Abstract
Many antineoplastic drugs kill tumor cells by inducing apoptosis. This highly controlled mechanism of cell death is thought to be physiologically advantageous because apoptotic cells are removed by phagocytosis before they lose their permeability barrier, thus preventing induction of an inflammatory response to the dying cells. In contrast, necrotic cells lyse and release their contents into the extracellular space, thus inducing inflammation. In this report, we examine the effects of oxidative stress on chemotherapy-induced cell killing. We find that H2O2 inhibits the ability of 4 different chemotherapy drugs (VP-16, doxorubicin, cisplatin, and AraC) to induce apoptosis in human Burkitt lymphoma cells. H2O2 shifts the form of cell death from apoptosis to pyknosis/necrosis, which occurs after a significant delay compared with chemotherapy-induced apoptosis. It can also lower the degree of cell killing by these drugs. These effects of H2O2 can be prevented by the antioxidant agents Desferal, Tempol, and dimethylsulfoxide. Phagocytosis by monocyte-derived macrophages of VP-16–treated lymphoma cells is also inhibited by H2O2. Cells killed with H2O2 (with or without VP-16) do ultimately undergo phagocytosis, but this occurs only after they have lost their permeability barrier. Thus, membrane-intact apoptotic cells are recognized and phagocytosed by monocyte-derived macrophages, but membrane-intact pyknotic/necrotic cells are not. The results suggest that chemotherapy-induced apoptosis and phagocytosis of cancer cells may be enhanced by including certain antioxidant agents in the treatment protocol.
Chemotherapy is one of the mainstays of medical intervention for cancer. Many antineoplastic drugs kill tumor cells by inducing a form of cell death called apoptosis,1 which is characterized by unique biochemical and morphologic features.2 Most notably, cells dying by apoptosis fragment into small, subcellular, membrane-bound “apoptotic bodies.”3 In contrast, cells that die by necrosis swell and then lyse, releasing their contents into the extracellular space. It is thought that death by apoptosis is physiologically advantageous because apoptotic cells are cleared by phagocytosis and subsequent intracellular degradation.4 5 In this manner, apoptotic cells are removed without causing damage to the surrounding tissue. In contrast, necrotic cells are thought to promote an inflammatory response caused by the leakage of intracellular proteins and nucleic acids. Agents that interfere with the ability of an antineoplastic agent to induce apoptosis may limit drug efficacy and induce a tissue-damaging inflammatory response.
This work examines the possible influence of oxidants on the effects of cancer chemotherapy. Oxidants such as superoxide, hydrogen peroxide (H2O2), and hydroxyl radical are generated by activated phagocytes (eg, neutrophils and macrophages) as part of the inflammatory response.6 Under normal circumstances, oxidants serve a protective function by killing invading bacteria and tumor cells.7 However, they can have detrimental side effects as well, causing tissue damage and contributing to the development or progression of numerous different diseases including cancer.8 Solid tumors are often infiltrated by inflammatory phagocytes,9 which can generate oxidative stress within the tumor tissue. In support of this possibility, cancer patients are reported to have higher levels both of generalized oxidative stress10-12 and of oxidative damage within tumor tissues as compared with normal tissues.13-15
In previous studies with Burkitt lymphoma cells, we found that relatively low concentrations of H2O2 (50-100 μmol/L) inhibited the induction of apoptosis by the chemotherapy drug VP-16 (etoposide) and the calcium ionophore A23187.16 Cell death still occurred, but it was by a nonapoptotic mechanism. Thus, nuclear condensation and fragmentation of the cells into apoptotic bodies were prevented, as were many of the biochemical steps that are known to mediate apoptosis, such as oligonucleosomal degradation of the DNA and caspase activation. The goal of the present work was to determine whether oxidants such as H2O2interfere with the actions of cancer chemotherapeutic agents in general. Using Burkitt lymphoma cells as a model, we found that H2O2 inhibited the induction of apoptosis by 4 different antineoplastic agents. The presence of H2O2 could also reduce the overall level of cell killing by 3 of these drugs. Because of its inhibition of apoptosis, H2O2 also inhibited the phagocytosis of VP-16–treated lymphoma cells by human monocyte-derived macrophages. Induction of apoptosis could be restored by coadministration of selected antioxidants which did not, by themselves, induce apoptosis. The potential ramifications of these findings for cancer chemotherapy are discussed.
Materials and methods
Cells and treatments
The Burkitt lymphoma cell lines JLP 119, ST-486, and BL-41 were provided by Kishor Bhatia (National Cancer Institute, National Institutes of Health, Bethesda, MD). Cells were grown in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 2 mmol/L L-glutamine, and 50 μmol/L β-mercaptoethanol (“medium”) at 37°C in 5% CO2 in air. Exponentially growing cells were harvested by centrifugation and resuspended in fresh medium to achieve a culture density of 5 × 105 cells/mL. H2O2 was added 30 minutes after chemotherapy drugs. This time point was chosen to minimize the possibility of any direct interactions between H2O2 and the chemotherapeutic agents. In previous studies with VP-16, we found that H2O2does not have any direct chemical effect on VP-16,16 but similar experiments were not carried out for all of the other drugs used in the present studies. To avoid any possible interactions, we allow the drug to enter the cells before adding the H2O2. The time of addition of H2O2 is not critical as long as it is added before the onset of apoptosis; it inhibits the induction of apoptosis equally well if added 30 minutes before or after adding the chemotherapy drugs. When antioxidants were tested, they were added 30 to 60 minutes before the chemotherapy drugs. Cell incubations were for 4 to 22 hours, as indicated in the text.
Quantification of cell death using Hoechst/propidium iodide nuclear staining and fluorescence microscopy
Cells were stained with Hoechst 33342 and propidium iodide (PI) and were visualized using fluorescence microscopy, as described previously.17 A minimum of 200 cells were counted and classified as follows: (1) live cells (normal nuclei: blue chromatin with organized structure); (2) membrane-intact apoptotic cells (bright blue chromatin that is highly condensed, marginated, or fragmented); (3) membrane-permeable apoptotic cells (bright red chromatin, highly condensed, or fragmented); (4) necrotic cells (red, enlarged nuclei with smooth normal structure); and (5) pyknotic/necrotic cells (bright red, slightly condensed nuclei sometimes divided into 2-3 spheres). All experiments were repeated at least 3 times.
Phagocytosis assay
A fluorescence microscope–based assay was developed for quantifying phagocytosis of lymphoma cells by macrophages. Monocyte-derived macrophages were prepared by incubating normal human monocytes (prepared by elutriation) for 7 to 14 days in RPMI containing 10% FCS, penicillin/streptomycin, and recombinant human macrophage colony-stimulating factor (rhM-CSF; 100 ng/mL). Monocytes were seeded at a starting density of 2 × 105 cells/well in 24-well plates. Burkitt lymphoma cells were stained with the stable, cell-permeable green fluorescent dye CFDA [5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester] (0.16 mg/mL stock in dimethylsulfoxide [DMSO]) by suspending 8 × 105 cells in 1 mL phosphate-buffered saline (PBS) in a test tube and adding 0.75 μL CFDA. The dye becomes modified by esterases once it enters cells so that it gains fluorescence, binds to intracellular proteins, and becomes trapped inside the cell. Cells were incubated for 20 minutes at 37°C, mixed with one-half volume of medium, centrifuged at 4°C, and resuspended to a cell density of 5 × 105cells/mL in medium. Treatments with H2O2 and chemotherapy drugs were performed as described earlier. The intensity of green staining of the target cells did not diminish over the course of any of the incubations. At the end of the incubations, cells were added at a 2:1 ratio to the macrophages, and the cells were allowed to coincubate for 1 hour at 37°C. Nonphagocytosed lymphoma cells were removed by thorough washing with medium. The macrophages were stained with phycoerythrin (PE)-labeled anti-CD14 antibody (1:15 dilution in buffer containing 1% albumin and azide) at 4°C for 45 minutes. Cells were detached from the wells by adding 1% lidocaine/0.5% serum in PBS and incubating for 10 minutes at 37°C. The wells were mixed vigorously to remove all cells (verified by phase contrast microscopy), which were then centrifuged and resuspended in PBS. Cells were analyzed by fluorescence microscopy using a 2-color (FITC/TRITC) fluorescence cube (λex 450-490 nm, λem520-650 nm). The percent phagocytosis is the percentage of total macrophages (red/orange cells, at least 200 per measurement) containing internalized Burkitt lymphoma cells (green spheres). Macrophages containing multiple green cells were counted only once. The presence of free (nonphagocytosed) Burkitt lymphoma cells was rare (≤ 1 per measurement) and was not quantified.
Measurement of H2O2 production by Burkitt lymphoma cells
To determine whether the Burkitt lymphoma cells used in these experiments produce their own H2O2, we suspended JLP 119 or ST-486 cells (5 × 105/mL) in PBS/1 mg/mL glucose ± 1 mmol/L azide to inhibit endogenous catalase activity. At various time points (10 minutes to 2 hours), samples were removed and centrifuged, and cell-free supernatants were assayed for the presence of H2O2 by the phenol red assay, performed as described previously.17 Because H2O2 can diffuse freely in and out of cells, the concentration of H2O2 outside of the cells should reflect the intracellular concentration as well.
Reagents
VP-16, doxorubicin, AraC (cytosine β-D-arabinofuranoside), cisplatin (cis-Platinum(II)diammine dichloride), Desferal (deferoxamine mesylate), Tempol (4-hydroxy-Tempo), 1,10-o-phenanthroline, ascorbic acid, ebselen (2-phenyl-1,2-benzisoselenazol-3[2H]-one), DMSO, Hoechst 33342, and PI were purchased from Sigma Chemical, Inc. (St. Louis, MO). CFDA (catalog no. 1157) was from Molecular Probes, Inc. (Eugene, OR). PE-labeled anti-CD14 antibody was from PharMingen (San Diego, CA). rhM-CSF was kindly provided by Valerie Calvert (Food and Drug Administration/Center for Biologics Evaluation and Research [CBER]).
Results
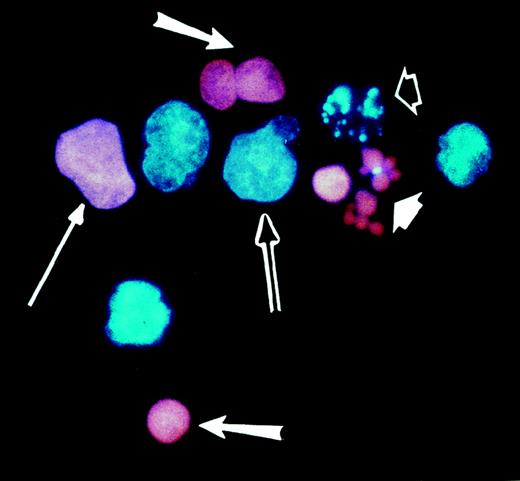
VP-16 kills Burkitt lymphoma cells almost entirely by inducing apoptosis, and this mode of cell death is inhibited by the presence of H2O2.16 Cells treated either with H2O2 alone or with VP-16 in the presence of H2O2 are mostly nonapoptotic, showing cytoplasmic swelling, mild pyknosis of the nucleus, and little or no DNA fragmentation, caspase activation, or annexin-V binding.16 Because these cells resemble necrotic cells except for the pyknosis of the nucleus, they are referred to as pyknotic/necrotic. Using cell morphology as the criterion for assessing the mode of cell death, we quantified cell death by fluorescence microscopy after staining the cells with the nuclear dyes Hoechst 33342 and PI. This method allowed us to distinguish apoptotic from nonapoptotic cells and also revealed whether the cells had lost their plasma-membrane permeability barrier (see “Materials and methods”). Examples of the different cell death morphologies identified with this method are shown in Figure1.
Morphologies of live and dead Burkitt lymphoma cells as determined by fluorescence microscopy.
JLP 119 cells were treated with 50 μmol/L H2O2. Cells were harvested after 22 hours, stained with Hoechst 33342 and PI, and examined by fluorescence microscopy as described in “Materials and methods.” Characteristic live and dead cell morphologies are identified by the arrows as follows: double arrow, live cell; long thin arrow, necrotic cell; long fat arrows, pyknotic/necrotic cells; short white arrow, membrane-permeable apoptotic cells; short open arrow, membrane-intact apoptotic cell.
Morphologies of live and dead Burkitt lymphoma cells as determined by fluorescence microscopy.
JLP 119 cells were treated with 50 μmol/L H2O2. Cells were harvested after 22 hours, stained with Hoechst 33342 and PI, and examined by fluorescence microscopy as described in “Materials and methods.” Characteristic live and dead cell morphologies are identified by the arrows as follows: double arrow, live cell; long thin arrow, necrotic cell; long fat arrows, pyknotic/necrotic cells; short white arrow, membrane-permeable apoptotic cells; short open arrow, membrane-intact apoptotic cell.
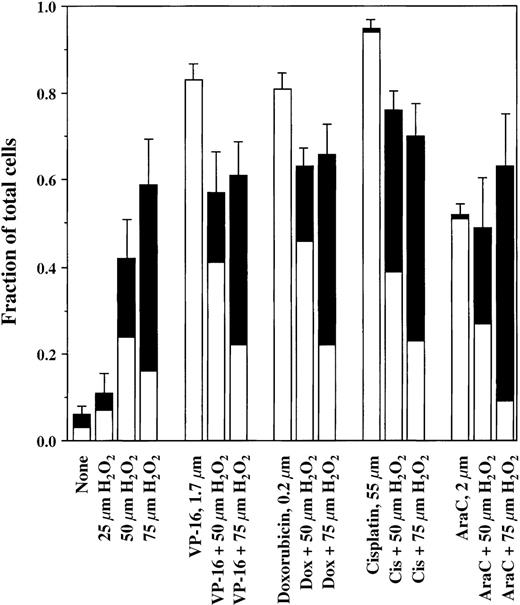
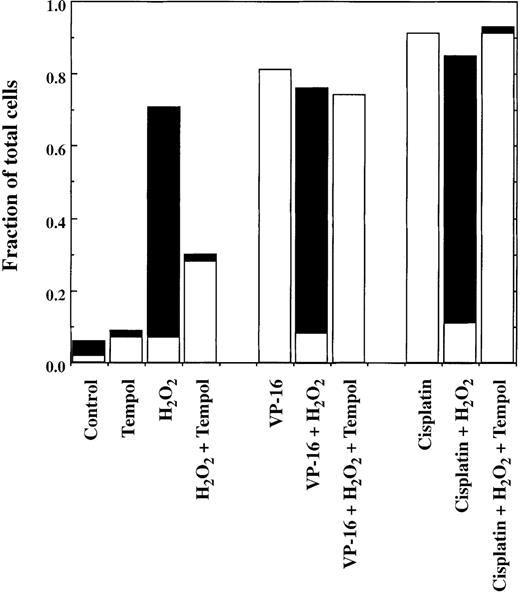
The results in Figure 2 show the effects of H2O2 on the induction of apoptosis by 4 different antineoplastic drugs with diverse mechanisms of action: VP-16 (1.7 μmol/L), doxorubicin (0.2 μmol/L), cisplatin (50 μmol/L), and AraC (2 μmol/L). H2O2 was added 30 minutes after each drug addition. As shown, all of these drugs killed the lymphoma cells by inducing apoptosis. The presence of increasing concentrations of H2O2 progressively inhibited the occurrence of apoptosis and converted the mode of cell death to pyknosis/necrosis. In addition, decreased overall cell killing by VP-16, doxorubicin, and cisplatin occurred in the presence of H2O2. For the representative experiments shown in Figure 2, the fraction of cells appearing alive and healthy after VP-16 treatment increased from 16% ± 4% (mean ± SD, n = 3) in the absence of H2O2 to 43% ± 11% in the presence of 50 μmol/L H2O2. With doxorubicin, the respective values were 18% ± 4% without H2O2 and 36% ± 5% with 50 μmol/L H2O2(n = 4). For cisplatin, values were 4% ± 1% and 24% ± 5% without and with H2O2, respectively (n = 4). Because the level of cell killing by 75 μmol/L H2O2 alone exceeded the level of killing by AraC, cells treated with 75 μmol/L H2O2 and AraC together incurred a level of cell death that was comparable to the higher level induced by H2O2 alone. Control experiments with VP-16 showed that H2O2 had no direct chemical effect on the drug.16
Induction of cell death by chemotherapy drugs in the presence and absence of H2O2.
JLP 119 cells were treated with either VP-16, doxorubicin, cisplatin, or AraC followed 30 minutes later by the addition of the indicated concentrations of H2O2. After 22 hours of incubation, cells were harvested and analyzed by fluorescence microscopy, as described in “Materials and methods.” The fraction of total cells representing each form of cell death is shown. For ease of interpretation, the numbers of blue (membrane-intact) and red (PI-permeable) apoptotic cells were added together (□), as were the numbers of pyknotic/necrotic and necrotic cells (▪). The number of typical necrotic cells generally represented less than 10% of the number of pyknotic/necrotic cells. Each experiment was repeated at least 3 times. Error bars show the SD for the total number of dead cells.
Induction of cell death by chemotherapy drugs in the presence and absence of H2O2.
JLP 119 cells were treated with either VP-16, doxorubicin, cisplatin, or AraC followed 30 minutes later by the addition of the indicated concentrations of H2O2. After 22 hours of incubation, cells were harvested and analyzed by fluorescence microscopy, as described in “Materials and methods.” The fraction of total cells representing each form of cell death is shown. For ease of interpretation, the numbers of blue (membrane-intact) and red (PI-permeable) apoptotic cells were added together (□), as were the numbers of pyknotic/necrotic and necrotic cells (▪). The number of typical necrotic cells generally represented less than 10% of the number of pyknotic/necrotic cells. Each experiment was repeated at least 3 times. Error bars show the SD for the total number of dead cells.
Further experiments were carried out to determine whether the decreased cell killing seen after incubation with a chemotherapy drug plus H2O2 can translate into increased long-term survival of tumor cells. For these studies, JLP 119 cells were treated with 1.7 μmol/L VP-16 in the presence or absence of 50 μmol/L H2O2, and the cells were allowed to incubate for 12 hours. Live cells were then counted by trypan blue exclusion, diluted in fresh growth medium, and plated at 3 cells/well in 96-well plates (288 wells per treatment). After 3 weeks, the number of wells containing growing cells was counted. The cell densities in each positive well were equivalent, suggesting that each growth was derived from a single cell (clone). In 2 independent experiments (Table1), we found that 1.5- to 2-fold more cells survived VP-16 treatment when H2O2 was also present, consistent with the results obtained at 22 hours.
Clonal outgrowth of lymphoma cells treated with either VP-16 alone or VP-16 in the presence of H2O2
| Treatment . | No. of surviving clones (%) . |
|---|---|
| Experiment 1 | |
| VP-16 alone | 2 (0.7) |
| VP-16 + 50 μmol/L H2O2 | 4 (1.4) |
| Experiment 2 | |
| VP-16 alone | 6 (2.1) |
| VP-16 + 50 μmol/L H2O2 | 9 (3.1) |
| Treatment . | No. of surviving clones (%) . |
|---|---|
| Experiment 1 | |
| VP-16 alone | 2 (0.7) |
| VP-16 + 50 μmol/L H2O2 | 4 (1.4) |
| Experiment 2 | |
| VP-16 alone | 6 (2.1) |
| VP-16 + 50 μmol/L H2O2 | 9 (3.1) |
JLP 119 cells were treated for 12 h with 1 μg/mL VP-16 alone or in the presence of 50 μmol/L H2O2. Remaining live cells were counted, diluted in fresh culture medium, and plated in 96-well plates at 3 cells/well (288 wells per treatment). The number and percentage of wells containing cellular outgrowth after 3 weeks of culture are shown.
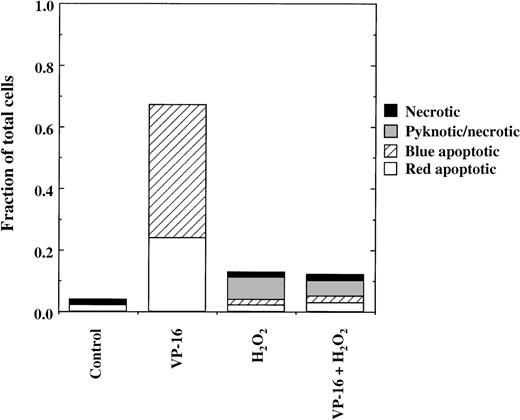
In the experiments shown in Figure 2, we used concentrations of chemotherapy drugs (eg, 1.7 μmol/L VP-16) that killed 80% of the cells over the course of 22 hours of incubation. With increased drug concentrations (eg, 8.5 μmol/L VP-16), apoptosis occurs much more rapidly, such that 60% to 70% of JLP 119 cells are killed within 4 hours.16 Under these conditions, the presence of 75 μmol/L H2O2 completely inhibited induction of apoptosis (Figure 3). However, these cells will ultimately die from H2O2 toxicity over the course of an overnight incubation, and most of this delayed cell death is pyknotic/necrotic (eg, as seen in Figure 2). Identical results have been obtained with 2 additional Burkitt lymphoma cell lines, ST-486 and BL-41 (see following text).
Rapid inhibition of VP-16–induced apoptosis by H2O2.
JLP 119 cells were treated for 4 hours with 8.5 μmol/L VP-16 in the presence or absence of 75 μmol/L H2O2, added 30 minutes after the VP-16. Cell death was assayed by fluorescence microscopy, as described in “Materials and methods.”
Rapid inhibition of VP-16–induced apoptosis by H2O2.
JLP 119 cells were treated for 4 hours with 8.5 μmol/L VP-16 in the presence or absence of 75 μmol/L H2O2, added 30 minutes after the VP-16. Cell death was assayed by fluorescence microscopy, as described in “Materials and methods.”
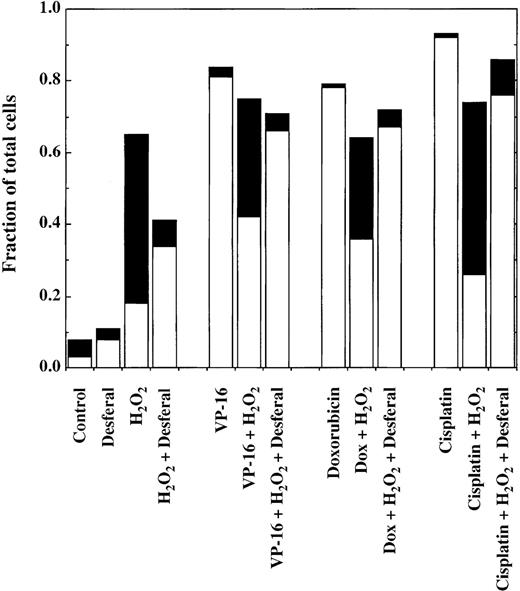
A series of agents known to counteract the actions of reactive oxygen species were tested for their ability to prevent H2O2 from inhibiting chemotherapy-induced apoptosis. Three compounds were found to restore the induction of apoptosis by antineoplastic drugs: the iron chelator Desferal (desferrioxamine), the nitroxide spin trap Tempol, and the nonspecific radical scavenger DMSO. The results of experiments carried out using Desferal with VP-16, doxorubicin, and cisplatin are shown in Figure4. Cells were pretreated for 1 hour with Desferal to provide time for uptake of the compound into the cells. At concentrations of 5 or 10 μmol/L, the chelator lowered the overall level of cell killing induced by 50 μmol/L H2O2alone. The cell death that remained was primarily apoptotic instead of pyknotic/necrotic. When administered together with chemotherapy drugs, Desferal inhibited the antiapoptotic effects of H2O2, and the cells died by apoptosis instead of pyknosis/necrosis. Similar results were observed in experiments using Tempol and 75 μmol/L H2O2 (Figure5) and DMSO (data not shown). Like Desferal, Tempol also reduced the overall level of cell killing induced by 50 μmol/L H2O2 alone from 70% to 30%, converting the mode of cell death from pyknosis/necrosis to apoptosis. Neither Desferal nor Tempol had a significant effect on the level or type of cell death induced by the chemotherapy drugs alone (data not shown).
Effect of Desferal on cell killing induced by chemotherapy drugs in the presence and absence of H2O2.
After a 1-hour preincubation with Desferal (10 μmol/L), cells were treated for 22 hours with 1.7 μmol/L VP-16, 0.2 μmol/L doxorubicin, or 50 μmol/L cisplatin in the presence or absence of 50 μmol/L H2O2 (added 30 minutes after the chemotherapy drugs) and analyzed as described in the legend to Figure 2. Pyknotic/necrotic cells are indicated by ▪, and apoptotic cells are indicated by □.
Effect of Desferal on cell killing induced by chemotherapy drugs in the presence and absence of H2O2.
After a 1-hour preincubation with Desferal (10 μmol/L), cells were treated for 22 hours with 1.7 μmol/L VP-16, 0.2 μmol/L doxorubicin, or 50 μmol/L cisplatin in the presence or absence of 50 μmol/L H2O2 (added 30 minutes after the chemotherapy drugs) and analyzed as described in the legend to Figure 2. Pyknotic/necrotic cells are indicated by ▪, and apoptotic cells are indicated by □.
Effect of Tempol on cell killing induced by chemotherapy drugs in the presence and absence of H2O2.
Cells were treated for 18 hours with 1.7 μmol/L VP-16 or 50 μmol/L cisplatin in the presence or absence of 75 μmol/L H2O2 and 0.5 mmol/L Tempol and analyzed as described in the legend to Figure 2. The order of addition of reagents was Tempol first, followed by chemotherapy drug, followed by H2O2 (30-minute delay). Pyknotic/necrotic cells are indicated by ▪, and apoptopic cells are indicated by □.
Effect of Tempol on cell killing induced by chemotherapy drugs in the presence and absence of H2O2.
Cells were treated for 18 hours with 1.7 μmol/L VP-16 or 50 μmol/L cisplatin in the presence or absence of 75 μmol/L H2O2 and 0.5 mmol/L Tempol and analyzed as described in the legend to Figure 2. The order of addition of reagents was Tempol first, followed by chemotherapy drug, followed by H2O2 (30-minute delay). Pyknotic/necrotic cells are indicated by ▪, and apoptopic cells are indicated by □.
Four other antioxidant compounds that were tested failed to inhibit the effects of H2O2 on the induction of apoptosis by VP-16 (0.85-1.7 μmol/L) or doxorubicin (0.2 μmol/L) during overnight incubations. These were the divalent metal cation chelator 1,10-o-phenanthroline (1-4 μmol/L), which may have failed to inhibit the effects of H2O2 because of its inherent cytotoxicity to the cells at concentrations greater than 2 μmol/L; vitamin C (10-500 μmol/L), which was toxic at concentrations of 100 μmol/L or greater; ebselen (10 μmol/L, an inhibitor of lipid peroxidation), which was also toxic to the cells; and trolox (a vitamin E analog), tested at 10 to 500 μmol/L.
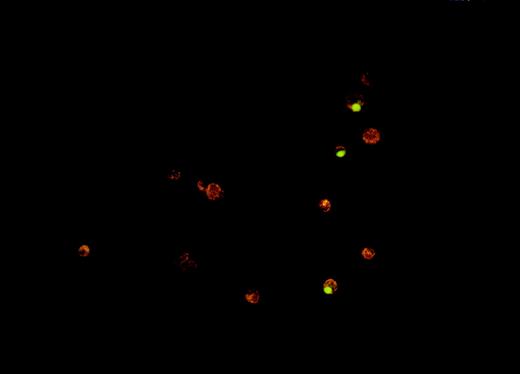
One of the main physiologic advantages of apoptotic death compared with necrotic death derives from how the cells are cleared from tissues. It is believed that apoptotic cells are taken up and removed from tissues by phagocytosis, either by professional phagocytes (eg, tissue macrophages) or by other neighboring cells.18 We sought to determine whether oxidative stress interferes with phagocytosis of dying tumor cells, given that it inhibits the induction of apoptosis. A fluorescence microscopy–based in vitro assay was developed for measuring phagocytosis of apoptotic Burkitt lymphoma cells by human monocyte-derived macrophages. A photograph of a representative result from this assay is shown in Figure 6.
Fluorescence microscopy assay for phagocytosis of Burkitt lymphoma cells by human monocyte-derived macrophages.
The phagocytosis assay was performed as described in Materials and methods. The photograph shows 3 macrophages (red-orange cells), which have engulfed VP-16–treated ST-486 (green) cells.
Fluorescence microscopy assay for phagocytosis of Burkitt lymphoma cells by human monocyte-derived macrophages.
The phagocytosis assay was performed as described in Materials and methods. The photograph shows 3 macrophages (red-orange cells), which have engulfed VP-16–treated ST-486 (green) cells.
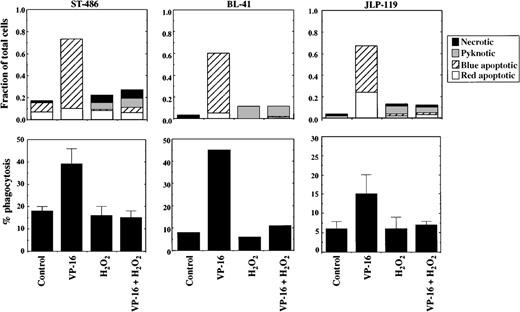
Phagocytosis of cells treated with VP-16 in the presence or absence of H2O2 was tested using 3 different Burkitt lymphoma cell lines: JLP 119, ST-486, and BL-41. Cells were treated with VP-16 at concentrations that induce morphologic apoptosis within 4 hours of treatment. H2O2 was used at 75 μmol/L, which is sufficient to convert most of the VP-16–induced phagocytosis to pyknosis/necrosis. As shown in Figure7, all 3 cell lines underwent significantly increased phagocytosis after VP-16–induced apoptosis as compared with control cells. Note that the apoptotic cells underwent phagocytosis even though the membranes were still intact (impermeable to PI). H2O2inhibited apoptosis and subsequent phagocytosis of all 3 cell lines. The inhibition of phagocytosis was not due to a direct effect of H2O2 on the macrophages because there is no residual H2O2 present at the time when the lymphoma cells are added to the macrophages; the H2O2 is removed by cellular peroxidases and other consumption mechanisms within 2 hours after addition to the tumor cells.17
Effect of H2O2 on chemotherapy-induced apoptosis and phagocytosis of Burkitt lymphoma cells.
The Burkitt lymphoma cell lines JLP 119, BL-41, and ST-486 were treated for 4 hours with VP-16 (8.5, 340, or 85 μmol/L, respectively) in the presence or absence of 75 μmol/L H2O2 and then subjected to phagocytosis by human monocyte-derived macrophages, as described in “Materials and methods.” The fractions of cells dying by apoptosis, pyknosis/necrosis, or typical necrosis are shown in the top row of graphs. Percent phagocytosis for these cell populations is shown in the lower row of graphs. The results represent the mean ± range or SD for 2 to 3 separate experiments for JLP 119 and ST-486 cells. The data for BL-41 represent 1 experiment.
Effect of H2O2 on chemotherapy-induced apoptosis and phagocytosis of Burkitt lymphoma cells.
The Burkitt lymphoma cell lines JLP 119, BL-41, and ST-486 were treated for 4 hours with VP-16 (8.5, 340, or 85 μmol/L, respectively) in the presence or absence of 75 μmol/L H2O2 and then subjected to phagocytosis by human monocyte-derived macrophages, as described in “Materials and methods.” The fractions of cells dying by apoptosis, pyknosis/necrosis, or typical necrosis are shown in the top row of graphs. Percent phagocytosis for these cell populations is shown in the lower row of graphs. The results represent the mean ± range or SD for 2 to 3 separate experiments for JLP 119 and ST-486 cells. The data for BL-41 represent 1 experiment.
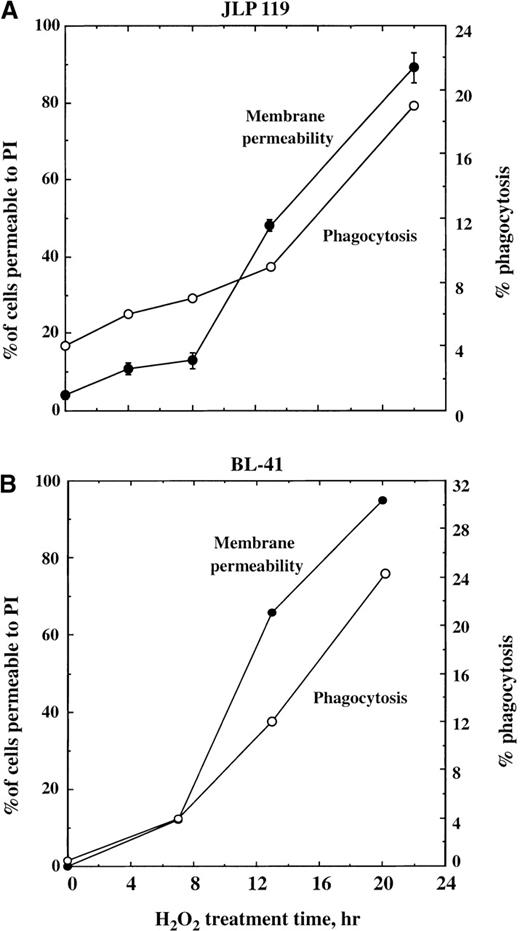
Cells killed with H2O2 (with or without VP-16) do ultimately undergo phagocytosis, but this occurs only after they have lost their permeability barrier. As shown in Figure8A, JLP 119 cells treated with 75 μmol/L H2O2 remained mostly impermeable to PI during the first 8 hours of incubation. After this time, PI permeability increased sharply, rising at a steady rate thereafter. However, a shift in the rate of phagocytosis does not occur until 13 hours after adding H2O2. A similar result was obtained when BL-41 cells were treated with H2O2; increased phagocytosis followed the loss of membrane integrity (Figure 8B).
Time course of lymphoma cell permeabilization and phagocytosis following H2O2 treatment.
JLP 119 cells (A) or BL-41 cells (B) were treated with 75 or 250 μmol/L H2O2, respectively, and allowed to incubate for the times indicated in the figure. Cells were then harvested and incubated with monocyte-derived macrophages for 1 hour. Percent phagocytosis (open circles) was analyzed as described in “Materials and methods.” Cell death was quantified by Hoechst/PI staining. The total percentage of cells that had become permeable to PI is shown (closed circles). Under these conditions, at least 82% of the PI-permeable cells at each time point were pyknotic/necrotic. The results for JLP 119 are averaged from 2 separate experiments, and those for BL-41 are from a single experiment.
Time course of lymphoma cell permeabilization and phagocytosis following H2O2 treatment.
JLP 119 cells (A) or BL-41 cells (B) were treated with 75 or 250 μmol/L H2O2, respectively, and allowed to incubate for the times indicated in the figure. Cells were then harvested and incubated with monocyte-derived macrophages for 1 hour. Percent phagocytosis (open circles) was analyzed as described in “Materials and methods.” Cell death was quantified by Hoechst/PI staining. The total percentage of cells that had become permeable to PI is shown (closed circles). Under these conditions, at least 82% of the PI-permeable cells at each time point were pyknotic/necrotic. The results for JLP 119 are averaged from 2 separate experiments, and those for BL-41 are from a single experiment.
Discussion
These results demonstrate that H2O2interferes with the ability of antineoplastic drugs to kill tumor cells. This oxidant shifts the form of cell death from apoptosis to pyknosis/necrosis, which occurs after a significant delay compared with chemotherapy-induced apoptosis. H2O2 also lowers the degree of cell killing by antineoplastic agents. This finding provides a mechanism whereby cancer cells may escape killing from chemotherapy agents. Tumors are often infiltrated with inflammatory phagocytes, which can generate large levels of reactive oxygen species within the tumor tissue. Tumor cells themselves have also been found to generate oxidants,19 although the Burkitt lymphoma cells used in these studies did not generate significant levels of H2O217 (data not shown). Cancer patients are reported to have higher levels of generalized oxidative stress.10-15 Thus, some tumor tissues may contain significant levels of reactive oxygen species. H2O2 is pivotal among the array of oxidants generated in vivo because it is produced by virtually every form of oxidative stress (eg, by activated phagocytes, reperfusion of ischemic tissues, ionizing radiation) and because it is required for the generation of secondary reactive oxygen species (eg, the highly reactive hydroxyl radical and hypochlorous acid). Our findings suggest that if this H2O2 is present at the time of administration of cancer chemotherapy, some neoplastic cells that would otherwise be killed may survive drug treatment.
An important consequence of the effect of H2O2on cell death was the delay of phagocytosis of the tumor cells by human monocyte-derived macrophages. Cells treated with VP-16 undergo the morphologic changes associated with apoptosis before losing the membrane permeability barrier.16 These early apoptotic cells are capable of being recognized and phagocytosed by monocyte-derived macrophages within 4 hours of drug treatment. If the cells are treated with VP-16 in the presence of H2O2, this phagocytosis is lost even though the cells have been lethally damaged; ie, they will go on to die by pyknosis/necrosis over the course of an overnight incubation. Thus, membrane-intact apoptotic cells are recognized and phagocytosed by monocyte-derived macrophages, but membrane-intact pyknotic/necrotic cells are not. The finding that pyknotic/necrotic cells become phagocytosed after they have lost their membrane permeability barrier may derive from macrophage recognition of intracellular or inner membrane markers, which are exposed only upon cell lysis. To our knowledge, the extent and time course of phagocytosis of nonapoptotic cells have not been documented previously. Our results show that pyknotic/necrotic cells are taken up only after they have undergone drastic changes, unlike apoptotic cells, which are recognized early in the death process. One potential physiologic ramification of this finding is that cells killed in vivo in the presence of H2O2 (with or without chemotherapy drugs) are not phagocytosed until after they have begun to leak their contents into the extracellular space, thus allowing an inflammatory response to ensue.18 This may induce a cycle of chronic inflammation and further interference with chemotherapy action.
If oxidants have the same effect on drug-induced apoptosis in vivo as they do in vitro, then the overall effectiveness of chemotherapy might be improved by coadministering antioxidants with the chemotherapeutic agents. Support for this concept first came from a report by Chinery et al,20 who found that administration of vitamin E and pyrrolidinedithiocarbamate (PDTC) together with the chemotherapy agents 5-fluorouracil and doxorubicin enhanced the tumor-reduction capacity of the drugs. In this model for colon cancer treatment, the proposed mechanism of action for the antioxidants was significantly different from that shown here because vitamin E and PDTC alone also induced apoptosis in the colon tumor cells when tested in vitro. Under our experimental conditions, the antioxidants that were effective in preventing H2O2 from inhibiting apoptosis—Desferal, Tempol, and DMSO—did not by themselves induce any cell death. In addition, we did not find inhibition of the effects of H2O2 by 2 lipid-active compounds, trolox (a vitamin E analog) and ebselen (which inhibits lipid peroxidation reactions). Vitamin C was similarly ineffective, possibly because of its inherent toxicity to these cells. Although Desferal and Tempol have very different activity profiles in biologic systems, they share the ability to prevent redox reactions of iron in the presence of H2O2; Desferal is a potent iron chelator21 and Tempol has the ability to keep iron in an oxidized state, thus preventing catalysis of the Haber-Weiss reaction with H2O2.22 Our finding that both Desferal and Tempol prevented the effects of H2O2 implies that free radicals mediate the effects of H2O2. These results differ from but are complimentary to those of Chinery et al,20 suggesting that there may be different mechanisms whereby antioxidants can improve the efficacy of chemotherapy. Additional animal-based studies to verify the applicability of these findings in vivo are merited.
In the studies presented here, 4 different drugs were examined. With all drugs, the mode of cell death was diverted away from apoptosis to pyknosis/necrosis in the presence of 50 to 75 μmol/L H2O2. The drugs were VP-16, doxorubicin, cisplatin, and AraC, which represent 4 different classes of chemotherapeutic agents: a topoisomerase II inhibitor (VP-1623), an anthracycline (doxorubicin24), a bulky adduct (cisplatin25), and a metabolic inhibitor (AraC26). Thus, H2O2 acts independently of the mechanism of action of the drug; ie, it acts by preventing downstream events initiated by the drugs and does not block a specific action of any of the drugs. This is consistent with our previous finding16 that H2O2 acts by depleting the cells of adenosine triphosphate (ATP), which is required for apoptosis to proceed.27,28 In the absence of ATP, numerous markers of VP-16–induced apoptosis are blocked, including activation of caspases, oligonucleosomal DNA fragmentation, and exposure of phosphatidylserine on the cell surface.16Other researchers have suggested that ATP must be maintained above a critical threshold level of approximately 25% to 50% of control steady-state levels for apoptosis to occur.29,30 Of note, we have found that both Tempol and Desferal inhibit the H2O2 (75 μmol/L)-induced depletion of intracellular ATP, such that ATP levels are maintained at 60% or more of control levels in the presence of these compounds (data not shown). Desferal and Tempol act by inhibiting the induction of DNA single-strand breaks by H2O2,31,32thus preventing activation of poly(ADP)-ribosylation and the subsequent depletion of ATP.33 The protection of ATP levels can explain why Tempol and Desferal overcome the antiapoptotic effects of H2O2 and may also clarify why the residual cell death induced by H2O2 alone in the presence of these compounds is primarily apoptotic; that is, H2O2 by itself has the capacity to induce apoptosis in Burkitt lymphoma cells, but this apoptosis is prevented because of the irreversible drop in ATP induced by concentrations of H2O2 greater than 50 μmol/L.16 By reducing the drop in ATP levels, Desferal and Tempol allow apoptosis to occur.
Acknowledgments
We are grateful to Valerie Calvert (Food and Drug Administration/Center for Biologics Evaluation and Research) for supplying human monocytes. We also thank Boon Chock and Giovanna Tosato for careful reading of the manuscript and for many useful suggestions.
Supported by a grant from The Food and Drug Administration Office of Women's Health.
Reprints:Emily Shacter, Bldg 29A, Rm 2A-11, 29 Lincoln Dr, Bethesda, MD 20892; e-mail: shacter@cber.fda.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal