Abstract
The proteins encoded by RAG1 and RAG2 can initiate gene recombination by site-specific cleavage of DNA in immunoglobulin and T-cell receptor (TCR) loci. We identified a new homozygous RAG1 gene mutation (631delT) that leads to a premature stop codon in the 5′ part of the RAG1 gene. The patient carrying this 631delT RAG1 gene mutation died at the age of 5 weeks from an Omenn syndrome-like T+/B−severe combined immunodeficiency disease. The high number of blood T-lymphocytes (55 × 106/mL) showed an almost polyclonal TCR gene rearrangement repertoire not of maternal origin. In contrast, B-lymphocytes and immunoglobulin gene rearrangements were hardly detectable. We showed that the 631delT RAG1 gene can give rise to an N-terminal truncated RAG1 protein, using an internal AUG codon as the translation start site. Consistent with the V(D)J recombination in T cells, this N-terminal truncated RAG1 protein was active in a plasmid V(D)J recombination assay. Apparently, the N-terminal truncated RAG1 protein can recombine TCR genes but not immunoglobulin genes. We conclude that the N-terminus of the RAG1 protein is specifically involved in immunoglobulin gene rearrangements.
Severe combined immunodeficiency disease (SCID) is clinically characterized by failure to thrive and by opportunistic infections, usually starting within the second month of life.1 SCID consists of a heterogeneous group of diseases, including an X-linked form2 and multiple autosomal recessive forms.3 It can be immunologically classified by the absence or presence of T, B, and natural killer (NK) cells, a phenomenon that is associated with different disease categories defined on molecular bases. For instance the non-B, non-T form of SCID is frequently caused by mutations in the recombination activating genes (RAG1 or RAG2).4 During recombination of an immunoglobulin or a T-cell receptor (TCR) gene, a combination of the available variable (V), diversity (D), and joining (J) gene segments is made, resulting in a V-D-J exon. The RAG1–RAG2 protein complex first cleaves the DNA at specific sites, called recombination signal sequences (RSS), which are characterized by a heptamer–nonamer sequence separated by a spacer of 12 or 23 base pairs (bp). The cleaved DNA is finally linked together by factors involved in double-strand break repair, such as Ku70, Ku80, and DNA-PKcs.5-7
The absence of functionally rearranged immunoglobulin and TCR genes blocks the B- and T-cell differentiation in an early stage, resulting in the absence of mature B- and T-lymphocytes in the peripheral blood (PB).8,9 However, some patients with SCID have T-lymphocytes because of intrauterine transfer from mother to child.10,11 These maternal T-lymphocytes can hamper the diagnosis of non-B, non-T SCID. Careful proof or exclusion of the maternal origin of T-lymphocytes is needed for correct diagnosis because, in another form of SCID known as the Omenn syndrome (OS), patients have oligoclonal (nonmaternal), activated T cells in their PB and very low numbers of B cells accompanied by hypogammaglobulinemia and high levels of IgE.1 T cells from patients with OS have a T-helper (Th)2-phenotype, which can account for the high IgE levels.12,13 Generally, OS is characterized by failure to thrive, erythrodermia, eosinophilia, hepatosplenomegaly, lymphadenopathy, and graft–versus–host (GVH)-like disease.1 Recently, Villa et al14 described OS patients with oligoclonal T cells who had mutations in theirRAG genes, implying that these mutations do not necessarily completely abolish the function of the RAG proteins. However, it remained unclear why the B-cell lineage seems to be more affected than the T-cell lineage by these partially functional RAG proteins.15
The essential parts of the murine RAG1 and RAG2 genes have been characterized by studies in cell lines on the residual function of deletion constructs.16-22 The RAG1 core domain consists of amino acid (aa) 384 to aa 1008 of the 1040 aa long murine RAG1 protein. Most deletions in this core domain abolish recombination activity. The N-terminal part of the RAG1 protein is not essential, though its presence may enhance the recombination activity.20,21 The portion of the N-terminus responsible for this enhancement is localized in a small region between aa 216 and 238 (basic aa motif BIIa).20 Although the murine and human RAG1 proteins have an overall 90% amino acid sequence identity,23 the N-terminal first 350 aa have a homology of only 77%, whereas the core domain has a high homology of 95%.
Here we describe a patient with OS-like T+/B− SCID with a homozygous T nucleotide deletion in the RAG1 gene (631delT). We showed that the 631delTRAG1 gene can give rise to an N-terminal truncated RAG1 protein that was active in a plasmid V(D)J recombination assay. The patient had large numbers of (nonmaternal) T cells in her PB, but no B cells could be detected. The N-terminal truncated RAG1 protein was apparently able to direct TCR but not immunoglobulin gene rearrangements.
Patient, materials, and methods
The patient was the first-born girl from consanguineous, healthy Moroccan parents. Six hours after birth she was admitted to a local hospital with an erythematous skin rash and tachypnea of 60 to 80 breaths per minute (O2 saturation rate, 92%). First laboratory results showed leukocytosis of 32.4 × 109/L with the following differential blood count: 11% eosinophils, 20% metamyelocytes, 5% band forms, 12% polymorphonuclear leukocytes, 51% lymphocytes, and 1% monocytes. Neonatal sepsis was considered and antibiotic treatment was started, but no improvement was observed. Gradually, hepatosplenomegaly and generalized lymphadenopathy developed. On the ninth day of life, the patient experienced a generalized convulsion. After this event she was admitted to the neonatal intensive care unit of the university hospital for further diagnosis and treatment.
The differential diagnosis included metabolic disorder, sepsis or toxic shock–like syndrome, histiocytosis, neonatal leukemia, autoimmune disease, or GVHD. Laboratory results showed that the leukocytosis had increased to 68.5 × 109/L. The differential blood count showed 1% eosinophils, 1% metamyelocytes, 1% band forms, 8% polymorphonuclear leukocytes, 87% lymphocytes, and 2% monocytes. Serum immunoglobulin levels were as follows: IgG, 2.73 g/L; IgA, < 0.10 g/L; and IgM, 0.11 g/L. IgE levels were not determined.
Immunophenotyping with triple labeling of membrane and intracellular markers was performed as described before24 25 and showed complete absence of B lymphocytes in the PB (less than 0.01% lymphocytes). Sensitive B-cell detection could be achieved by a double lymphocyte and exclusion gate, using CD3, CD14, CD15, CD16, and CD56 to exclude T cells, NK cells, monocytes, and granulocytes from the lymphogate (Figure 1). In the bone marrow (BM), virtually no precursor B cells could be detected (< 1% CD34+, < 0.5% CD117+, < 0.5% TdT+, 3% CD10+, < 1% CD19+). On the other hand, 66% of PB leukocytes consisted of CD3+T lymphocytes with the following immunophenotypes—35% CD4+, 59% CD8+, 89% TCRαβ+, 4% TCRγδ+, and 66% CD45RO+—thereby showing substantial immunophenotypic heterogeneity within the expanded T-cell population. The origin of PB T lymphocytes was determined by human leukocyte antigen typing, which showed that the T lymphocytes were not of maternal origin.
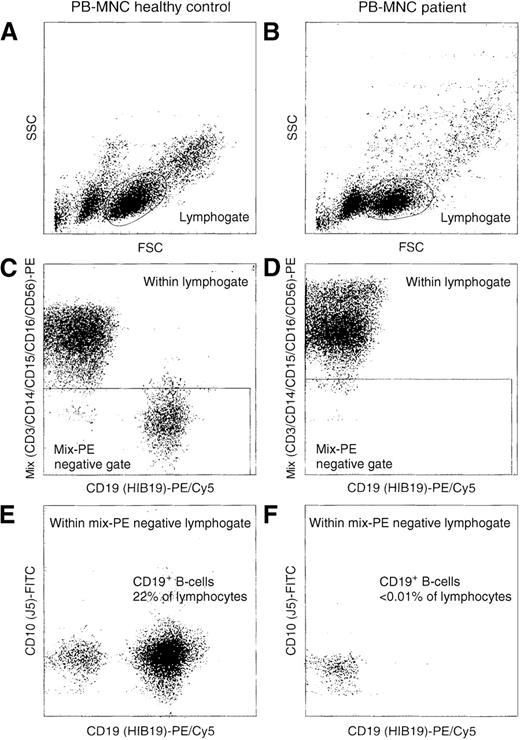
Sensitive flow cytometric analysis of PBMC of a healthy control and the patient with the 631delT RAG1 gene.
Based on scatter characteristics, gating was performed on lymphocytes (A and B). To further reduce background staining in our attempts to detect rare events (CD19+ B cells), we used a so-called exclusion gate with negativity for labeling with the PE-conjugated CD3, CD14, CD15, CD16, and CD56 antibodies (C and D). This exclusion of T-lymphocytes, monocytes, granulocytes, and NK cells resulted in the sensitive detection of CD19+ B-lymphocytes. The patient had less than 0.01% CD19+ B-lymphocytes (F); the healthy control had 22% (E).
Sensitive flow cytometric analysis of PBMC of a healthy control and the patient with the 631delT RAG1 gene.
Based on scatter characteristics, gating was performed on lymphocytes (A and B). To further reduce background staining in our attempts to detect rare events (CD19+ B cells), we used a so-called exclusion gate with negativity for labeling with the PE-conjugated CD3, CD14, CD15, CD16, and CD56 antibodies (C and D). This exclusion of T-lymphocytes, monocytes, granulocytes, and NK cells resulted in the sensitive detection of CD19+ B-lymphocytes. The patient had less than 0.01% CD19+ B-lymphocytes (F); the healthy control had 22% (E).
Despite all supportive care the child gradually deteriorated. Respiratory insufficiency required artificial ventilation. Because of an interstitial pulmonary inflammatory reaction, high ventilation pressures were needed to obtain sufficient PO2and SaO2 values. Treatment with methylprednisolone and cyclosporin, on the tentative diagnosis of autoimmune disease or GVHD, could not reverse the inflammatory reaction. She died at the age of 32 days because of severe hypoxemia despite maximal respiratory support. The clinical picture, together with the immunophenotyping results, suggested an OS-like form of SCID.14
Omenn syndrome is characterized by a dominance of Th2 cells, which in turn are characterized by high levels of interleukin (IL)-4 and IL-5, compared with Th1 cells, which produce interferon (IFN)-γ. IL-4, IL-5, and IFN-γ levels were not detectable in the plasma or the supernatant of unstimulated PB mononuclear cells (PBMC). PBMC stimulated with Ca-ionophore and phorbol myristate acetate produced 4430 ng/mL IFN-γ, which was comparable to the amount of IFN-γ produced by polyclonally stimulated PBMC isolated from neonatal cord blood of control patients (range, 235-9900 ng/mL).26,27 In addition, the IL-4 levels were normal (patient, 32 pg/mL; range, 1-72 pg/mL in neonatal cord blood), whereas the IL-5 levels were raised slightly (patient, 145 pg/mL; range, 2-82 pg/mL in neonatal cord blood).26 27 Thus, we did not find a typical Th2 profile in this patient with OS-like T+/B− SCID.
DNA and RNA extraction and reverse transcriptase reaction
Granulocytes, mononuclear cells, or both were isolated from PB or BM by Ficoll–Paque (density, 1.077 g/mL; Pharmacia, Uppsala, Sweden) density centrifugation. DNA was extracted from PBMC, PB granulocytes, and BM mononuclear cells using the QIAamp Blood kit (Qiagen, Chatsworth, CA).28 Total RNA was isolated from PBMC according to the method of Chomczynski29 using RNAzol B (Tel-Test, Friendswood, TX). cDNA was prepared from mRNA, as described before, using oligo(dT) and AMV reverse transcriptase.30
PCR amplification and analysis of immunoglobulin and TCR gene rearrangements
Polymerase chain reaction (PCR) was performed as described previously.31 In each 100 μL PCR reaction, 0.1 to 1 μg DNA sample, 12.5 pmol of 5′ and 3′ oligonucleotides, and 1 U AmpliTaq gold polymerase (PE Biosystems, Foster City, CA) were used. The TCRB reverse transcription (RT)-PCR amplification used multiple Vβ family primers in combination with a single Cβ primer, as was described before.32 Most oligonucleotides for amplification of the IGH, IGK, IGL, TCRB, TCRG, and TCRD genes were published before.33-35 (RT)-PCR conditions were 2 to 10 minutes at 94°C, followed by 45 seconds at 92°C, 90 seconds at 57 to 65°C, 2 minutes at 72°C for 40 cycles, and a final extension step for 7 minutes at 72°C. Heteroduplex analysis of PCR products was used to analyze the monoclonal, oligoclonal, or polyclonal nature of the amplified rearrangements, as described before.32 The PCR products were cloned in pGEM-T easy vector (Promega, Madison, WI) and subsequently sequenced. The minimal numbers of nucleotides used for the identification of a D gene segment was 3 for Dδ1 and Dδ2, 4 for Dδ3 and Dβ1, and 5 for Dβ2.36,37 Immunoglobulin gene rearrangements were identified using IMGT, the international ImMunoGeneTics database http://imgt.cnusc.fr:8104 (initiator and coordinator: Marie–Paule Lefranc, Montpellier, France,lefranc@ligm.igh.cnrs.fr).38
Long-range PCR for amplification of RAG genes
The entire RAG1 or RAG2 gene was amplified in 1 long-range (LR)-PCR reaction (100 μL). When the LR-PCR product was generated for sequencing, 4 U rTth DNA polymerase XL (PE Biosystems) was used. In cloning the LR-PCR product, 5.25 U Expand enzyme mix (Boehringer Mannheim, Mannheim, Germany) was used, and 30 pmol of 5′ and 3′ oligonucleotides was used. (Note: The sequences of the oligonucleotides used for the LR-PCR of RAG1and RAG2 will be made available on request.) LR-PCR conditions were 2 minutes at 94°C, followed by 15 seconds at 94°C, 30 seconds at 60°C, and 3 minutes at 68°C for 25 cycles using a 15-second auto-extension from cycle 11 onward. After the last cycle, an additional step of 10 minutes at 72°C was performed for the final extension.
Fluorescent sequencing reaction and analysis
LR-PCR products of RAG1 and RAG2 were purified using QIAquick PCR purification kit (Qiagen). Then 5 to 9 μL purified PCR product was sequenced with 5 μL rhodamine dye terminator mix (PE Biosystems) using 3.3 pmol internal sequencing primers. All sequencing was performed as described before33 and run on an ABI Prism 377 fluorescent sequencer (PE Biosystems).
Cloning of the mutated and wild-type RAG1 genes
LR-PCR products of the RAG1 gene were cloned into the pGEM-T easy vector. The DNA fragment containing the entire RAG1 open reading frame was isolated after digestion with MluI and partial cleavage with XhoI, and cloned into theXhoI–MluI cut vector pMSE1 (a pCDM8-based vector22). Protein expression will result in a C-terminal fusion of a myc epitope tag to the RAG1 protein, which can be used for easy detection. The cloned wt and 631delT RAG1 genes were sequenced to exclude the presence of any additional mutations.
In vitro transcription and translation
pGEM-T easy construct (0.5 μg) was added in a 25 μL reaction volume of TNT Coupled Reticulocyte Lysate System (Promega), using 0.5 μL of T7 RNA polymerase and 1 μL of 35S-labeled methionine. In vitro transcription and translation took place at 30°C for 90 minutes. Protein products were separated on a 7.5% polyacrylamide gel and visualized by autoradiography.
Western blotting
COS cells (5 × 105; 40% confluent) were transfected with 2 μg expression construct for 631delT or wtRAG1 using SuperFect Transfection Reagent (Qiagen) and cultured for 2 days at 37°C. Proteins were separated on a 6% polyacrylamide gel and blotted onto a nylon membrane (Schleicher & Schuell, Dassel, Germany). The RAG1 protein was detected by the anti-c-myc 9E10 murine monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized by enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
V(D)J recombination assay
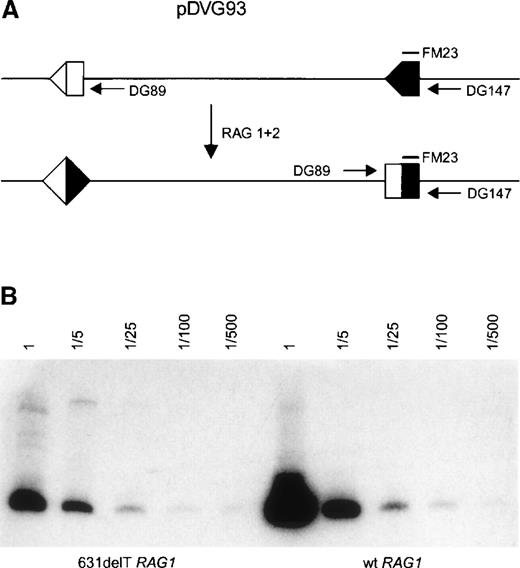
Two micrograms of pMSE1-RAG1 (631delT or wt), together with 2 μg pMSE1-RAG2 wt and 1 μg of the recombination substrate (pDVG93) containing 2 RSS elements, was transfected into Chinese hamster ovary cells using SuperFect Transfection Reagent (Qiagen). Transfected cells were cultured for 2 days at 37°C and 5% CO2 before they were harvested. On V(D)J recombination, the sequence between the RSS elements was inverted, which could be detected by PCR (Figure 5A). The level of recombination activity of the 631delT RAG1 was compared to the wt RAG1. DNA recovered from these transfection experiments was diluted, as indicated, and used as a template for PCR. The PCR products were detected by blotting onto a nylon membrane (Schleicher & Schuell) and hybridization with the32P-labeled oligonucleotide FM23, and they were visualized by phosphor imaging.
Results
Mutation detection in the RAG1 gene
Based on the clinical presentation and immunodiagnostic results, this patient was classified as having SCID. Because it is known that both T−/B− SCID and OS with oligoclonal T cells can be caused by mutations in the RAGgenes,4,14 we analyzed the RAG1 and RAG2genes. Fluorescent sequencing of LR-PCR products of RAG1and RAG2 revealed a homozygous deletion of 1 T-nucleotide inRAG1 at position 631 (631delT) (numbering according to Schatz et al23; Genbank accession number M29474). This point mutation leads to a frameshift at codon 173, giving rise to a polypeptide of 199 aa. The consanguineous parents of the patient were both heterozygous for this mutation. We did not detect any mutations in the RAG2 gene of the patient.
Analysis of T-cell receptor gene rearrangements
The maternal origin of the PB T lymphocytes was excluded by human leukocyte antigen typing. Furthermore, after Ficoll–Paque density centrifugation, the PB granulocyte fraction was immunophenotyped and appeared to consist of more than 80% T cells, probably because of the unusually high T-cell counts. DNA isolated from these cells was used for mutation detection and revealed a homozygousRAG1 mutation, whereas the mother of the child was heterozygous for this mutation, again excluding the maternal origin of the T cells.
Because of the extremely elevated PB T-lymphocyte counts (55.5 × 106/mL), T-cell leukemia was suspected. We therefore investigated the clonality of the PB T-lymphocytes by PCR amplification and then conducted heteroduplex analysis of TCRB, TCRG, andTCRD gene rearrangements. This technique depends on the denaturation and renaturation of PCR products, which results in the formation of homoduplexes and heteroduplexes. Single homoduplex bands indicate monoclonality, whereas heteroduplex “smears” or “staircase” patterns indicate polyclonality or oligoclonality, respectively.32 Figure 2 shows the result of the heteroduplex analysis, indicating full usage of the TCR repertoire with some oligoclonal patterns. Because the leukocyte count in the PB was extremely elevated, with 81% CD3+ T lymphocytes, we assumed that the oligoclonal pattern was largely caused by the expansion of several T-lymphocyte clones in an otherwise polyclonal background. This assumption was supported by flow cytometric analysis of TCRVβ protein expression using a panel of 22 different Vβ antibodies39 that showed elevated percentages of Vβ1 (within CD8+ T lymphocytes) and Vβ14 and Vβ5.1 (within CD4+ T lymphocytes). Percentages of Vβ3, Vβ5.2/5.3, Vβ7, Vβ8.1/8.2, Vβ9.1, Vβ13.1/13.3, Vβ13.6, Vβ16, Vβ17, Vβ22, and Vβ23 levels were decreased (data not shown) in comparison with those in healthy neonates and children.46
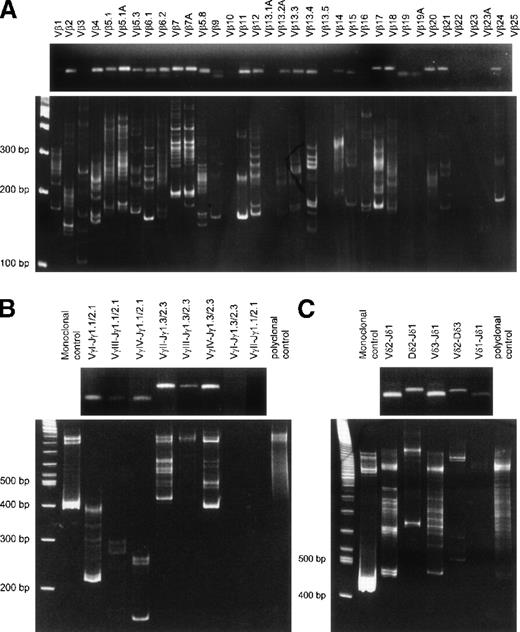
Agarose gel and heteroduplex PCR analysis ofTCR gene rearrangements.
TCRB gene rearrangements (RT-PCR with Vβ-Cβ primer) (A), TCRG gene rearrangements (B), and TCRD gene rearrangements (C) showing oligoclonal rearrangement patterns in an otherwise polyclonal background. The upper part of each panel shows the presence of PCR products in agarose gels for virtually each primer combination, whereas the lower part of each panel shows heteroduplex analysis of the obtained PCR products. The oligoclonal patterns are probably related to the high T-cell counts with expansion of several T-lymphocyte clones.
Agarose gel and heteroduplex PCR analysis ofTCR gene rearrangements.
TCRB gene rearrangements (RT-PCR with Vβ-Cβ primer) (A), TCRG gene rearrangements (B), and TCRD gene rearrangements (C) showing oligoclonal rearrangement patterns in an otherwise polyclonal background. The upper part of each panel shows the presence of PCR products in agarose gels for virtually each primer combination, whereas the lower part of each panel shows heteroduplex analysis of the obtained PCR products. The oligoclonal patterns are probably related to the high T-cell counts with expansion of several T-lymphocyte clones.
To evaluate the deletion and insertion of nucleotides and the usage of D gene segments, cloned TCRB and TCRD gene rearrangements were sequenced (Table 1). The sequenced rearrangements consisted of V, D, and J gene segments. In addition, the insertion and deletion of nucleotides had taken place in most rearrangements.
Analysis of TCRB, TCRD, IGH, and IGLgene rearrangements
| Analyzed Gene . | V Gene Segment . | Del . | Junctional Region . | Del . | D Gene Segment . | Del . | Junctional Region . | Del . | D Gene Segment . | Del . | Junctional Region . | Del . | J Gene Segment . | Frame . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCRB | Vβ13.4 | ? | TATCTTG | −3 | Dβ1 | −4 | CC | — | — | — | — | −2 | Jβ2.7 | + |
| Vβ17 (S1A1T) | −3 | CGGTG | −4 | Dβ1 | −4 | ACGGG | — | — | — | — | −6 | Jβ1.1 | + | |
| Vβ17 (S1A1T) | −3 | CCT | — | — | — | — | — | — | — | — | −3 | Jβ2.1 | + | |
| Vβ12 (S1A1N2) | 0 | G | 0 | Dβ1 | −6 | AAAA | — | — | — | — | −2 | Jβ2.1 | + | |
| TCRD | Vδ2 | −11 | — | −5 | Dδ1 | 0 | GGGGGGGG | −2 | Dδ3 | — | — | — | — | NA |
| Vδ2 | −6 | ATGGGA | — | — | — | — | −3 | Dδ3 | — | — | — | — | NA | |
| — | — | — | — | Dδ2 | −1 | GCGTTAGATC | −4 | Dδ3 | 0 | CGT | 0 | Jδ1 | NA | |
| — | — | — | — | Dδ2* | −14 | GGG† | — | — | — | — | −4 | Jδ1 | NA | |
| — | — | — | — | Dδ2 | −3 | CGAGGAG | −2 | Dδ3 | −5 | CTAT | −4 | Jδ1 | NA | |
| Vδ1 | 0 | AC | — | — | — | — | −5 | Dδ3 | −4 | CT | 0 | Jδ1 | + | |
| Vδ1 | −5 | CCACCCGTACTGG | −2 | Dδ2 | −3 | CCTGGG | −8 | Dδ3 | 0 | CGGGTTTGTC | −7 | Jδ1 | + | |
| Vδ3 | 0 | A | −3 | Dδ2 | −2 | GGT | 0 | Dδ3 | −1 | CGGGCGG | −1 | Jδ1 | + | |
| Vδ3 | −2 | GT | — | — | — | — | 0 | Dδ3 | −5 | — | 0 | Jδ1 | + | |
| Vδ3 | −4 | CG | −3 | Dδ2 | −3 | — | −1 | Dδ3 | −5 | TTATCG | 0 | Jδ1 | + | |
| Vδ2 | 0 | GGAAC | 0 | Dδ2 | −4 | AGC | −3 | Dδ3 | −5 | TTTGG | −4 | Jδ1 | + | |
| Vδ2 | −1 | — | — | — | — | — | −3 | Dδ3 | −6 | — | −2 | Jδ1 | + | |
| Vδ2 | −2 | TG | — | — | — | — | −3 | Dδ3 | −2 | ATGT | −3 | Jδ1 | − | |
| Vδ2 | 0 | GGTTGG | 0 | Dδ1 | −5 | CCCG | −2 | Dδ3 | −6 | — | −2 | Jδ1 | + | |
| IGH | — | — | — | — | — | — | — | — | DH2-15 | −5 | T | −2 | JH4 | NA |
| IGL | Vλ2 | −4 | CCT | — | — | — | — | — | — | — | — | 0 | Jλ3 | + |
| Analyzed Gene . | V Gene Segment . | Del . | Junctional Region . | Del . | D Gene Segment . | Del . | Junctional Region . | Del . | D Gene Segment . | Del . | Junctional Region . | Del . | J Gene Segment . | Frame . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCRB | Vβ13.4 | ? | TATCTTG | −3 | Dβ1 | −4 | CC | — | — | — | — | −2 | Jβ2.7 | + |
| Vβ17 (S1A1T) | −3 | CGGTG | −4 | Dβ1 | −4 | ACGGG | — | — | — | — | −6 | Jβ1.1 | + | |
| Vβ17 (S1A1T) | −3 | CCT | — | — | — | — | — | — | — | — | −3 | Jβ2.1 | + | |
| Vβ12 (S1A1N2) | 0 | G | 0 | Dβ1 | −6 | AAAA | — | — | — | — | −2 | Jβ2.1 | + | |
| TCRD | Vδ2 | −11 | — | −5 | Dδ1 | 0 | GGGGGGGG | −2 | Dδ3 | — | — | — | — | NA |
| Vδ2 | −6 | ATGGGA | — | — | — | — | −3 | Dδ3 | — | — | — | — | NA | |
| — | — | — | — | Dδ2 | −1 | GCGTTAGATC | −4 | Dδ3 | 0 | CGT | 0 | Jδ1 | NA | |
| — | — | — | — | Dδ2* | −14 | GGG† | — | — | — | — | −4 | Jδ1 | NA | |
| — | — | — | — | Dδ2 | −3 | CGAGGAG | −2 | Dδ3 | −5 | CTAT | −4 | Jδ1 | NA | |
| Vδ1 | 0 | AC | — | — | — | — | −5 | Dδ3 | −4 | CT | 0 | Jδ1 | + | |
| Vδ1 | −5 | CCACCCGTACTGG | −2 | Dδ2 | −3 | CCTGGG | −8 | Dδ3 | 0 | CGGGTTTGTC | −7 | Jδ1 | + | |
| Vδ3 | 0 | A | −3 | Dδ2 | −2 | GGT | 0 | Dδ3 | −1 | CGGGCGG | −1 | Jδ1 | + | |
| Vδ3 | −2 | GT | — | — | — | — | 0 | Dδ3 | −5 | — | 0 | Jδ1 | + | |
| Vδ3 | −4 | CG | −3 | Dδ2 | −3 | — | −1 | Dδ3 | −5 | TTATCG | 0 | Jδ1 | + | |
| Vδ2 | 0 | GGAAC | 0 | Dδ2 | −4 | AGC | −3 | Dδ3 | −5 | TTTGG | −4 | Jδ1 | + | |
| Vδ2 | −1 | — | — | — | — | — | −3 | Dδ3 | −6 | — | −2 | Jδ1 | + | |
| Vδ2 | −2 | TG | — | — | — | — | −3 | Dδ3 | −2 | ATGT | −3 | Jδ1 | − | |
| Vδ2 | 0 | GGTTGG | 0 | Dδ1 | −5 | CCCG | −2 | Dδ3 | −6 | — | −2 | Jδ1 | + | |
| IGH | — | — | — | — | — | — | — | — | DH2-15 | −5 | T | −2 | JH4 | NA |
| IGL | Vλ2 | −4 | CCT | — | — | — | — | — | — | — | — | 0 | Jλ3 | + |
Del, deletion of germline gene segment nucleotides; P nucleotides are in boldface; NA, not applicable.
Only 5′ sequence of Dδ2 present.
Four nucleotides are required to identify Dδ3.
Overall, the combinatorial TCR repertoire in this patient was comparable to that of healthy individuals,36 as deduced from the finding that almost all tested V, D, and J gene segments were used (Figure 2). The junctional region repertoire seemed to be somewhat reduced because some primer combinations resulted in PCR products with oligoclonal heteroduplex patterns (Figure 2), but this was probably caused by the increased T-cell counts.
Analysis of immunoglobulin gene rearrangements
To study the effect of the 631delT RAG1 mutation on immunoglobulin gene rearrangements, we could use only DNA from BM mononuclear cells (with less than 1% CD19+ precursor B cells) because no B lymphocytes were detected in the PB (less than 0.01% of lymphocytes). Owing to the limited amount of available BM cells, we could study only a limited number of potential immunoglobulin gene rearrangements. PCR amplifications of IGH rearrangements in BM mononuclear cells were negative (DH3–JH, DH6–JH, DH7–JH, and VH3–JH), except for a DH2–JH rearrangement (Table 1), which appeared monoclonal on heteroduplex analysis. The same DH2–JH rearrangement was amplified from PB DNA. Because no B cells were detectable in PB, we concluded that the incomplete DH2–JH rearrangement was probably derived from T cells, occurring as a cross-lineage immunoglobulin gene rearrangement.33
PCR amplification of IGK and IGL rearrangements in BM mononuclear cells showed faint bands on agarose gels. These amounts of PCR products were not sufficient for heteroduplex analysis. In addition, cloning and fluorescent sequencing was hardly possible, and only a single VλII–Jλ3 rearrangement was identified (Table 1).
Expression of N-terminal truncated RAG1 protein
The human wt RAG1 protein has a molecular weight of 119 kd.23 Usage of a second (codon position 183) or third (codon position 202) AUG codon as an alternative translation start site would theoretically lead to an N-terminal truncated RAG1 protein of approximately 100 kd; the third AUG codon is in a Kozak consensus context. We did not have sufficient BM cells to perform Western blot analysis for the detection of RAG1 proteins. Therefore, we decided to clone the 631delT and the wt RAG1 gene in 2 different expression vectors, pGEM-T easy and pMSE1, which were used for in vitro transcription and translation and for transfection of COS cells followed by Western blot analysis, respectively. The pGEM-T easy expression vector carries the T7 promotor for initiation of transcription. The in vitro transcription and translation experiment with the wt RAG1 construct generated a protein of the expected size, whereas the 631delT RAG1 construct generated a smaller protein (Figure 3). The same smaller protein band was also visible in the wt lane, suggesting that the alternative translation start site can be used in the wt RAG1gene.
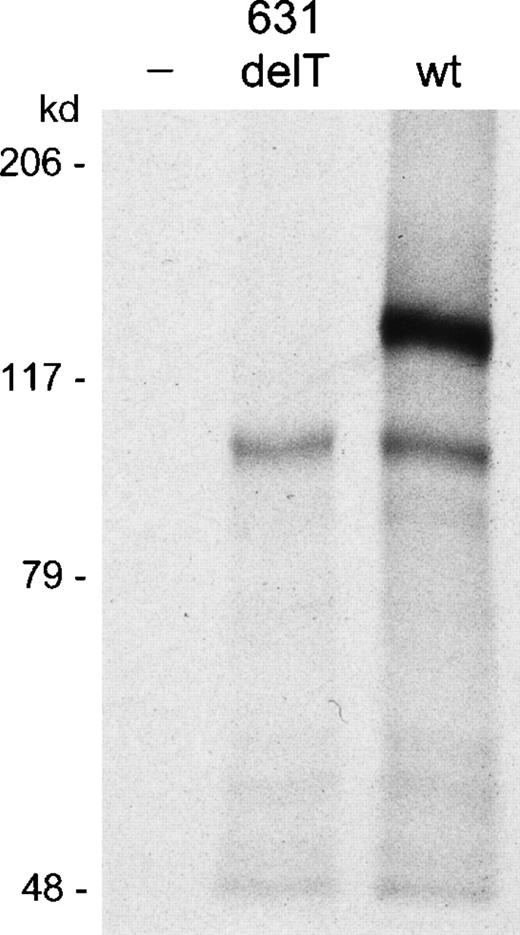
In vitro transcription and translation assay.
The 631delT RAG1 gene and the wt RAG1 gene were both cloned in a pGEM-T easy expression vector using the T7 promotor. Transcription and translation of the 631delT RAG1 gene showed absence of the 119-kd wt protein band, which was present in the lane of the wt RAG1 gene. The 631delT showed only the smaller 100-kd N-terminal truncated protein band, which was also present in the wtRAG1 gene lane.
In vitro transcription and translation assay.
The 631delT RAG1 gene and the wt RAG1 gene were both cloned in a pGEM-T easy expression vector using the T7 promotor. Transcription and translation of the 631delT RAG1 gene showed absence of the 119-kd wt protein band, which was present in the lane of the wt RAG1 gene. The 631delT showed only the smaller 100-kd N-terminal truncated protein band, which was also present in the wtRAG1 gene lane.
RAG1 protein expression from the pMSE1 vector results in a C-terminal fusion of a myc epitope tag to the RAG1 protein, which can be used for easy detection. RAG1 protein expression was analyzed after transfection of the expression constructs into COS cells. Western blot analysis showed a number of protein products (Figure4). In the lane of the N-terminal truncated RAG1 protein, the same pattern is observed as in the wt lane, except for the absence of the 119-kd wt product. The 100-kd polypeptide, present in both lanes, represents the N-terminal truncated RAG1 protein, thereby suggesting once more that the wt RAG1 gene can use this alternative translation start site. Furthermore, some smaller bands were present in both lanes at the same positions, probably representing degradation products, which is consistent with the short half-life of the RAG1 protein.22
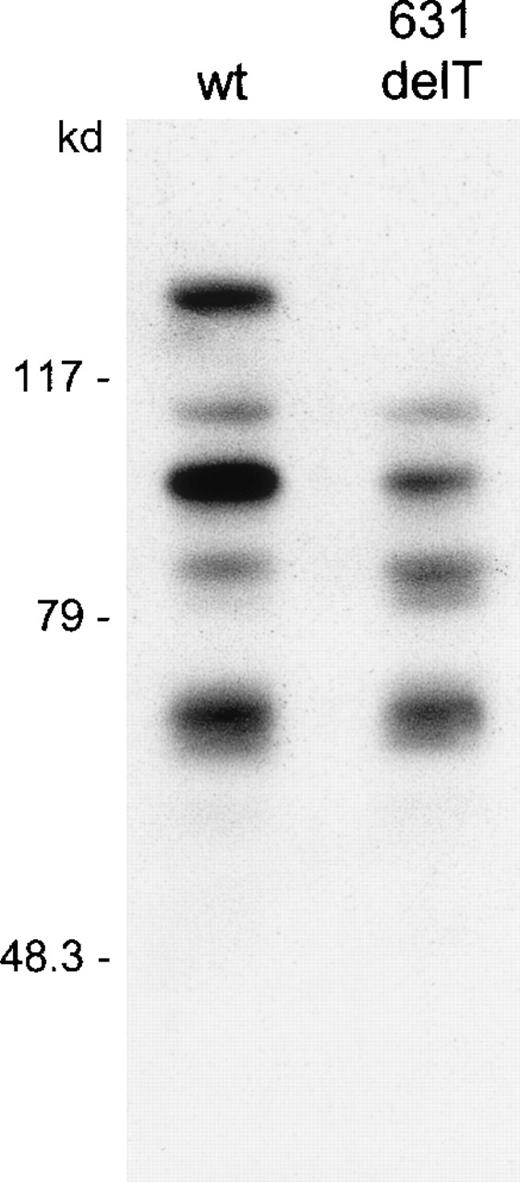
Western blotting after transfection of COS cells with wt RAG1 and 631delT RAG1 constructs.
RAG1 proteins with c-myc tag were detected using a c-mycantibody and were visualized by enhanced chemiluminescence. The upper band in the wt lane represents the 119-kd wt RAG1 protein, which is absent in the 631delT RAG1 lane. Both the wt RAG1 and the 631delT RAG1 gene express the 100-kd N-terminal truncated protein. In both lanes additional protein products are seen, representing degradation products of the RAG1 protein, which is in line with the short half-life of this protein.
Western blotting after transfection of COS cells with wt RAG1 and 631delT RAG1 constructs.
RAG1 proteins with c-myc tag were detected using a c-mycantibody and were visualized by enhanced chemiluminescence. The upper band in the wt lane represents the 119-kd wt RAG1 protein, which is absent in the 631delT RAG1 lane. Both the wt RAG1 and the 631delT RAG1 gene express the 100-kd N-terminal truncated protein. In both lanes additional protein products are seen, representing degradation products of the RAG1 protein, which is in line with the short half-life of this protein.
Analysis of V(D)J recombination activity
V(D)J recombination activity of the wt and N-terminal truncated RAG1 proteins was tested using the recombination substrate pDVG93 (Figure5A). On recombination, the sequence between the RSS elements was inverted, which can be detected by PCR. As shown in Figure 5B, both the wt and the N-terminal truncated RAG1 protein were able to recombine this substrate, though the activity of the truncated RAG1 protein may have been slightly reduced.
Plasmid recombination assay using construct pDVG93.
Transfection of pDVG93, RAG1, and RAG2 in CHO cells leads to an inversion rearrangement of pDVG93, which can be detected by primers DG89 and DG147 (A). PCR reactions with primers DG89 and DG147 were performed on serial dilutions of recombination products, as indicated. PCR products were visualized by hybridization with 32P-labeled oligonucleotide FM23 followed by phosphor imaging. The N-terminal truncated RAG1 protein is still able to perform inversion rearrangement of pDVG93, although the activity may be slightly reduced as compared to wt RAG1 protein (B).
Plasmid recombination assay using construct pDVG93.
Transfection of pDVG93, RAG1, and RAG2 in CHO cells leads to an inversion rearrangement of pDVG93, which can be detected by primers DG89 and DG147 (A). PCR reactions with primers DG89 and DG147 were performed on serial dilutions of recombination products, as indicated. PCR products were visualized by hybridization with 32P-labeled oligonucleotide FM23 followed by phosphor imaging. The N-terminal truncated RAG1 protein is still able to perform inversion rearrangement of pDVG93, although the activity may be slightly reduced as compared to wt RAG1 protein (B).
Discussion
We identified a novel mutation in the RAG1 gene (631delT) of a newborn with SCID; her parents were consanguineous. In line with the presence of high blood T-cell counts with an almost complete polyclonal TCR repertoire, we observed that this 631delT RAG1 gene could direct V(D)J recombination on plasmid recombination substrates. Western blotting and in vitro transcription and translation showed the usage of a second translation start site in the 631delT RAG1 gene, leading to an N-terminal truncation of the RAG1 protein. This N-terminal truncation apparently had a major effect on B-cell differentiation because sensitive flow cytometric analysis showed virtually no B cells in the PB (less than 0.01% of lymphocytes) and less than 1% CD19+ precursor B cells in the BM of the patient.
Schwarz et al4 found that a number of patients with B− SCID had RAG mutations that resulted in severely decreased RAG activity (recombination frequency ofRAG1 mutants, less than 0.7%). Villa et al14observed that OS patients with oligoclonal T cells and diminished numbers of B cells had RAG gene defects with residual RAG activity (recombination frequency of RAG1 mutants 5% to 23%). Our patient, with a homozygous 631delT RAG1 mutation, appeared to have high blood T-cell counts with an almost complete TCR repertoire but with undetectable numbers of B cells, whereas the RAG activity was only slightly diminished. She had some clinical symptoms characteristic of OS, such as GVH-like disease, erythrodermia, hepatosplenomegaly, lymphadenopathy, and agammaglobulinemia. However, the clinical picture was unusually severe, considering the early onset and her death before the fifth week of life. Other OS characteristics, such as a predominance of Th2 cells, were not present. IgE levels were not determined, but the complete absence of B lymphocytes in the PB makes high IgE levels unlikely. Eosinophilia was present at first admission but was absent on referral to the university hospital. The strongly increased T-lymphocyte counts (55.5 × 106/mL) suggested the presence of T-cell leukemia, but only limited TCR oligoclonality was observed in an otherwise polyclonal background as demonstrated by Vβ antibody studies, heteroduplex PCR, and sequencing of TCR gene rearrangements. This is not in line with studies on OS patients, which show that the peripheral T-cell repertoire in OS is highly restricted.40 41 The combined clinical and laboratory data are not in line with genuine OS but are in line with OS-like T+/B− SCID.
Despite the polyclonal TCR repertoire, B-lymphocytes and immunoglobulin gene rearrangements were hardly detectable in our patient. As reported before, deletion of part of the N-terminus of the RAG1 protein did not have a major effect on recombination activity in a V(D)J recombination assay.16,22 Furthermore, the basic aa motif BIIa, responsible for enhancement of the recombination activity, was still present in the N-terminal truncated RAG1 protein.20 These combined data indicate that the N-terminus of the RAG1 protein is probably particularly important for immunoglobulin gene rearrangements. Similarly, the deletion constructs of Kirch et al17suggested that the C-terminus of the RAG2 protein is essential for efficient VH to DJH rearrangement. Thus, the N-terminal part of RAG1 and the C-terminus of RAG2 are dispensable for basic recombination activity, but they may have a role in targeting immunoglobulin loci.
We considered the following explanations for the absence of immunoglobulin gene rearrangements in our patient: (1) The RSS might differ in sequence between immunoglobulin and TCR genes, and the RAG1 N-terminus would be required only for immunoglobulin RSS recognition or cleavage. However, published data on RSS do not support this suggestion.42,43 (2) The N-terminal truncated RAG1 protein might be able to mediate “1-step” rearrangements (V to J joining), but not “2-step” rearrangements (V to D-J joining). Positioning of the 12- and 23-bp spacer length in the RSS of the V, D, and J segments of the IGH, TCRB, and TCRD genes differs in such a way that the TCRB and TCRD genes can skip D gene segments and thereby produce 1-step V-J joinings. IGH gene rearrangements in principle always include D segments and thus have to be “2-step” rearrangements. Sequencing of TCRB andTCRD gene rearrangements showed usage of D gene segments, proving the occurrence of 2-step rearrangements in our patient (Table1). (3) Recruitment of the RAG1 protein to the immunoglobulin genes might be established by its N-terminus, implying that the N-terminal truncated RAG1 protein is hampered in reaching immunoglobulin loci. (4) The N-terminus of the RAG1 protein might be involved in opening of the chromatin structure of the immunoglobulin loci before recombination, implying that an N-terminal truncation of the RAG1 protein would prevent a complete rearrangement of immunoglobulin genes. (5) The N-terminus of the RAG1 protein might interact with a B-cell–specific factor, which would form a complex necessary for the rearrangement of immunoglobulin genes. (6) Alternatively, immunoglobulin gene rearrangements might require higher levels of RAG activity than TCR gene rearrangements. This hypothesis is supported by the observation that cross-lineage TCRB, TCRG, and TCRD gene rearrangements occur at high frequency in precursor B-ALL (more than 90%), whereas cross-lineage IGH, IGK, and IGL gene rearrangements are rare in T-ALL or do not occur at all.33,44 45 Explanations 3 to 6 are not mutually exclusive, and the development of model systems will be required to clarify this issue.
Acknowledgments
We thank Drs A. W. Langerak, M. C. M. Verschuren, M. J. Willemse, M. van der Burg, and T. Szczepañski for fruitful discussions and for technical assistance, Mrs S. de Bruin–Versteeg for flow cytometric analysis, Dr A. W. Langerak for critical reading of the manuscript, and T. M. van Os for preparation of the figures.
Reprints:Jacques J. M. van Dongen, Department of Immunology, Erasmus University Rotterdam, PO Box 1738, 3000 DR Rotterdam, The Netherlands; e-mail: vandongen@immu.fgg.eur.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal