Abstract
The idiotypic determinant (Id) of the immunoglobulin expressed by a B-cell malignancy can serve as an effective tumor-specific antigen but is only weakly immunogenic. This study demonstrates that the immunogenicity of the tumor Id protein can be dramatically increased by directing it to antigen-presenting cells (APCs). Cytotoxic T-lymphocyte antigen 4 (CTLA-4) present on activated T cells has a strong binding affinity to both B7-1 and B7-2 molecules, which are primarily expressed on APCs. After construction of a fusion protein consisting of Id and CTLA-4 (Id-CTLA4), mice immunized with the fusion protein induced high titers of Id-specific antibody and T-cell proliferative responses without adjuvants and were protected from lethal tumor challenge. The Id-CTLA4 fusion protein was so potent that even low doses (down to 0.1 μg) of the immunogen were able to elicit strong antibody responses. By using an Id-CTLA4 mutant protein, the ability to bind B7 molecules on APCs was shown to be required for the enhanced immunogenicity of Id-CTLA4. These findings demonstrate that fusing CTLA-4 to a potential tumor antigen represents an effective approach to prime antitumor immunities in vivo and may be applicable to the design of vaccines for a variety of other diseases.
Introduction
The idiotypic protein (Id) of the surface immunoglobulin (Ig) expressed by malignant B lymphocytes can serve as a unique tumor-specific antigen (Ag) and as a model for the development of cancer vaccines. Vaccination with tumor-derived Id induces protective immunity against lymphoma in a number of mouse models.1-6 Moreover, in combination with chemotherapy, Id vaccination was reported to cure animals with established tumors.7,8 In a clinical trial for low-grade follicular lymphoma, Id immunization induced humoral responses against the specific Id expressed on each patient's tumor.9 However, the autologous Id proteins, like many other tumor Ags, are only weakly immunogenic and must be chemically coupled to a strongly immunogenic carrier protein and mixed with an adjuvant to induce an immune response. Alternatively, genetic fusion of the Id protein with various cytokine molecules, including interleukin (IL)-2, IL-4, and granulocyte-macrophage colony-stimulating factor (GM-CSF), converts this self tumor Ag into a strong immunogen capable of inducing anti-Id antibody (Ab) and protective immunities without carrier proteins or adjuvants. This adjuvant effect is dependent on the biologic activity of the cytokine as well as the covalent linkage of the Id and cytokine.10,11 Vaccination with fusion proteins between a single-chain Id and chemokine was also recently reported to induce protective immune responses against a large tumor challenge.12
Another strategy to increase the immunogenicity of a protein Ag is by directing immunogens to antigen-presenting cells (APCs) for more efficient Ag processing and presentation. Previous experiments have shown that coupling immunogens to Abs against the class II major histocompatibility complex (MHC), the Fcγ receptor (FcγR), the 33D1 dendritic cell-specific Ag on APCs,13,14 or surface IgM or IgG on B cells15 can substantially increase immune responses. Boyle and colleagues16 showed that vaccination with DNA encoding a model Ag (human IgG) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) dramatically increased both Ab and T-cell proliferation responses compared with the control plasmid expressing the Ag alone. CTLA-4 is a glycoprotein expressed on activated T cells that has a strong binding affinity to both B7-1 (CD80) and B7-2 (CD86) molecules, which are primarily expressed on APCs.17Presumably, the direct targeting of the Ag to APCs through the interaction of CTLA-4 and B7 causes the Ag to be processed and presented to T cells with much higher efficiency, therefore leading to a stronger immune response.
In the present study, we investigated whether the immunogenicity of the tumor Id protein could be improved by targeting to APCs through B7-1 and B7-2 molecules. We constructed a fusion protein consisting of 38C13 Id and CTLA-4 and found that the resulting Id-CTLA4 fusion protein induces significant antitumor immunity. The Id-CTLA4 fusion protein was so potent that even a dose as low as 0.1 μg was able to elicit high titers of anti-Id Abs. These results demonstrate that fusion of a weak tumor Ag to CTLA-4 represents a powerful means to increase the immunogenicity of the Ag and may be applicable to the design of vaccines for immunotherapy of other types of tumors as well as for other pathogens and disease states.
Materials and methods
Mice
Female C3H/HeN mice, 6 to 8 weeks old, were purchased from the National Laboratory Animal Breeding and Research Center, Taipei, Taiwan, and housed at the Laboratory Animal Facility, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan. All animal studies were approved by the Animal Committee of Institute of Biomedical Sciences, Academia Sinica and performed according to their guidelines.
Celllines
The carcinogen-induced 38C13 B-cell lymphoma has been described18 previously and was a gift from R. Levy (Stanford University, Stanford, CA). Transfectoma cell lines producing a human-mouse chimeric Id or Id-murine GM-CSF fusion protein (Id-GM) were described previously.10 FO (CRL-1646, American Type Culture Collection, Manassas, VA) is a murine plasmacytoma cell line negative for Ig production. DC2.4 (courtesy of K. Rock, Dana Farber Cancer Institute, Boston, MA) is an immortalized murine bone marrow–derived dendritic cell line and is positive in the expression of B7-1 and B7-2.19 DG44 (courtesy of L. Chasin, Columbia University, New York, NY) is a dihydrofolate reductase–deficient mutant cell line of Chinese hamster ovary (CHO) cells.20
Plasmid construction
The complementary DNAs (cDNA) of murine B7-1 (CD80) and B7-2 (CD86) were kindly provided by G. Freeman (Dana-Farber Cancer Institute) and separately cloned into a eukaryotic expression vector, pTCAE,21 under the transcriptional control of a cytomegalovirus promoter. The resulting plasmids containingB7-1 or B7-2 genes were designated as p369 and p391, respectively.
The Ig heavy and light chain expression vectors containing 38C13 Id and a Cla I/Not I cloning cassette at the end of the CH3 exon were constructed as previously described.10 To construct the Id-CTLA4 fusion protein, we used overlap polymerase chain reaction (PCR) to replace the signal sequence of the human CTLA-4 gene with a sequence encoding the GGGGSGGGGS peptide linker and introduced a stop codon upstream of the transmembrane domain. The forward primer 5′-GGAGGCGGGGGCTCGATGCACGTGGCCCAGCCTG-3′ and the reverse primer 5′-GGATCGCGGCCGCTCAGTCAGAATCTGGGCACGGTTC-3′ were used in the first round PCR on the human CTLA-4 gene. The resulting PCR product was used as template to perform a second round PCR using the same reverse primer and a second forward primer 5′-CTTATCGATGGAGGCGGGGGCTCGGGAGGCGGGGGCTCG-3′. The 5′ side of the first forward primer and the 3′ side of the second forward primer have a 15 nucleotide overlap. The final PCR product was ligated into pCR-Blunt vector (Invitrogen, San Diego, CA) to produce plasmid pBlunt-CTLA4 for sequencing. To create a mutant CTLA-4 protein, we used the Quick Change Site Directed Mutagenesis Kit (Stratagene, La Jolla, CA) to introduce a point mutation at CTLA-4 residue 104 (Tyr→Ala) in pBlunt-CTLA4. A pair of overlapping primers, 5′-CCACCGCCAGCCTACCTGGGC-3′ and 5′-GCCCAGGTAGGCTGGCGGTGG-3′, was used to create the point mutation. The pBlunt plasmids were digested at the Cla I and Not I sites, which were introduced in the PCR primers, to release the DNA fragments containing the wild-type or mutant CTLA-4gene. The resulting fragments were inserted into the heavy-chain plasmid at the end of the CH3 exon. The resulting plasmids, p362 and p497, contain the coding regions for 38C13 Id joined to the complete human γ1 constant region, followed by a GGGGSGGGGS linker and the extracellular domain of CTLA-4 or CTLA-4Y104A, respectively.
Production and purification of idiotype fusion proteins
The heavy-chain vectors p362 and p497 were cotransfected with the light-chain vector p307710 into an Ig-nonproducing plasmacytoma cell line, FO, by electroporation. Transfectomas were selected by resistance to G418 and the highest producer was selected for protein production. Transfectoma clones were expanded for large-scale production in Dulbecco modified Eagle medium (DMEM) containing 1% low IgG fetal calf serum (FCS) (HyClone Laboratories, Logan, UT) and purified by protein A chromatography as previously described.22
Transfection of Chinese hamster ovary cells
Stable transfectants expressing B7-1 or B7-2 were isolated following electrotransfection of DG44 cells with plasmids p369 and p391, respectively. Electroporation was performed at 210 V, 400 μF, 13 ohm. Transfectants were selected by resistance to G418 (0.5 mg/mL) and screened for expression of B7 molecules by fluorescence-activated cell sorting (FACS) analysis. Lines expressing high levels of B7 (CHO/B7-1, CHO/B7-2) were isolated by sorting on a FACStarPlus (Becton Dickinson, Mountain View, CA).
Flow cytometry analysis
To screen for expression of B7-1 or B7-2, transfected CHO cells were removed from their culture vessels by incubation in phosphate-buffered saline (PBS) containing 10 mmol/L EDTA. Cells were reacted with biotin-conjugated hamster antimouse B7-1 (16-10A1, PharMingen, San Diego, CA) monoclonal antibody (mAb) at 1:800 or rat antimouse B7-2 (GL1, PharMingen) mAb at 1:800. The bound biotinylated Abs were detected with fluorescein isothiocyanate (FITC)-labeled avidin (Cappel, ICN Biomedicals, Costa Mesa, CA) at 1:500 and analyzed on a FACSCallibur (Becton Dickinson).
To detect the reactivity of various Id proteins to DC2.4 and B7 transfected CHO cells (CHO/B7-1, CHO/B7-2), 5 × 105cells were reacted at 4°C for 30 minutes with 20 μg/mL of each Id fusion protein. The bound Id proteins were detected with FITC-labeled goat antihuman κ Ab (Cappel) at 1:200 and subjected to FACS analysis.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblot analyses
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transfer of proteins to nitrocellulose membrane by semidry electroblotting were performed as previously described.23 Blots were probed with either a biotinylated anti-Id mAb, S5A8 (IgG2b)24 at 1:1000, or mouse antihuman CTLA-4 mAb (BNI3; IgG2a; PharMingen) at 1:500. The bound biotinylated Abs were then reacted with horseradish peroxidase (HRP)-conjugated avidin (Cappel) at 1:2000 and detected with the enhanced chemiluminescence (ECL) system (Amersham, Arlington Heights, IL), according to the manufacturer's directions.
Immunization
Mice were immunized 2 times at 2-week intervals by intraperitoneal or subcutaneous injection of an appropriate amount of Id fusion proteins in 200 μL PBS. For some experiments, mice were immunized twice at 2-week intervals with a mixture of 2 μg hepatitis B surface Ag (HBsAg) and 10 μg of the various Id proteins. Immune sera were collected right before and 2 weeks after the booster immunization.
Enzyme-linked immunosorbent assay
Serum samples were collected by tail bleeding. Anti-Id and anti-HBsAg levels in the immune sera were quantitated by titering sera on enzyme-linked immunosorbent assay (ELISA) plates coated with purified 38C13 Id or yeast-derived recombinant HBsAg, respectively. Bound proteins were detected with HRP-conjugated goat antimouse IgG Fc (1:1000; Cappel). Color was generated by adding 2,2′-azino-bis(ethylbenzthiazoline sulfonic acid) (Sigma Chemical, St Louis, MO), and the absorbance at 405 nm was measured on an ELISA reader. For measurement of IgG anti-Id Abs, the readings were referenced to a mixture of purified monoclonal anti-38C13 Id Abs containing IgG1, IgG2a, and IgG2b isotypes24 in a 2:1:1 ratio. For measurement of IgG anti-HBsAg Abs, the readings were referenced to a standard serum pooled from 5 mice given injections of 2 μg recombinant HBsAg with complete Freund adjuvant, and the results were expressed as arbitrary units per milliliter (1 U = 50% maximum optical density).
Lymphocyte proliferation assay
One week after the booster immunization, splenocytes were collected and passed through a nylon wool column to enrich T lymphocytes. To perform the lymphoproliferative assay, 100 μL of 2 × 106 cells was added to each well in 96-well flat-bottomed plates and stimulated with 50 μg/mL 38C18 Id or transferrin (Sigma). Cells stimulated with 5 μg/mL concanavalin A served as a positive mitogenic control. Negative control wells received cells only. After 7 days in culture, the cells were pulsed with [3H]thymidine (0.5 μCi/well) for 16 hours and incorporated radioactivity was determined using Top Count (Packard, Meriden, CT). The stimulation index was calculated as the mean counts per minute of the stimulated wells divided by the mean counts per minute of the negative control wells.
Tumor challenge
Mice were challenged intraperitoneally with 200 38C13 cells or subcutaneously with 1000 38C13 cells 2 weeks after the booster immunization. It was previously shown that these are lethal inocula that killed all unprotected mice. For subcutaneous challenge, tumors were measured every other day, and the tumor volume (in cubic millimeters) was approximated using the ellipsoidal formula: length × width × height × 0.52. The mean volume and SD of mice bearing measurable tumor mass in each group were calculated. Animals were killed when subcutaneous tumors measured more than 3000 mm3 or until any mouse appeared to be moribund. For intraperitoneal challenge, the survival of challenged mice was followed and results were analyzed for significance by the Student ttest. Data were considered statistically significant atP ≤ .05.
Results
Construction of Id-CTLA4 fusion protein
The genetic constructs to make heavy and light chains of the chimeric Id protein were previously reported.10 Briefly, the light-chain expression vector contains the 38C13 light-chain variable region gene joined to the human κ constant region gene. The heavy-chain expression vector contains the 38C13 heavy-chain variable region gene joined to the human γ1 constant region gene. The resulting Id protein retained the original Id determinants expressed on the native murine 38C13 Id protein. To make the Id-CTLA4 fusion protein, a PCR fragment encoding a flexible linker (GGGGSGGGGS) and the extracellular domain of human CTLA-4 was ligated to the end of the CH3 exon of the heavy-chain gene. This modified Id heavy-chain vector was cotransfected with the Id light-chain vector to make Id-CTLA4.
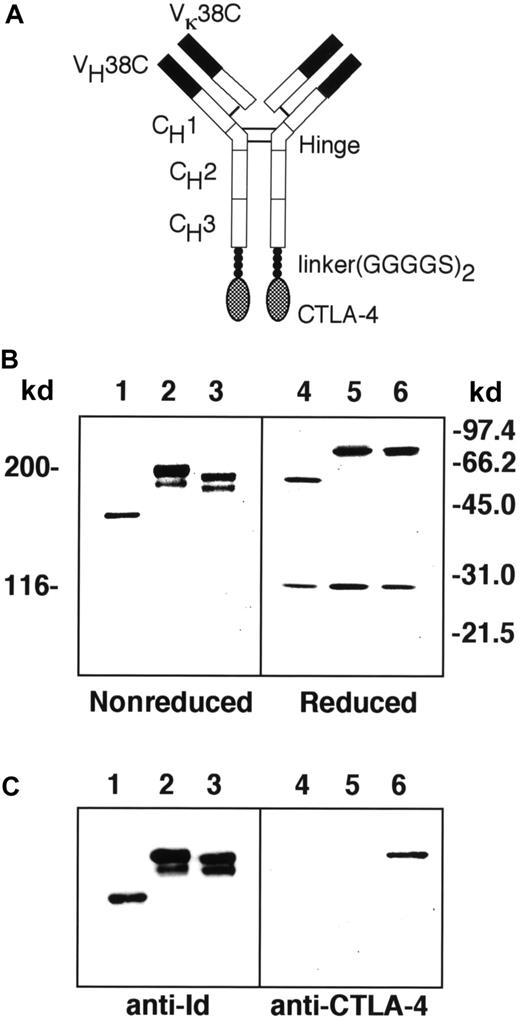
The Id-CTLA4 fusion protein is expected to consist of a mouse–human chimeric molecule with CTLA-4 attached to each of the carboxy-terminal ends of the heavy chain, as shown in Figure1A. SDS-PAGE and immunoblotting techniques were applied to analyze the purified Id, the fusion protein Id-CTLA4, and a previously described fusion protein consisting of Id and murine GM-CSF (Id-GM).10 Under reducing conditions, the light chains of the Id and both Id fusion proteins all migrated at an apparent molecular weight of 28 kd (Figure 1B, lanes 4-6). However, the heavy chains of Id-CTLA4 migrated at an apparent molecular weight of 74 kd, which is similar to the 75-kd heavy chain obtained from Id-GM but not the smaller heavy chain (60 kd) from Id protein, indicating that the Id-CTLA4 fusion protein contained the CTLA-4 tail. This speculation was confirmed by an immunoblot analysis that showed that a human CTLA-4–specific Ab recognized the Id-CTLA4 fusion protein but not Id or Id-GM (Figure 1C, lanes 4-6). The heavy and light chains of the Id and both Id fusion proteins were properly assembled to give tetrameric proteins, as observed on a nonreducing gel (Figure 1B, lanes 1-3). However, unlike the Id protein, which was secreted as a single molecule, Id-CTLA4 and Id-GM migrated as doublets in the nonreducing gel. The double bands of the fusion proteins were also present in the reducing gel (Figure 1B, lanes 5 and 6), suggesting that they were the result of proteolytic cleavage of CTLA-4 of Ig and GM-CSF of Ig, or perhaps due to the involvement of glycosylation.
Construction of tumor Id and CTLA-4 fusion protein.
(A) Schematic diagram of Id-CTLA4. Solid areas represent variable regions from the 38C13 tumor. Open areas represent human γ1 and κ constant regions. Checkered regions represent the human CTLA-4 sequence. (B) SDS-PAGE of Id (lanes 1 and 4), Id-GM (lanes 2 and 5), and Id-CTLA4 (lanes 3 and 6) under nonreducing (lanes 1-3) or reducing (lanes 4-6) conditions. The molecular weight is determined by marker proteins. (C) Immunoblot analysis of Id fusion proteins. Id (lanes 1 and 4), Id-GM (lanes 2 and 5), and Id-CTLA4 (lanes 3 and 6) under nonreducing (lanes 1-3) or reducing (lanes 4-6) conditions were subjected to SDS-PAGE followed by electroblotting to nitrocellulose membrane. The strips were reacted with S5A8, a monoclonal anti-38C13 Id (lanes 1-3) or mouse antihuman CTLA-4 (lanes 4-6) Abs and detected with HRP-conjugated second-step reagents.
Construction of tumor Id and CTLA-4 fusion protein.
(A) Schematic diagram of Id-CTLA4. Solid areas represent variable regions from the 38C13 tumor. Open areas represent human γ1 and κ constant regions. Checkered regions represent the human CTLA-4 sequence. (B) SDS-PAGE of Id (lanes 1 and 4), Id-GM (lanes 2 and 5), and Id-CTLA4 (lanes 3 and 6) under nonreducing (lanes 1-3) or reducing (lanes 4-6) conditions. The molecular weight is determined by marker proteins. (C) Immunoblot analysis of Id fusion proteins. Id (lanes 1 and 4), Id-GM (lanes 2 and 5), and Id-CTLA4 (lanes 3 and 6) under nonreducing (lanes 1-3) or reducing (lanes 4-6) conditions were subjected to SDS-PAGE followed by electroblotting to nitrocellulose membrane. The strips were reacted with S5A8, a monoclonal anti-38C13 Id (lanes 1-3) or mouse antihuman CTLA-4 (lanes 4-6) Abs and detected with HRP-conjugated second-step reagents.
Reactivity of Id-CTLA4 with B7-1 and B7-2
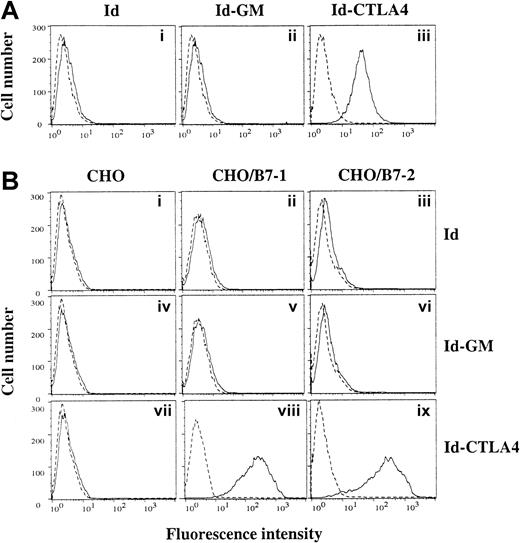
To investigate the functional activity of Id-CTLA4, we first tested its binding to a murine dendritic cell line, DC2.4, which expresses high levels of B7-1 and B7-2.19 As shown in Figure 2A, the Id-CTLA4 fusion protein clearly demonstrated its ability to bind to DC2.4 cells. We ruled out the possibility that interaction of Id-CTLA4 and DC2.4 cells was through the Fcγ receptor because under the same condition the Id and Id-GM did not show significant binding to DC2.4 cells. We also used B7-1– or B7-2–transfected CHO cell lines in FACS analysis to directly demonstrate the binding activity of Id-CTLA4. As shown in Figure 2B, Id-CTLA4 was bound by CHO/B7-1 and CHO/B7-2, but not by untransfected CHO cells. In contrast, no binding of Id and Id-GM was detected with either CHO/B7-1 or CHO/B7-2 cells. Thus, the Id-CTLA4 fusion protein retains the binding activity for both of its counter receptors on APCs.
Id-CTLA4 retains the binding activity for B7-1 and B7-2.
(A) DC2.4, a murine dendritic cell line, was stained with Id, Id-GM, or Id-CTLA4. (B) CHO (panels i,iv, and vii), CHO/B7-1 (panels ii,v, and viii), and CHO/B7-2 (panels iii,vi, and ix) cells were stained with Id (i-iii), Id-GM (iv-vi), or Id-CTLA4 (vii-ix). Cells were then washed and incubated with FITC-conjugated goat antihuman κ Ab. Dashed lines represent fluorescence from cells without addition of the tested Id fusion proteins.
Id-CTLA4 retains the binding activity for B7-1 and B7-2.
(A) DC2.4, a murine dendritic cell line, was stained with Id, Id-GM, or Id-CTLA4. (B) CHO (panels i,iv, and vii), CHO/B7-1 (panels ii,v, and viii), and CHO/B7-2 (panels iii,vi, and ix) cells were stained with Id (i-iii), Id-GM (iv-vi), or Id-CTLA4 (vii-ix). Cells were then washed and incubated with FITC-conjugated goat antihuman κ Ab. Dashed lines represent fluorescence from cells without addition of the tested Id fusion proteins.
Immune responses induced by Id-CTLA4 fusion protein
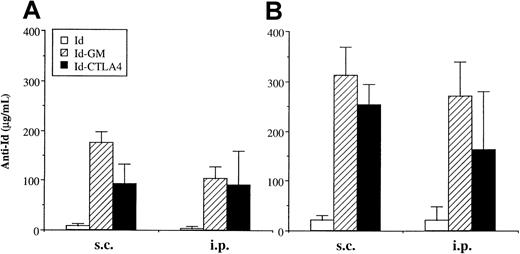
An initial experiment was designed to compare the immunogenicity of Id-CTLA4 with Id-GM, which was previously shown to be an excellent immunogen.10 11 Mice were immunized subcutaneously or intraperitoneally with 50 μg of Id, Id-CTLA4, or Id-GM and boosted 2 weeks later with the same amount of Ag. After a single immunization, the anti-Id Ab titers induced by Id-CTLA4 were comparable to those obtained by Id-GM (Figure 3A). Immunization with Id alone barely induced any detectable anti-Id Abs in the primary reaction. Following one booster immunization, a further increase of anti-Id titers was observed in mice that received Id-CTLA4 or Id-GM (Figure 3B). High levels of anti-Id Abs were present in every immunized animal of these 2 groups. The booster immunization also slightly increased the anti-Id Ab responses in the Id-immunized group but the titers remained low. Analysis of sera from each animal in this group revealed that 60% of animals receiving Id by intraperitoneal immunization remained seronegative, whereas all animals in the subcutaneous group had seroconverted. We measured anti-Id isotypes in the sera of mice treated with Id, Id-CTLA4, or Id-GM. Regardless of the type of Id proteins used for vaccination or the route of Ag delivery (subcutaneous or intraperitoneal), the predominance of IgG1 over IgG2a was observed in all animals (data not shown). The specificity of the anti-Id antibodies induced by the Id fusion proteins was confirmed by their reacting with 38C13 tumor cells but not with V1-1 (Id negative) or V2 (Ig negative) variant cells (data not shown).
Anti-Id titer induced by immunization with Id fusion proteins.
Mice were immunized twice by subcutaneous or intraperitoneal injection of 50 μg of Id, Id-GM, or Id-CTLA4, and bled 2 weeks after each immunization. Anti-Id titers in sera after the first (A) or second (B) immunization were determined by ELISA, as described in “Materials and methods.” The data are presented as the mean ± SD for 5 animals of each group.
Anti-Id titer induced by immunization with Id fusion proteins.
Mice were immunized twice by subcutaneous or intraperitoneal injection of 50 μg of Id, Id-GM, or Id-CTLA4, and bled 2 weeks after each immunization. Anti-Id titers in sera after the first (A) or second (B) immunization were determined by ELISA, as described in “Materials and methods.” The data are presented as the mean ± SD for 5 animals of each group.
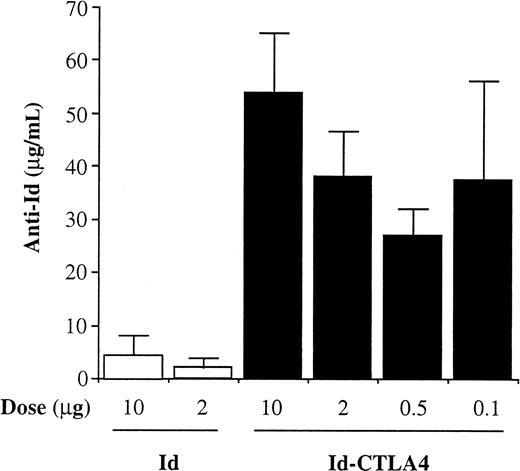
To determine the minimal amount of Id-CTLA4 necessary for induction of Ab responses, the animals were given 2 injections of various doses (10, 2, 0.5, or 0.1 μg) of Id-CTLA4 and the anti-Id Ab levels were assayed at 2 weeks after injection. Mice immunized with 10 or 2 μg Id served as controls. As shown in Figure 4, mice immunized with Id alone at a dose of either 10 or 2 μg produced only low titers of anti-Id Ab. In contrast, as little as 0.1 μg Id-CTLA4 was effective in inducing high titers of anti-Id Ab. In fact, the anti-Id titer induced by 0.1 μg Id-CTLA4 was comparable to titers induced by up to 2 μg Id-CTLA4 and was only a little lower than that induced by 10 μg Id-CTLA4 (Figure 4). These results clearly demonstrate that the vaccine efficacy in terms of dosage used in immunization can be dramatically increased by fusing Id to the CTLA-4 molecule.
Effect of Id-CTLA4 dose on anti-Id titer.
Mice were immunized twice subcutaneously with various doses of Id or Id-CTLA4 and bled 2 weeks after the second immunization. Anti-Id titers in immune sera were determined by ELISA. The data are presented as the mean ± SD for 5 animals of each group.
Effect of Id-CTLA4 dose on anti-Id titer.
Mice were immunized twice subcutaneously with various doses of Id or Id-CTLA4 and bled 2 weeks after the second immunization. Anti-Id titers in immune sera were determined by ELISA. The data are presented as the mean ± SD for 5 animals of each group.
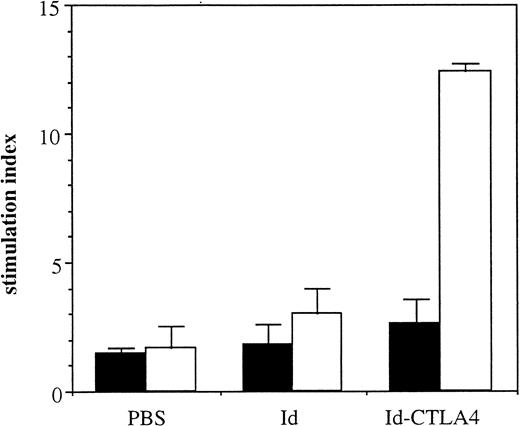
The enhancing effect of Id-CTLA4 on T cells was then examined. Mice were intraperitoneally immunized with Id or Id-CTLA4 and boosted 2 weeks later with the same Ag. Mice that received PBS served as negative controls. At 1 week after the second immunization, splenocytes were examined for proliferation in response to specific Ag stimulation. Splenic lymphocytes derived from Id-immunized animals did not respond to 38C13 Id stimulation, with a stimulation index of about 3.0, which was not much different from that produced by splenocytes from PBS-control animals (Figure 5). In contrast, Id-CTLA4 fusion protein enhanced the cellular proliferation, with the stimulation index increasing to about 12.4. All mice failed to respond to transferrin included as a control Ag. These results demonstrated that immunization with Id-CTLA4 fusion protein also induced the Id-specific T-cell arm of the immune response.
Id-specific T-cell responses induced by Id-CTLA4 immunization.
C3H/HeN mice were immunized twice intraperitoneally with 10 μg Id or Id-CTLA4. Mice receiving PBS only served as controls. Splenocytes pooled from 3 immunized mice were stimulated with 50 μg 38C13 Id (■) or transferrin (▪). Values are presented as mean stimulation index for triplicate wells ± SD. The mean counts per minute for the mitogenic control (5 μg/mL concanavaline A) of the PBS, Id, and Id-CTLA4 groups were 32 305 ± 4196, 25 088 ± 3123, and 51 125 ± 4307, respectively. The mean counts per minute for the negative control wells (receiving cells only) of the PBS, Id, and Id-CTLA4 groups were 2151 ± 372, 1105 ± 168, and 1922 ± 274, respectively.
Id-specific T-cell responses induced by Id-CTLA4 immunization.
C3H/HeN mice were immunized twice intraperitoneally with 10 μg Id or Id-CTLA4. Mice receiving PBS only served as controls. Splenocytes pooled from 3 immunized mice were stimulated with 50 μg 38C13 Id (■) or transferrin (▪). Values are presented as mean stimulation index for triplicate wells ± SD. The mean counts per minute for the mitogenic control (5 μg/mL concanavaline A) of the PBS, Id, and Id-CTLA4 groups were 32 305 ± 4196, 25 088 ± 3123, and 51 125 ± 4307, respectively. The mean counts per minute for the negative control wells (receiving cells only) of the PBS, Id, and Id-CTLA4 groups were 2151 ± 372, 1105 ± 168, and 1922 ± 274, respectively.
Tumor protection associated with Id-CTLA4 immunization
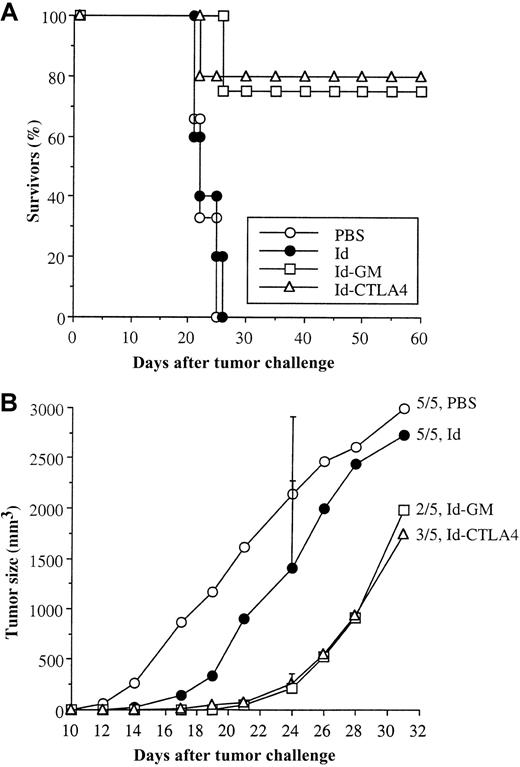
The various Id proteins were next compared for their efficacy in inducing protection against a lethal tumor challenge. Mice were subcutaneously immunized twice with 50 μg Id, Id-CTLA4, or Id-GM and challenged intraperitoneally with 38C13 tumor cells 2 weeks following the second immunization. The control mice (injection of PBS alone) or mice immunized with Id did not show any protection; all of these mice died within 4 weeks of the tumor challenge (Figure6A). In contrast, mice immunized with Id-CTLA4 significantly suppressed tumor growth and resulted in 80% long-term survivors (P < .025 versus medium control group), which was comparable to the 70% protection rate achieved by Id-GM (P < .05 versus medium control group). These long-term survivors remained disease free for an observation period of 120 days. In a separate experiment, mice were intraperitoneally immunized with 10 μg Id and Id fusion proteins and subcutaneously inoculated with 38C13 tumor cells. Compared with the PBS control group, Id-CTLA4 and Id-GM immunization resulted in 40% (2 of 5 mice) and 60% (3 of 5 mice) tumor-free animals (> 60 days), whereas all animals in the control group had detectable tumors by day 17. In addition, objective tumor growth suppression was observed in tumor-bearing animals in the Id-CTLA4 and Id-GM groups (Figure 6B). By day 24, the mean tumor volume of the tumor-bearing animals in the Id-CTLA4 and Id-GM groups was 265 ± 99 mm3 and 221 ± 142 mm3, respectively, as compared with 2140 ± 771 mm3 in the PBS-control group. Immunization with Id alone led to tumor suppression to some extent; however, no tumor-free animals were observed in this group.
Survival of mice challenged with 38C13 tumor cells.
(A) C3H/HeN mice were immunized twice subcutaneously with 50 μg of the tested Id fusion proteins or PBS alone and intraperitoneally challenged with 38C13 cells 2 weeks after the second immunization. The percentage of survivors in each group was recorded. (B) C3H/HeN mice were immunized twice intraperitoneally with 10 μg of the tested Id fusion proteins or PBS alone and subcutaneously challenged with 38C13 cells 2 weeks after the second immunization. The mean tumor volume of mice bearing a measurable tumor mass in each group was calculated. SD (bars) are only given at day 24 for clarity. The ratio of animals in each group that succumbed to tumor death is indicated.
Survival of mice challenged with 38C13 tumor cells.
(A) C3H/HeN mice were immunized twice subcutaneously with 50 μg of the tested Id fusion proteins or PBS alone and intraperitoneally challenged with 38C13 cells 2 weeks after the second immunization. The percentage of survivors in each group was recorded. (B) C3H/HeN mice were immunized twice intraperitoneally with 10 μg of the tested Id fusion proteins or PBS alone and subcutaneously challenged with 38C13 cells 2 weeks after the second immunization. The mean tumor volume of mice bearing a measurable tumor mass in each group was calculated. SD (bars) are only given at day 24 for clarity. The ratio of animals in each group that succumbed to tumor death is indicated.
Mechanisms involved in the high immunogenicity of Id-CTLA4
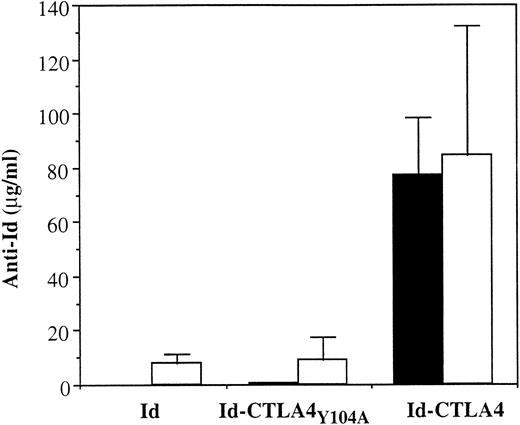
We next asked whether the biologic activity of CTLA-4, that is, the ability to bind B7 molecules on APCs, was required for the enhanced immunogenicity of Id-CTLA4. We constructed an Id fusion protein containing a mutant form of human CTLA-4 (CTLA4Y104A) with a point mutation at residue 104 (Tyr→Ala) in the MYPPPY motif (residue 99-104). The MYPPPY motif located in the CDR3-like region of CTLA-4 is critical for its binding to B7 molecules, and the mutation of Tyr 104 in this region to Ala completely abolishes the interaction of the mutant CTLA-4 to both B7-1 and B7-2.25 The purified Id-CTLA4Y104A retained the idiotypic determinant as demonstrated by its interaction with the specific anti-Id mAb (data not shown). In FACS analysis using B7-1– or B7-2–transfected CHO cell lines, we confirmed that Id-CTLA4Y104A lost its binding activity to both B7-1 and B7-2 molecules (data not shown). We then intraperitoneally immunized mice with 10 μg Id, Id-CTLA4, or Id-CTLA4Y104A and analyzed the presence of specific anti-Id Ab in the immune sera. As shown in Figure7, mice receiving 2 Id-CTLA4Y104A inoculations produced only low titers of anti-Id Ab similar to the titers achieved by Id immunization, whereas a single immunization with Id-CTLA4 was able to produce high titers of anti-Id Ab. When the mice were injected intraperitoneally with a lethal dose of 38C13 tumors, none of the animals in the Id and Id-CTLA4Y104A groups survived the challenge, whereas immunization with Id-CTLA4 resulted in 60% long-term survivors. Taken together, these results strongly suggest that the interaction of CTLA4 and B7 on APCs is required for the enhanced antitumor immunity of Id-CTLA4.
Reduction of anti-Id Ab response by immunization with Id-CTLA4Y104A mutant protein.
Mice were immunized twice by intraperitoneal injection of 10 μg Id, Id-CTLA4Y104A, or Id-CTLA4 and bled 2 weeks after each immunization. First immunization, ▪; 2nd immunization, ■. Anti-Id titers in immune sera were determined by ELISA, as described in “Materials and methods.” The data are presented as the mean ± SD for 5 animals in each group.
Reduction of anti-Id Ab response by immunization with Id-CTLA4Y104A mutant protein.
Mice were immunized twice by intraperitoneal injection of 10 μg Id, Id-CTLA4Y104A, or Id-CTLA4 and bled 2 weeks after each immunization. First immunization, ▪; 2nd immunization, ■. Anti-Id titers in immune sera were determined by ELISA, as described in “Materials and methods.” The data are presented as the mean ± SD for 5 animals in each group.
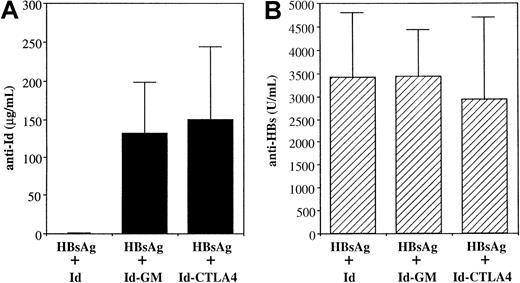
We further investigated whether the physical linkage between Ag and CTLA-4 was important for the adjuvant effect of CTLA-4. We mixed Id, Id-GM, or Id-CTLA4 with a model Ag, in this case HBsAg, and twice intraperitoneally injected the mixture into mice. Although much stronger anti-Id Abs were generated in the HBsAg plus Id-GM and HBsAg plus Id-CTLA4 groups compared with those generated in the HBsAg plus Id group (Figure 8A), all groups produced similar levels of anti-HBsAg Abs (Figure 8B). Thus, the CTLA-4 or cytokine molecules must be physically linked to the Ag to achieve increased immune responses.
Coimmunization of mice with HBsAg and various Id proteins.
Mice were immunized twice intraperitoneally with a mixture of 2 μg HBsAg and 10 μg Id, Id-GM, or Id-CTLA4. Sera were collected 2 weeks after the second immunization. Anti-Id (A) and anti-HBsAg (B) titers were determined by ELISA, as described in “Materials and methods.” The data are presented as the mean ± SD for 5 animals in each group.
Coimmunization of mice with HBsAg and various Id proteins.
Mice were immunized twice intraperitoneally with a mixture of 2 μg HBsAg and 10 μg Id, Id-GM, or Id-CTLA4. Sera were collected 2 weeks after the second immunization. Anti-Id (A) and anti-HBsAg (B) titers were determined by ELISA, as described in “Materials and methods.” The data are presented as the mean ± SD for 5 animals in each group.
Discussion
Most tumor malignancies express tumor-associated or tumor-specific Ags but do not elicit an efficient immune response. Various experimental strategies have been explored to enhance the immunogenicity of tumor vaccines. In the present study, we used B-cell lymphoma as a model system to test a new strategy for inducing antitumor immunity by specifically targeting APCs with fusion proteins consisting of tumor-derived Id protein and CTLA-4. We found that the Id-CTLA4 fusion protein elicited strong Id-specific T-cell responses and anti-Id Abs that specifically bind to Id-positive tumor cells. Mice immunized with Id-CTLA4 were significantly protected against a lethal tumor challenge. The immunogenicity of Id-CTLA4 is so potent that as little as 0.1 μg induced high titers of anti-Id Abs.
Previous experiments have shown that targeting immunogens to various APC populations with Abs against class II MHC,13,14FcγR,13 33D1,14 or surface Ig15can substantially increase Ab responses. Immunoconjugated Ag was demonstrated to be more efficiently presented to T cells than Ag alone in an in vitro assay.26 Compared to the previous studies using immunogenic proteins as Ags, we found that fusion with CTLA-4 was able to convert a low- to nonimmunogenic tumor Ag into a strong immunogen. CTLA-4 likely provides such a strong adjuvant activity due to its strong binding affinity to B7 molecules on dendritic cells. Among the professional APCs, dendritic cells express high levels of both B7-1 and B7-2 and are particularly important in initiating primary immune responses.27 CTLA-4 binds to both B7-1 and B7-2 with a 20- to 50-fold higher affinity than CD28,28 another B7-binding receptor on T cells. Using flow cytometric analysis, we found that the Id-CTLA4 fusion protein retained its ability to bind to both B7-1 and B7-2 molecules (Figure 2B) and interacted strongly with a murine dendritic cell line (Figure 2A). Another feature of Id-CTLA4 that might contribute to its strong binding to B7-expressing APCs is the presence of 2 CTLA-4 molecules in its construct. The dimeric CTLA-4 in the Id-CTLA4 fusion protein can bind 2 B7 molecules29and thus increase its binding avidity.
Another possible explanation for the high immunogenicity of Id-CTLA4 involves the carrier effect of the human Ig constant region or human CTLA-4 used to construct the fusion protein. Indeed, we observed anti-Id Ab responses in mice that received the Id protein, although the magnitude was much weaker than responses induced by Id-CTLA4 or Id-GM (Figures 3 and 4). We also noted the presence of antihuman IgG Ab in all immunized groups and antihuman CTLA-4 Ab in the group immunized with Id-CTLA4 (data not shown). In a previous study, it was also reported that the xenogenic human IgG constant region was required for a DNA vaccine to induce anti-Id responses.30 However, for several reasons we believe that the contribution of xenogeneic carrier effect to the immunogenicity of Id-CTLA4 observed in our study was minimal. First, the presence of a xenogenic determinant is not absolutely required for creating immunogenicity of the Id protein. One example is that recombinant Id proteins containing only the lymphoma Ig variable region and chemokines were able to elicit high levels of specific anti-Id Abs and protective immunity.12 Second, we previously made an Id fusion protein containing the xenogenic human Ig constant region and human GM-CSF that has a size similar to Id-CTLA4, but that has no GM-CSF activity in mice.10 In contrast to the high immunogenicity of Id-CTLA4, the Id-human GM-CSF fusion protein failed to produce anti-Id Abs. This finding strongly suggests that the contribution of the xenogenic carrier protein to the immune-enhancing function of Id-CTLA4 is minimal; instead, the biologic activity of CTLA-4, that is, the ability to bind B7 molecules, is critical for its enhanced immunogenicity. To more clearly address this issue, we constructed a mutant Id-CTLA4 fusion protein (Id-CTLA4Y104A) that has no binding activity to either B7-1 or B7-2 molecules. We demonstrated that Id-CTLA4Y104A, like the control Id protein, produced much less anti-Id Ab than Id-CTLA4 (Figure 7) and failed to provide protection against 38C13 tumor challenge. Together, these results indicate that the strong adjuvant activity of Id-CTLA4 is largely due to its more efficient targeting to APCs but not the classic carrier effect of the xenogenic Ig or CTLA-4 in the fusion protein.
In several previous studies using Id-cytokine or Id-chemokine fusion proteins as vaccines, it was demonstrated that vaccine efficacy depended on covalent linkage of the Id protein and cytokine or chemokine.10-12 In the present study, we performed an experiment coimmunizing a stronger Ag (HBsAg) with Id-CTLA4 and saw no enhancement of immune responses to HBsAg (Figure 8B). Therefore, the physical linkage between Id and CTLA-4 is likely required for the increased immunogenicity of Id-CTLA4. We hypothesize that the presence of CTLA-4 moiety in the fusion protein would ensure its targeting to APCs and, therefore, leads to more efficient uptake and processing of Id-CTLA4. This assumption is supported by a recent study that a CTLA4-Ig fusion protein composed of CTLA-4 and IgG Fc was present at 4-fold higher levels in draining lymph nodes than a nontargeted protein within 24 hours of administration.31
In contrast to the enhanced immunogenicity of Id-CTLA4 found in the present study, CTLA4-Ig was reported to suppress formation of Ab responses to sheep red blood cells and keyhole limpet hemocyanin.32 CTLA4-Ig has also been used as an immunosuppressive drug in animal models of transplantation and autoimmune diseases.33,34 The immune suppression mediated by CTLA4-Ig is likely through its inhibition of CD28-B7 interactions, which provide an important positive signal for T-cell proliferation and cytokine release.35 The discrepancy between the immunomodulating effects of CTLA-4 in our study and in previous studies is likely due to the number of treatment doses of CTLA-4 administered. In the previous studies, to achieve maximal immune suppression, a high dose (50-500 μg) of CTLA4-Ig was applied before Ag treatment and was continued several days thereafter. In our study, Id-CTLA4 was administered only twice with a 2-week interval between immunizations and at significantly low doses. Using our protocol, it is likely that the B7 molecules on APCs are not completely blocked, even at a relatively high dose of 50 μg, and thus remain available to engage with CD28 and thereby provide a signal for T-cell activation. Indeed, we found that mice immunized with Id-CTLA4 elicited Id-specific and human Ig-specific T-cell proliferation responses (Figure 5 and data not shown). Vaccination with DNA encoding CTLA4-Ig provides additional evidence that CTLA4-Ig at low levels increases both Ab and T-cell proliferation responses.16,36 Another possible strategy to overcome the potential immune-suppressive effect of CTLA-4 may be to apply a mutant CTLA-4 (CTLA4Y100A). The mutation of the first Tyr to Ala at residue 100 in the important MYPPPY motif completely abolishes CTLA-4 binding to B7-2, whereas its B7-1 reactivity is retained.28 Thus, the Id-CTLA4Y100A fusion protein would be directed to APCs through interaction with B7-1, whereas the remaining B7-2 molecules on APCs would serve to activate T cells.
Other immune-enhancing molecules used to increase the immunogenicity of Id in the fusion context include cytokines (GM-CSF, IL-1β, IL-2, IL-4),10,11,30,37 chemokines (IP-10, monocyte chemotactic protein 3),12 as well as a nontoxic fragment of tetanus toxin.38 Among these, the adjuvant effect of GM-CSF is unique and the best characterized. GM-CSF is known to be a critical stimulatory cytokine for a variety of APCs by increasing expression of class II MHC, B7-1, and B7-2, as well as other adhesion molecules.39-41 Recombinant GM-CSF was shown to provide strong adjuvant activity to Id proteins in animal studies42and human clinical trials.43 We demonstrated in this study that Id-CTLA4 and Id-GM were almost identical in their ability to induce primary and secondary anti-Id Ab responses, as well as protective immunity. Because the adjuvant effects of Id-CTLA4 and Id-GM appeared to work on APCs but are dependent on different mechanisms, the combination of these 2 fusion proteins in vaccine formulation might have additive effects. Studies in this regard are currently under investigation in our laboratory.
In summary, we demonstrated that fusion of CTLA-4 to a weak Id Ag dramatically increases its immunogenicity and substantially promotes specific Ab responses and protective immunity. This approach is simple and does not require the use of immunologic adjuvants. The general approach of CTLA-4 fusion vaccines may be applicable to tumor Ags of other cancers as well as infectious diseases.
Acknowledgments
We thank Drs Sherie L. Morrison (UCLA, Los Angeles, CA) and Chou-Chik Ting (National Cancer Institute, National Institutes of Health, Bethesda, MD) for critical reading of this manuscript and many helpful suggestions. We also thank Mr Douglas Platt for the English editing of this manuscript.
Supported by Academia Sinica and by grant DOH89A1-PPLABAD01 from the National Health Research Institute, Taiwan.
T.H.H. and P.Y.W. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mi-Hua Tao, Institute of Biomedical Sciences, Academia Sinica, Taipei 115, Taiwan; e-mail:bmtao@ccvax.sinica.edu.tw.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal