Abstract
Vascular development and response to injury are regulated by several cytokines and growth factors including the members of the fibroblast growth factor and vascular endothelial cell growth factor (VEGF) families. Fibrinogen and fibrin are also important in these processes and affect many endothelial cell properties. Possible specific interactions between VEGF and fibrinogen that could play a role in coordinating vascular responses to injury are investigated. Binding studies using the 165 amino acid form of VEGF immobilized on Sepharose beads and soluble iodine 125 (125I)–labeled fibrinogen demonstrated saturable and specific binding. Scatchard analysis indicated 2 classes of binding sites with dissociation constants (Kds) of 5.9 and 462 nmol/L. The maximum molar binding ratio of VEGF:fibrinogen was 3.8:1. Further studies characterized binding to fibrin using 125I-labeled VEGF- and Sepharose-immobilized fibrin monomer. These also demonstrated specific and saturable binding with 2 classes of sites havingKds of 0.13 and 97 nmol/L and a molar binding ratio of 3.6:1. Binding to polymerized fibrin demonstrated one binding site with a Kd of 9.3 nmol/L. Binding of VEGF to fibrin(ogen) was independent of FGF-2, indicating that there are distinct binding sites for each angiogenic peptide. VEGF bound to soluble fibrinogen in medium and to surface immobilized fibrinogen or fibrin retained its capacity to support endothelial cell proliferation. VEGF binds specifically and saturably to fibrinogen and fibrin with high affinity, and this may affect the localization and activity of VEGF at sites of tissue injury.
Introduction
Activation of the coagulation system leads to fibrin formation, which both stabilizes the hemostatic plug and provides the temporary matrix required for subsequent cellular responses of wound and vessel repair. In these processes the role of fibrin is not passive, but rather it actively directs cellular responses through specific receptor-mediated interactions with cells of the blood and vessel wall. These result in fibrin-specific responses of endothelial cells including adhesion and spreading,1proliferation,2 protein synthesis3 and secretion,4 and angiogenesis.5 Fibrin is also a component of the stroma of many tumors and is deposited as a result of increased vascular permeability, extravasation of plasma protein, and the presence of tissue factor and other procoagulants associated with tumors.6,7 Fibrinogen and fibrin are also implicated in the development, progression, and thrombotic complications of atherosclerosis.8
The angiogenic peptide vascular endothelial growth factor (VEGF), also known as vascular permeability factor, stimulates both increased microvascular permeability, which leads to local fibrin formation, and also endothelial cell proliferation needed for angiogenesis.9 There are 5 different human VEGF isoforms consisting of monomers of 121, 145, 165, 189, and 206 amino acids that are derived from a single gene by alternative splicing.10The active forms of VEGF are homodimers, and the best characterized VEGF species is the heparin-binding 165 amino acid form.10,11 VEGF is a multifunctional cytokine for endothelial cells expressing VEGF receptor-1 (flt-1)12 and receptor-2 (flk-1/KDR),13,14 and it exerts several effects including von Willebrand factor release,15induction of the expression of integrins,16,17interstitial collagenase,18 plasminogen activators, plasminogen activator receptor, plasminogen activator inhibitor-1,19-21 and stimulating migration of endothelial cells.22 VEGF also induces enhanced tissue factor expression23 and stimulates migration of monocytes expressing the VEGF receptor-1.24 The role of VEGF in vessel injury and repair is further supported by evidence that VEGF synthesis and release from vessel wall cells increase after injury25 and that VEGF messenger RNA (mRNA) is upregulated in atherosclerotic arteries.26 Recent in vitro and in vivo studies have revealed that the transformation of endothelial cells to an angiogenic phenotype is induced by several factors, including VEGF, fibroblast growth factors (FGFs), hepatocyte growth factor, interleukin-8, and others,27 but the interaction between VEGF and its receptors is the most important angiogenic event in the physiological and pathological angiogenesis.
The need for fibrin to support endothelial cell spreading, migration, and angiogenesis and the potent stimulation of similar angiogenic responses by VEGF suggest that these processes may be interrelated. This concept is supported by evidence that fibrin clots provide a good matrix to support VEGF-stimulated angiogenesis in vitro28and that angiogenic peptide FGF-2 binds specifically to fibrinogen and fibrin.29 We have, therefore, investigated the association of VEGF with fibrinogen and fibrin. The results demonstrate specific and saturable high-affinity binding of functionally active VEGF with implications for colocalization of angiogenic peptides at sites of injury and for distribution of pharmacologically administered VEGF.
Materials and methods
Protein preparation
Plasminogen-free human fibrinogen was purchased from Enzyme Research Laboratories (South Bend, IN), and fibronectin in the preparation was depleted by chromatography on gelatin-Sepharose30 (Pharmacia, Inc, Piscataway, NJ). Fibrinogen eluting from the gelatin-Sepharose column was further depleted of fibronectin by immunoaffinity chromatography, as described elsewhere,1 and the final preparation contained less than 0.02 ng/mL fibronectin as determined by enzyme-linked immunosorbent assay (ELISA) (American Diagnostica, Greenwich, CT) at 1 mg/mL fibrinogen. Radio-iodination of fibrinogen to a specific activity of 1.9 × 108 cpm/mg was performed using the iodogen method,31 and unbound iodine 125 (125I) was removed following chromatography on Sephadex G-10 (Pharmacia). The study used the following: human thrombin (3250 National Institutes of Health U/mg) (Calbiochem-Novabiochem, San Diego, CA); human recombinant VEGF and polyclonal antibody to human VEGF (Peprotech, Inc, Princeton, NJ); an ELISA for VEGF (R&D Systems, Minneapolis, MN); and 0.148 MBq/μg (4 μCi/μg)125I-VEGF (NEN Life Science Products, Boston, MA).
Binding of fibrinogen to immobilized VEGF
VEGF was immobilized on Affigel-15 beads (Bio-Rad, Hercules, CA) as described previously.29 Briefly, 1 mL purified 200 μg/mL polyclonal anti-VEGF antibody was incubated with 1 mL Affigel-15, which consisted of a derivatized cross-linked agarose gel bead support with active N-hydroxy succinimide esters, in 0.1 mol/L sodium phosphate buffer (SPB) (pH 7.4) containing 0.25 mol/L sodium chloride (NaCl) and gently mixed at 25°C for 2 hours. Over 97% of the antibody was bound to the beads. Residual active ester sites were then blocked by the addition of 1 mol/L ethanolamine (pH 8.0), and the suspension was washed several times with 0.1 mol/L SPB (pH 7.4) containing 0.25 mol/L NaCl. We then added 50 μg/mL VEGF to this suspension and gently mixed at 25°C for 1 hour, following which unbound VEGF was removed by washing with 0.1 mol SPB (pH 7.4) containing 0.25 mol/L NaCl. The amount of VEGF immobilized on the beads was 7.7 μg/mL, as determined by ELISA. For binding studies,125I-fibrinogen at concentrations from 2.5-300 nmol/L was incubated at 37°C with a 0.02 mL suspension of immobilized VEGF in a final volume of 0.1 mL. Non-specific binding was determined in parallel experiments using a 10-fold molar excess of unlabeled fibrinogen. Preliminary experiments demonstrated maximum specific binding after 30 minutes incubation in 0.1 mol/L SPB (pH 7.4) containing 0.25 mol/L NaCl, and these conditions were used for all subsequent experiments. Following incubation, the beads were separated by centrifugation at 5000g for 10 minutes, after which the supernatant was removed, and the beads were then washed rapidly twice with 0.1 mol/L SPB (pH 7.4) containing 0.25 mol/L NaCl at 4°C to minimize non-specific association. The amount of bound fibrinogen was calculated from the radioactivity associated with the beads.
To characterize the protein that was bound to immobilized VEGF, 1 mg/mL125I-fibrinogen was passed through a column of immobilized VEGF. The column was washed with 0.1 mol/L SPB (pH 7.4) containing 0.25 mol/L NaCl to remove unbound radioactivity. Bound protein was then eluted by the addition of 2 mg/mL unlabeled fibrinogen or ovalbumin as a control, and 200 μL aliquots were collected and counted. Aliquots of selected fractions were electrophoresed on sodium dodecyl sulfate (SDS) 7% polyacrylamide gels after disulfide bond reduction, dried, and used to prepare autoradiograms.
Binding of 125I-VEGF to fibrin monomer
A similar approach was used with incubation of125I-VEGF with fibrin monomer immobilized on Sepharose beads. Affigel-15 beads were first incubated with 1 mg/mL purified monoclonal antibody J88B, which is directed against a site within the sequence arganine 63–methionine 78 (Arg63-Met78) of the human fibrinogen γ-chain,32 in 0.2 mol/L sodium bicarbonate buffer (pH 8.3) and gently mixed at 25°C for 2 hours. Residual sites were blocked by incubation in 1 mol/L ethanolamine (pH 8.0), and suspension was washed several times with 0.2 mol/L sodium bicarbonate buffer (pH 8.3) containing 0.25 mol/L NaCl. Gel containing bound antibody was then incubated with 200 μg/mL fibrinogen in SPB (pH 7.4) containing 0.25 mol/L NaCl to remove unbound fibrinogen. This was continued until no further fibrinogen was removed, as determined by monitoring the optical density at 280 nmol/L. To convert bound fibrinogen to fibrin monomer, beads were incubated with 0.5 U/mL thrombin at 37°C for 90 minutes. Characterization of binding of125I-VEGF to fibrin monomer was performed in the same way as 125I-fibrinogen binding to immobilized VEGF (above).125I-VEGF at concentrations from 0.1-100 nmol/L was incubated with a 0.02-mL suspension of beads containing 0.1 μg fibrin in a final volume of 0.1 mL. Non-specific binding was determined in parallel experiments using a 10-fold molar excess of unlabeled VEGF. Specificity of the binding of VEGF to fibrin was confirmed by competition experiments in which 0.1 nmol/L 125I-VEGF was incubated with 1 μg/mL immobilized fibrin monomer in a final volume of 0.1 mL, and the binding was competitively inhibited by unlabeled VEGF at concentrations from 0.1-100 nmol/L.
Binding of VEGF to polymerized fibrin
125I-VEGF at concentrations of 0.1-100 nmol/L was added to 100 μg/mL fibrinogen in 0.1 mol/L Tris (tris[hydroxymethyl] aminomethane) buffer containing 0.25 mol/L NaCl. Thrombin was then added to a final concentration of 0.5 U/mL, which resulted in clotting of the solution. Following incubation at 37°C for 30 minutes, the clot and supernatant were separated by vacuum filtration using GF/C glass micro fiber filters (Sigma Chemical, St Louis, MO) previously soaked overnight in a solution of 0.5% polyvinylpyrolidone and 0.1% Tween-20 to reduce non-specific binding. The clot on the filter was washed quickly with cold 0.1 mol/L Tris buffer containing 0.25 mol/L NaCl, and associated radio-label was measured. Non-specific binding was determined in parallel experiments incorporating a 10-fold molar excess of unlabeled VEGF.
Preparation of fibrinogen- and fibrin-coated surfaces
Cell culture wells were coated by incubation for 1 hour at 25°C with 0.4 mL of 10 μg/mL fibrinogen in McCoy 5A medium (Flow Laboratories, McLean, VA). Excess fibrinogen solution was aspirated, and the wells were washed twice with McCoy 5A medium before the cells were plated. Fibrin-coated wells were prepared using 1 mg/mL fibrinogen in McCoy 5A medium to which 1 U/mL thrombin (Calbiochem-Novabiochem) was added, mixed, and using a pipette, rapidly added to 12-well nontissue culture-treated cell culture plates. The solution was aspirated after 45 seconds and before polymerization, thereby leaving a thin coating of fibrin on the surface. Wells coated with fibrinogen or fibrin with VEGF were prepared in the same way except that 20 ng/mL VEGF was added to the fibrinogen solution and incubated for 20 minutes at 37°C prior to coating wells. Fibrin-coated wells were treated with 1 μg/mL D-phenylalanyl-L-prolyl-L-arginylchloromethyl ketone (Bachem, Torrance, CA), a synthetic specific thrombin inhibitor, for 30 minutes to inhibit any remaining thrombin, and this was followed by 2 washes with McCoy 5A medium before plating the cells.
Cell culture
Primary endothelial cells were obtained from human umbilical veins as described previously;33 seeded on 0.2% wt/vol gelatin-coated 25-cm2 tissue culture flasks; and cultured in McCoy 5A medium containing 20% fetal bovine serum (FBS), 50 μg/mL endothelial cell growth supplement (ECGS) (Collaborative Research, Inc, Bedford, MA), and 100 μg/mL heparin (Sigma) until they reached confluence, typically within 4 to 5 days. The cells were passaged up to 2 times before use and then placed in suspension by trypsinization of monolayers. Cells were suspended by rinsing in Hanks balanced salt solution (HBSS) followed by brief incubation with trypsin-EDTA (ethylenediamine tetraacetic acid) (Gibco Life Technologies, Inc, Grand Island, NY). The cells were pelleted by centrifugation for 10 minutes at 500g and resuspended in McCoy 5A medium in the absence of serum. This wash procedure was repeated twice prior to use in experimental protocols.
Hydrogen 3–thymidine incorporation
Approximately 2 × 104 human umbilical vein endothelial cells suspended in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL endothelial cell growth supplement, and 100 μg/mL heparin were plated in gelatin-coated 12-well plates (Becton Dickinson & Company, Rutherford, NJ) and allowed to adhere for 6 hours. The medium was then removed, and the cells were washed twice with McCoy 5A medium. We then added serum-free medium containing 1% Nutridoma (Boehringer Mannheim, Indianapolis, IN), 20 ng/mL VEGF, or 25 ng/mL FGF-2 and 0.037 MBq/mL (1 μCi/mL) hydrogen 3 (3H)–thymidine (New England Nuclear, Boston, MA) in the presence or absence of 10 μg/mL fibrinogen. After incubation at 37°C for 24 hours, nonadherent cells were removed by washing twice with ice-cold phosphate-buffered saline. Then 500 μL 10% ice-cold trichloroacetic acid was added to each well, and precipitates were collected on a filter using a filtration manifold. Filters were washed twice with ice-cold 5% trichloroacetic acid (TCA) followed by 95% ethanol, allowed to air dry, and then suspended in scintillation fluid. Acid precipitable counts per minute were quantitated using a scintillation counter.
Data analysis
Unless otherwise indicated, the data are expressed as the mean ± SD. Scatchard analysis of the data was performed using the Ligand program34 (Biosoft, Ferguson, MO). Each experiment was performed at least 3 times, and either triplicate or quadruplicate wells were used in each experiment. The SD in means was determined using a 2-tailed Student t test.
Results
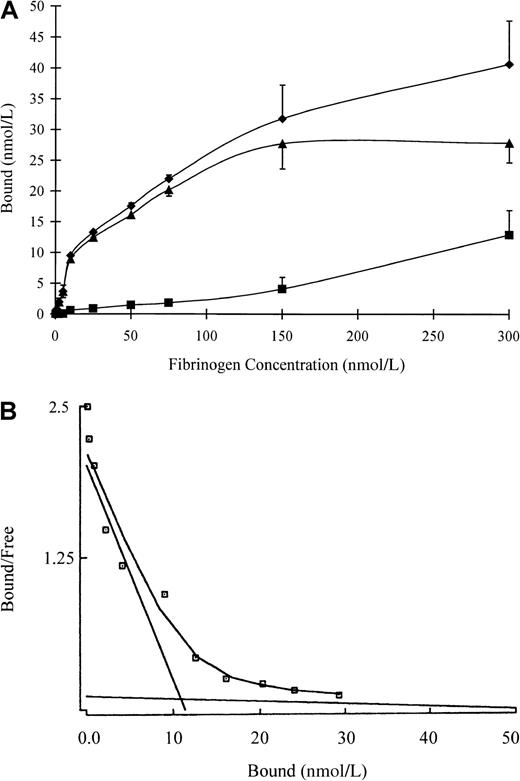
Binding of fibrinogen to immobilized VEGF was saturable and specific, with non-specific binding representing less than 20% of the total (Figure 1A). Saturation of specific binding occurred at a fibrinogen concentration of 150 nmol/L, and only an increase in non-specific binding was observed at higher concentrations. In control experiments there was a maximum of 5% binding of 125I-fibrinogen over the same range of concentrations to beads with immobilized anti-VEGF immunoglobulin only or to beads with no protein-bound and active sites blocked with ethanolamine. A plot of bound versus bound-free fibrinogen (Figure 1B) was nonlinear, thereby suggesting the presence of more than one binding site. This was confirmed by Scatchard analysis, which indicated that binding was best described by a 2-site model with apparentKds of 5.9 and 462 nmol/L. Bmax was 12 and 53 nmol/L for the high- and low-affinity sites, respectively, and the maximum molar binding ratio of VEGF:fibrinogen was 3.8:1.
Binding of fibrinogen to VEGF.
(A) 125I-fibrinogen was incubated with VEGF immobilized on Sepharose beads, and the amount of bound protein was determined as radioactivity associated with the beads following centrifugation and washing. Non-specific binding (squares) was determined in the same way in the presence of a 10-fold molar excess of unlabeled VEGF. Specific binding (triangles) was calculated by subtracting the non-specific from the total bound (diamonds). Each point represents the mean ± SD of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined by analysis using the Ligand program, and it is consistent with the presence of 2 binding sites of different affinities.
Binding of fibrinogen to VEGF.
(A) 125I-fibrinogen was incubated with VEGF immobilized on Sepharose beads, and the amount of bound protein was determined as radioactivity associated with the beads following centrifugation and washing. Non-specific binding (squares) was determined in the same way in the presence of a 10-fold molar excess of unlabeled VEGF. Specific binding (triangles) was calculated by subtracting the non-specific from the total bound (diamonds). Each point represents the mean ± SD of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined by analysis using the Ligand program, and it is consistent with the presence of 2 binding sites of different affinities.
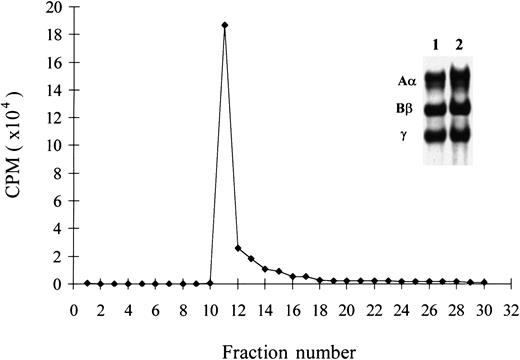
To further characterize the protein that bound to VEGF,125I-fibrinogen was passed over a column of immobilized VEGF. Following washing, the bound protein was eluted with 2 mg/mL unlabeled fibrinogen (Figure 2) and approximately 90% of bound radio-labeled protein rapidly eluted in 2 fractions. SDS-PGE (polyacrylamide gel electrophoresis) of the eluted protein showed bands consistent with the Aα, Bβ, and γ-chains of fibrinogen (Figure 2, inset), which establishes that the bound protein was fibrinogen and not a minor contaminant. In control experiments, less than 5% of bound radioactivity was eluted from the column with 2.0 mg/mL ovalbumin, which demonstrates specificity of the elution.
Elution of bound protein from immobilized VEGF.
125I-fibrinogen (1.0 mg/mL) was passed through a 1-mL column of Sepharose-immobilized VEGF. Following washing, the column was eluted with 2 mg/mL unlabeled fibrinogen, and fractions of 200 μL were collected. Approximately 90% of the bound radioactivity eluted in fraction 10 to 12. These fractions were pooled, and an aliquot was electrophoresed on a 7% SDS-PAGE gel and used to prepare autoradiograms (inset). The polypeptide chain pattern in the eluted pool showed Aα, Bβ, and γ chains of fibrinogen and was similar to that in the starting material.
Elution of bound protein from immobilized VEGF.
125I-fibrinogen (1.0 mg/mL) was passed through a 1-mL column of Sepharose-immobilized VEGF. Following washing, the column was eluted with 2 mg/mL unlabeled fibrinogen, and fractions of 200 μL were collected. Approximately 90% of the bound radioactivity eluted in fraction 10 to 12. These fractions were pooled, and an aliquot was electrophoresed on a 7% SDS-PAGE gel and used to prepare autoradiograms (inset). The polypeptide chain pattern in the eluted pool showed Aα, Bβ, and γ chains of fibrinogen and was similar to that in the starting material.
Conversion of fibrinogen to fibrin is mediated by thrombin that cleaves fibrinopeptides A and B from the Aα and Bβ chains, respectively, forming fibrin monomer which then polymerizes to form a branching network of fibers. To characterize the association of VEGF with fibrin in the absence of polymerization, we incubated Sepharose-immobilized fibrinogen with thrombin. The antibody J88B mediated the immobilization of fibrinogen to the Sepharose beads, thereby limiting polymerization of the resulting fibrin and forming a surface with immobilized fibrin monomer. Binding of VEGF to fibrin monomer was found to be of high affinity, with specific binding approaching saturation at concentrations between 25 and 50 nmol/L VEGF (Figure3A). Non-specific binding was low at VEGF concentrations below 25 nmol/L and increased at higher concentrations. The binding curve at concentrations below 1 nmol/L VEGF suggested the presence of a high-affinity–binding site of low capacity. This was confirmed by Scatchard analysis (Figure 3B), which indicated the presence of 2 binding sites with apparent Kds of 0.13 and 97 nmol/L and Bmax of 1.0 and 65 nmol/L for low- and high-affinity sites, respectively. Competitive inhibition of the binding was performed to further characterize the specificity and the degree of non-specific association. 125I-VEGF at a concentration of 0.5 nmol/L was incubated with Sepharose-immobilized fibrin, and varying concentrations of unlabeled VEGF were then added. The binding of 125I-VEGF to fibrin monomers was progressively reduced with increasing concentrations of unlabeled VEGF (Figure 3C). FGF-2, another angiogenic peptide, also binds with high affinity to fibrinogen and fibrin.29 To determine if the binding of FGF-2 is independent of VEGF binding, we used FGF-2 to compete with VEGF in this experiment. No competition was observed at a FGF-2 concentration of up to 250 nmol/L, which indicates the presence of separate binding sites on fibrin(ogen).
Binding of VEGF to fibrin monomer.
(A) Fibrinogen was immobilized on Sepharose beads and then converted to fibrin monomer by incubation with thrombin. 125I-VEGF was incubated with the beads, and bound and free ligand were then separated by centrifugation. Non-specific binding (squares) was measured in the presence of a 10-fold molar excess of unlabeled VEGF, and specific binding (triangles) was determined by subtraction of non-specific binding from total binding (diamonds). Each point represents the mean ± SD of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined using the Ligand program and indicated the presence of 2 distinct binding sites. (C) Competitive inhibition of binding. Increasing concentrations of unlabeled VEGF were used to competitively inhibit the binding of 125I-VEGF to fibrinogen. Each point represents mean ± SD of 3 different experiments.
Binding of VEGF to fibrin monomer.
(A) Fibrinogen was immobilized on Sepharose beads and then converted to fibrin monomer by incubation with thrombin. 125I-VEGF was incubated with the beads, and bound and free ligand were then separated by centrifugation. Non-specific binding (squares) was measured in the presence of a 10-fold molar excess of unlabeled VEGF, and specific binding (triangles) was determined by subtraction of non-specific binding from total binding (diamonds). Each point represents the mean ± SD of 3 different experiments. (B) Scatchard plot. The best fit of the data was determined using the Ligand program and indicated the presence of 2 distinct binding sites. (C) Competitive inhibition of binding. Increasing concentrations of unlabeled VEGF were used to competitively inhibit the binding of 125I-VEGF to fibrinogen. Each point represents mean ± SD of 3 different experiments.
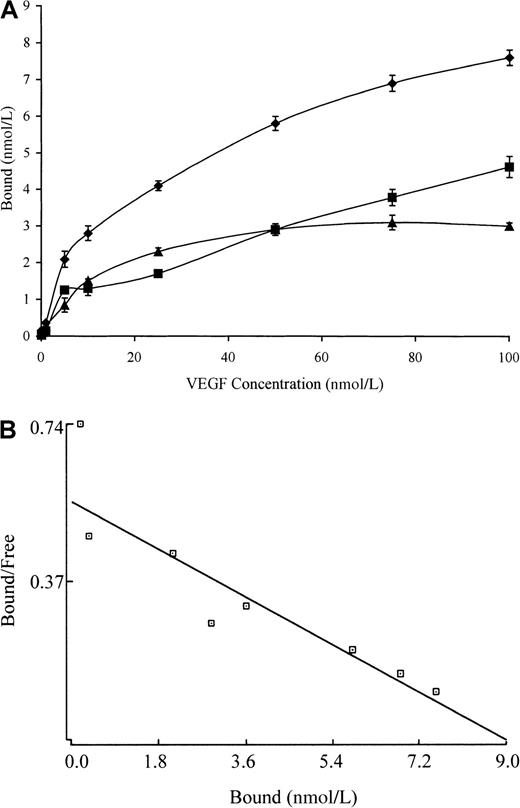
Characterization of binding to polymerized fibrin presents technical and interpretive problems because transport of VEGF into the gel may be slow or incomplete, and access to potential binding sites within individual fibrin fibers may also be restricted. We chose, therefore, to add 125I-VEGF to a solution of fibrinogen, which was then clotted with thrombin to limit problems of transport of VEGF into a preformed gel. Total binding was measured with this clotting system in the absence of an unlabeled competitor, whereas non-specific binding was measured in the presence of 10-fold molar access of unlabeled VEGF (Figure 4A). Non-specific binding represented up to 50% of the total (Figure 4A), and this was higher than seen with binding to fibrinogen or fibrin monomer (Figures 1A and3A), which possibly reflects entrapment of radio-label within the fibrin gel. A plot of bound versus bound-free 125I-VEGF was linear (Figure 4B), and Scatchard analysis identified a single binding site with an apparent Kd of 9.3 nmol/L. The maximum molar binding ratio of VEGF:polymerized fibrin was 0.1:1.
Binding of 125I-VEGF to polymerized fibrin.
(A) 125I-VEGF was added to a solution of 100 μg/mL fibrinogen and then clotted by the addition of 0.5 U/mL thrombin. Bound and unbound VEGF were then separated by vacuum filtration, and non-specific binding (squares) was determined in the presence of 10-fold molar excess of VEGF. Specific binding (triangles) was calculated by subtracting non-specific from total binding (diamonds). Each point represents the mean ± SD of 3 different experiments. (B) Scatchard analysis. The best fit of the data was determined using the Ligand program and indicated the presence of a single binding site.
Binding of 125I-VEGF to polymerized fibrin.
(A) 125I-VEGF was added to a solution of 100 μg/mL fibrinogen and then clotted by the addition of 0.5 U/mL thrombin. Bound and unbound VEGF were then separated by vacuum filtration, and non-specific binding (squares) was determined in the presence of 10-fold molar excess of VEGF. Specific binding (triangles) was calculated by subtracting non-specific from total binding (diamonds). Each point represents the mean ± SD of 3 different experiments. (B) Scatchard analysis. The best fit of the data was determined using the Ligand program and indicated the presence of a single binding site.
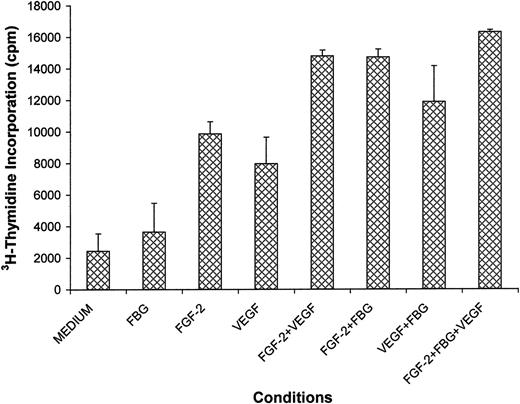
It has been shown previously that VEGF is a potent mitogen for endothelial cells.35 To determine whether VEGF retains its activity when bound to fibrinogen, human umbilical vein endothelial cells were cultured in medium containing 20 ng/mL VEGF in the presence or absence of 10 μg/mL fibrinogen, and proliferation of cells was measured by 3H-thymidine incorporation after 24 hours. In the presence of VEGF alone there was a 3.6 ± 1.3-fold increase in3H-thymidine incorporation with medium alone containing no VEGF (Figure 5). A similar 4.3 ± 1.8-fold increase in proliferation was observed with fibrinogen-bound VEGF, which demonstrated no loss of activity with fibrinogen binding. It has been demonstrated previously that a combination of VEGF and FGF-2 stimulates greater endothelial cell proliferation than either alone.36 We confirmed this finding with a 6.1-fold increase with VEGF plus FGF-2 compared to a 3.3-fold increase with VEGF or a 4.1-fold increase with FGF-2 alone (P < .05 and P < .03, respectively). The addition of fibrinogen resulted in no additional proliferation compared to VEGF plus FGF-2. Of note, endothelial cell proliferation was almost the same with FGF-2 in combination with fibrinogen (6.5-fold), which was similar to FGF-2 plus VEGF.
Endothelial cell proliferation in the presence and absence of VEGF, FGF-2, and fibrinogen.
Endothelial cells were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma, 0.037 MBq/mL (1 μCi/mL) 3H-thymidine with or without 20 ng/mL VEGF, 25 ng/mL FGF-2, and 10 μg/mL fibrinogen (FBG) for 24 hours. Isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. Results are shown as mean ± SD of 4 different experiments.
Endothelial cell proliferation in the presence and absence of VEGF, FGF-2, and fibrinogen.
Endothelial cells were plated on gelatin-coated wells in McCoy 5A medium supplemented with 20% FBS, 50 μg/mL ECGS, and 100 μg/mL heparin and allowed to adhere for 6 hours. The cells were then washed twice with McCoy medium and incubated in serum-free medium containing 1% Nutridoma, 0.037 MBq/mL (1 μCi/mL) 3H-thymidine with or without 20 ng/mL VEGF, 25 ng/mL FGF-2, and 10 μg/mL fibrinogen (FBG) for 24 hours. Isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. Results are shown as mean ± SD of 4 different experiments.
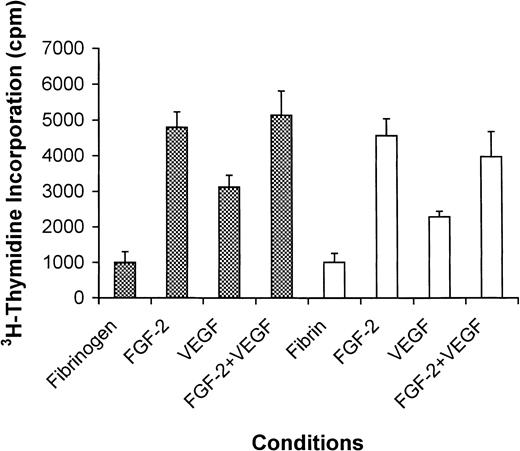
Thrombin converts fibrinogen to fibrin, which provides the initial matrix required for cell adhesion and wound healing after tissue injury. To determine whether VEGF was active when bound to fibrinogen or fibrin as adhesive substrate, surfaces were coated with fibrinogen or fibrin with or without VEGF and FGF-2. As assessed visually, cells grown on the surface of fibrinogen or fibrin in the absence of growth factors were very sparse, but incorporation of VEGF into the matrix resulted in more robust growth. As quantitated by3H-thymidine incorporation, proliferation was minimal on fibrinogen or fibrin without VEGF or FGF-2 (Figure6). Proliferation was increased 4.8-fold by the addition of FGF-2 on fibrinogen-coated surfaces (P < .001) and 4.5-fold on fibrin-coated surfaces (P < .005). Similarly, VEGF increased endothelial cell proliferation 3.1 ± 0.9-fold on fibrinogen-coated surfaces (P < .02) and 2.3 ± 0.5-fold on fibrin-coated surfaces (P < .04). The combination of FGF-2 and VEGF did not increase proliferation over FGF-2 alone on either surface.
Endothelial cell proliferation on fibrinogen- or fibrin-coated surfaces in the presence and absence of VEGF and FGF-2.
Endothelial cells were plated in wells coated with fibrinogen (filled bars) or fibrin (open bars) in the absence or presence of 20 ng/mL VEGF, 25 ng/mL FGF-2, or both. After 6 hours the medium was removed and replaced with serum-free medium containing 0.037 MBq/mL (1 μCi/mL)3H-thymidine, and the cultures were incubated for an additional 24 hours. No growth factor was present in the medium. Isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. Results are shown as mean ± SD of 3 different experiments.
Endothelial cell proliferation on fibrinogen- or fibrin-coated surfaces in the presence and absence of VEGF and FGF-2.
Endothelial cells were plated in wells coated with fibrinogen (filled bars) or fibrin (open bars) in the absence or presence of 20 ng/mL VEGF, 25 ng/mL FGF-2, or both. After 6 hours the medium was removed and replaced with serum-free medium containing 0.037 MBq/mL (1 μCi/mL)3H-thymidine, and the cultures were incubated for an additional 24 hours. No growth factor was present in the medium. Isotope incorporated into DNA was precipitated with TCA, collected by vacuum filtration, and measured by scintillation counting. Results are shown as mean ± SD of 3 different experiments.
Discussion
The results presented demonstrate that VEGF binds specifically and saturably to both fibrinogen and fibrin and that fibrinogen-bound VEGF retains functional activity. Two distinct binding sites for VEGF were identified with both Sepharose-immobilized fibrinogen and fibrin monomer with similar Kds, and the maximum molar-binding ratios were 3.8 and 3.6, respectively. Considering that fibrinogen is a dimerically symmetric molecule37 and that 2 binding sites with different Kds were identified, the ratio of 4 VEGF to 1 fibrinogen would be expected and consistent with the presence of 2 structurally distinct and independent sites on each half-molecule. Caution is needed in interpreting curvilinear Scatchard plots as indicative of receptor heterogeneity, as low-affinity or non-specific binding can cause artifact.38This problem was approached by estimating non-specific binding with experiments using an excess of unlabeled ligand and by computer modeling of the data. However, the relatively high amounts of non-specific binding could affect estimates ofKd for low-affinity sites. Binding of VEGF to polymerized fibrin differed from fibrinogen or fibrin monomer, as only a single binding site was identified, and the maximum molar-binding ratio of VEGF:fibrin was 0.1:1, indicating that VEGF bound to only 1 in 10 monomeric units. This is most likely explained by structural changes in fibrin after polymerization, which results in formation of highly organized fibers composed of multiple protofibril units aggregated laterally.39 This polymerization may prevent VEGF from binding to sites near to those involved in intermolecular association and may also restrict binding to less accessible molecules within aggregated fibers.
FGF-2, another angiogenic peptide, also binds with high affinity to fibrin and fibrinogen,29 and this binding increases the capacity of FGF-2 to stimulate endothelial cell proliferation.40 The current results demonstrate that the binding of VEGF to fibrinogen is not inhibited by FGF-2, thereby indicating that the binding sites on fibrinogen for these 2 angiogenic peptides are independent and separate. This is further supported by differences in the maximum molar binding ratios for fibrin, which were 3.8 for VEGF but 2.0 for FGF-2.29 The binding affinity for both FGF-2 and VEGF increased after conversion of Sepharose-immobilized fibrinogen to fibrin monomer, suggesting that thrombin-mediated conformational changes affect the binding sites and result in increased affinity for both angiogenic peptides. Insulin-like growth factor–1, another peptide important in wound healing, also binds to fibrinogen and fibrin through insulin-like growth factor–1 binding protein–3 with Kd values of 0.67 and 0.7 nmol/L, respectively.41 These high-affinity interactions support a role for fibrinogen and fibrin as a reservoir of growth factors at the site of injury.
The significance of VEGF binding to fibrinogen and fibrin must be considered in relation to both the tissue distribution of VEGF and the availability of other sites for binding within the vasculature. VEGF is present in normal serum at a concentration of 0.9 pmol/L, and patients with cancer may have elevated levels of up to 12 pmol/L.42At a normal fibrinogen concentration of 7 μmol/L, essentially all VEGF in plasma should be bound to fibrinogen considering theKds in the nmol/L range. VEGF also binds to α-2 macroglobulin in a slow, covalent reaction, which inhibits receptor interaction.43 This was, however, characterized in experiments using purified α-2 macroglobulin or serum so that any effects of fibrinogen on binding could not be assessed. Characterization of the binding of VEGF to plasma fibrinogen and to localized fibrin deposits will be important in understanding tissue distribution, metabolism, and clearance as VEGF is used pharmacologically to stimulate angiogenesis.44
Vessel wall cells, including endothelial cells, synthesize and secrete VEGF,45,46 which then remains closely associated and bound to the cell membrane or matrix.47 Membrane binding is mediated in part by specific surface receptors of the tyrosine kinase family including flt-1 with a Kd of 10 to 12 pmol/L12 and flk-1/KDR, which has aKd of 75 to 125 pmol/L.14 VEGF also binds specifically and with high affinity to intact bovine endothelial cells with Kds of 10 pmol/L and 100 pmol/L.48 The higher affinities for specific receptors compared to fibrin would result in preferential binding to cell receptors at a site of injury, although the total capacity of fibrin binding could be important because of the variable but potentially large capacity of the fibrin deposit. There are some parallels in this interaction between VEGF and heparan sulfate,49 which is also considered important in determining its association with vascular cells and extracellular matrix. VEGF binds to heparan sulfate and to heparin as an inactive complex that resists proteolytic degradation. The interaction of VEGF with abundant cell surface heparan sulfate may promote its binding to high-affinity transmembrane signaling receptors,50 and the affinity of VEGF for specific receptors on endothelial cells is increased 8-fold in the presence of heparin.50 Gitay-Goren et al50 have shown that cell-associated heparan sulfate enhances the binding of VEGF to high-affinity receptors in a manner similar to that reported for FGF-2. The binding of VEGF to heparan sulfate proteoglycans in the extracellular matrix may provide a protected reservoir of biologically active VEGF that is available following enzymatic release. Houck et al47 have demonstrated in vitro that plasmin can release VEGF, which is biologically active both as an endothelial cell mitogen and as a vascular permeability enhancing agent.47
Integration of cell responses to VEGF and fibrinogen may occur at the receptor and the signal transduction level. Binding of endothelial cells to matrix glycoproteins, including fibrinogen and fibrin, occurs through integrin receptors, and this alters their sensitivity to growth factor–induced signaling.51 In turn, VEGF stimulates endothelial cell surface integrin expression,16,17 thereby regulating the cell response to fibrinogen and fibrin. Both fibrinogen and fibrin can support endothelial cell attachment through occupancy of αvβ3, which results in the formation of a focal adhesion complex that colocalizes integrin and VEGF receptors52 and fosters signal integration.53 Further, fibrin binds specifically to VE-cadherin through the amino terminus of the β-chain54supporting fibrin-specific endothelial cell responses.
VEGF and FGF-2, both of which bind to fibrinogen and fibrin, have distinct and complementary actions in angiogenesis. VEGF is a direct-acting secreted polypeptide45 that can serve as an initiator of angiogenesis. This is in contrast to FGF-2, which lacks a signal peptide and requires prolonged exposure to cells for maximum effect. Pepper et al55 have shown that the greatest proliferative and angiogenic effects of VEGF were observed with FGF-2, and the combination was synergistic. Optimum angiogenesis requires the presence of both. The binding of both VEGF and FGF-2 to fibrinogen or fibrin may provide needed colocalization and spatial organization of both angiogenic peptides for maximum effect at sites of inflammation or injury.
Supported in part by grants HL-30616 and HL-07152 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Charles W. Francis, Vascular Medicine Unit, PO Box 610, University of Rochester Medical Center, 601 Elmwood Avenue, Rochester, NY 14642; e-mail: charles_francis@urmc.rochester.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal