Abstract
Detailed studies of tumor cell–associated procoagulants and fibrinolytic factors have implied that local thrombin generation and fibrin deposition and dissolution may be important in tumor growth and dissemination. To directly determine whether fibrin(ogen) or plasmin(ogen) are determinants of the metastatic potential of circulating tumor cells, this study examined the impact of genetic deficits in each of these key hemostatic factors on the hematogenous pulmonary metastasis of 2 established murine tumors, Lewis lung carcinoma and the B16-BL6 melanoma. In both tumor models, fibrinogen deficiency strongly diminished, but did not prevent, the development of lung metastasis. The quantitative reduction in metastasis in fibrinogen-deficient mice was not due to any appreciable difference in tumor stroma formation or tumor growth. Rather, tumor cell fate studies indicated an important role for fibrin(ogen) in sustained adhesion and survival of tumor cells within the lung. The specific thrombin inhibitor, hirudin, further diminished the metastatic potential of circulating tumor cells in fibrinogen-deficient mice, although the inhibitor had no apparent effect on tumor cell proliferation in vitro. The absence of plasminogen and plasmin-mediated fibrinolysis had no significant impact on hematogenous metastasis. The authors concluded that fibrin(ogen) is a critical determinant of the metastatic potential of circulating tumor cells. Furthermore, thrombin appears to facilitate tumor dissemination through at least one fibrin(ogen)-independent mechanism. These findings suggest that therapeutic strategies focusing on multiple distinct hemostatic factors might be beneficial in the containment of tumor metastasis.
Introduction
A specific association between human cancer and the hemostatic system has been recognized for more than a century. Many significant hemostatic abnormalities have been described in patients with cancer, including disseminated intravascular coagulation, hemorrhagic events, and migratory thrombophlebitis. Indeed, hemostatic complications are a common cause of death in patients with cancer.1-3 Many tumor cells possess strong procoagulant activities that promote the local activation of the coagulation system.2,4 Furthermore, tumor-mediated activation of the coagulation cascade has been implicated in both the formation of tumor stroma and the promotion of hematogenous metastasis.4-8Most solid tumors in humans and experimental animals contain considerable amounts of fibrinogen-related products, mostly cross-linked fibrin, suggesting that fibrin(ogen) is important in tumor stroma formation.1,3,4,9-13 Fibrin matrices promote the migration of a substantial number of distinct cell types, including both transformed cells, endothelial cells, macrophages, and fibroblasts.4,14,15 Fibrin matrices also promote neovascularization, supporting the notion that fibrin may facilitate tumor stroma formation by mechanisms that are analogous to wound repair.10,16,17 Fibrin degradation products (FDPs) have also been shown to display powerful chemotactic, immune-modulatory, as well as angiogenic properties.18-23 Taken together, substantial indirect evidence points to an important role for fibrin(ogen) in tumor progression.
Hematogenous metastasis represents a second aspect of tumor biology in which activation of the coagulation system has been implicated. Following their entrance into the circulation, tumor cells must adhere in the microvasculature of a target organ prior to growth.24 It has been suggested that the formation of platelet-fibrin-tumor cell aggregates may be causally related to endothelial adhesion and metastatic potential.5,25,26Deposition of fibrin within adherent tumor cell–platelet aggregates can be detected as early as 5 minutes after tumor cell inoculation and persists for more than 9 hours.25,27 Furthermore, antibody or drug-induced thrombocytopenia or inhibition of tissue factor (TF), factor Xa, or thrombin has been shown to substantially reduce experimental hematogenous metastasis.6,7 28-32
In this study, we used 2 transplantable murine tumor cell lines, Lewis lung carcinoma (LLC) and B16-BL6 melanoma, and fibrinogen-deficient transgenic mice to directly determine whether fibrin(ogen) contributes to metastatic potential. We report that fibrin(ogen) is an important determinant of metastatic potential, but thrombin facilitates tumor progression through at least one fibrinogen-independent mechanism.
Materials and methods
Transgenic mice
The generation of fibrinogen- and plasminogen-deficient mice has been described previously.33,34 All mice enrolled in these studies were inbred into the C57BL/6J background (Jackson Laboratories, Bar Harbor, ME) for 6 generations. These mice were shown to be uniformly histocompatible with the transplantable tumors by monitoring tumor growth following subcutaneous injection of either LLC or B16-BL6 melanoma. The genotypes of the mice were determined by multiplex polymerase chain reaction analysis of DNA obtained from ear biopsies as described previously.33 34 Age- and sex-matched cohorts of fibrinogen-deficient (Fib−), plasminogen-deficient (Plg−), and control (heterozygous) mice were used in all experiments. The study protocols were approved by the Children's Hospital Research Foundation Institutional Animal Care and Use Committee and were in accordance with the guidelines of the National Institutes of Health.
Tumor cell inoculation
The LLC cells were a gift from Dr Michael S. O'Reilly (Boston, MA). Single cell suspensions of these tumor cells were prepared as described previously.35 The B16-BL6 melanoma cell line was kindly provided by Dr Isaiah Fidler (Houston, TX). Both tumor cell lines were cultured in vitro by subconfluent passage in Dulbecco modified Eagle medium (DMEM) (Bio Whittaker, Walkersville, MD) containing 10% fetal calf serum (FCS), penicillin, and streptomycin. Subconfluent tumor cells were washed with phosphate-buffered saline (PBS), detached by a brief exposure to a 0.25% trypsin, 0.53 mmol/L EDTA solution (Life Technologies, Rockville, MD), washed in serum-containing media, and then resuspended in cold serum-free medium. The viability of the tumor cells was determined by trypan blue exclusion and was always more than 95%. The cells were kept in an ice bath until transplanted into mice. Mice were anesthetized by inhalation of 2% isoflurane (Ohmeda PPD, Liberty Corner, NJ), and 200 μL of tumor cell suspension was injected into the lateral tail vein using a 27-gauge needle.
Quantitation of surface pulmonary metastatic foci
Tumor-bearing mice were killed 17 to 21 days after tumor cell injection. The lungs were removed, rinsed in PBS, and placed in Bouin fixative for at least 24 hours. The lungs were separated into individual lobes and the number of surface metastatic foci was counted with a stereomicroscope by an investigator unaware of animal genotype.
Histologic analysis
Fixed tissue was processed into paraffin, sectioned, and stained with hematoxylin and eosin. Fibrin(ogen) immunostaining was performed using a rabbit antimouse polyclonal antiserum as described previously.36
Labeling of cells with [125I] iododeoxyuridine
To prepare labeled cells for tail vein injection, tumor cells were plated on 100-mm dishes at a density of 106 cells per dish, and grown for 24 hours in DMEM containing 10% FCS. Then, 1 μCi/ml 5-(125I)iodo-2′-deoxyuridine (ICN Biomedicals, Costa Mesa, CA) was added to the medium and the cells were incubated for an additional 24 hours. The cells were washed in PBS, detached with trypsin-EDTA, washed in serum-containing media, and suspended in cold serum-free media at a concentration of 1 × 106 live cells/mL.
Determination of the fate of tumor cells after introduction to the circulation
The fate of labeled tumor cells was determined essentially as described previously.6,37 Briefly, (125I)iododeoxyuridine-labeled tumor cells (2 × 105 cells/77 000 cpm) in 200 μL serum-free medium were introduced into the circulation by injection into the lateral tail vein as described above. At 15 minutes, 1 hour, 4 hours, and 24 hours the mice were anesthetized by isoflurane inhalation and approximately 400 μL blood was collected from the inferior vena cava into 10 μL 0.5 mol/L EDTA anticoagulant using a 27-gauge needle. In addition, lungs, liver, and spleen were collected, rinsed in PBS, and placed in 70% ethanol. The organs were washed extensively in 70% ethanol for 4 days to liberate any free iodine.6 37Radioisotope levels in blood, organs, and in cell suspensions were measured with a Cobra gamma counter (Packard, Canberra, Canada). Radioactivity in blood and organs was expressed as a percentage of radioactivity of the input dose. The input dose was determined by measuring the radioactivity of three 200-μL aliquots of the cell suspension in parallel with the organ and blood samples to adjust for radioactive decay.
Determination of the rate of growth of intradermally transplanted LLC cells
A single cell suspension of 5 × 105 LLC cells/100 μL serum-free media was injected subcutaneously into the dorsal midscapular skin of anesthetized fibrinogen-deficient mice and controls. The mice were monitored daily for the development of visible tumors. Once a tumor was clearly visible, it was calipated daily and the volume estimated by the formula V = (LW2)π/6, where V = volume, L = longest diameter, and W = shortest diameter.38 After 12 days, the mice were killed and the tumors removed and weighed.
Determination of the effect of hirudin on the in vitro growth of B16-BL6 melanoma cells
The B16-BL6 cells (1 × 105) were plated onto replicate 60-mm dishes in serum-containing media. After 24 hours the cells from 3 of the plates were removed by trypsinization and viable cells counted by trypan blue exclusion to provide a baseline cell count per plate. Saline carrier or deshirudin (Rhône-Poulenc Rorer, Collegeville, PA) at a final concentration of either 30 μg/mL or 100 μg/mL was added to each plate. Every other day media changes included the same concentrations of hirudin. At each time point, 3 plates from each group were trypsinized and the total cell number counted.
Determination of tumor metastasis in the presence of hirudin
Cohorts of fibrinogen-deficient and control mice were anesthetized with isoflourane and injected subcutaneously with either saline or deshirudin at a dose of 10 mg/kg as previously described.6 Twenty minutes after injection with either hirudin or saline, 200 μL of a single cell suspension of B16-BL6 melanoma (3.5 × 105 cells/mL) was injected into the lateral tail vein. The animals were killed 21 days after injection and the pulmonary metastatic foci quantitated as described earlier.
Results
Fibrinogen increases the metastatic potential of circulating tumor cells
To directly test the hypothesis that fibrin(ogen) is a determinant of the metastatic potential of circulating tumor cells, the formation of pulmonary metastatic foci was compared in immunocompetent control and fibrinogen-deficient mice following the intravenous injection of LLC and B16-BL6 melanoma. These specific tumor lines were selected for detailed study because both are highly metastatic and various inhibitors of the coagulation and fibrinolytic systems have been reported previously to inhibit their metastatic potential.6 30 Control experiments using mice carrying the fibrinogen knockout allele that had been inbred to C57BL/6J for 6 generations indicated that both LLC and B16-BL6 melanoma formed tumors when injected subcutaneously (5 × 105 cells/mouse) with 100% penetrance (n = 40 for LLC, n = 20 for B16-BL6). Therefore, no evidence of histoincompatibility or tumor rejection was observed based on the rapid development of visible tumors after injection (100% of mice within 3 days with both LLC and B16-BL6) and the steady growth of the tumors (representative data, Figure1). Both LLC and B16 melanoma cells were also capable of establishing pulmonary metastatic foci in fibrinogen-deficient mice that were qualitatively comparable to those observed in fibrinogen-expressing mice in macroscopic and microscopic appearance (Figures 2 and3). Thus, fibrinogen is not strictly required for hematogenous metastasis. However, fibrinogen deficiency strongly diminished the metastatic capacity of both tumors. Quantitative analyses in a series of independent experiments with LLC and B16 melanoma demonstrated a consistent and significant reduction in surface pulmonary metastases in fibrinogen-deficient mice (Table1).
Fibrin(ogen) deficiency does not reduce the growth rate of subcutaneously transplanted LLC.
A single cell suspension of 5 × 105 LLC cells was injected subcutaneously into the dorsal, midscapular skin of Fib− (n = 16) and control (n = 19) mice. The individual tumor volumes were measured daily by calipation. The data presented are mean values and SDs for each time point. No significant difference was noted in tumor growth between Fib− and control mice (P > .6, random coefficient mixed model).
Fibrin(ogen) deficiency does not reduce the growth rate of subcutaneously transplanted LLC.
A single cell suspension of 5 × 105 LLC cells was injected subcutaneously into the dorsal, midscapular skin of Fib− (n = 16) and control (n = 19) mice. The individual tumor volumes were measured daily by calipation. The data presented are mean values and SDs for each time point. No significant difference was noted in tumor growth between Fib− and control mice (P > .6, random coefficient mixed model).
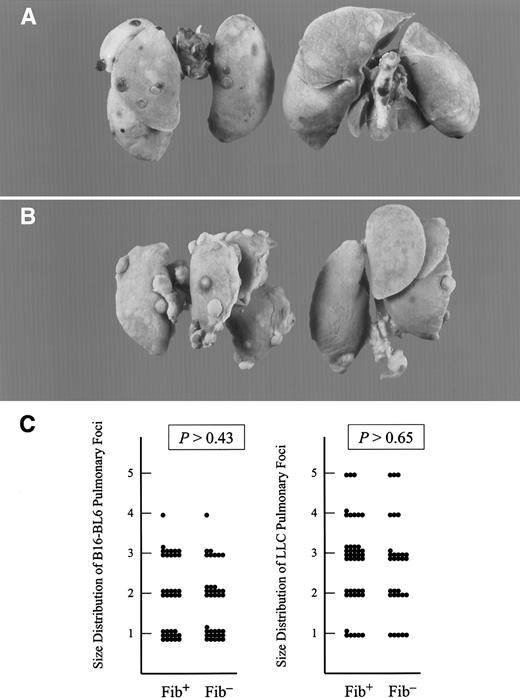
Fibrinogen deficiency diminished the metastatic potential of circulating tumor cells but not the growth of metastatic foci.
Representative examples of metastatic pulmonary foci produced 21 days after intravenous injection of 1.7 × 104 B16-BL6 cells (A) and 1 × 105 LLC cells (B) into the lateral tail vein. Lungs from fibrinogen-deficient mice are pictured on the right and controls on the left. (C) Scatter plot of the size distribution of metastatic foci with transplanted B16-BL6 (left) and LLC (right) in fibrinogen-deficient (Fib−) and control (Fib+) mice. See text for definitions of arbitrary units used for size distribution. (P values were generated using the Studentt test.)
Fibrinogen deficiency diminished the metastatic potential of circulating tumor cells but not the growth of metastatic foci.
Representative examples of metastatic pulmonary foci produced 21 days after intravenous injection of 1.7 × 104 B16-BL6 cells (A) and 1 × 105 LLC cells (B) into the lateral tail vein. Lungs from fibrinogen-deficient mice are pictured on the right and controls on the left. (C) Scatter plot of the size distribution of metastatic foci with transplanted B16-BL6 (left) and LLC (right) in fibrinogen-deficient (Fib−) and control (Fib+) mice. See text for definitions of arbitrary units used for size distribution. (P values were generated using the Studentt test.)
Comparative microscopic analysis of metastatic foci in fibrinogen-deficient and control mice.
Representative examples show lung metastases (∗) of B16-BL6 melanoma (A and B) and LLC (C and D) in control mice (A and C) and Fib− mice (B and D). Fibrin(ogen) deposition (appearing as red reaction product in panels A and B and brown staining in panels C and D) is apparent in the lung tissue surrounding the metastatic nodule of the control mice but not in the Fib− mice. The metastases from both genotypes were often pleural based and associated with blood vessels (arrow). Note the dark material in panels A and B is melanin produced by the B16-BL6 cells.
Comparative microscopic analysis of metastatic foci in fibrinogen-deficient and control mice.
Representative examples show lung metastases (∗) of B16-BL6 melanoma (A and B) and LLC (C and D) in control mice (A and C) and Fib− mice (B and D). Fibrin(ogen) deposition (appearing as red reaction product in panels A and B and brown staining in panels C and D) is apparent in the lung tissue surrounding the metastatic nodule of the control mice but not in the Fib− mice. The metastases from both genotypes were often pleural based and associated with blood vessels (arrow). Note the dark material in panels A and B is melanin produced by the B16-BL6 cells.
Effect of fibrinogen deficiency on pulmonary metastasis of circulating tumor cells
| Tumor cell line . | Mouse genotype . | Number of cells injected . | Pulmonary metastatic foci median (range) . | P (Mann-Whitney U 2-tailed) . |
|---|---|---|---|---|
| Lewis lung carcinoma | ||||
| Experiment 1 | Control (n = 16) | 1 × 105 | 13 (2-24) | < .007 |
| Fib− (n = 16) | 0.5 (0-6) | |||
| Experiment 2 | Control (n = 15) | 1 × 105 | 22 (0-142) | < .029 |
| Fib− (n = 10) | 5 (0-16) | |||
| Experiment 3 | Control (n = 16) | 2 × 105 | 46 (5-110) | < .004 |
| Fib− (n = 10) | 11 (0-37) | |||
| B16-BL6 melanoma | ||||
| Experiment 1 | Control (n = 17) | 1.7 × 104 | 36 (3-163) | < .01 |
| Fib− (n = 18) | 9 (2-66) | |||
| Experiment 2 | Control (n = 20) | 1.7 × 104 | 9 (0-30) | < .009 |
| Fib− (n = 14) | 3 (0-13) |
| Tumor cell line . | Mouse genotype . | Number of cells injected . | Pulmonary metastatic foci median (range) . | P (Mann-Whitney U 2-tailed) . |
|---|---|---|---|---|
| Lewis lung carcinoma | ||||
| Experiment 1 | Control (n = 16) | 1 × 105 | 13 (2-24) | < .007 |
| Fib− (n = 16) | 0.5 (0-6) | |||
| Experiment 2 | Control (n = 15) | 1 × 105 | 22 (0-142) | < .029 |
| Fib− (n = 10) | 5 (0-16) | |||
| Experiment 3 | Control (n = 16) | 2 × 105 | 46 (5-110) | < .004 |
| Fib− (n = 10) | 11 (0-37) | |||
| B16-BL6 melanoma | ||||
| Experiment 1 | Control (n = 17) | 1.7 × 104 | 36 (3-163) | < .01 |
| Fib− (n = 18) | 9 (2-66) | |||
| Experiment 2 | Control (n = 20) | 1.7 × 104 | 9 (0-30) | < .009 |
| Fib− (n = 14) | 3 (0-13) |
Although the total number of metastatic foci was dependent on animal genotype, there was no obvious difference in the size or distribution of the metastatic foci that formed in control and fibrinogen-deficient mice. A random sampling of LLC and B16-BL6 foci from 4 mice of each genotype was measured and scored from 1 to 5 as follows: 1 = < 0.5 mm; 2 = 0.6-0.9 mm; 3 = 1-1.4 mm; 4 = 1.5-1.9 mm; 5 = ≥ 2 mm. No significant difference between genotypes was noted in the size distribution of individual metastastic foci from LLC or B16-BL6 (Figure2C). This suggests that initial establishment of metastatic foci, and not tumor growth, was impaired in fibrinogen-deficient mice.
To explore in greater detail whether the presence of fibrinogen has any impact on tumor growth, we compared the growth of LLC tumors following subcutaneous injection into control and fibrinogen-deficient mice. All mice developed palpable tumors within 3 days of injection, regardless of genotype. Furthermore, the tumor volumes, as measured by calipation,38 increased in parallel over a 12-day study period (Figure 1) (P > .6, random coefficient mixed model). Therefore, fibrin(ogen) appears to have a significant impact on metastatic potential. However, it is not critical for tumor cell proliferation and has no major impact on overall tumor expansion.
Plasmin-mediated fibrinolysis does not have a critical impact on the metastasis of circulating tumor cells
Because fibrin(ogen) is a determinant of metastatic potential, it follows that the primary physiologic fibrinolytic enzyme, plasmin, might influence metastasis in the same assay system. To determine the importance of plasmin-mediated fibrinolysis in hematogenous metastasis, LLC and B16-BL6 melanoma cells were injected into the tail veins of plasminogen-deficient and control mice in a separate series of experiments. Contrary to expectations, plasminogen deficiency had no appreciable impact on the number of surface pulmonary metastases observed, regardless of the tumor model used (Table2).
Effect of plasminogen deficiency on pulmonary metastasis of circulating tumor cells
| Tumor cell line . | Mouse genotype . | Number of cells injected . | Pulmonary metastatic foci median (range) . | P (Mann-Whitney U 2-tailed) . |
|---|---|---|---|---|
| Lewis lung carcinoma | ||||
| Experiment 1 | Control (n = 12) | 2 × 105 | 19 (2-42) | > .18; NS |
| Plg− (n = 12) | 23 (11-46) | |||
| Experiment 2 | Control (n = 9) | 2 × 105 | 34 (0-63) | > .65; NS |
| Plg− (n = 10) | 36 (17-52) | |||
| B16-BL6 melanoma | ||||
| Experiment 1 | Control (n = 30) | 1.7 × 104 | 39 (2-106) | > .32; NS |
| Plg− (n = 16) | 47 (16-255) | |||
| Experiment 2 | Control (n = 13) | 1.7 × 104 | 53 (17-101) | > .50; NS |
| Plg− (n = 14) | 61 (11-172) |
| Tumor cell line . | Mouse genotype . | Number of cells injected . | Pulmonary metastatic foci median (range) . | P (Mann-Whitney U 2-tailed) . |
|---|---|---|---|---|
| Lewis lung carcinoma | ||||
| Experiment 1 | Control (n = 12) | 2 × 105 | 19 (2-42) | > .18; NS |
| Plg− (n = 12) | 23 (11-46) | |||
| Experiment 2 | Control (n = 9) | 2 × 105 | 34 (0-63) | > .65; NS |
| Plg− (n = 10) | 36 (17-52) | |||
| B16-BL6 melanoma | ||||
| Experiment 1 | Control (n = 30) | 1.7 × 104 | 39 (2-106) | > .32; NS |
| Plg− (n = 16) | 47 (16-255) | |||
| Experiment 2 | Control (n = 13) | 1.7 × 104 | 53 (17-101) | > .50; NS |
| Plg− (n = 14) | 61 (11-172) |
NS indicates not significant.
Histologic analysis of pulmonary metastatic foci in control and fibrinogen-deficient mice
Tumor-associated fibrin deposition has been proposed to be essential for tumor stroma formation and angiogenesis. A detailed microscopic analysis of lung tissue from tumor-bearing fibrinogen-deficient and control mice failed to reveal any obvious qualitative differences in metastatic lesions other than the distinct absence of peritumoral fibrin(ogen) in the Fib− mice (representative data, Figure 3). The metastatic foci in mice of both genotypes grew as discreet nodules composed primarily of tumor cells with little associated stromal tissue. However, examination at higher magnification revealed the presence of capillaries in foci from both genotypes. The nodules from both genotypes were often pleural based, but also showed evidence of extension into underlying pulmonary parenchyma. These data demonstrate that fibrinogen is not required for tumor stroma formation. However, a more detailed quantitative analysis of tumors at several stages of development will be required to determine if fibrinogen deficiency has more subtle effects on the formation, stability, or composition of the tumor stroma.
Fibrinogen deficiency does not affect initial arrest but impairs the sustained adherence of tumor cells in the lungs
To study the immediate fate of circulating tumor cells in fibrinogen-deficient mice, we used an assay originally described by Fidler and colleagues37 in which tumor cells were labeled in vitro with (125I)iododeoxyuridine before introduction into the circulation. This method of labeling permits the direct determination of the distribution and fate of circulating tumor cells, because the label is stable in living tumor cells, but rapidly cleared from dead tumor cells and excreted from the body.37Labeled tumor cells were injected into the tail vein of fibrinogen-deficient and control mice. Blood, lungs, livers, and spleens were collected from the mice from 15 minutes to 24 hours after the introduction of the radiolabeled cells, and the distribution of tumor cells in the blood and organs of the 2 groups of mice was determined (Figure 4). As described previously,37 the labeled tumor cells were rapidly cleared from the circulation, with less than 25% of the initial inoculum remaining in circulation 15 minutes after injection. The majority of the tumor cells (approximately two-thirds) were arrested in the lungs (Figure 4), whereas a minor fraction of the cells was associated with the liver and spleen (10% and < 1%, respectively.) There were no quantitative differences in the initial arrest of labeled tumor cells between the fibrinogen-deficient and control mice in any of the 3 organs. However, 4 hours after injection there was a significant reduction in the apparent number of tumor cells in the lungs of the fibrinogen-deficient mice relative to fibrinogen-expressing animals. At 24 hours after injection, a 4-fold difference in the number of tumor cells was apparent between genotypes (Figure 4).
Fibrinogen deficiency does not prevent the initial arrest of circulating tumor cells in lung tissue but diminishes sustained adherence or survival.
LLC cells labeled with 5-(125I)iodo-2′-deoxyuridine were injected into the lateral tail vein of fibrin(ogen)-deficient (white bars) and control mice (hatched bars). The mice were killed at the specified time points and the amount of radioisotope in the lungs was measured. The data presented are median values and are expressed as percent of injected dose (1 × 105 cells/77 000 cpm); n = number of mice. The P values were determined by the Mann-Whitney U test, 2 tailed.
Fibrinogen deficiency does not prevent the initial arrest of circulating tumor cells in lung tissue but diminishes sustained adherence or survival.
LLC cells labeled with 5-(125I)iodo-2′-deoxyuridine were injected into the lateral tail vein of fibrin(ogen)-deficient (white bars) and control mice (hatched bars). The mice were killed at the specified time points and the amount of radioisotope in the lungs was measured. The data presented are median values and are expressed as percent of injected dose (1 × 105 cells/77 000 cpm); n = number of mice. The P values were determined by the Mann-Whitney U test, 2 tailed.
Thrombin promotes the metastatic potential of circulating tumor cells by at least one fibrinogen-independent mechanism
The specific thrombin inhibitor, hirudin, inhibits the metastasis of circulating tumor cells.6 To determine whether the inhibition of metastasis achieved with thrombin inhibition was related primarily to the thrombin substrate, fibrinogen, we injected 2 groups of fibrinogen-deficient mice and controls with either saline or 10 mg/kg hirudin 20 minutes prior to intravenous injection of 7 × 104 B16-BL6 melanoma cells.
Consistent with earlier reports,6 hirudin diminished the number of pulmonary metastatic foci by more than 20-fold in fibrinogen-expressing mice relative to saline-treated controls (P < .0001 Mann-Whitney U test) (Figure5). Interestingly, a significant diminution in pulmonary metastasis was also observed with hirudin in fibrinogen-deficient mice (Figure 5). The median number of pulmonary foci was 2.5 for the saline-treated Fib− mice and 0 for the hirudin-treated Fib− mice (P < .01 Mann Whitney U Test). This experiment was repeated with similar results (data not shown).
Thrombin promotes the metastasis of circulating tumor cells by at least one fibrinogen-independent mechanism.
Fibrinogen-sufficient mice (A) and fibrinogen-deficient mice (B) were pretreated with 10 mg/kg hirudin or saline 20 minutes before injection of 7 × 104 B16-BL6 melanoma cells into the lateral tail vein. The mice were killed 21 days later and the surface pulmonary metastases quantitated. The data presented are the number of surface pulmonary metastases observed in individual mice. The horizontal bars represent the median value for each group. The median value for the hirudin-treated Fib− mice was 0 (B). The Pvalues were determined by the Mann-Whitney U test, 2 tailed.
Thrombin promotes the metastasis of circulating tumor cells by at least one fibrinogen-independent mechanism.
Fibrinogen-sufficient mice (A) and fibrinogen-deficient mice (B) were pretreated with 10 mg/kg hirudin or saline 20 minutes before injection of 7 × 104 B16-BL6 melanoma cells into the lateral tail vein. The mice were killed 21 days later and the surface pulmonary metastases quantitated. The data presented are the number of surface pulmonary metastases observed in individual mice. The horizontal bars represent the median value for each group. The median value for the hirudin-treated Fib− mice was 0 (B). The Pvalues were determined by the Mann-Whitney U test, 2 tailed.
To confirm that hirudin has no direct inhibitory effect on cultured B16 melanoma, 1 × 105 B16-BL6 cells were plated onto 60-mm plates and cultured in the presence of either saline carrier or hirudin at a final concentration of either 30 μg/mL or 100 μg/mL. No difference in the rate of cell growth or the morphology of the cells was observed, regardless of the presence or absence of hirudin (data not shown).
Discussion
A critical role for hemostatic factors in malignancy has been suspected for decades and is a concept that is supported by a substantial body of correlative and indirect evidence. The recent generation of viable mouse lines with selected deficits in key hemostatic factors has provided an opportunity to directly test this long-standing hypothesis. The studies of fibrinogen-deficient mice presented here directly demonstrate that fibrin(ogen) plays an important role in cancer pathophysiology and is a determinant of metastatic potential. Fibrin(ogen) appears to facilitate metastasis by enhancing the sustained adherence and survival of individual tumor cell emboli in the vasculature of target organs. However, fibrin(ogen) does not appear to play a critical role in the growth of established metastases. Tumor cell–associated thrombin generation, and the local conversion of fibrinogen to fibrin, may be mechanistically related to metastasis (see below), but it appears that thrombin promotes tumor cell metastasis through at least one fibrinogen-independent mechanism. Interestingly, despite the powerful effect of fibrin(ogen) on metastasis, plasmin-mediated fibrinolysis does not appear to be a critical determinant of the metastatic potential of circulating tumor cells.
Although these studies firmly establish that fibrinogen is important in tumor dissemination in vivo, 2 important and still unresolved questions are: (1) How does fibrin(ogen) sustain tumor cell adhesion/survival? and (2) What is the relative biologic importance of soluble fibrinogen, insoluble fibrin polymer, and FDPs in tumor progression? Conceivably, all 3 of these fibrinogen derivatives could influence tumor dissemination and could do so in ways that are not mutually exclusive. Soluble fibrinogen has many functional properties that might promote metastatic potential, including the ability to support cell-cell adhesion through integrin (eg, αIIbβ3,39αvβ3,40α5β1,41αMβ242) and nonintegrin (eg, ICAM-143) receptors, and the ability to interact with other soluble factors and matrix components, including factor XIII and fibronectin.44,45 Soluble fibrinogen is a dimeric molecule that could support the stable adherence of tumor cells in the lung by acting as a “molecular bridge” between specific receptors on tumor cells and vascular endothelium or adherent platelets and leukocytes. The concept that platelets and platelet-tumor cell microemboli contribute to tumor dissemination is particularly attractive based on microscopy data showing the colocalization of platelets and adherent tumor cells in vascular beds.26,27Furthermore, a variety of antiplatelet agents appear to reduce tumor cell metastatic potential.28,29 Detailed studies of tumor progression in mice with single and combined deficits in fibrinogen and platelet function, including deficits in integrin β3,46 protease-activated receptors (PARs),47-49 glycoprotein (GP)1b,50 and Gαq,51 should help illuminate the interplay between platelets and fibrinogen in tumor dissemination. The hypothesis that soluble fibrinogen and platelets contribute significantly to the exit of tumor cells from the vasculature is consistent with recent findings that fibrinogen- and platelet-mediated adhesion may be important in stable leukocyte adhesion and transendothelial cell migration.20
Fibrin polymer formation might also be key to tumor dissemination by several distinct mechanisms. In the context of circulating tumor cells, local deposition of an insoluble fibrin matrix could stabilize the adhesion of tumor cells or tumor cell–associated emboli to the vessel wall, particularly in a high-shear stress environment. In addition, just as fibrin provides an important provisional matrix supporting the growth and organization of cells within wound fields, local fibrin deposition might support initial tumor cell proliferation and migration. Finally, fibrin matrices could support the formation of the stroma tissue (eg, tumor vasculature) that provides nutrient and gas exchange for rapidly growing malignant cells. Indeed, the general similarities between stromal tissues formed within wound fields and tumor tissue have been well documented, and in both contexts fibrin or FDPs has been proposed to direct neovascularization.4,10,16,17 However, consistent with the established capacity of fibrinogen-deficient mice to generate and remodel their vasculatures in physiologic contexts, including embryonic development and wound repair,52 53 there appears to be no obvious defect in either the formation of tumor stroma or tumor growth rate in fibrinogen-deficient mice. Although subtle differences in tumor stroma in fibrinogen-deficient mice have not been excluded, the mechanism(s) linking fibrin(ogen) with metastatic potential does not appear to be related to tumor growth.
Fibrin degradation products have been reported to have angiogenic, chemoattractant, and anti-inflammatory activities18-23 and these proteolytic derivatives of fibrin might also be of biologic relevance to tumor progression. Although the data presented here do not exclude a role for FDPs in metastatic disease, based on the absence of any significant impact of plasminogen deficiency on hematogenous metastasis, it is clear that FDPs generated by plasmin-mediated fibrinolysis are not critical for the development of pulmonary metastasis.
If fibrin formation is a determinant of metastatic potential, then it follows that the conversion of prothrombin to thrombin is also a critical factor in tumor dissemination. The failure of prothrombin-deficient mice to survive beyond the neonatal period54 precludes a direct genetic test of this concept, but substantial indirect evidence supports this view. Notably, the metastatic potential of transplanted tumors in experimental animals is greatly diminished by specific inhibitors of coagulation system components, including the specific thrombin inhibitor, hirudin.6,31 In addition, gene-transfer studies have shown that the presence of tissue factor on the surface of tumor cells markedly increases the number of pulmonary metastases observed following intravenous inoculation into mice.7 In contrast, the expression of mutant forms of tissue factor that do not support factor VIIa binding or activity does not increase metastatic potential, implying that coagulation system activation, and ultimately thrombin formation, promote metastatic phenotype. However, it should be noted that the cytoplasmic domain of TF has also been shown to be important to tumor cell metastatic potential,7 suggesting a more complex relationship between TF and tumor dissemination than solely thrombin generation.
Several targets of thrombin-mediated proteolysis other than fibrinogen might be related to tumor progression, including factor XIII, factor XI, protein C, and thrombin-activated fibrinolysis inhibitor (TAFI). However, the thrombin substrates that are perhaps the most likely determinants of tumor metastatic potential are the G-protein–coupled signal molecules, PAR-1, -3, and -4. Because mice with genetic deficits in several PARs have now been described,47 48 the role and interplay of thrombin, PARs, and fibrinogen in tumor biology can be explored in detail.
The impact of fibrinogen deficiency on spontaneous tumor metastasis has not yet been established. Conceivably, the absence of fibrin matrices within primary tumor tissue might promote the escape of tumor cells by removing at least one physical barrier that must be negotiated to enter the circulation. On the other hand, the absence of fibrin might impede primary tumor cell dissemination by destabilizing the tumor stroma or removing a suitable matrix for tumor cell migration. Indeed, fibrin might be simultaneously an asset and a liability to tumor cells with the net effect on metastatic potential determined by local matrix composition at the site of tumor growth and the functional properties of individual tumor cells related to the binding and infiltration of fibrin matrices.
In summary, these studies directly demonstrate that the key hemostatic factor, fibrinogen, is a critical determinant of tumor cell metastatic potential. Fibrinogen and thrombin may work cooperatively in facilitating metastatic disease, but these studies have shown that thrombin contributes to metastatic potential by at least one mechanism that is independent of fibrinogen. These data suggest that therapeutic interventions directed at multiple distinct hemostatic factors might be effective in clinically limiting tumor cell dissemination. Finally, a more detailed understanding of the mechanism(s) by which hemostatic factors influence metastatic potential might suggest advantageous therapeutic targets for adjunct cancer treatment.
Acknowledgments
The authors would like to thank Dr David Witte for his expert assistance in interpreting the histologic data. We would also like to than Alicia Emley for her help with the photographs, Alan A. Thomay for his technical assistance, and Drs Robert J. Arceci and Beth A. Myers for critically reviewing the manuscript.
Supported in part by an award from the American Heart Association, Ohio Affiliate (A.F.D.) and grants (HL47826 and HL63194) from the National Institutes of Health (J.L.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jay L. Degen, Children's Hospital Research Foundation, Children's Hospital Medical Center, IDR-NRB Rm 2042, 3333 Burnet Ave, Cincinnati, OH 45229-3039; e-mail: degenjl@chmcc.org.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal