Abstract
Peripheral blood B cells in patients with paroxysmal nocturnal hemoglobinuria (PNH) comprise variable mixtures of normal B cells produced before the onset of disease and glycosylphosphatidylinositol (GPI)-deficient B cells derived from the PNH hematopoietic stem cell. In a detailed phenotypic analysis of 29 patients with PNH, this study shows consistent phenotypic differences between PNH B cells and residual normal B cells. In the majority of patients with active disease, PNH B cells comprised mainly naive cells with a CD27−IgM+IgDstrong+IgG−phenotype. The proportion of CD27+ memory cells within this compartment was related to disease duration (Spearman [rs] 0.403; P = .030). In PNH patients with predominantly GPI-deficient hematopoiesis, that is, a large granulocyte PNH clone, the residual normal B cells had a predominantly memory (CD27+) phenotype. Furthermore, the majority of these memory B cells were not immunoglobulin (Ig) class switched and had an IgM+IgD+IgG− phenotype. Using PNH as a novel model with which to study B lymphopoiesis, this study provides direct evidence that production of new naive B cells occurs throughout life and that the major population of long-lived memory B cells are IgM+IgD+. Moreover, studies of GPI− B cells in 2 patients in remission from PNH suggest that the life span of a B-cell clone can be more than 24 years.
Introduction
Recent studies have shown that coexpression of the tumor necrosis factor receptor superfamily member CD27 and the receptor-type protein tyrosine phosphatase CD148 by human peripheral blood B cells defines the memory B-cell compartment generated by germinal center reaction.1-3 These CD19+CD27+ cells show somatically mutated immunoglobulin (Ig) variable gene regions and can be subdivided into 2 phenotypically distinct populations of memory B cells: (1) Ig class-switched cells expressing surface IgG or IgA and (2) IgM only and IgM/IgD-expressing memory cells.2-4 Further evidence that CD27 is a marker for memory B cells is shown by the findings that cord blood B cells are predominantly CD27− and that the proportion of CD27+ B cells increases with age.5 The recent discovery that memory B cells not only comprise Ig class-switched (IgG, IgA, or IgE) but also IgM+cells clearly shows that somatic hypermutation of immunoglobulin genes on exposure to antigen does not always coincide with Ig class switching as depicted in classical models of the immune response.4,6 7 What remains poorly defined is the relative contribution of non–class-switched (IgM+CD27+) and class-switched (IgM−CD27+) subsets to the memory B-cell compartment and an indication of the life span of naive and memory B-cell subsets.
Paroxysmal nocturnal hemoglobinuria (PNH) is a unique condition in which somatic mutation of the X-linked PIG-A gene within a hematopoietic stem cell results in a partial or absolute deficiency of glycosylphosphatidylinositol (GPI)-linked membrane proteins.8-13 Following onset of the disorder and failure of normal hematopoiesis, new blood cell formation in patients with large PNH clones is almost entirely derived from the PNH stem cell. Nevertheless, in the majority of patients, residual populations of normal myeloid and erythroid cells produced by the few remaining normal stem cells can be detected. The majority of T and B lymphocytes are usually normal with only relatively small PNH populations present even in patients with over 90% of their hematopoiesis derived from the PNH clone. This is due to the fact that many of these are long-lived cells produced before the onset of PNH.
We have shown previously that PNH can be used as a model with which to study the immune system and that the absence of GPI-linked antigens can be used as a biologic marker with which to examine production of cells following the onset of PNH.14 We have used this approach to study peripheral blood B cells to examine the definition of naive and memory B cells, the phenotypic characteristics of the memory B-cell compartment in the peripheral blood, and the effect of aging on B-cell production. A more detailed understanding of peripheral memory B-cell compartment and the humoral immune response is important for a number of reasons including monitoring B-cell recovery after bone marrow transplantation or after chemotherapy and potentially monitoring the effectiveness of vaccination.
Using sensitive multicolor flow cytometry techniques, GPI-deficient (GPI−) B cells can be detected in the peripheral blood of a high proportion of patients with PNH.15,16 These PNH B cells are identified by their lack of GPI-anchored membrane antigens such as CD48, CD55, and CD59 and can be clearly separated from residual normal GPI+ B cells generated before the onset of PNH.16,17 In general terms, a low absolute number of B cells is a feature of PNH, though the proportion of GPI− B cells is highly variable from patient to patient. What remains poorly characterized are the phenotypic characteristics of both PNH B cells and residual normal B cells, specifically with respect to the newly described markers of CD27 and CD148 that identify memory B cells.1-3 By examining the differential expression of CD27 and Ig heavy-chain isotype by normal and GPI− B-cell populations in a series of patients with PNH, we provide some important new insights into B-lymphocyte biology and the memory B-cell compartment.
Patients, materials, and methods
Patients
Peripheral blood samples from a series of 29 consecutive adult patients with PNH were screened for GPI− B cells using established protocols.16 Informed consent was obtained from the patients before specimen collection. The initial diagnosis of PNH was made either by demonstrating absence of GPI-linked proteins from red cells or granulocytes by flow cytometry or by a positive Ham test.18 19 PNH B-cell clones were detected in all patients studied. The 14 men and 15 women had an age range of 18 to 78 years. Further analyses undertaken on all patients for the purposes of this study included (1) determination of the absolute numbers of B cells by flow cytometry, (2) flow cytometric analysis of B cells using a 3-color combination of monoclonal antibodies to CD48/CD27/CD19, and (3) full blood counts including absolute lymphocyte counts performed using a Sysmex K1000 cell counter (Sysmex, Milton Keynes, UK).
Isolation of leukocytes by differential erythrolysis with ammonium chloride
One-milliliter volumes of peripheral blood were added to 9 mL of 0.16 mol/L ammonium chloride, incubated at 37°C for 5 minutes, then centrifuged at 300g for 4 minutes and the supernatant discarded. The white cell pellet was resuspended and washed twice with 10-mL volumes of FACSFlow (Becton Dickinson, San Jose, CA) supplemented with 0.5% bovine serum albumin (FACSFlow/BSA) (Sigma Chemicals, St Louis, MO) and the white cell count adjusted to 50 × 109/L for subsequent immunophenotyping studies.
Determination of the absolute numbers of B lymphocytes
An initial whole blood lysis screen was performed to assess the proportions and absolute numbers of B cells using a prestandardized combination of directly conjugated CD3/HLA-DR/CD19 monoclonal antibodies (fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE:Cy5) and an established protocol.16 The cells were analyzed using a FACScan flow cytometer (Becton Dickinson) and CellQuest software (Becton Dickinson) collecting a minimum of 1 × 104 gated lymphocyte events identified by low forward scatter (FSC) and low side scatter (SSC) characteristics. Absolute numbers of B cells were calculated using the Sysmex lymphocyte count multiplied by the percentage of lymphocytes expressing CD19 divided by 100.
Analysis of CD27 expression on normal and paroxysmal nocturnal hemoglobinuria B cells
Volumes (100 μL) of whole blood were stained as for absolute B-cell counts with 30-μL volumes of the following combination of fluorochrome-conjugated monoclonal antibodies: CD48Fitc/CD27PE/CD19PE:Cy5. Cells were analyzed by flow cytometry using a FACScan flow cytometer with CellQuest software. A minimum of 1 × 103 gated events were collected on B-lymphocyte populations identified by low SSC characteristics and CD19 positivity. Data were stored as list mode files for subsequent analysis. Quadrant statistical analyses of dot-plots of CD48 versus either CD27 were undertaken, and the proportions of normal and PNH lymphocytes expressing CD27 calculated. Absolute numbers of GPI− naive B cells were calculated by multiplying the absolute numbers of B cells by the percentage of CD48−CD27− cells, divided by 100.
Analysis of Ig heavy-chain expression on normal and paroxysmal nocturnal hemoglobinuria B cells
Immunoglobulin heavy-chain expression (IgM and IgG) was examined on normal and GPI− B-cell subsets of 10 patients using 3-color flow cytometry. Leukocytes (1 × 106) were stained in microtiter plate wells with 30 μL of the following combinations of fluorochrome-conjugated monoclonal antibodies: (Fitc/PE/PE:Cy5) IgM/CD48/CD19 and IgG/CD48/CD19. The cell-antibody combinations were mixed, incubated at room temperature for 30 minutes, and then centrifuged at 400g for 15 seconds to pellet the cells. After removal of excess antibody and washing twice with 150-μL volumes of FACSFlow/BSA, the cells were resuspended to 500 μL in FACSFlow prior to flow cytometry studies and analyzed by flow cytometry. A minimum of 1 × 104 gated B cells was collected on the basis of low SSC characteristics and CD19 positivity. The proportions of normal and PNH B cells expressing IgM or IgG were derived from quadrant statistical analysis of CD48 versus Ig heavy-chain dot-plots. In addition, analysis of CD48/IgD/CD19 expression was undertaken in 4 cases.
Monoclonal antibodies
The sources and specificities of all monoclonal antibodies used for immunophenotyping were as follows: CD3 (OKT3 Fitc) and HLA-DR (L243 PE) from American Type Culture Collection (ATCC); CD48 (MEM102 Fitc and PE) from Cymbus Biotechnology Limited, Chandler's Ford, Hampshire, UK; CD19 (FMC63 PE:Cy5) from Professor Zola, Flinders Medical Centre, Australia; and CD27 (M-T271 PE), anti-IgD (IA6-2 PE), anti-IgG (G18-145 Fitc), anti-IgM (G20-127 Fitc) from Pharmingen, San Diego, CA.
Results
The clinical and laboratory features of the 29 patients are presented in Table 1. Fifteen patients had hemolytic PNH, 11 had hypoplastic disease, and 2 patients presented with thrombotic disease. Two of the hemolytic patients also had concurrent thrombotic disease, and 2 patients had morphologic evidence of myelodysplastic syndrome. Two patients (PNH104 and PNH008) no longer had detectable PNH red cells or granulocyte clones in the peripheral blood. Patient PNH104 originally presented in 1962 with severe hemolytic PNH; this persisted for a number of years with attacks of macroscopic hemoglobinuria ceasing in 1976. He currently has myelodysplastic syndrome. Expression of GPI-linked antigens on red cells and granulocytes was abnormal, although the flow cytometry profiles of CD55 and CD59 expression were not those seen in classical PNH. Patient 008 originally presented with hemolytic PNH 12 years ago and is currently in clinical remission. She has no detectable granulocyte or red cell PNH clones by flow cytometry in the peripheral blood.
Clinical and laboratory features of patients studied
| Patient ID . | Age (y)/sex . | Clinical features† . | Duration (mo)‡ . | GPI-deficient clone (%)* . | B cell count/ (109/L)1-153 . | ||
|---|---|---|---|---|---|---|---|
| Granulocytes . | RBCs . | B cells . | |||||
| PNH006 | 45/F | Hemolytic/thrombotic | 195 | 99.4 | 32.9 | 0.8 | 0.18 |
| PNH008 | 32/F | Hemolytic | 144 | 0.0 | 0.0 | 13.8 | 0.07 |
| PNH018 | 31/M | Hypoplastic | 96 | 1.9 | 0.4 | 4.6 | 0.04 |
| PNH033 | 36/F | Hemolytic | 200 | 99.7 | 57.8 | 54.4 | 0.02 |
| PNH042 | 56/M | Hemolytic | 144 | 97.7 | 60.6 | 2.2 | 0.10 |
| PNH043 | 46/M | Hypoplastic | 60 | 99.3 | 49.7 | 0.5 | 0.03 |
| PNH048 | 30/F | Hypoplastic | 59 | 27.7 | 13.3 | 0.1 | 0.06 |
| PNH055 | 45/F | Hypoplastic | 45 | 80.2 | 17.1 | 3.7 | 0.06 |
| PNH056 | 32/M | Hemolytic | 78 | 31.3 | 34.8 | 9.6 | 0.10 |
| PNH064 | 24/M | Hypoplastic | 34 | 29.7 | 13.1 | 3.1 | 0.04 |
| PNH065 | 23/F | Hypoplastic/MDS | 0 | 79.2 | 40.9 | 6.5 | 0.14 |
| PNH069 | 50/F | Hemolytic | 2 | 93.3 | 24.3 | 28.6 | 0.06 |
| PNH075 | 31/M | Hypoplastic | 25 | 9.6 | 8.7 | 4.7 | 0.09 |
| PNH077 | 52/M | Hemolytic | 40 | 98.4 | 36.5 | 3.8 | 0.10 |
| PNH090 | 37/F | Hemolytic | 30 | 93.5 | 54.3 | 6.0 | 0.01 |
| PNH094 | 34/F | Hypoplastic | 0 | 0.9 | 3.5 | 0.2 | 0.24 |
| PNH098 | 32/M | Hemolytic/thrombotic | 93 | 57.3 | 36.0 | 3.5 | 0.02 |
| PNH102 | 78/M | Hypoplastic | 0 | 55.5 | 5.2 | 1.2 | 0.12 |
| PNH104 | 69/M | MDS | 480 | — | — | 1.3 | 0.02 |
| PNH105 | 36/M | Hemolytic to hypoplastic | 6 | 77.0 | 28.3 | 2.5 | 0.02 |
| PNH107 | 37/M | Thrombotic | 8 | 94.6 | 37.6 | 1.8 | 0.07 |
| PNH110 | 56/M | Hemolytic | 18 | 75.9 | 51.8 | 5.6 | 0.22 |
| PNH111 | 43/F | Hemolytic | 10 | 95.2 | 47.9 | 14.7 | 0.03 |
| PNH115 | 52/F | Hypoplastic | 14 | 5.0 | 1.1 | 0.2 | 0.33 |
| PNH119 | 45/F | Thrombotic | 0 | 15.3 | 6.8 | 4.0 | 0.03 |
| PNH120 | 51/F | Hemolytic | 11 | 83.3 | 45.8 | 1.6 | 0.03 |
| PNH121 | 42/F | Hemolytic | 0 | 92.6 | 31.3 | 0.6 | 0.05 |
| PNH122 | 23/F | Hemolytic | 12 | 57.7 | 23.3 | 2.0 | 0.12 |
| PNH124 | 18/M | Hemolytic | 92 | 72.3 | 55.0 | 2.7 | 0.07 |
| Patient ID . | Age (y)/sex . | Clinical features† . | Duration (mo)‡ . | GPI-deficient clone (%)* . | B cell count/ (109/L)1-153 . | ||
|---|---|---|---|---|---|---|---|
| Granulocytes . | RBCs . | B cells . | |||||
| PNH006 | 45/F | Hemolytic/thrombotic | 195 | 99.4 | 32.9 | 0.8 | 0.18 |
| PNH008 | 32/F | Hemolytic | 144 | 0.0 | 0.0 | 13.8 | 0.07 |
| PNH018 | 31/M | Hypoplastic | 96 | 1.9 | 0.4 | 4.6 | 0.04 |
| PNH033 | 36/F | Hemolytic | 200 | 99.7 | 57.8 | 54.4 | 0.02 |
| PNH042 | 56/M | Hemolytic | 144 | 97.7 | 60.6 | 2.2 | 0.10 |
| PNH043 | 46/M | Hypoplastic | 60 | 99.3 | 49.7 | 0.5 | 0.03 |
| PNH048 | 30/F | Hypoplastic | 59 | 27.7 | 13.3 | 0.1 | 0.06 |
| PNH055 | 45/F | Hypoplastic | 45 | 80.2 | 17.1 | 3.7 | 0.06 |
| PNH056 | 32/M | Hemolytic | 78 | 31.3 | 34.8 | 9.6 | 0.10 |
| PNH064 | 24/M | Hypoplastic | 34 | 29.7 | 13.1 | 3.1 | 0.04 |
| PNH065 | 23/F | Hypoplastic/MDS | 0 | 79.2 | 40.9 | 6.5 | 0.14 |
| PNH069 | 50/F | Hemolytic | 2 | 93.3 | 24.3 | 28.6 | 0.06 |
| PNH075 | 31/M | Hypoplastic | 25 | 9.6 | 8.7 | 4.7 | 0.09 |
| PNH077 | 52/M | Hemolytic | 40 | 98.4 | 36.5 | 3.8 | 0.10 |
| PNH090 | 37/F | Hemolytic | 30 | 93.5 | 54.3 | 6.0 | 0.01 |
| PNH094 | 34/F | Hypoplastic | 0 | 0.9 | 3.5 | 0.2 | 0.24 |
| PNH098 | 32/M | Hemolytic/thrombotic | 93 | 57.3 | 36.0 | 3.5 | 0.02 |
| PNH102 | 78/M | Hypoplastic | 0 | 55.5 | 5.2 | 1.2 | 0.12 |
| PNH104 | 69/M | MDS | 480 | — | — | 1.3 | 0.02 |
| PNH105 | 36/M | Hemolytic to hypoplastic | 6 | 77.0 | 28.3 | 2.5 | 0.02 |
| PNH107 | 37/M | Thrombotic | 8 | 94.6 | 37.6 | 1.8 | 0.07 |
| PNH110 | 56/M | Hemolytic | 18 | 75.9 | 51.8 | 5.6 | 0.22 |
| PNH111 | 43/F | Hemolytic | 10 | 95.2 | 47.9 | 14.7 | 0.03 |
| PNH115 | 52/F | Hypoplastic | 14 | 5.0 | 1.1 | 0.2 | 0.33 |
| PNH119 | 45/F | Thrombotic | 0 | 15.3 | 6.8 | 4.0 | 0.03 |
| PNH120 | 51/F | Hemolytic | 11 | 83.3 | 45.8 | 1.6 | 0.03 |
| PNH121 | 42/F | Hemolytic | 0 | 92.6 | 31.3 | 0.6 | 0.05 |
| PNH122 | 23/F | Hemolytic | 12 | 57.7 | 23.3 | 2.0 | 0.12 |
| PNH124 | 18/M | Hemolytic | 92 | 72.3 | 55.0 | 2.7 | 0.07 |
GPI-deficient clones as measured by flow cytometry.
Hypoplastic patients defined by an absence of macroscopic hemoglobinuria.
Time in months since the onset of PNH.
Reference ranges: B cells 0.06-0.6 × 109/L.
The median size of the granulocyte clone for the 27 patients with active PNH was 77.0%. This was considered as a reliable indicator that the majority of hematopoiesis was clonally derived from the PNH stem cell. Absolute B-cell counts were low (< 0.06 × 109/L) in 15 of 29 patients (52%) and borderline low (> 0.06 × 109/L but < 0.1 × 109/L) in an additional 7 patients. The median B-cell count was 0.06 × 109/L (observed range 0.01-0.33 × 109/L).
Detection of paroxysmal nocturnal hemoglobinuria B-cell clones and relationship with the granulocyte paroxysmal nocturnal hemoglobinuria clone size
Of the 29 patients studied, PNH B cells were detected in all cases including the 2 patients with spontaneous remission. The percentage of GPI− B cells showed a wide variation (0.14%-54.4%, median 3.05%) though in 86% of cases the clone comprised less than 10% of total B cells. To determine whether this variability was related to the activity of the PNH stem cell, correlations between the levels of PNH B cells and PNH granulocytes were examined. No significant correlations were found between the proportions (Spearman [rs] 0.131; P = .45) or absolute numbers (Spearman (rs) 0.16; P = .42) of PNH B cells and PNH granulocytes.
Relationships between age and absolute numbers of paroxysmal nocturnal hemoglobinuria B cells
One explanation for the wide variation in the proportion of PNH B cells is that patient age may affect the ability to produce B cells. Because PNH B cells are unequivocally derived from a bone marrow hematopoietic stem cell, we examined the relationship between absolute numbers of PNH B cells versus patient age as a measure of B-cell production. No significant correlation was found (Spearman [rs] 0.017; P = .93). In a more specific analysis of the 19 patients where the majority of hematopoiesis was derived from the PNH stem cell (ie, a > 50% PNH granulocyte clone) again no significant correlation was found (Spearman [rs] 0.062; P = .80). These findings show that the proportion of PNH B cells present in any individual patient is not directly related to the size of the granulocyte PNH clone nor to patient age. Furthermore, these studies confirm that new B cells are continually produced throughout adult life and that the levels of production appear not to decline with age (Figure 1).
Relationship between patient age and absolute numbers of GPI− B cells in patients with PNH.
Although there is no direct correlation between age and absolute numbers of PNH B cells, it is clear that production of B cells occurs throughout adult life with even the oldest patient PNH102 (age 78) producing detectable levels of PNH B cells.
Relationship between patient age and absolute numbers of GPI− B cells in patients with PNH.
Although there is no direct correlation between age and absolute numbers of PNH B cells, it is clear that production of B cells occurs throughout adult life with even the oldest patient PNH102 (age 78) producing detectable levels of PNH B cells.
CD27 expression by CD48+and CD48− subpopulations of B cells
Results comparing CD27 expression on PNH (CD48−) B cells with residual normal B cells (CD48+) in the same patient (Table2) showed absent or reduced levels of CD27 expression within the PNH component. These differences were statistically significant (paired t test: t = −9.96; degrees of freedom [df], 28; P < .0001). A series of representative flow cytometry dot-plots of CD48 versus CD27 for CD19+ gated B cells are shown in Figure2 and illustrate differential CD27 expression between GPI− B cells and normal B cells. The GPI− B cells detected in patients with a recent onset of PNH were predominantly of a naive (CD27−) phenotype consistent with recent production. The coexisting GPI+ B cells in these patients showed normal proportions of CD27+cells. In marked contrast to this, the residual normal B-cell components in patients with large PNH granulocyte clones and a long history of PNH were predominantly of a memory (CD27+) phenotype. There are 2 possible mechanisms that contribute to this: (1) the long-term failure in production of new naive GPI+ B cells and (2) conversion of the existing normal CD27− B cells, produced before the onset of PNH, to a memory phenotype. The highest proportions of CD27+ PNH B cells were found in patients with long duration of disease and in the 2 patients in whom spontaneous remission of PNH had occurred.
Absolute numbers of PNH B cells and CD27 expression by normal and GPI-deficient B-cell populations
| Case no. . | % CD27 expression* . | Abs PNH B cells/(106/L) . | |
|---|---|---|---|
| PNH . | Normal . | ||
| PNH006 | 23.4 | 82.6 | 1.48 |
| PNH008 | 9.8 | 59.7 | 9.47 |
| PNH018 | 12.9 | 57.1 | 1.85 |
| PNH033 | 15.5 | 91.8 | 10.9 |
| PNH042 | 3.2 | 89.4 | 2.2 |
| PNH043 | 11.6 | 78.6 | 0.15 |
| PNH048 | 0.0 | 66.4 | 0.08 |
| PNH055 | 3.3 | 82.2 | 2.19 |
| PNH056 | 1.1 | 31.3 | 9.64 |
| PNH064 | 3.6 | 22.4 | 1.22 |
| PNH065 | 2.0 | 22.3 | 9.16 |
| PNH069 | 5.0 | 33.2 | 17.16 |
| PNH075 | 3.4 | 29.3 | 42.12 |
| PNH077 | 20.8 | 70.0 | 3.75 |
| PNH090 | 5.4 | 76.7 | 0.60 |
| PNH094 | 0.0 | 15.7 | 0.46 |
| PNH098 | 5.1 | 27.5 | 0.7 |
| PNH102 | 2.5 | 27.5 | 1.44 |
| PNH104 | 58.0 | 84.2 | 0.25 |
| PNH105 | 6.0 | 32.7 | 0.498 |
| PNH107 | 14.9 | 30.2 | 1.27 |
| PNH110 | 2.2 | 34.7 | 12.41 |
| PNH111 | 5.2 | 31.9 | 5.02 |
| PNH115 | 22.2 | 33.8 | 0.59 |
| PNH119 | 17.9 | 62.3 | 1.19 |
| PNH120 | 6.9 | 72.5 | 0.49 |
| PNH121 | 0.0 | 45.2 | 0.31 |
| PNH122 | 3.0 | 38.0 | 2.4 |
| PNH124 | 16.4 | 33.7 | 1.9 |
| Median | 5.2 | 38.0 | — |
| Normal range† | — | 22.6-54.3 | |
| Case no. . | % CD27 expression* . | Abs PNH B cells/(106/L) . | |
|---|---|---|---|
| PNH . | Normal . | ||
| PNH006 | 23.4 | 82.6 | 1.48 |
| PNH008 | 9.8 | 59.7 | 9.47 |
| PNH018 | 12.9 | 57.1 | 1.85 |
| PNH033 | 15.5 | 91.8 | 10.9 |
| PNH042 | 3.2 | 89.4 | 2.2 |
| PNH043 | 11.6 | 78.6 | 0.15 |
| PNH048 | 0.0 | 66.4 | 0.08 |
| PNH055 | 3.3 | 82.2 | 2.19 |
| PNH056 | 1.1 | 31.3 | 9.64 |
| PNH064 | 3.6 | 22.4 | 1.22 |
| PNH065 | 2.0 | 22.3 | 9.16 |
| PNH069 | 5.0 | 33.2 | 17.16 |
| PNH075 | 3.4 | 29.3 | 42.12 |
| PNH077 | 20.8 | 70.0 | 3.75 |
| PNH090 | 5.4 | 76.7 | 0.60 |
| PNH094 | 0.0 | 15.7 | 0.46 |
| PNH098 | 5.1 | 27.5 | 0.7 |
| PNH102 | 2.5 | 27.5 | 1.44 |
| PNH104 | 58.0 | 84.2 | 0.25 |
| PNH105 | 6.0 | 32.7 | 0.498 |
| PNH107 | 14.9 | 30.2 | 1.27 |
| PNH110 | 2.2 | 34.7 | 12.41 |
| PNH111 | 5.2 | 31.9 | 5.02 |
| PNH115 | 22.2 | 33.8 | 0.59 |
| PNH119 | 17.9 | 62.3 | 1.19 |
| PNH120 | 6.9 | 72.5 | 0.49 |
| PNH121 | 0.0 | 45.2 | 0.31 |
| PNH122 | 3.0 | 38.0 | 2.4 |
| PNH124 | 16.4 | 33.7 | 1.9 |
| Median | 5.2 | 38.0 | — |
| Normal range† | — | 22.6-54.3 | |
Percentage values in the table are the proportion of either CD48+ (normal) or CD48− (PNH) B cells expressing CD27.
Derived from an analysis of 10 normal adults.
Expression of CD27 by normal and PNH B cells.
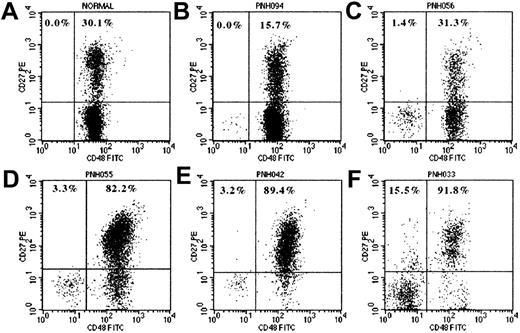
Flow cytometry dot-plots show CD27 expression by normal B cells (A) and by normal (CD48+) and PNH (CD48−) components of CD19+ B cells in 5 patients with PNH (B-F). B cells were identified by CD19+SSClow characteristics. The percentage values represent the proportions of PNH and normal B cells expressing CD27. Plot A is from a normal healthy adult donor and shows no GPI− B cells and 30.1% CD27+ (memory) B cells. Plots B, C, and D are from 3 patients with PNH, with GPI− B cell clones of 0.19%, 9.64%, and 3.65% and granulocyte PNH clones of 0.9%, 31.3%, and 80.2%, respectively. These show that GPI− B cells are detectable in most patients with PNH regardless of the size of the granulocyte clone and that they have a predominantly naive (CD27−) phenotype. Plots D, E, and F are from patients with large PNH granulocyte clones (ie, predominantly GPI− hematopoiesis) and show that the residual normal B cells are predominantly CD27+ (memory phenotype). Plot F shows CD27 expression for the normal and PNH fractions of B cells from patient PNH033 who developed hemolytic PNH over 16 years ago. The residual normal B cells are almost all CD27+ and furthermore a significant proportion of the PNH B cells (15.5%) have also become CD27+, reflecting the long duration of disease in this patient.
Expression of CD27 by normal and PNH B cells.
Flow cytometry dot-plots show CD27 expression by normal B cells (A) and by normal (CD48+) and PNH (CD48−) components of CD19+ B cells in 5 patients with PNH (B-F). B cells were identified by CD19+SSClow characteristics. The percentage values represent the proportions of PNH and normal B cells expressing CD27. Plot A is from a normal healthy adult donor and shows no GPI− B cells and 30.1% CD27+ (memory) B cells. Plots B, C, and D are from 3 patients with PNH, with GPI− B cell clones of 0.19%, 9.64%, and 3.65% and granulocyte PNH clones of 0.9%, 31.3%, and 80.2%, respectively. These show that GPI− B cells are detectable in most patients with PNH regardless of the size of the granulocyte clone and that they have a predominantly naive (CD27−) phenotype. Plots D, E, and F are from patients with large PNH granulocyte clones (ie, predominantly GPI− hematopoiesis) and show that the residual normal B cells are predominantly CD27+ (memory phenotype). Plot F shows CD27 expression for the normal and PNH fractions of B cells from patient PNH033 who developed hemolytic PNH over 16 years ago. The residual normal B cells are almost all CD27+ and furthermore a significant proportion of the PNH B cells (15.5%) have also become CD27+, reflecting the long duration of disease in this patient.
Previous studies have established that CD27 expression on normal peripheral blood B cells increases with age.5 To determine whether CD27 expression on PNH B cells was related to disease duration, we examined the correlation between percentage CD27 expression by the PNH B cells and time from the date of onset of clinical symptoms of PNH. Statistical analysis (Figure 3), not unexpectedly, revealed a significant correlation (Spearman [rs] 0.403; P = .030) between disease duration and the proportion of CD27+ PNH B cells (ie, memory PNH B cells).
Time-dependent relationship between disease duration and the proportion of PNH B cells expressing CD27.
The proportion of CD27+ (memory) PNH B cells increases with disease duration. Correlation determined by the Spearman rank correlation.
Time-dependent relationship between disease duration and the proportion of PNH B cells expressing CD27.
The proportion of CD27+ (memory) PNH B cells increases with disease duration. Correlation determined by the Spearman rank correlation.
Surface IgM and IgG expression by normal (CD48+) and paroxysmal nocturnal hemoglobinuria (CD48−) subpopulations of B cells
Having demonstrated clear differences in CD27 expression between normal and PNH B cells, the analysis was extended to examine surface Ig heavy-chain isotype expression by normal and GPI− B cells. IgM and IgG expression was studied in 10 patients, 8 with active disease and 2 in remission, using 3-color flow cytometry. This analysis (Table 3 and Figure4) showed that the PNH B-cell component in patients with active disease (hemolytic or hypoplastic) were predominantly IgM+IgG−(CD27−), that is, a naive phenotype consistent with recent production. Furthermore, analysis of membrane IgD expression in 3 patients (PNH008, PNH042, and PNH069) showed that the strength of expression of IgD was considerably higher on PNH B cells compared to residual normal B cells (Figure 4). The PNH B cells in patient PNH104, who developed PNH 36 years ago, showed a high proportion of memory cells with significant populations of non–class-switched (IgM+) and class-switched (IgG+) cells.
Comparative expression of surface IgM, IgG and CD27 by normal (CD48+) and GPI-deficient (CD48−) B cells in patients with PNH3-150
| Case no. . | PNH B cells (CD48−) % positive . | Normal B cells (CD48+) % positive . | ||||
|---|---|---|---|---|---|---|
| CD27+ . | IgM+ . | IgG+ . | CD27+ . | IgM+ . | IgG+ . | |
| PNH0063-151 | 23.4 | — | — | 82.6 | 68.8 | 36.93 |
| PNH0083-152 | 9.8 | 91.5 | 5.8 | 59.7 | 77.3 | 17.1 |
| PNH018 | 12.9 | 62.8 | 2.6 | 57.1 | 37.1 | 26.9 |
| PNH0423-151,3-153 | 3.2 | 88.4 | 0.0 | 89.4 | 75.2 | 12.4 |
| PNH0433-151 | 11.2 | 88.9 | 7.4 | 78.6 | 80.4 | 7.3 |
| PNH0693-151,3-155 | 7.2 | 99.9 | 8.2 | 54.4 | 98.3 | 27.94 |
| PNH0903-151 | 5.4 | 64.0 | 1.4 | 76.7 | 76.8 | 0.8 |
| PNH1043-154 | 58.0 | 41.4 | 27.0 | 84.2 | 44.6 | 22.7 |
| PNH110 | 2.18 | 69.8 | 2.1 | 34.7 | 47.0 | 15.1 |
| PNH1113-151 | 5.16 | 95.8 | 0.4 | 31.9 | 86.9 | 6.9 |
| Case no. . | PNH B cells (CD48−) % positive . | Normal B cells (CD48+) % positive . | ||||
|---|---|---|---|---|---|---|
| CD27+ . | IgM+ . | IgG+ . | CD27+ . | IgM+ . | IgG+ . | |
| PNH0063-151 | 23.4 | — | — | 82.6 | 68.8 | 36.93 |
| PNH0083-152 | 9.8 | 91.5 | 5.8 | 59.7 | 77.3 | 17.1 |
| PNH018 | 12.9 | 62.8 | 2.6 | 57.1 | 37.1 | 26.9 |
| PNH0423-151,3-153 | 3.2 | 88.4 | 0.0 | 89.4 | 75.2 | 12.4 |
| PNH0433-151 | 11.2 | 88.9 | 7.4 | 78.6 | 80.4 | 7.3 |
| PNH0693-151,3-155 | 7.2 | 99.9 | 8.2 | 54.4 | 98.3 | 27.94 |
| PNH0903-151 | 5.4 | 64.0 | 1.4 | 76.7 | 76.8 | 0.8 |
| PNH1043-154 | 58.0 | 41.4 | 27.0 | 84.2 | 44.6 | 22.7 |
| PNH110 | 2.18 | 69.8 | 2.1 | 34.7 | 47.0 | 15.1 |
| PNH1113-151 | 5.16 | 95.8 | 0.4 | 31.9 | 86.9 | 6.9 |
As determined by 3-color flow cytometry using CD48/CD27/CD19, IgM/CD48/CD19, and IgG/CD48/CD19 combinations of monoclonal antibodies as described in “Materials and methods.” Values in the table are the percentage of either CD48− or CD48+ cells expressing CD27, IgM, or IgG.
Patients with >90% PNH granulocyte clone.
Patient developed hemolytic PNH 12 years ago and currently has no detectable PNH red cells or granulocytes. Analysis of IgD expression showed 94.7% positivity (mean fluorescent intensity [MFI], 1248) for the PNH B cells and 70.2% positivity for normal B cells (MFI 941).
Analysis of IgD expression showed 95.5% positivity (MFI 2607) for the PNH B cells and 77.0% positivity for normal B cells (MFI 644).
Analysis of IgD expression showed 97.6% positivity (MFI 2466) for the PNH B cells and 68.9% positivity for normal B cells (MFI 1142).
Patient developed hemolytic PNH 36 years ago, with attacks of macroscopic hemoglobinuria ceasing in 1976.
Analysis of cell surface immunoglobulin (Ig) M, IgD, IgG, and CD27 expression by normal (CD48+) and PNH (CD48−) B cells in patients with PNH.
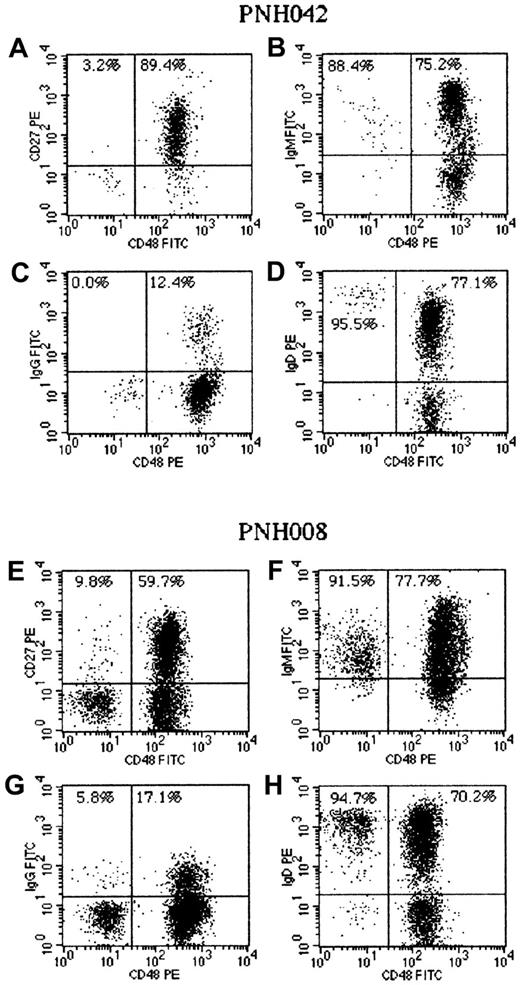
Patient PNH042 (A-D) had a large PNH granulocyte clone (> 95%) and a disease duration of 12 years. The residual normal B cells comprised mainly CD27+ memory cells (A). The majority of these were IgM+ (B) with only a small proportion class-switched (IgG+) cells (C). The PNH B cells had a naive phenotype (CD27−) and expressed IgM and IgD. The strength of IgD expression on these naive (CD48−) B cells was significantly higher than on the corresponding (CD48+) IgD+CD27+ memory B cells (D). Patient PNH008 (E-H) first developed hemolytic PNH 12 years ago and had no detectable granulocyte or red cell PNH clones at the time of analysis. The large PNH B-cell clone in this patient showed a small proportion of CD27+ cells (E) and a small population of class-switched (IgG+) PNH B cells (G). The majority of PNH B cells had a naive phenotype, with mainly strong IgD expression (H). The normal B-cell component in this patient comprised naive B cells and both non–class-switched (F, H) and class-switched (G) memory B cells.
Analysis of cell surface immunoglobulin (Ig) M, IgD, IgG, and CD27 expression by normal (CD48+) and PNH (CD48−) B cells in patients with PNH.
Patient PNH042 (A-D) had a large PNH granulocyte clone (> 95%) and a disease duration of 12 years. The residual normal B cells comprised mainly CD27+ memory cells (A). The majority of these were IgM+ (B) with only a small proportion class-switched (IgG+) cells (C). The PNH B cells had a naive phenotype (CD27−) and expressed IgM and IgD. The strength of IgD expression on these naive (CD48−) B cells was significantly higher than on the corresponding (CD48+) IgD+CD27+ memory B cells (D). Patient PNH008 (E-H) first developed hemolytic PNH 12 years ago and had no detectable granulocyte or red cell PNH clones at the time of analysis. The large PNH B-cell clone in this patient showed a small proportion of CD27+ cells (E) and a small population of class-switched (IgG+) PNH B cells (G). The majority of PNH B cells had a naive phenotype, with mainly strong IgD expression (H). The normal B-cell component in this patient comprised naive B cells and both non–class-switched (F, H) and class-switched (G) memory B cells.
The residual normal B-cell component in patients where the majority of hematopoiesis was derived from the PNH stem cell (ie, > 90% of granulocytes GPI−) and a long disease duration were predominantly of a memory phenotype. Surface Ig isotype expression on these B cells was primarily IgM, although minor populations of IgG+ were also present. It is clear from these results that the major population of circulating memory B cells are not class switched and are in fact IgM+.
Discussion
Analysis of CD27 expression by normal and GPI−subsets of B cells showed a significantly lower proportion of CD27+ memory components within the PNH B-cell fraction when compared to coexisting normal B cells in the same patients. In general terms, the GPI− B cells in patients with a recent onset of PNH were predominantly CD27− (ie, a naive phenotype), almost certainly reflecting their recent production. In the patients with a longer duration of disease, the proportion of GPI−B cells expressing CD27 was much higher, reflecting exposure to antigen, Ig gene mutation, and memory cell formation. This gradual, time-dependent, accumulation of memory B cells within the PNH B-cell subset was further emphasized from studies of 2 patients (PNH008 and PNH104) who originally presented with hemolytic PNH 12 and 40 years ago, respectively, and now had no detectable granulocyte or red cell PNH clones at the time of analysis. In both instances PNH B cells were still present and showed the highest proportions of GPI−CD27+ cells. This relationship between disease duration and CD27 expression on PNH B cells reached statistical significance (Spearman [rs] 0.403; P = .030).
Analysis of the normal (GPI+) B-cell component was more complex, particularly in patients with only small PNH granulocyte clones. In these patients hematopoiesis is variably maintained from both normal and PNH stem cells, and the normal B cells generally comprised normal proportions of CD27+ components. In cases with a longer duration of disease and a large PNH granulocyte clone (ie, hematopoiesis maintained largely from the PNH stem cells), the residual normal B cells comprised mainly B cells generated before the onset of PNH. The majority of these were CD27+ (memory phenotype) with very few detectable CD27− B cells. In contrast to this was the group of patients whose GPI+ B cells comprised normal distributions of CD27+ and CD27− components, which could be separated into 2 distinct groups: (1) patients with only small PNH granulocyte clones whose residual normal hematopoietic stem cells continue to produce GPI+ naive B cells and (2) presentation cases of PNH with large granulocyte clones, whose normal B cell production is minimal, but sufficient time has not yet elapsed for the remaining pool of normal naive B cells to encounter their specific antigen and convert to a memory phenotype.
A more detailed analysis of Ig heavy-chain isotype expression in 10 patients (8 with active PNH and 2 in remission) showed significant phenotypic differences between PNH B cells and normal B cells. In 4 patients, all with a long duration of active disease and granulocyte clones of more than 90%, the residual normal B-cell component was predominantly CD27+, with the majority of cells expressing IgM and only small proportions of class-switched (IgG+) cells. These results demonstrate that the majority of circulating memory B cells in fact comprise non–class-switched cells. It is also evident from these patients that in the absence of any new naive B-cell formation, over a period of 3 to 5 years the majority of normal B cells convert to a memory phenotype. Sequential studies of the residual normal B-cell component in a cohort of patients with large PNH granulocyte clones and a recent onset of disease should further clarify this. In contrast to these findings for GPI+ B cells, the PNH B-cell component in all patients with active PNH was predominantly CD27−IgM+IgG−. Furthermore, in 3 cases where membrane IgD expression was also studied, the fluorescent intensity was significantly higher on PNH B cells than on the corresponding population of normal B cells. These results provide definitive evidence that naive peripheral blood B cells have a CD27−IgM+IgDstrong+ phenotype.
In the absence of a simple in vitro functional assay to test whether these CD27− GPI− lymphocytes would undergo germinal center formation, somatic hypermutation, and Ig class switching, we relied on phenotypic data from patients with long duration of disease to establish this. To determine whether PNH B cells had the capability to undergo conversion to a memory cell phenotype in vivo, we studied peripheral blood B cells in patient PNH104, previously diagnosed as hemolytic PNH in 1962 with subsequent clinical remission of disease occurring in 1976. Although the patient currently has myelodysplastic syndrome (agranular neutrophils in the peripheral blood), there was no clinical or laboratory evidence of PNH. A small but discrete population of GPI− PNH B cells was identified in the peripheral blood, and significant proportions of these were of a memory phenotype (CD27+, 58%) and Ig class switched (IgG+, 27%). Based on these data, we speculate that these PNH B cells were produced sometime between 24 to 38 years ago. Furthermore, prospective monitoring of the residual PNH B-cell clone in patient PNH008, who recently attained both clinical and laboratory remission of PNH (no detectable red cell or granulocyte PNH clones by flow cytometry), should not only provide a means of studying long-term remission in PNH, but also provide further insights into the life span of B cells and the process of memory cell formation.
Using multicolor flow cytometry we were able to detect GPI− B cells in all 29 patients in this study, although the proportion of PNH B cells showed wide variation from patient to patient. Previous studies by a number of authors have shown, not unexpectedly because PNH is a stem cell disorder, significant correlation between the size of the PNH granulocyte clone and other myeloid PNH components.19-21 In this study, no significant statistical relationship was found between the size of the PNH granulocyte and B-cell clone. Although these cells originate from a common hematopoietic stem cell, a multitude of specific factors influence B-cell ontogeny, with many negative selection steps and large numbers of self-reactive B cells deleted in the bone marrow.22 To determine whether patient age had a direct influence on the ability to produce PNH B cells, statistical relationships between patient age and absolute numbers of PNH B cells were examined. No significant correlations were found for either all patients with PNH or, in a more specific analysis, for those patients with more than 50% PNH granulocyte clones (ie, hematopoiesis predominantly maintained from the PNH stem cell). Moreover, what was clearly evident from a plot of age versus absolute numbers of PNH B cells for these specific patients was that production of new naive B cells occurs throughout life, and that even the oldest patient (PNH102, age 78) had significant numbers of PNH B cells detectable at presentation. Statistical analysis of these data showed that the ability to produce naive B cells does not decline with age (as seen with T cells14) and these findings confirm directly that B-cell production is a lifelong process.23 24 In summary, we provide convincing evidence that the majority of circulating memory B cells are not class switched (CD27+IgM+IgD+) and that naive B cells have a CD27−IgM+IgDstrong+phenotype.
Acknowledgments
We are indebted to the many clinicians throughout the United Kingdom for providing patient samples. The authors gratefully acknowledge the support of Cymbus Biosciences for generous donation of monoclonal antibodies used in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen J. Richards, Haematological Malignancy Diagnostic Service, The Algernon Firth Building, Leeds General Infirmary, Leeds, LS1 3EX, United Kingdom; e-mail:stephenr@pathology.leeds.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal