Abstract
Platelets play roles in both thrombosis and inflammation, and chemokines that are released at sites of inflammation could potentially activate platelets. Among the chemokine receptors expressed on platelets, the CXCR4 is the receptor for chemokine stromal cell-derived factor-1 (SDF-1), and the CCR4 is the receptor for macrophage-derived chemokine (MDC). Of the chemokines tested, SDF-1 and MDC were the only 2 that activated platelets. Both are weak agonists, but they enhanced response to low-dose adenosine 5′-diphosphate (ADP), epinephrine, or serotonin. When SDF-1 and MDC were added together, full and brisk platelet aggregation occurred. Platelet activation by these 2 chemokines appears to involve distinct pathways: SDF-1 inhibited an increase in cyclic adenosine monophosphate (cAMP) following prostaglandin (PG) I2, while MDC had no effect. In contrast, MDC, but not SDF-1, lead to Ca++mobilization by platelets. Further, second-wave aggregation induced by MDC in platelet-rich plasma was inhibited by aspirin, ADP scavenger creatine phosphate/creative phosphokinase (CP/CPK), and ARL-66096, an antagonist of the ADP P2TAC receptor involved in adenylyl cyclase inhibition. But the aggregation was not affected by A3P5PS, an inhibitor of the ADP P2Y receptor. SDF-1–induced aggregation was inhibited by aspirin, but it was only slightly affected by CP/CPK, ARL-66096, or A3P5PS. Finally, the presence of chemokines in platelets was determined. Reverse transcriptase–polymerase chain reaction studies with platelet RNA did not detect the presence of SDF-1 or MDC. In summary, SDF-1 and MDC are platelet agonists that activate distinct intracellular pathways. Their importance in the development of thrombosis at sites of inflammation needs to be further evaluated.
Chemokines are small cytokines that are important as intermediates in the inflammatory response.1,2The chemokines fall into 2 major families: the CXC (or α-chemokines) and the CC (or β-chemokines). The designation of CC indicates that the first 2 of 4 conserved cysteine residues fall immediately adjacent to each other; in CXC, there is an intervening additional amino acid residue between the first 2 cysteine residues. Membrane receptors for these chemokines are members of the G protein-coupled family of receptors and have been designated CCR and CXCR, depending on the family of chemokines to which they bind. Some receptors bind to multiple related chemokines, while others, like CXCR4, bind to a single ligand, in this case SDF-1.3-5
In addition to their importance in inflammation, chemokines have other biological roles. In 1989 it was shown that platelet factor 4 (PF4) inhibits megakaryocyte formation.6 Since then it has been shown that in addition to PF4, other chemokines, specifically interleukin-8 (IL-8), macrophage inflammatory protein–1α (MIP-1α), and MIP-1β, also inhibit megakaryopoiesis.7 It appears that maturing megakaryocytes express CXCR1 and CXCR2, the known IL-8 receptors.7 Further studies have shown the presence of other chemokine receptors on the surface of hematopoietic cells.8 Of great interest was the demonstration by our group10 and others9,11that CXCR4, a coreceptor for T-tropic human immunodeficiency virus–1 (HIV-1) infection, was present on CD34+ cells, early megakaryocyte progenitors, megakaryocytes, and platelets. The presence of CXCR4 on marrow progenitor cells was not unexpected, as CXCR4−/− knockout mice do not properly populate their adult marrow space.12-14 This suggests that the SDF-1 ligand/receptor axis is central to hematopoietic progenitor cell homing. Indeed, studies with megakaryocyte progenitor cells demonstrated that SDF-1 can induce chemotaxis of these cells via CXCR4.9-11
The role of SDF-1 on mature megakaryocytes and platelets is unclear. In our hands, mature megakaryocytes did not show a similar chemotactic response as megakaryocyte progenitors.10 SDF-1 did not inhibit or stimulate megakaryopoiesis, and we could not demonstrate a Ca++ flux in cells stimulated with SDF-1. Others have found similar results.11 However, Wang et al9 have reported a modest but consistent stimulation of megakaryocyte colony formation by SDF-1 and suggested that SDF-1, along with thrombopoietin, is involved in the normal regulation of megakaryopoiesis. Also data showing that SDF-1 increases the growth of murine megakaryocytic colonies in the presence of thrombopoietin have been published recently.15 In addition, the ability of SDF-1 to attract maturing megakaryocytes may be important for platelet formation.16
The presence of CXCR4 on platelets and the ability of SDF-1 to activate platelets were also studied.10 There were approximately 2000 copies of CXCR4 per platelet, with an SDF-1 KD of 24 nmol/L. Similar values have been reported on other cells lines. Using washed platelets, platelet activation was not detected, possibly because the CXCR4 receptor was residually present on the surface of the platelet, and the lack of platelet activation was due to an intracellular signaling block in circulating platelets. However, with platelets in platelet-rich plasma (PRP), SDF-1 acted as a weak platelet agonist.
Other chemokines were tested to see if they could activate washed platelets or platelets in PRP. These include: the CXC chemokines IL-8, PF4, neutrophil-activating peptide–2 (NAP-2), and epithelial cell–derived neutrophil attractant–78 (ENA-78); the CC chemokines human MDC, RANTES (regulated on activation normal T expressed and secreted), MIP-1α, monocyte chemoattractant protein–3 (MCP-3), and thymus- and activation-related chemokine (TARC); and the CX3C chemokine fractalkine. Among these chemokines, only MDC stimulated Ca++ flux in washed platelets and induced aggregation in both washed platelets and platelets in PRP. The platelet effects of these chemokines were further characterized by their interactions with other weak agonists and by the intracellular pathways involved in their activation. The study examined the possibility that these 2 chemokines may be present in platelets. The potential biological consequences of the chemokines' ability to activate platelets is discussed.
Materials and methods
Materials
Chemokines SDF-1 α and MDC were purchased from 2 sources (PeproTech Inc, Rocky Hill, NJ, and R&D Systems, Minneapolis, MN), and there was no difference in chemokine potency between these 2 sources. Other chemokines were also used (R&D Systems), and PF4 and NAP-2 were synthesized in our laboratory as previously described.17
All other chemokines were purchased from R&D Systems. Rabbit anti-human MDC IgG was purchased from PeproTech Inc. Adensosine diphosphate (ADP) was purchased from Sigma Diagnostics (St. Louis, MO) and thrombin receptor PAR1 activating peptide SFLLRN (TRAP) was from Bachem (Torrance, CA). Carbacycline, a stable analog of prostaglandin PGI2, was purchased from BIOMOL Research Laboratories, Inc. (Plymouth Meeting, PA). All common reagents including acetylsalicylic acid (aspirin), apyrase (Grade VII), theophylline and inhibitor of P2Y1PLC ADP receptor A3P5PS were from Sigma Co. The inhibitor of P2YAC receptor, ARL 66096, was a gift from Fisons (now Astra Zeneca, Loughborough, UK). All molecular biology enzymes and tissue culture medium for growing megakaryocytes were from GibcoBRL (Gaithersburg, MD). Delipidated, deionized, and charcoal-treated BSA, iron-saturated transferrin, insulin, and 2 L-glutamine for megakaryocyte growth were from Sigma Co. Recombinant human (rH) thrombopoietin (TPO) and rH interleukin-3 (IL-3) were obtained from R&D Systems, Minneapolis, MN.
Isolation of human blood platelets
The platelets were isolated by differential centrifugation of whole blood. Blood, obtained by venipuncture from healthy volunteers for studies approved by the institutional human subject's review board, was anticoagulated with 3.8% sodium citrate. PRP used for the study of the platelets was isolated from plasma by blood centrifugation at 150g for 20 minutes at room temperature. To obtain washed platelets, blood was collected on acid-citrate-dextrose (ACD) and centrifuged as above, and PGE1 was added to PRP to a final concentration of 1 μmol/L. The platelets were then pelleted by PRP centrifugation at 800g for 20 minutes at room temperature. This pellet was resuspended and washed twice in a Tyrode's buffer: 134 mmol/L sodium chloride (NaCl), 3 mmol/L potassium chloride (KCl), 0.3 mmol/L sodium phosphate (NaH2 PO4) 2 mmol/L magnesium dichloride (MgCl2), 5 mmol/L N-[2-Hydroxyethyl] piperazine-N′-[2-ethane-sulfonic acid (HEPES), 5 mmol/L D-(+) glucose containing 1 mg/mL albumin, 5 U/mL apyrase, and 1 mmol/L EGTA (ethyleneglycotetraacetic acid). After each wash, the platelets were sedimented by centrifugation at 900g for 15 minutes at room temperature. The final pellet was resuspended in Tyrode's buffer (pH 7.5) to a concentration of 3 × 108 platelets per mL. Following each preparation, the platelets were tested for their ability to aggregate with ADP in the presence of fibrinogen and 1 mmol/L calcium dichloride (CaCl2) and used within 5 hours. When indicated, the platelets were incubated with 1 mmol/L aspirin for at least 10 minutes at room temperature.
Measurements of platelet activation
Platelet activation was measured using 3-4 × 108 platelets per mL of either freshly washed platelets or platelets in PRP. Four different measurements were completed: (1) extent of platelet aggregation, (2) activation of αIIb/β3 platelet surface expression, (3) P-selectin expression on the platelet surface, and (4) amount of PF4 released from the platelet α-granules.
For the first measurement, the extent of platelet aggregation was quantified using an aggregometer (Chrono-Log, Havertown, PA), as a light transmission change in platelet suspension, stirred at 37°C in the presence of a specific agonist. The results were expressed as a percentage of light transmission compared with unstimulated platelets. The second measurement, activation of αIIb/β3, was detected using the fluorescein isothiocyanate–labeled (FITC-labeled) monoclonal antibody (mAb) PAC-118 and a fluorescence activated cell sorter (FACS) (FACS Star Plus II; Becton Dickinson, San Jose, CA).
The third measurement quantified surface expression on the platelets of activation-dependent α-granule membrane protein (CD62 or P-selectin).19 The binding of phycoerythrin-conjugated (PE-conjugated) mouse antihuman CD62P mAb (Becton Dickinson) to the platelets was also measured using the FACS sorter. We also measured the amount of PF4 released from platelet α-granules (Assera-Chrom PF4 kit; American Bioproduct Co, Parsippany, NJ). Aliquots of platelets in PRP were stimulated for 5 minutes in an aggregometer cuvette with various agonists followed by centrifugation at 4000 rpm for 3 minutes, and the supernatants were measured for the amount of PF4 released from the platelet α-granules.
Measurement of thromboxane B2 formation in intact platelets
Aliquots of washed platelets or platelets in PRP were stimulated for 5 minutes in an aggregometer cuvette with various agonists. This was then followed by freezing and thawing. The amount of thromboxane B2 (TxB2) formed in platelets was measured using the BIOTRAK Thromboxane B2 Enzymeimmunoassay System (Amersham International, Little Chalfont, England).
Measurement of cyclic AMP formation in intact platelets
Washed platelets were stimulated with 10 nmol/L carbacycline, the stable analogue of PGI2, in the presence of 7 mmol/L theophylline. We added 1 μmol/L ADP, 100 nmol/L SDF-1α, or 100 nmol/L MDC for 5 minutes. The reaction was stopped by the addition of an equal amount of ice-cold 10% trichloroacetic acid. The samples were centrifuged at 15 000 rpm for 5 minutes. The supernatants were extracted with 5 volumes of water-saturated ether and then lyophilized. Platelet cAMP concentrations were measured in the supernatants using the 125I-cAMP radioimmunoassay kit (NEN; Life Science Products, Inc, Boston, MA). These assays were completed in the presence of 1 mmol/L aspirin to inhibit the cyclooxygenase pathway and to abolish generation of TxA2.
Measurement of cytoplasmic Ca++ in platelets
Cytosolic-free Ca++ was determined after the platelets were loaded with Fura-2/AM (Molecular Probes, Eugene, OR) for 30 minutes at room temperature.17 Excess Fura-2/AM was removed by washing cells in wash buffer, and the platelets were resuspended in resuspension buffer as described above. Fluorescence was recorded with an Aminco-Bowman Series-2 Luminescence Spectrometer (SLM Instruments, Inc, Urbana, IL). We stirred 1 mL aliquots of cells continuously in a warmed holder during the period of changes in fluorescence recording. Fluorescence was monitored at 340 and 380 nm for excitation and 510 nm for emission. The data were recorded as the relative ratio of fluorescence excited at 340 and 380 nm, and concentration of mobilized Ca++ was calculated using 224 nmol/L, the known dissociation constant of the Fura-2:Ca++ complex.
Binding of 125I-MDC to platelets
We resuspended 8 × 107 platelets in 75 μL of binding buffer (50 mmol/L HEPES (pH 7.4), 150 mmol/L sodium chloride (NaCl), 5 mmol/L magnesium dichloride (MgCl2), 1 mmol/L calcium dichloride (CaCl2), and 5% BSA. MDC was radiolabeled (IODO-Bead method; Pierce, Rockford, IL) according to the manufacturer's instructions. Next, 5 nmol/L125I-MDC (specific activity, 429.2 × 1010 Bq/mmol [116 Ci/mmol]) was added to the cells with more cold MDC in an additional 25 μL binding buffer. The platelets were incubated at room temperature for 1 hour, and then unbound radioactivity was removed by filtering cells through a Whatman GF/C filter (Whatman International, Maidstone, England) presoaked in polyethylenimine. The filters were washed 3 times with 4 mL wash buffer comprising 50 mmol/L HEPES (pH 7.4), 150 mmol/L NaCl, 5 mmol/L MgCl2, and 1 mmol/L CaCl2. The filters were counted in a Beckman Gamma 5500 Counter (Beckman Instruments, Palo Alto, CA); the KD and the number of binding sites per platelet were calculated as described earlier.10
Reverse transcriptase–polymerase chain reaction
Reverse transcriptase–polymerase chain reaction (RT-PCR) was carried out as previously reported using messenger RNA (mRNA) that was isolated from cells and platelets (Quick-Pre mRNA purification Kit; Pharmacia, Piscataway, NJ).20 The total RNA was prepared using platelets obtained from 100 mL human blood, and the entire preparation was used for a single set of experiments. For these studies, the platelet-to-white-cell ratio was greater than 104:1. In addition, RNA was extracted from whole buffy coat cells so that total RNA was rich in white cells. CD34+cells were expanded in a serum-free liquid system as described.20 Briefly, CD34+A−T− mononuclear cells were resuspended in 104 cells per mL Iscove's Modified Dulbecco's medium (IMDM) supplemented with 25% artificial serum containing 1% delipidated, deionized, and charcoal-treated BSA; 270 mg/mL iron-saturated transferrin; 20 mg/mL insulin; and 2 mmol/L L-glutamine. The megakaryocyte colony-forming unit (CFU-Meg) growth was stimulated with 50 ng/mL rHTPO and 10 ng/mL rHIL-3. Cultures were incubated at 37°C in humidified atmosphere supplemented with 5% carbon dioxide (CO2). Under these conditions, after 14 days, approximately 85% of the expanded cells were positive for αIIb/β3.20 For RT-PCR analysis, cells were further enriched for a population of positive αIIb/β3 cells (purity, greater than 97%) by additional selection with immunomagnetic beads (Miltenyl Biotec, Auburn, CA). Stromal cells were obtained as described earlier.20
We reverse transcribed 0.5 μg mRNA with 500 U Moloney murine leukemia virus RT by using 50 pmol 3′-antisense oligonucleotide of each of the respective primer pairs. The resulting first-strand complementary DNA (cDNA) was PCR-amplified using 5 unitsThermus aquaticus (Taq) polymerase and the primer pairs indicated: CCR4 (sense [S]), 5′-ATG AAC CCC ACG GAT ATSA GCA GAT-3′; CCR4 (antisense [AS]), 5′-CGA ACA CAG CCA CTG ACC ATG TAG-3′; LFA-1 β2 chain (S), 5′-ATC GAC CTG TAC TAT CTG ATG GAC-3′; LFA-1 β2 chain (AS), 5′-GCG ACC TGC ATC ATG GCG TCC AGC-3′; SDF-1 (S), 5′-AAC GCC AAG GTC GTG GTC GTG CTG-3′; SDF-1 (AS), 5′-CAC ATG TTG AAC CTC TTG TTT AAA AGC-3′; MDC (S), 5′-GCT CGC CTA CAG ACT GCA CTC CTG-3′; MDC (AS), 5′-GCT TAT TGA GAA TCA TCT TCA CCC AGG-3′; MIP-1α (S), 5′-CCT TGC TGT CCT CCT CTG CAC-3′; MIP-1α (AS), 5′-CAC TCA GCT CCA GGT CGC TGA-3′; RANTES (S), 5′-TCA TTG CTA CTG CCC TCT GCG-3′; and RANTES (AS), 5′-CTC ATC TCC AAA GAG TTG ATG-3′.
Results
Activation of human washed platelets by chemokines
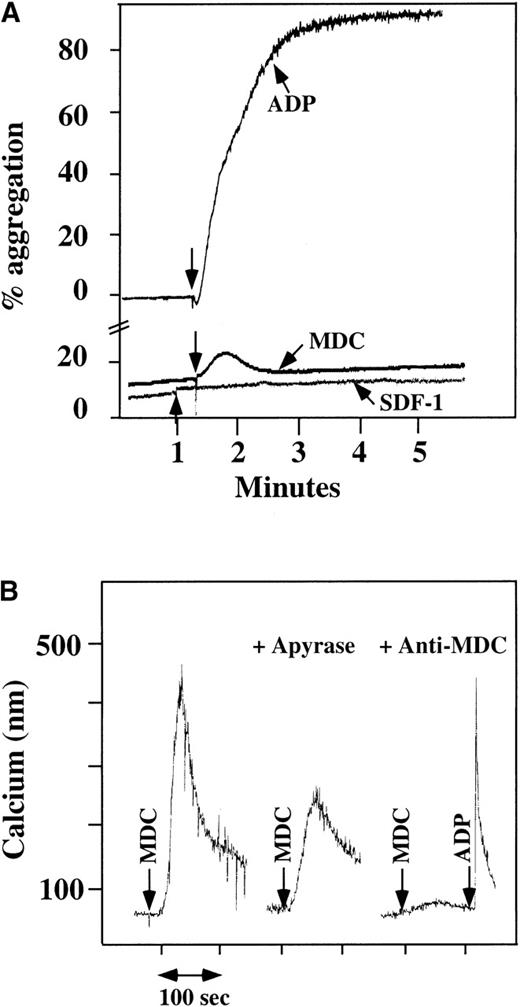
We had previously found that washed platelets could not be activated by SDF-1,10 and repeat studies confirmed this finding (Figure 1A). Using human washed platelets, platelet aggregation studies were also done with other chemokines. The CC chemokine MDC stimulated washed platelets with a small but reproducible response (Figure 1A). A number of other CXC chemokines (IL-8, NAP-2, PF4, and ENA-78) and CC chemokines (MIP-1 and MCP-3) did not activate platelets, even at concentrations greater than that needed to activate other cell lines (data not shown).
Platelet aggregation using chemokine agonists.
(A) Platelet aggregation data using human washed platelets. Platelets were stimulated with 100 nmol/L chemokine (as shown) or 10 μmol/L ADP in the presence of 1 mmol/L CaCl2 and 200 μg/mL fibrinogen. (B) Ca++–flux data using Fura-2–loaded washed platelets involving 30 nmol/L MDC activation. This activation was inhibited by prior incubation of the platelets with 20 U/mL apyrase, as shown in the middle of the figure. The activation specificity is shown by the ability of 20 μg/mL anti-MDC antibody to block MDC-induced platelet activation, as shown on the right side of the figure.
Platelet aggregation using chemokine agonists.
(A) Platelet aggregation data using human washed platelets. Platelets were stimulated with 100 nmol/L chemokine (as shown) or 10 μmol/L ADP in the presence of 1 mmol/L CaCl2 and 200 μg/mL fibrinogen. (B) Ca++–flux data using Fura-2–loaded washed platelets involving 30 nmol/L MDC activation. This activation was inhibited by prior incubation of the platelets with 20 U/mL apyrase, as shown in the middle of the figure. The activation specificity is shown by the ability of 20 μg/mL anti-MDC antibody to block MDC-induced platelet activation, as shown on the right side of the figure.
Using human washed platelets loaded with Fura-2, the activation of platelets by MDC was also seen with mobilization of intracellular Ca++ (Figure 1B, left side). SDF-1 did not induce intracellular Ca++ mobilization in washed platelets at a concentration at which we were able to induce such changes in neutrophils.10 In parallel studies, MDC did not induce Ca++ mobilization in neutrophils, as also demonstrated by others22 (data not shown). MDC-induced Ca++mobilization was inhibited partially by apyrase, an inhibitor of ADP23 (Figure 1B, middle), thereby demonstrating the importance of ADP release in MDC activation of platelets. The response to MDC was specific: 20 μg/mL antihuman MDC antibody blocked the MDC induction of Ca++ mobilization (Figure 1B, right side).
Platelet-binding studies with MDC
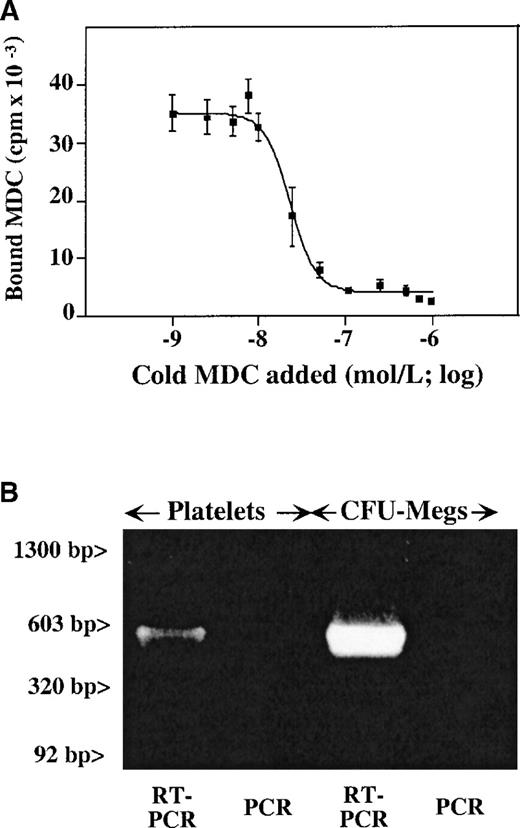
We had previously shown that SDF-1 binds to the CXCR4 receptor on the surface of platelets, with approximately 2000 sites per platelet and a KD of 24 nmol/L.10 We now examined whether CCR4, the chemokine receptor which was shown to bind MDC,24 is present on platelets. The CCR4-specific antibody (a gift from R&D Systems) does not bind to CCR4 on primary cells. Therefore, 125I-radiolabeled MDC was used to detect MDC binding to platelet membrane,25 and the binding characteristics of 125I-MDC were determined using displacement studies with increasing concentrations of unlabeled MDC (Figure 2A). It appears that there are approximately 4000 MDC binding sites per platelet, with a KD of 30 nmol/L. This number of sites and affinity is similar to that reported for SDF-1 and its receptor on platelets.10 While the 125I-MDC binding was completely inhibited by 1.25 μmol/L unlabeled MDC, the addition of cold SDF-1 at the same concentration did not affect MDC binding (data not shown).
Presence of CCR4 on megakaryocytes and platelets.
(A) Unlabeled MDC competition study of 125I-MDC binding to nonactivated platelets. The number of binding sites (approximately 4000 sites per platelet), with a kd of 30 nmol/L, were determined as described in “Material and methods.” (B) RT-PCR data from megakaryocytes and platelets shows the presence of a detectable CCR4 message in both. The anticipated 500–base pair (500-bp) band is seen in both platelet and megakaryocyte RT-PCR. Platelet preparation was the same for both Figures 2 and 3.
Presence of CCR4 on megakaryocytes and platelets.
(A) Unlabeled MDC competition study of 125I-MDC binding to nonactivated platelets. The number of binding sites (approximately 4000 sites per platelet), with a kd of 30 nmol/L, were determined as described in “Material and methods.” (B) RT-PCR data from megakaryocytes and platelets shows the presence of a detectable CCR4 message in both. The anticipated 500–base pair (500-bp) band is seen in both platelet and megakaryocyte RT-PCR. Platelet preparation was the same for both Figures 2 and 3.
Expression of CCR4 and chemokines in platelets
Using oligonucleotides specific for CCR4 receptor, we were able to show the presence of the CCR4 message in platelets and megakaryocytes. RT-PCR of platelet and megakaryocyte total RNA demonstrated the presence of a 500–base pair (500-bp) band corresponding to the predicted product of CCR4 cDNA amplification (Figure 2B). There were no observed bands in the same condition when the reverse transcription step was omitted. Contamination with white cell RNA was ruled out by the absence of an LFA-1 β2 chain message, which was negative for the same platelet RNA preparation (Figure3).
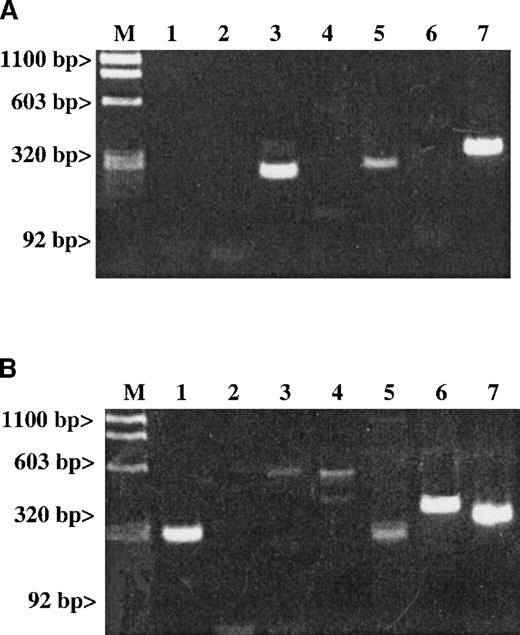
Studies examining whether the messages of either SDF-1 or MDC are detectable in platelet total RNA.
Results are given for (A) RT-PCR of human platelets and (B) RT-PCR of stromal cells. The expected size for each band is indicated. Lane 1: An SDF-1 message (281 bp) is present in stromal cells but not in platelets. Lane 2: An MDC message (270 bp) is also absent in platelets. Lanes 3-5 represent the following messages: Lane 3: A RANTES message (238 bp); Lane 4: An IL-8 message (536 bp); and Lane 5: An MIP-1α message (254 bp). Lane 6: The LFA-1 message (353 bp) is a white cell–specific message showing that the preparation is clean of contaminating white cell messages at the level of RT-PCR examined. Line 7: The ubiquitous message from β-actin (309 bp) was used as a general positive control for the RT-PCR studies.
Studies examining whether the messages of either SDF-1 or MDC are detectable in platelet total RNA.
Results are given for (A) RT-PCR of human platelets and (B) RT-PCR of stromal cells. The expected size for each band is indicated. Lane 1: An SDF-1 message (281 bp) is present in stromal cells but not in platelets. Lane 2: An MDC message (270 bp) is also absent in platelets. Lanes 3-5 represent the following messages: Lane 3: A RANTES message (238 bp); Lane 4: An IL-8 message (536 bp); and Lane 5: An MIP-1α message (254 bp). Lane 6: The LFA-1 message (353 bp) is a white cell–specific message showing that the preparation is clean of contaminating white cell messages at the level of RT-PCR examined. Line 7: The ubiquitous message from β-actin (309 bp) was used as a general positive control for the RT-PCR studies.
A number of chemokines are stored in platelet α-granules. In particular, PF4 and platelet basic protein (PBP), which is cleaved into β-thromboglobulin (βTG) and further cleaved into NAP-2, are platelet-specific. They are made and stored at high concentrations in the α-granules of maturing megakaryocytes.26 In addition, platelet α-granules contain demonstrable amounts of other chemokines such as IL-8,27 ENA-78, MCP-3, and RANTES.28 It is possible that the release of these chemokines is one mechanism by which platelets contribute to inflammation. We wondered whether SDF-1 and MDC could also be synthesized by megakaryocytes. RT-PCR studies with gel-filtered platelet total RNA did not detect the presence of SDF-1 or MDC (Figure 3A), while it did detect the presence of RANTES and MIP1-α messages. The SDF-1 messages were detected in a positive control of total RNA extracted from hematopoietic stromal cells (Figure3B).
Characterization of platelets in PRP activated by chemokines
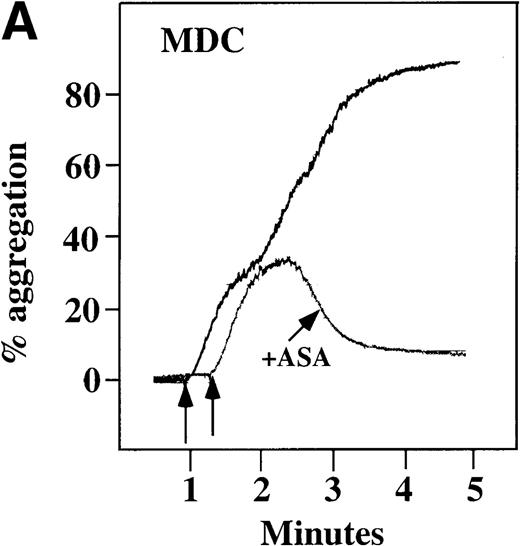
To examine the effect of these chemokines on platelet activation in a more physiological environment, platelet activation in PRP was studied. Full aggregation was achieved with 100 nmol/L MDC (Figure4A). At concentrations of at least 10 nmol/L, MDC induced a dose-dependent biphasic activation of platelets with full aggregation, although the minimal concentration of MDC required for full activation was donor-dependent. The related chemokine TARC, which binds to the same receptor as MDC,24induced only a primary wave of platelet aggregation, even at concentrations of 100 nmol/L (data not shown).
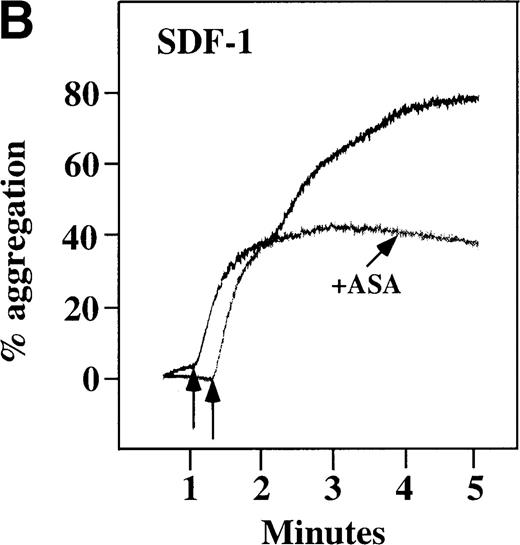
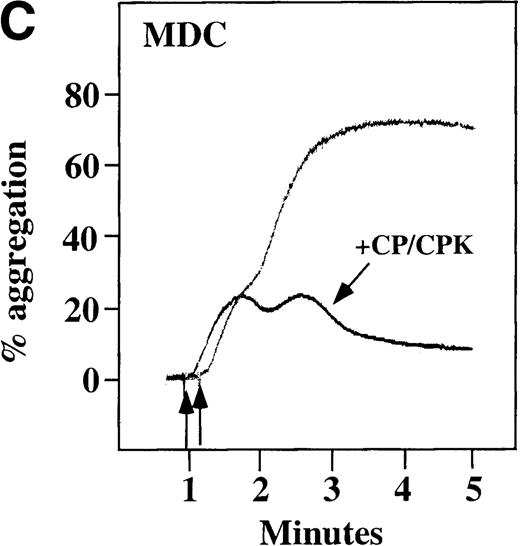
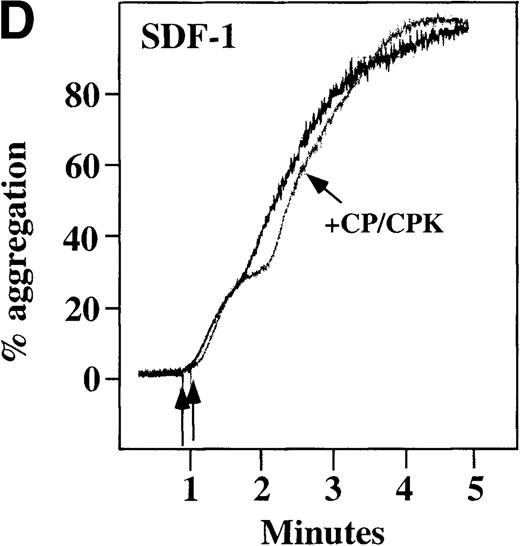
Characterization of the intracellular mechanism(s) by which SDF-1 and MDC activate platelets in PRP.
The figure demonstrates the ability of 500 μmol/L aspirin to inhibit the secondary wave of plasma platelet aggregation stimulated by 100 nmol/L (A) MDC (B) or SDF-1. The inclusion of the ADP scavenger, 10 mmol/L creatine phosphate, plus 10 U/mL creatine phosphokinase (CP/CPK) inhibits platelet activation stimulated by (C) 100 nmol/L MDC but not by (D) 100 nmol/L SDF-1.
Characterization of the intracellular mechanism(s) by which SDF-1 and MDC activate platelets in PRP.
The figure demonstrates the ability of 500 μmol/L aspirin to inhibit the secondary wave of plasma platelet aggregation stimulated by 100 nmol/L (A) MDC (B) or SDF-1. The inclusion of the ADP scavenger, 10 mmol/L creatine phosphate, plus 10 U/mL creatine phosphokinase (CP/CPK) inhibits platelet activation stimulated by (C) 100 nmol/L MDC but not by (D) 100 nmol/L SDF-1.
With the exception of SDF-1, none of the other chemokines tested activated platelets in PRP (data not shown). Although SDF-1 did not cause aggregation of washed platelets, it appears that in platelets in PRP, 100 nmol/L SDF-1 induced a biphasic curve of aggregation (Figure 4B). This discrepancy may be due to the fact that full aggregation of platelets in PRP by MDC or SDF-1 may result from a synergistic effect of TxA2, released ADP, and/or the presence of these or other agonists (eg, epinephrine or serotonin) in the plasma. To test this possibility, platelet stimulation by MDC was carried out in the presence of aspirin, which inhibits TxA2formation. Preincubation of platelets in PRP with aspirin led to reversible platelet aggregation after MDC stimulation, similar to the effect in washed platelets (Figure 4A). Preexposure of the platelets to aspirin did not effect initial platelet activation by SDF-1 in PRP, and a full second wave was not present. However, in contrast to MDC, primary aggregation was not completely reversible, and small aggregates with less variability in the tracing were observed (Figure 4B). Thus, it appears that chemokine secondary aggregation of platelets in PRP is dependent upon TxA2formation.29
We tested whether ADP release is important for platelet activation by either MDC or SDF-1. The presence of the ADP scavenger, creatinine phosphate 10 mmol/L plus 10 U/mL creatinine phosphokinase (CP/CPK), markedly inhibited MDC activation (Figure 4C), but only modestly decreased SDF-1 stimulation (Figure 4D) in PRP. This suggests that the presence of released ADP is an important part of the response to MDC stimulation only. When both TxA2 and ADP were eliminated by the simultaneous addition of aspirin and CP/CPK, the initial platelet response to the stimulation by SDF-1 and MDC was the same as the platelet response in the presence of aspirin only (data not shown). Thus, it appears that the presence of TxA2 and ADP in PRP may at least partially explain the difference seen between these 2 chemokines regarding platelet activation using washed platelets and platelets in PRP. For SDF-1, the blockage of the ADP effect and TxA2 generation did not result in a loss of first-wave aggregation, as seen with the washed platelets in Figure1A. It may be that either TxA2 was already present or that some other agonist in the plasma, such as serotonin, was responsible for the enhanced response of platelets in PRP following SDF-1 stimulation in the presence of both CP/CPK and aspirin.
Additional measures of platelet activation
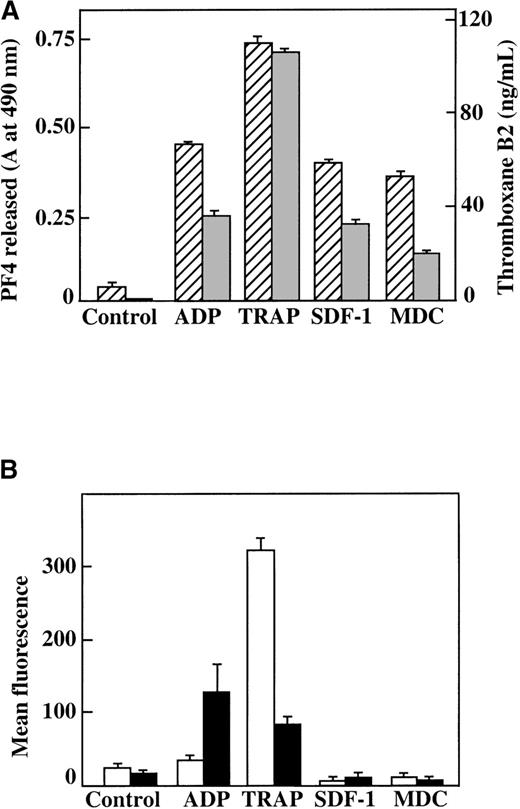
We also measured other parameters of platelet activation in both washed platelets and platelets in PRP. Neither SDF-1 nor MDC could stimulate TxB2 formation in washed platelets, further supporting the fact that these chemokines are weak agonists (data not shown). When PRP was stimulated under aggregating conditions by various agonists, including 100 nmol/L SDF-1 and MDC, there was formation of TxB2, and PF4 was released from α-granules (Figure5A). However, platelet stimulation by the 2 chemokines in nonaggregating conditions (unstirred platelets in PRP) did not lead to activation, as indicated by measurements of either surface expression of P-selectin or activation of the αIIb/β3 receptor by the active-complex dependent mAb PAC-117 (Figure 5B). This contrasts with the results from the stronger agonist, the TRAP peptide, which activates the PAR-1 thrombin receptor on human platelets.30 These findings further demonstrate that these chemokines are weak platelet agonists. In addition, the secondary aggregation and release reaction observed in PRP require close platelet-to-platelet contact, which takes place as aggregates form in a rapidly stirred system and may be due in part to the synergistic effects of TxA2 and released ADP.31
Characterization of platelet activation by SDF-1, MDC, ADP, and TRAP.
(A) TxB2 formation (gray bars) and PF4 release from platelets (hatched bars) after plasma platelet stimulation with agonists under aggregating conditions. (B) Surface P-selectin expression (white bars) and αII/β3 receptor activation (black bars) after plasma platelet stimulation with various agonists under nonaggregating conditions. Each chemokine shown was tested at 100 nmol/L; ADP was tested at 10 μmol/L, and TRAP was tested at 25 μmol/L. Data represents the mean ± SEM for 3 experiments completed in duplicate.
Characterization of platelet activation by SDF-1, MDC, ADP, and TRAP.
(A) TxB2 formation (gray bars) and PF4 release from platelets (hatched bars) after plasma platelet stimulation with agonists under aggregating conditions. (B) Surface P-selectin expression (white bars) and αII/β3 receptor activation (black bars) after plasma platelet stimulation with various agonists under nonaggregating conditions. Each chemokine shown was tested at 100 nmol/L; ADP was tested at 10 μmol/L, and TRAP was tested at 25 μmol/L. Data represents the mean ± SEM for 3 experiments completed in duplicate.
Effect of chemokines on the inhibition of adenylyl cyclase in platelets
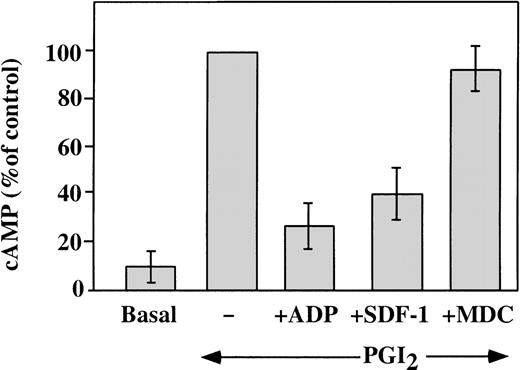
Elevated concentrations of cAMP can inhibit platelet response to stimulation with various agonists. Agents, such as PGI2, stimulate adenylyl cyclase and increase platelet cAMP levels.32 A major intracellular effect of ADP on platelets is the inhibition of the cAMP levels, which leads to platelet aggregation.33 This is achieved by activation of the Gαi protein, which leads to the inhibition of adenylyl cyclase.34 We investigated the ability of SDF-1 and MDC to inhibit the PG-stimulated adenylyl cyclase activity in washed platelets (Figure 6). Platelet cAMP concentrations measured in the presence of theophylline rose after stimulation with 10 nmol/L carbacycline, the stable analog of PGI2, and this increase was inhibited by 1 μmol/L ADP. Like ADP, 100 nmol/L SDF-1 inhibited cAMP levels, but 100 nmol/L MDC had little effect on this assay. Other chemokines tested, including RANTES, MCP-3, ENA-78, PF4, and Fractalkine2 (all at a concentration of 100 nmol/L), had no significant effect on the cAMP inhibition in platelets (data not shown). Thus, comparing chemokines tested, SDF-1 appears to be unique in strongly inhibiting adenylyl cyclase activity in platelets. Most of the chemokine receptors are coupled through pertussis toxin–sensitive Gαi proteins, although coupling can also occur through the Gαq proteins.1 Our results suggest that, in platelets, the SDF-1 receptor CXCR4 is coupled through a Gαi protein. On the other hand, the MDC receptor CCR4 may be coupled to a different G protein, which does not signal by inhibiting adenylyl cyclase.
Effect of SDF-1 and MDC on PGI2-stimulated adenylyl cyclase activity.
Washed platelets were stimulated with 10 nmol/L carbacycline, a stable analog of PGI2, resulting in an elevated level of cAMP in the treated platelets (represented as 100%). ADP (1 μmol/L) inhibited 70% of this increase. Similarly, 100 nmol/L SDF-1 inhibited 60% of the increased cAMP levels, while the same concentration of MDC had no significant affect on this assay. Data presented is the mean ± SEM for 5 experiments.
Effect of SDF-1 and MDC on PGI2-stimulated adenylyl cyclase activity.
Washed platelets were stimulated with 10 nmol/L carbacycline, a stable analog of PGI2, resulting in an elevated level of cAMP in the treated platelets (represented as 100%). ADP (1 μmol/L) inhibited 70% of this increase. Similarly, 100 nmol/L SDF-1 inhibited 60% of the increased cAMP levels, while the same concentration of MDC had no significant affect on this assay. Data presented is the mean ± SEM for 5 experiments.
Chemokine enhancement of agonist-stimulated platelet activation
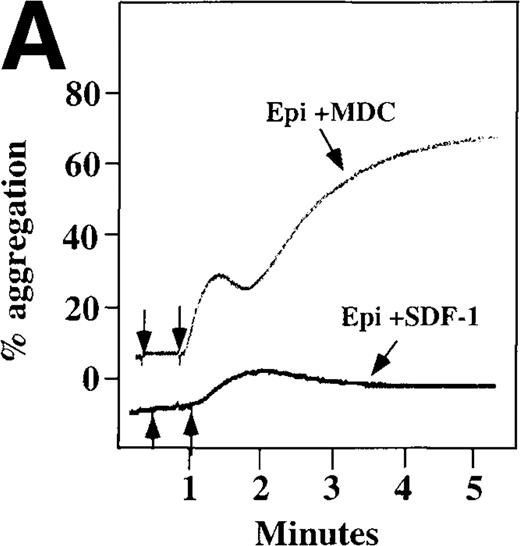
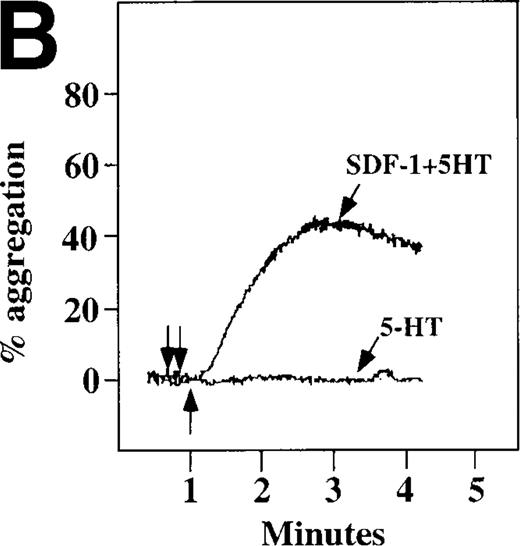
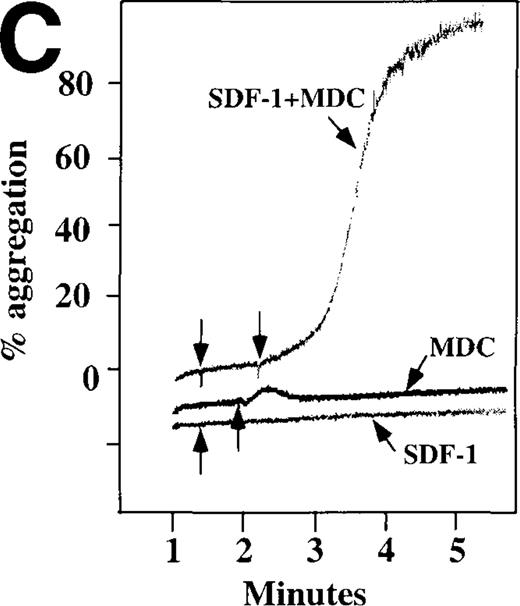
Because SDF-1 and MDC appear to activate different signal transduction pathways, we examined whether MDC can synergize with other weak platelet agonists. Aggregation of washed platelets induced by MDC was potentiated by epinephrine, a weak platelet agonist coupled to the α2A receptor, which mediates inhibition of adenylyl cyclase35 (Figure 7A). MDC could also synergize with low concentrations of ADP, although using washed platelets, neither 100 nmol/L MDC nor 1 μmol/L ADP alone resulted in full aggregation. However, the addition of a chemokine with the same amount of ADP resulted in vigorous platelet aggregation (data not shown). The synergism between MDC and ADP contrasts with our previously published observation that low concentrations of ADP do not potentiate SDF-1 aggregation.10 However, as shown in Figure7B, SDF-1 can synergize with serotonin (5HT), a weak platelet agonist. Serotonin activates a platelet receptor that is coupled to activation of phospholipase C (PLC).36 Our results using SDF-1 and 5HT are similar to the findings of Jin and Kunapuli37 (Figure7B). They showed that weak agonists, such as low concentrations of ADP and 5HT, can synergize in platelet activation. This suggests that SDF-1 and 5HT activate platelets, in part, by nonoverlapping pathways.38 In fact, our studies suggest that SDF-1 and MDC may be such a pair of nonoverlapping weak agonists. While 100 nmol/L of each chemokine alone cannot activate washed platelets, the 2 together resulted in platelet activation (Figure 7C).
Synergistic effect of SDF-1 and MDC on washed platelet aggregation.
(A) The synergistic effect of MDC and epinephrine (Epi) on platelet aggregation. At the first arrow, 1 μmol/L epinephrine was added, followed by 100 nmol/L MDC or SDF-1. (B) The synergistic effect of 100 nmol/L SDF-1 and 5 μmol/L 5HT. (C) Similarly, neither 100 nmol/L SDF-1 nor MDC induced platelet aggregation, but the 2 agonists added together induced full aggregation of washed platelets.
Synergistic effect of SDF-1 and MDC on washed platelet aggregation.
(A) The synergistic effect of MDC and epinephrine (Epi) on platelet aggregation. At the first arrow, 1 μmol/L epinephrine was added, followed by 100 nmol/L MDC or SDF-1. (B) The synergistic effect of 100 nmol/L SDF-1 and 5 μmol/L 5HT. (C) Similarly, neither 100 nmol/L SDF-1 nor MDC induced platelet aggregation, but the 2 agonists added together induced full aggregation of washed platelets.
Effect of ADP receptor antagonists on chemokine-induced aggregation in platelets in PRP
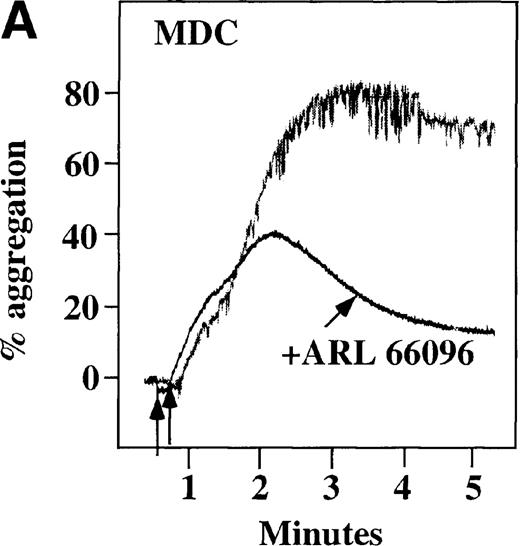
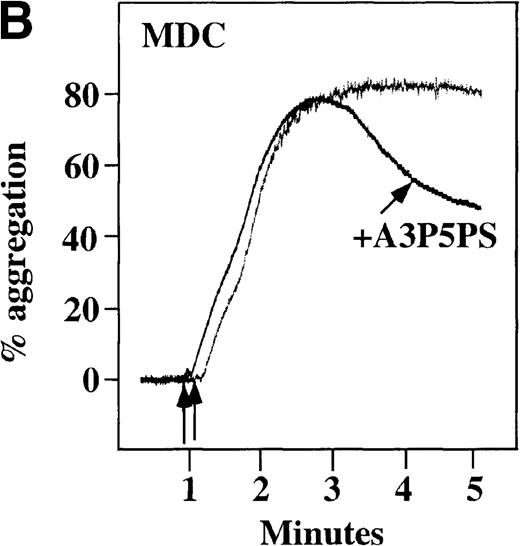
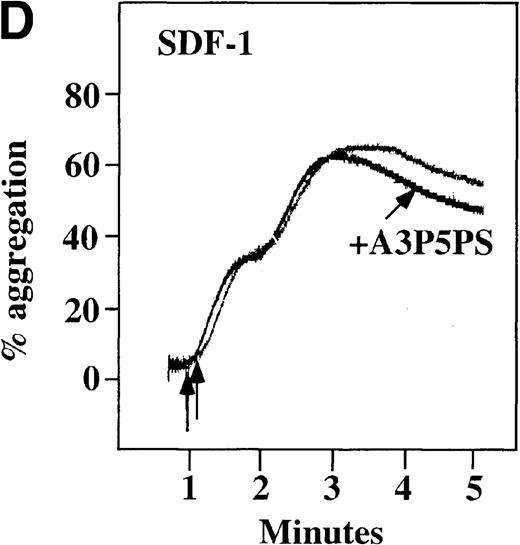
CP/CPK, a scavenger of ADP, inhibited the secondary wave of plasma platelet aggregation stimulated by MDC. We therefore tested MDC-stimulated platelet aggregation in the presence of inhibitors of the other pathways of ADP-induced signal transduction. The second wave of MDC-induced plasma platelet aggregation was inhibited by 1 μmol/L ARL-66096, an inhibitor of the proposed ADP P2TAC receptor involved in adenylyl cyclase inhibition (Figure8A).37 However, aggregation was not affected by 100 μmol/L A3P5PS, an inhibitor of the P2Y1 ADP receptor involved in PLC activation (Figure 8B). In contrast, SDF-1–induced aggregation was only slightly affected by either ADP antagonists ARL-66096 or A3P5PS (Figure 8C and D). These data are consistent with the ability of SDF-1 to primarily activate platelets through decreasing cAMP levels, which requires granular release to lead to PLC activation and full platelet aggregation. MDC primarily activates aggregation through PLC and Ca++ mobilization and requires other agonists to inhibit adenylyl cyclase and fully aggregate platelets. Thus, it appears that initial platelet activation by SDF-1 and MDC involves distinct pathways. We hypothesize that part of this complementary activation of platelets (Figure 7C) is due to the fact that SDF-1 predominantly activates platelets by inhibiting adenylyl cyclase activity, and MDC predominantly activates platelets by stimulating PLC activity.
Effect of ADP antagonists ARL 66096 and A3P5PS on plasma platelet aggregation induced by SDF-1 and MDC.
Platelets in PRP were preincubated with a specific ADP antagonist and stimulated with 100 nmol/L MDC. (A) The second wave of MDC-induced platelet aggregation was inhibited by 1 μmol/L ARL 66096, (B) while 100 μmol/L A3P5PS had only a slight inhibitory effect. In contrast, aggregation induced by SDF-1 is only slightly affected by (C) ARL 66096 or (D) A3P5PS.
Effect of ADP antagonists ARL 66096 and A3P5PS on plasma platelet aggregation induced by SDF-1 and MDC.
Platelets in PRP were preincubated with a specific ADP antagonist and stimulated with 100 nmol/L MDC. (A) The second wave of MDC-induced platelet aggregation was inhibited by 1 μmol/L ARL 66096, (B) while 100 μmol/L A3P5PS had only a slight inhibitory effect. In contrast, aggregation induced by SDF-1 is only slightly affected by (C) ARL 66096 or (D) A3P5PS.
Discussion
It has become clear that chemokine receptors are present on multiple hematopoietic lineages including the megakaryocytic lineage. We and others have demonstrated that a number of chemokine receptors, including the HIV coreceptor CXCR4, are present on developing megakaryocytes.9-11 The roles these receptors play during megakaryopoiesis and platelet formation and function are presently being explored by a number of laboratories. Such roles can include stimulation or inhibition of megakaryocyte formation, platelet formation, or platelet biology. In our previous studies, we were surprised to find that although the SDF-1 receptor CXCR4 is present on platelets, SDF-1 did not activate washed platelets in the presence of apyrase.10 We concluded that the CXCR4 receptor was important at a much earlier stage of megakaryopoiesis and that CXCR4 was only residually present on platelets. The loss or change of a cellular response to the binding of a chemokine by a particular chemokine receptor is well documented. For example, D'Apuzzo et al5 have shown that during B-cell differentiation, intracellular signaling through CXCR4 by SDF-1 changes. Ca++ mobilization by SDF-1 was observed in pro-B and pre-B cell lines, but cell lines representing higher stages of maturation were unresponsive.
Several chemokines were studied for their ability to activate platelets, thereby leading to platelet aggregation. Among the chemokines tested, only SDF-1 and MDC were platelet agonists. Both were effective in inducing aggregation of platelets in PRP. These chemokines bind to platelets by way of their respective receptors using similar dissociation constants and a similar number of binding sites. However, these 2 weak agonists did not appear to activate platelets in an identical fashion. MDC, but not SDF-1, induced intracellular Ca++ mobilization in Fura-2–loaded washed platelets (Figure 1B), thereby supporting an important PLC-dependent pathway.41 SDF-1, but not MDC, inhibited prostaglandin-induced cAMP levels in washed platelets (Figure 6).
In plasma platelets, both SDF-1 and MDC induced biphasic aggregation (Figure 4A and 4B). However, the presence of aspirin inhibited MDC from inducing full aggregation in platelets in PRP, and only reversible first-wave aggregation was detected (Figure 4A). This finding suggests a role for TxA2 formation in MDC-induced full platelet aggregation. In contrast, while TxA2 formation seems to be important for full secondary wave aggregation induced by SDF-1 in platelets in PRP, stable small aggregates were still observed in the presence of aspirin (Figure 4B). Further, ADP release appears to be important for MDC activation of platelets in PRP, much more so than for SDF-1 (Figure 4 C, D). Thus, our data are consistent with the fact that MDC primarily activates platelets through PLC and Ca++mobilization, thereby requiring other agonists to lead to adenylyl cyclase inhibition and full platelet aggregation. SDF-1 primarily activates platelets through decreasing cAMP levels, thereby requiring granular release to lead to PLC activation and full platelet aggregation.
Weak agonists that activate platelets by different pathways can synergize and result in vigorous platelet activation when used in a combination.37 For example, ADP and 5HT or epinephrine can activate platelets synergistically. We found that SDF-1 could act synergistically with 5HT, but not with epinephrine (Figure 5). On the other hand, MDC could act synergistically with low concentrations of ADP and epinephrine. Furthermore, SDF-1 and MDC could markedly synergize each other's response using washed platelets (Figure 7C). In washed platelets, SDF-1 alone had no effect, and MDC was only able to achieve a weak primary wave. Together, these 2 chemokines achieved full and brisk platelet aggregation.
Significant differences in G-protein coupling have been noted among the different chemokine receptors. Many of these receptors and other 7-transmembrane receptors are coupled only to the Gαiprotein, but some of them are coupled to other G proteins such as Gαq and/or Gα16.39,40 For example, in platelets, the thrombin receptor PAR1, another 7-transmembrane receptor, has been shown to couple to both the Gαq protein, which activates PLC, and to the Gαi protein, which inhibits adenylyl cyclase.34 A platelet-activation model has been proposed to explain the complex activation of platelets by ADP.37 In this model, intracellular Ca++mobilization and inositol triphosphate (IP3) formation is modulated by the 7-transmembrane purogenic P2Y1 receptor coupled through the Gαq protein to PLC, while inhibition of adenylyl cyclase is mediated through the P2YAC receptor coupled to the Gαi protein. In addition, the receptor-operated Ca++ channel P2X1 facilitates rapid Ca++ influx by ADP.41 Our results suggest that the SDF-1 and MDC receptors are also coupled to distinct G proteins and therefore trigger different signal transduction pathways. We propose that the SDF-1 receptor CXCR4 links to the Gαi protein and inhibits adenylyl cyclase activity, while the MDC receptor CCR4 is coupled to a different G protein, perhaps Gαq, and leads to mobilization of Ca++ from intracellular stores.
The biological importance of chemokine affects on platelets needs to be determined. SDF-1 was originally cloned from a bone marrow stromal cell line. It has been characterized as a pre–B-cell growth-stimulating factor and chemotactic factor for monocytes, T lymphocytes, and CD34+ human progenitor cells.3,42,43 Further studies clearly showed that SDF-1 can be expressed by a large number of tissues.44 On the other hand, MDC is a CC chemokine synthesized by macrophages and dendritic cells.45Therefore, both chemokines may exist at sites of vasculitis or at atherogenic lesions. The presence of either chemokine would enhance chemotactic attraction of inflammatory cells and contribute to the activation of platelets at sites of injury, thereby leading to either thrombosis and/or acceleration of injury to the vascular integrity. Additionally, details of platelet activation at an inflammation site may differ based on the chemokines being expressed. Thus it is possible that different vascular injuries with different pools of released chemokines may result in different degrees of local activation of circulating platelets and risks of developing thrombosis.
Acknowledgments
We thank Dr Benjamin Doranz of the Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, for the 125I-labeled MDC and R&D Systems, Minneapolis, MN, for the CCR4-specific antibody.
Supported in part by grants HL61796 (M.A.K., M.R., and M.P.) and HL40387 (L.B. and M.P.) from the National Institutes of Health, Bethesda, MD.
Reprints:M. Anna Kowalska, Children's Hospital of Philadelphia, 34th Street & Civic Center Blvd, ARC, Room 314I, Philadelphia, PA 19104; e-mail: kowalska@emailchop.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal