Filopodia—short, thick projections having tightly packed actin fibers—extend from a central 3-dimensional filamentous mass in cytoskeletons of CRP-activated cells. Lamellae fill in between the projections of CRP-activated cells and are composed of shorter actin filaments organized into an orthogonal network.
Actin cytoskeleton of human platelets activated on CRP-coated surfaces.
Cytoskeleton of a human platelet activated on a CRP-coated coverslip surface by electron microscopy. Human platelets were activated on a CRP-coated coverslip surface. Cells were permeabilized with Triton X-100 in PHEM buffer, rapidly frozen, freeze-dried, and metal-coated. Cytoskeletons have prominent central regions from which motile filopodia extend.
Actin cytoskeleton of human platelets activated on CRP-coated surfaces.
Cytoskeleton of a human platelet activated on a CRP-coated coverslip surface by electron microscopy. Human platelets were activated on a CRP-coated coverslip surface. Cells were permeabilized with Triton X-100 in PHEM buffer, rapidly frozen, freeze-dried, and metal-coated. Cytoskeletons have prominent central regions from which motile filopodia extend.
SLP-76–deficient platelets fail to form filopodia and spread lamellae in response to CRP
The availability of mice lacking specific signaling or actin regulatory proteins allows evaluation of their roles in these responses. Platelets obtained from WT mice behaved as do the human blood platelets when exposed on CRP-coated surfaces (Figure3A). CRP induced predominantly the formation of filopodia in mouse platelets, and platelets adhering to CRP-coated surfaces remained filopodial even after incubation for long periods of time. SLP-76–deficient platelets exposed to CRP failed to spread and for the most part retained their disc shapes, though a few short filopodia extended from their surfaces (Figure 3B,C). In contrast, spreading of SLP-76–deficient platelets in response to thrombin was unaffected relative to WT platelets (Figure 3D-F). Resting SLP-76–deficient platelets have a discoid shape similar to that of WT platelets (data not shown).
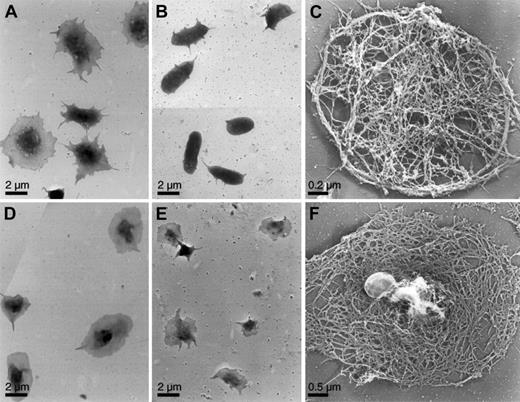
Surface topology and cytoskeleton of activated mouse platelets.
Mouse platelets were examined for shape change by electron microscopy. WT (A) and SLP-76–deficient (B, C) platelets were activated on a CRP-coated coverslip surface, as in Figure 2. WT (D) and SLP-76–deficient (E, F) platelets were attached to coverslips and activated with 1 U/mL thrombin. Cells were either fixed (A, B, D, E) or permeabilized with Triton X-100 in PHEM buffer (C, F), rapidly frozen, freeze-dried, and metal-coated. SLP-76–deficient platelets exposed to CRP retain their disc shapes, though they grow a small number of short filopodia. The cytoskeleton is similar to that of the normal resting mouse platelet cytoskeleton. SLP-76–deficient platelets activated with thrombin spread normally on the surface. The cytoskeleton has rearranged from the resting form and has a cortex composed of short filaments in an orthogonal network.
Surface topology and cytoskeleton of activated mouse platelets.
Mouse platelets were examined for shape change by electron microscopy. WT (A) and SLP-76–deficient (B, C) platelets were activated on a CRP-coated coverslip surface, as in Figure 2. WT (D) and SLP-76–deficient (E, F) platelets were attached to coverslips and activated with 1 U/mL thrombin. Cells were either fixed (A, B, D, E) or permeabilized with Triton X-100 in PHEM buffer (C, F), rapidly frozen, freeze-dried, and metal-coated. SLP-76–deficient platelets exposed to CRP retain their disc shapes, though they grow a small number of short filopodia. The cytoskeleton is similar to that of the normal resting mouse platelet cytoskeleton. SLP-76–deficient platelets activated with thrombin spread normally on the surface. The cytoskeleton has rearranged from the resting form and has a cortex composed of short filaments in an orthogonal network.
PI 3-kinase inhibition delays the mobilization of platelet Ca++ induced by CRP
PI 3-kinase is required for platelet spreading on fibrinogen-coated surfaces mediated by ADP.11 We therefore investigated the role of PI 3-kinase in the platelet shape changes mediated by CRP. We compared tyrosine phosphorylation of PLC-γ2 and Ca++ mobilization induced by CRP in mouse SLP-76–deficient platelets and in human platelets preincubated with 50 nmol/L wortmannin or 25 μmol/L LY294002 to inhibit PI 3-kinase. Similar results were found with wortmannin and LY294002; only the results obtained with wortmannin are presented.
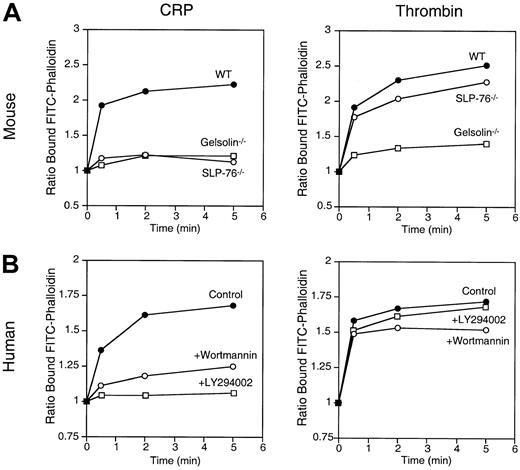
CRP failed to induce tyrosine phosphorylation of PLC-γ2 and Ca++ mobilization in SLP-76–deficient platelets (Figure4A,B). In contrast, wortmannin did not affect tyrosine phosphorylation of PLC-γ2 but delayed Ca++ mobilization induced by CRP (Figure 4D,E). However, it is noteworthy that SLP-76–deficient mouse platelets and human platelets preincubated with wortmannin mobilized Ca++normally in response to thrombin (Figure 4C,F).14 This is consistent with Ca++ mobilization through the activation of a G-protein–coupled PLC-β in platelets stimulated by thrombin.3 4
Platelet Ca++ mobilization induced by CRP requires SLP-76 and is delayed by PI 3-kinase inhibition.
The roles of SLP-76 and PI 3-kinase on PLC-γ2 tyrosine phosphorylation and Ca++ mobilization were studied in SLP-76–deficient mouse platelets (−/−) (upper panels) and in human platelets preincubated for 15 minutes with 50 nmol/L wortmannin (+Wort.) (lower panels). (A, D) Platelets were stimulated with 3 μg/mL CRP for 2 minutes. PLC-γ2 immunoprecipitates were resolved by 8% SDS-PAGE and probed with a 1:1 mixture of 4G10 and PY20 anti-phosphotyrosine antibodies. Equal loading of PLC-γ2 was verified by Western blotting (not shown). Results shown are representative of 2 experiments. Indo1-am–preloaded platelets were stimulated for 3 minutes with either 3 μg/mL CRP (B, E) or 1 U/mL thrombin (C, F). [Ca++]i was calculated as described in the experimental procedures section. Results shown are representative of 3 experiments.
Platelet Ca++ mobilization induced by CRP requires SLP-76 and is delayed by PI 3-kinase inhibition.
The roles of SLP-76 and PI 3-kinase on PLC-γ2 tyrosine phosphorylation and Ca++ mobilization were studied in SLP-76–deficient mouse platelets (−/−) (upper panels) and in human platelets preincubated for 15 minutes with 50 nmol/L wortmannin (+Wort.) (lower panels). (A, D) Platelets were stimulated with 3 μg/mL CRP for 2 minutes. PLC-γ2 immunoprecipitates were resolved by 8% SDS-PAGE and probed with a 1:1 mixture of 4G10 and PY20 anti-phosphotyrosine antibodies. Equal loading of PLC-γ2 was verified by Western blotting (not shown). Results shown are representative of 2 experiments. Indo1-am–preloaded platelets were stimulated for 3 minutes with either 3 μg/mL CRP (B, E) or 1 U/mL thrombin (C, F). [Ca++]i was calculated as described in the experimental procedures section. Results shown are representative of 3 experiments.
CRP-ligation of GPVI/FcRγ-chain causes platelets to spread lamellae and assemble actin filaments by mechanisms dependent on PI 3-kinase
Although wortmannin treatment does not prevent the growth of filopodia induced by CRP, it does inhibit the extension of lamellae between filopodia grown by CRP-activated platelets (Figure5A). Quantification of these data shows that the formation of lamellae is completely inhibited in human platelets first treated by wortmannin (Table1). Similar results were obtained with LY294002 (data not shown). As described for the spreading of platelets on fibrinogen-coated surfaces mediated by ADP,11 lamellae normally spread by CRP do not form between filopodia after the inhibition of PI 3-kinase.
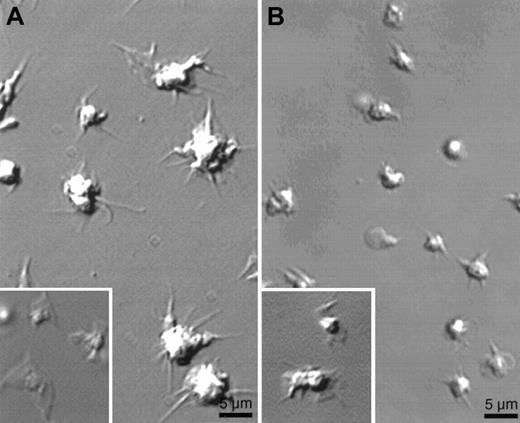
Absence of lamellae in human platelets preincubated with wortmannin or in gelsolin-deficient mouse platelets.
Platelets stimulated as in Figure 1 were examined for shape change by video microscopy. (A) Effect of 50 nmol/L wortmannin preincubated for 15 minutes on the shape change of human platelets exposed to a CRP-coated surface. (Inset) Shape change of human platelet on CRP-coated surface in the absence of wortmannin. (B) Gelsolin-deficient mouse platelets activated on a CRP-coated surface. (Inset) Mouse WT platelets on a CRP-coated surface. Images were taken after 15 minutes of activation. Results shown are representative of 3 experiments.
Absence of lamellae in human platelets preincubated with wortmannin or in gelsolin-deficient mouse platelets.
Platelets stimulated as in Figure 1 were examined for shape change by video microscopy. (A) Effect of 50 nmol/L wortmannin preincubated for 15 minutes on the shape change of human platelets exposed to a CRP-coated surface. (Inset) Shape change of human platelet on CRP-coated surface in the absence of wortmannin. (B) Gelsolin-deficient mouse platelets activated on a CRP-coated surface. (Inset) Mouse WT platelets on a CRP-coated surface. Images were taken after 15 minutes of activation. Results shown are representative of 3 experiments.
Quantification of the area of lamellae after platelet activation by CRP
| . | Lamellar area ± SD (μm2) . |
|---|---|
| Human | |
| Control | 6.38 ± 4.39 (n = 74) |
| +50 nmol/L wortmannin | 0.22 ± 0.02 (n = 81) |
| Mouse | |
| Gelsolin+/+ | 3.65 ± 2.45 (n = 99) |
| Gelsolin−/− | 0.55 ± 0.46 (n = 128) |
| . | Lamellar area ± SD (μm2) . |
|---|---|
| Human | |
| Control | 6.38 ± 4.39 (n = 74) |
| +50 nmol/L wortmannin | 0.22 ± 0.02 (n = 81) |
| Mouse | |
| Gelsolin+/+ | 3.65 ± 2.45 (n = 99) |
| Gelsolin−/− | 0.55 ± 0.46 (n = 128) |
Platelets stimulated as in Figure 5 were examined by video microscopy. Images were taken after 10 minutes of activation, and lamellar areas were measured in video frames using the NIH image analysis program. Results shown are the total of 3 experiments for each condition of activation.
Platelet shape change is associated with an increase in cellular F-actin content.5 Actin assembly begins immediately after the addition of CRP and reaches a maximum of 70% of the total actin within 60 seconds (Figure 6). Both CRP and thrombin stimulated a 2-fold increase in the F-actin content of both mouse WT and human platelets. Although PI 3-kinase inhibition does not prevent filopodial growth as discussed (Figure 5A), wortmannin or LY294002 prevents measurable actin assembly by CRP, suggesting that the bulk of the actin assembly measured derives from the lamellar assembly that fills the spaces between filopodia. Only thrombin, but not CRP, increased the F-actin content of SLP-76–deficient platelets. Actin assembly is markedly reduced in SLP-76–deficient platelets activated with CRP, consistent with the essential role of SLP-76 in early steps of the signaling pathway of GPVI/FcRγ-chain (Figure4).15
Actin assembly in mouse and human platelets activated by CRP.
(A) F-actin content of CRP- and thrombin-activated mouse platelets. Platelets from WT, SLP-76–deficient mice, and gelsolin-deficient mice activated with 3 μg/mL CRP or 1 U/mL thrombin were fixed with 3.4% formaldehyde, permeabilized with 0.1% Triton X-100 containing 2 μmol/L FITC-phalloidin, and analyzed by FACS. (B) F-actin content of CRP- and thrombin-activated human platelets preincubated with or without 50 nmol/L wortmannin or 25 μmol/L LY294002 for 15 minutes. Results are expressed as the ratio between the fluorescence of activated versus resting cells. Results shown are representative of 3 experiments.
Actin assembly in mouse and human platelets activated by CRP.
(A) F-actin content of CRP- and thrombin-activated mouse platelets. Platelets from WT, SLP-76–deficient mice, and gelsolin-deficient mice activated with 3 μg/mL CRP or 1 U/mL thrombin were fixed with 3.4% formaldehyde, permeabilized with 0.1% Triton X-100 containing 2 μmol/L FITC-phalloidin, and analyzed by FACS. (B) F-actin content of CRP- and thrombin-activated human platelets preincubated with or without 50 nmol/L wortmannin or 25 μmol/L LY294002 for 15 minutes. Results are expressed as the ratio between the fluorescence of activated versus resting cells. Results shown are representative of 3 experiments.
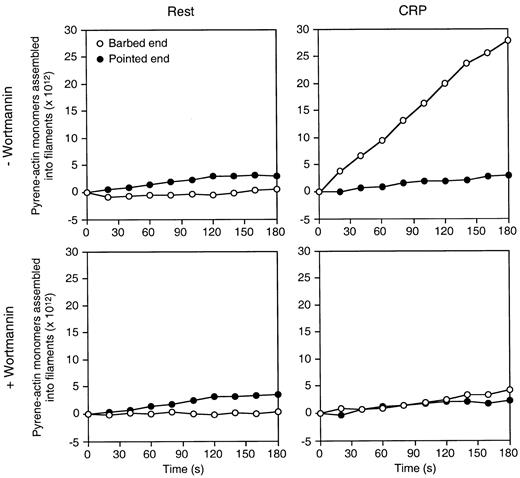
In the case of thrombin receptor, actin assembly after ligation correlates with the temporal exposure of actin filament barbed ends.5 CRP treatment of platelets also increases the number of barbed ends to a maximum of 400 per cell. Wortmannin decreases this number by 80% but fails to completely prevent barbed-end exposure after CRP (Figure 7). Wortmannin-insensitive nucleation sites may therefore drive the assembly of actin into filopodia, but, under these conditions, the actin disassembly and assembly rates are balanced.
Wortmannin inhibits the exposure of actin filament barbed ends in human platelets activated by CRP.
The rate of pyrene-actin assembly in lysates from resting and CRP-activated platelets was determined. Platelets were preincubated with or without 50 nmol/L wortmannin for 15 minutes. Resting or CRP-activated platelets for 2 minutes, permeabilized with Triton X-100, were added to 1 μmol/L pyrene-actin monomer to initiate actin assembly. Activity inhibited by 2 μmol/L cytochalasin B is defined as barbed-end actin assembly. Activity not inhibited by cytochalasin B is defined as pointed-end actin assembly. Results shown are representative of 4 experiments.
Wortmannin inhibits the exposure of actin filament barbed ends in human platelets activated by CRP.
The rate of pyrene-actin assembly in lysates from resting and CRP-activated platelets was determined. Platelets were preincubated with or without 50 nmol/L wortmannin for 15 minutes. Resting or CRP-activated platelets for 2 minutes, permeabilized with Triton X-100, were added to 1 μmol/L pyrene-actin monomer to initiate actin assembly. Activity inhibited by 2 μmol/L cytochalasin B is defined as barbed-end actin assembly. Activity not inhibited by cytochalasin B is defined as pointed-end actin assembly. Results shown are representative of 4 experiments.
CRP-induced lamellae formation and actin assembly require gelsolin expression
The efficient extension of lamellae in platelets activated by thrombin requires gelsolin.6 7 In similar fashion, filopods grow from the surfaces of gelsolin-deficient platelets when they contact a CRP-coated surface, but these platelets reproducibly fail to extend lamellae between the filopodia (Figure 5B). Table 1shows that mouse platelets lacking gelsolin and activated on CRP-coated surfaces spread poorly, and lamellae extension was reduced by 85% compared to WT platelets. Before filopods formed, the surfaces of gelsolin-deficient platelets rippled and undulated, and small blebs were formed and retracted. As for thrombin, actin assembly was also markedly reduced in gelsolin-deficient platelets activated with CRP (Figure 6).
Discussion
Platelet shape changes induced by trimeric G-protein–coupled receptors such as the thrombin receptor are widely studied.5,8 These changes result in lamellar extension and cell spreading by a mechanism requiring gelsolin for actin filament severing and actin assembly onto sites equivalent to filament barbed-end sites.6,7 In the current work, we studied how platelets change shape when activated by GPVI/FcRγ-chain, an ITAM-containing receptor coupled to Syk, SLP-76, and PI 3-kinase.13,15 29 The results show that platelets activated through GPVI/FcRγ-chain first rapidly round, and then grow dynamic projections that rotate around the periphery of the cell. Small, thin lamellae subsequently spread between the projections (Figures 1, 2).
To understand the signaling pathways leading to platelet shape changes downstream of GPVI/FcRγ-chain, we compared tyrosine phosphorylation of PLC-γ2 and Ca++ mobilization induced by CRP in normal and in SLP-76–deficient mouse platelets and in human platelets preincubated with and without wortmannin or LY294002. We found that CRP fails to induce tyrosine phosphorylation of PLC-γ2, Ca++mobilization, and shape change in SLP-76–deficient platelets (Figures3, 4). In contrast, Ca++ mobilization induced by CRP is delayed by wortmannin, but not inhibited. Most platelet responses are only partially affected in normal platelets treated with wortmannin. Therefore, we confirm that SLP-76 is essential for platelet activation by GPVI/FcRγ-chain and show that the activation of PLC-γ2 depends on SLP-76–dependent tyrosine phosphorylation.15 The lipid products of PI 3-kinase may only modulate PLC-γ2 activation, as described for the signaling pathway of FcγRIIA, another platelet ITAM-containing receptor.27,36 This is consistent with recent observations by Watson's group.26,37 However, the 2 signaling pathways leading to full activation of PLC-γ2, its tyrosine phosphorylation mediated by Syk and SLP-76, and its translocation to the membrane mediated by PI 3-kinase are not exclusive. PI 3-kinase may act downstream of SLP-76 in platelets stimulated by GPVI/FcRγ-chain. Syk is upstream of PI 3-kinase in B-cell receptor signaling.38 Direct measurements of the PI 3-kinase activity in CRP-activated SLP-76–deficient platelets are required to confirm this hypothesis.
Our study shows that the formation of lamellae normally mediated by CRP is blocked by wortmannin (Figure 5, Table 1). Hence, though PI 3-kinase is not required for the platelet spreading mediated by thrombin,10 it is required for lamellae spreading on CRP-coated surfaces. This requirement is similar to platelet activation by the fibrinogen receptor, the integrin αIIbβ3. PI 3-kinase is involved in spreading of lamellae on fibrinogen-coated surfaces mediated by ADP11 and in platelet actin assembly initiated by αIIbβ3.10 As for αIIbβ3, the extension of lamellae initiated by GPVI/FcRγ-chain requires net actin assembly and an exposure of actin filament barbed ends downstream of PI 3-kinase (Figures 5, 6, 7).
Formation of lamellae induced by GPVI/FcRγ-chain also requires gelsolin for maximal actin filament barbed-end exposure, actin assembly, and lamellae spreading (Figures 5, 6, Table 1). Our results suggest that gelsolin may act downstream of PI 3-kinase in the signaling pathway of GPVI/FcRγ-chain. Lamellae are composed of orthogonal arrays of short filaments. The extension of lamellae observed in platelets activated by thrombin requires the severing by gelsolin of actin filaments present in the resting cell, the formation of barbed-end nucleation sites, and the addition of actin monomers to these nucleation sites to double the F-actin content.5Moreover, 75% of the platelet actin nucleation activity in the active cell derives from Ca++-activated, gelsolin-based filament fragmentation and capping, subsequently uncapped by membrane ppIs.6,7 Production of ppIs requires the activation of the small GTPase Rac,8 whose rapid and robust activation follows ligation of the thrombin receptor and correlates with actin assembly kinetics.9 PI 3-kinase may be involved in the activation of Rac downstream of GPVI/FcRγ-chain, as has been shown for growth factor receptors.39-41 The lipid products of PI 3-kinase also interact with Rac and stimulate GDP dissociation from Rac.42
Filopodial growth induced by CRP is not inhibited in human platelets treated with wortmannin or in mouse gelsolin-deficient platelets (Figure 5). Wortmannin or LY294002 treatment, however, prevents a large amount of the actin assembly induced by CRP (Figure 6), suggesting that the bulk of measurable actin assembly derives from the lamellar assembly that fills the spaces between filopodia, but not from the formation of filopodia themselves. Although wortmannin decreases the number of nucleation sites by 80%, it fails to completely stop barbed-end exposure after CRP (Figure 7). Therefore, the wortmannin-insensitive nucleation sites may drive the assembly of filopodia under conditions in which actin filament disassembly and assembly rates balance.
Our observations that SLP-76–deficient platelets fail to spread and increase their F-actin content may also suggest that SLP-76 plays a role in the reorganization of the actin cytoskeleton triggered by engagement of GPVI/FcRγ-chain. SLP-76 interacts with Fyb/SLAP-130 after platelet stimulation by GPVI/FcRγ-chain,43 and Fyb/SLAP-130 is found exclusively at the front of lamellipodia of glass-activated platelets, where it co-localizes with VASP.44 After ligation of the T-cell receptor, SLP-76 also interacts with Fyb/SLAP-13023,24 and is present in a biochemical complex containing Nck and the Wiskott-Aldrich syndrome protein (WASP).44 Krause et al propose that SLP-76, Fyb/SLAP-130, Nck, and WASP bring together VASP and the Arp2/3 complex in T cells to nucleate actin and to remodel the actin cytoskeleton.44 The role of WASP and the Arp2/3 complex in platelet shape changes and actin assembly is under investigation in our laboratory.
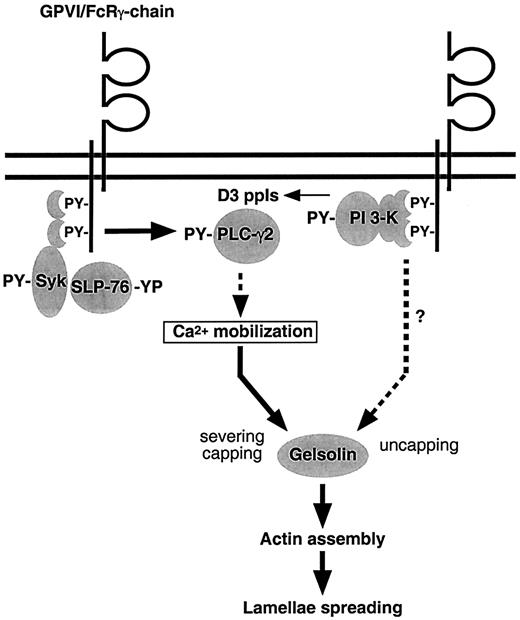
In summary, SLP-76 is essential for the signaling pathways leading to platelet shape changes initiated by GPVI/FcRγ-chain. Moreover, PI 3-kinase only modulates these events but is required for actin filament barbed end exposure and actin assembly (Figure8). As for activation through the thrombin receptor, gelsolin is required for actin assembly and formation of lamellae induced by CRP. The results suggest that, though GPVI/FcRγ-chain and the thrombin receptor differ in their signaling cascades, they share common pathways leading to platelet shape changes.
Scheme for signal transduction from the platelet collagen receptor GPVI/FcRγ-chain complex to the actin cytoskeleton.
Engagement of GPVI/FcRγ-chain activates Syk, which phosphorylates SLP-76. SLP-76 recruits PLC-γ2 to the plasma membrane, where Syk phosphorylates and activates it. This mediates Ca++mobilization, resulting in the activation of gelsolin, which severs the actin filaments and caps the barbed ends. Engagement of GPVI/FcRγ-chain also results in PI 3-kinase activation. PI 3-kinase mediates the uncapping of the actin filament barbed ends by a mechanism possibly requiring Rac and a new production of ppIs. This induces the actin assembly required for the formation of lamellae.
Scheme for signal transduction from the platelet collagen receptor GPVI/FcRγ-chain complex to the actin cytoskeleton.
Engagement of GPVI/FcRγ-chain activates Syk, which phosphorylates SLP-76. SLP-76 recruits PLC-γ2 to the plasma membrane, where Syk phosphorylates and activates it. This mediates Ca++mobilization, resulting in the activation of gelsolin, which severs the actin filaments and caps the barbed ends. Engagement of GPVI/FcRγ-chain also results in PI 3-kinase activation. PI 3-kinase mediates the uncapping of the actin filament barbed ends by a mechanism possibly requiring Rac and a new production of ppIs. This induces the actin assembly required for the formation of lamellae.
Acknowledgments
We thank Jenny Bandura and Laurice Salib for technical assistance. We thank Drs Thomas P. Stossel, Karin M. Hoffmeister, and Eric Krump for helpful discussions and critical reading of the manuscript and Drs Richard W. Farndale and C. Graham Knight (Cambridge University, UK) for preparation and shipping of CRP. H.F. dedicates this work to the memory of his father, Maurice Falet (1940-1999), and of his friend, Eric Krump (1966-1999).
Supported by National Institutes of Health grants HL-56262 and HL-56949 (J.H.H.) and AI-35714 (R.S.G.) and by grants from Baxter HealthCare and Centeon Corporations.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John H. Hartwig, Division of Hematology, Brigham and Women's Hospital, 221 Longwood Avenue, LMRC 301, Boston, MA 02115; e-mail: hartwig@calvin.bwh.harvard.edu.


![Fig. 4. Platelet Ca++ mobilization induced by CRP requires SLP-76 and is delayed by PI 3-kinase inhibition. / The roles of SLP-76 and PI 3-kinase on PLC-γ2 tyrosine phosphorylation and Ca++ mobilization were studied in SLP-76–deficient mouse platelets (−/−) (upper panels) and in human platelets preincubated for 15 minutes with 50 nmol/L wortmannin (+Wort.) (lower panels). (A, D) Platelets were stimulated with 3 μg/mL CRP for 2 minutes. PLC-γ2 immunoprecipitates were resolved by 8% SDS-PAGE and probed with a 1:1 mixture of 4G10 and PY20 anti-phosphotyrosine antibodies. Equal loading of PLC-γ2 was verified by Western blotting (not shown). Results shown are representative of 2 experiments. Indo1-am–preloaded platelets were stimulated for 3 minutes with either 3 μg/mL CRP (B, E) or 1 U/mL thrombin (C, F). [Ca++]i was calculated as described in the experimental procedures section. Results shown are representative of 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3786/5/m_h82300406004.jpeg?Expires=1769210318&Signature=Y2bVkmPQdx8q35ahSvbBKOkGPx-IKGc9h-3MJyvmM9BSW3K3~-6vaZvVIFVR5MV~OIQCrYAWq1lQxITBbeE73INU~KoNJDUay8Ray6sl1XRKNYnLcTV5~s90Ao~4OD9HL9Q2kvKDJNlK4fFA2IaCleVKdb1dkhlC0WYQfWB-LKwcinFaS2lkktjYAzmXYrQ5wBbGNNbTEwJdvfR2rHKyIJ0Ua2x-IE2fVs0-Yehc4o9bP1RX7VJI5L7zKYIbx61CWJKTI11W4wUzavI1ZwkM9cauM8sf7kq7hwI5a5EHER~y72da81Gh~dLuly9Ud~zsc2A1GO32SJBalJcme8IQTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal