Abstract
The activation status of a recently identified STAT (signal transducers and activators of transcription) factor, LIL-Stat (lipopolysaccharide [LPS]/IL-1–inducible Stat) in adult T-cell leukemia (ATL) cells was investigated by electrophoretic mobility shift assays using nuclear extracts of leukemic cells from 7 patients with ATL and a GAS (gamma interferon activation site)-like element termed LILRE (LPS/IL-1–responsive element), which is found in the human prointerleukin 1β (IL1B) gene. Spontaneous DNA binding of LIL-Stat was observed in all ATL cells examined. However, in normal human peripheral lymphocytes, DNA binding of LIL-Stat was detected only after stimulation with IL-1. These results demonstrated that LIL-Stat is constitutively activated in ATL cells. Furthermore, our transient transfection studies using LILRE chloramphenicol acetyltransferase (CAT) reporters argue that LIL-Stat in ATL cells functions as a transcriptional activator through binding to the LILRE in theIL1B gene.
Binding of many cytokines to their cognate receptors immediately activates Jak tyrosine kinases and their substrates; namely, STAT (signal transducers and activators of transcription) DNA binding proteins. The activated STAT proteins dimerize and are translocated to the nucleus to transactivate genes by binding to variations on the gamma interferon (IFN-γ) activation site (GAS), which was initially reported to bind the IFN-γ–induced Stat 1.1,2 Recently, transformation of T cells by human T-cell leukemia virus type I (HTLV-I) has been reported to be associated with constitutive activation of the Jak-STAT pathway.3,4 Stat3 and Stat5, which become activated in normal T cells in response to IL-2, have been demonstrated to be constitutively activated in HTLV-I–transformed cells.3 Furthermore, Takemoto et al5 have observed constitutive activation of Stat1, Stat3, and Stat5 in leukemic cells of patients with adult T-cell leukemia (ATL), an aggressive T-cell malignancy induced by HTLV-I infection.
We have recently demonstrated that both lipopolysaccharide (LPS) and IL-1 immediately activate a common novel STAT factor.6 This LPS/IL-1 inducible factor (LIL-Stat) binds a GAS-like sequence (TTCCTGAGA) termed LILRE (LPS and IL-1 responsive element) in the human prointerleukin 1β (IL1B) gene and is recognized by an antibody (Ab) raised to the N-terminus of Stat1 (anti-Stat1N Ab), but not by those specific for either the C terminus of Stat1 (anti-Stat1C Ab) or any other GAS-binding STAT. Moreover, the DNA-binding activity of this protein has been shown to be specifically inhibited by phosphotyrosine, suggesting that phosphotyrosine mediates the obligate dimerization required for STAT DNA binding. Analysis of DNA binding specificity has demonstrated that the LIL-Stat possesses a novel GAS-like binding activity that contrasts with that of other STATs in a requirement for a G residue at position 8 (LILRE; TTCCTGAGA). The existence of such a factor relates the signaling pathway for IL-1 and LPS receptors to other cytokine receptors that mediate signaling via the immediate activation of STAT transcriptional factors. Interestingly, a recent study7 has demonstrated constitutive activation of LIL-Stat in acute myelocytic leukemia (AML) cells.
In this study, we investigated LIL-Stat activation status in ATL cells by electrophoretic mobility shift assay (EMSA) using a radiolabeled LILRE probe and ATL cell nuclear extracts. The functional role of LIL-Stat in ATL cells was further assessed by transient transfection studies using LILRE chloramphenicol acetyltransferase (CAT) reporters.
Study design
Oligonucleotides
The nucleotide sequences of oligonucleotides used in this study were as follows (the core recognition sequence of each oligonucleotide is underlined): LILRE, 5′-AGCTTATAAGAGGTTTCACTTCCTGAGAGTCGA-3′; mutated LILRE (LILRE/mGAS), 5′-AGCTTATAAGAGGTT- TCACTTCCTGAGcGTCGA-3′; GAS derived from the FcγRI gene, 5′-CGATCGAGATGTATTTCCCAGAAAAGTCGA-3′; mutated GAS (mGAS),5′-CGATCGAGATGTATggCCCAGAcAAGTCGA-3′; hSIE (high-affinity sis-inducible element), 5′-AGCTTGTGCATTTCCCGTAAATCTTGTCGTCGA-3′; SIE, 5′-AGCTTGTGCAGTTCCCGTCAATCTTGTCGTCGA-3′. LILRE corresponds to positions −2863 to −2841 of the IL1Bgene (GenBank accession no. L06808).
Antibodies
The amino-terminus-specific Stat1N Ab (anti-Stat1N Ab) was purchased from Transduction Laboratories (Lexington, KY). This Ab was raised against the amino-terminal 194 amino acids of Stat1. The carboxyl terminus-specific Stat1C (anti-Stat1C Ab) and antiphosphotyrosine Ab (anti-p-tyr Ab) were purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Anti-Stat1C Ab was raised against a peptide containing amino acids 688 to 700 of Stat1.
Cells and nuclear extracts
Blood samples were obtained from 7 patients with acute ATL after they gave their informed consent. Their peripheral white blood cell (WBC) counts ranged from 35 000 to 73 500/μL. More than 90% of the peripheral WBCs were leukemic cells. The diagnosis of acute ATL was made on the basis of clinical features, hematologic findings, and the presence of the monoclonal integration of HTLV-I provirus in the leukemic cells. Peripheral blood mononuclear cells were separated from patients' blood by Ficoll-Hypaque density gradient centrifugation. The cell population consisted of more than 95% ATL cells, as determined by both the morphologic characteristics of cells on Wright-Giemsa–stained slides and flowcytometric cell surface marker analysis using fluorescein isothiocyanate (FITC)- or phycoerythrin-conjugated monoclonal antibodies. Normal human lymphocytes were obtained from healthy blood donors after they gave their informed consent.
Nuclear extracts were prepared from ATL cells, normal human peripheral lymphocytes, and human THP-1 monocytic leukemic cells, as previously described.8 Freshly isolated ATL cells and lymphocytes were used in this study. In some experiments, lymphocytes were stimulated with 10 ng/mL of IL-1β for 15 minutes. Lymphocytes preactivated by phytohemagglutinin (PHA) treatment were stimulated with 10 ng/mL of IL-2 for 15 minutes. THP-1 cells (JCRB 0112; Health Science Research Resources Bank, Osaka, Japan) were cultured as previously described.8 THP-1 cells were stimulated by incubating them in the presence of 1 μg/mL of LPS for 15 minutes. All nuclear extracts were prepared in the presence of 1 mmol/L ZnCl2, 1 mmol/L sodium orthovanadate, and 10 mmol/L NaF to inhibit phosphatase activities.
EMSA
32P-labeled LILRE, hSIE, and SIE probes were used in this study. Binding reactions were performed as previously described, followed by analysis on a 4% polyacrylamide gel using 0.5 × TBE (45 mmol/L Tris-borate and 1 mmol/L EDTA) as the running buffer.6 Unlabeled competitor oligonucleotides were used at a 50-fold molar excess over the radiolabeled probe. In EMSA experiments using Abs, nuclear extracts were incubated with either anti-Stat1N Ab, anti-Stat1C Ab, or anti–p-tyr Ab at 4°C for 1 hour.
Plasmids
Either LILRE or LILRE/mGAS was inserted into a CAT reporter containing a minimal (−59 to +105) murine c-fos promoter (fos/CAT).9 A plasmid 3MHT contains the IL1Bgene promoter sequence between −131 and +12 (HT fragment) ligated to CAT as previously described.8 Constructs LILRE/3MHT and LILREmGAS/3MHT were generated by inserting LILRE and LILRE/mGAS, respectively into the 3MHT. These constructs were verified by sequencing.
Transfection and chloramphenicol acetyltransferase assay
Transfection of ATL cells was carried out by the DEAE-dextran method as described previously.8 This DEAE-dextran method does not induce the endogenous IL1B gene in ATL cells (data not shown). ATL cells (1 × 107 cells per plate) were transfected with 10 μg of plasmids. At 36 hours after transfection, cells were harvested. The CAT assays were carried out by a liquid scintillation method as described previously.8
Results and discussion
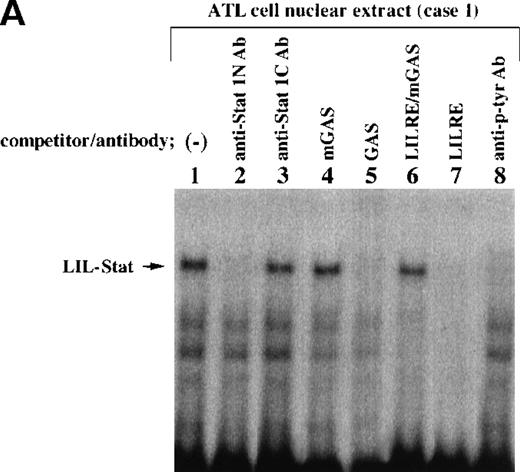
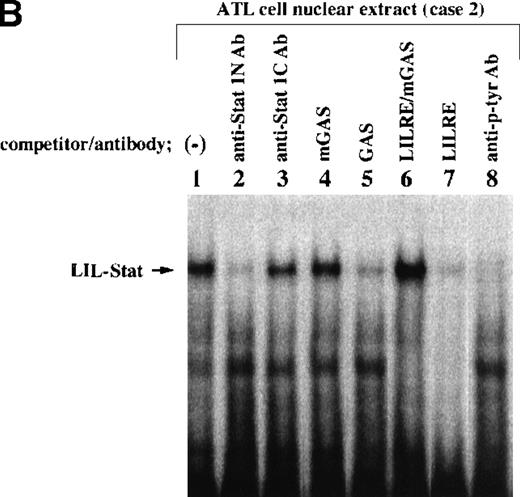
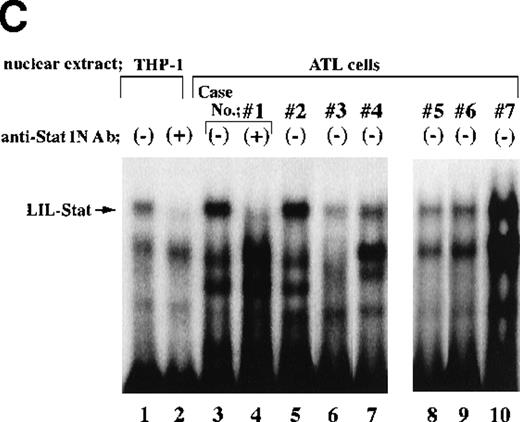
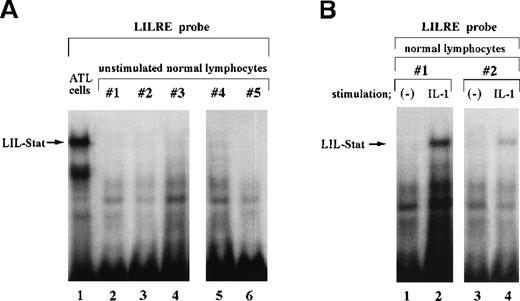
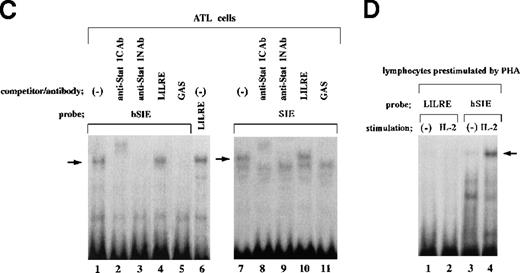
Nuclear extracts obtained from leukemic cells of 2 patients with ATL generated several complexes with the LILRE probe (Figure1A and B). One of these complexes (Figure1A and B; arrows) was recognized by anti-Stat1N Ab (lane 2), but not by anti-Stat1C Ab (lane 3). This complex was abrogated by the addition of antiphosphotyrosine Ab (anti–p-tyr Ab) (lane 8). Pearse et al10 reported that an A-to-C mutation at position 9 of the GAS sequence (TTCCNNNAA) resulted in a significant reduction in Stat 1 binding. In this study, a single point mutation at position 9 of the GAS-like sequence within the LILRE (LILRE/mGAS) significantly reduced the affinity of the LILRE for the complex (comparison of lane 6 with lane 7). In addition, a 50-fold molar excess of unlabeled bona fide GAS DNA competed for the complex (lane 5). These results indicated spontaneous binding of LIL-Stat to LILRE in ATL cells. We further investigated DNA binding of LIL-Stat in leukemic cells of 7 patients with ATL. As shown in lanes 3 to 10 of Figure 1C, spontaneous binding of LIL-Stat was observed in leukemic cells of all the patients with ATL examined. Moreover, the LIL-Stat/LILRE complex in LPS-stimulated THP-1 cells (lane 1 of Figure 1C) possessed the same electrophoretic mobility as that seen in ATL cells, supporting our argument that LIL-Stat is activated in ATL cells.
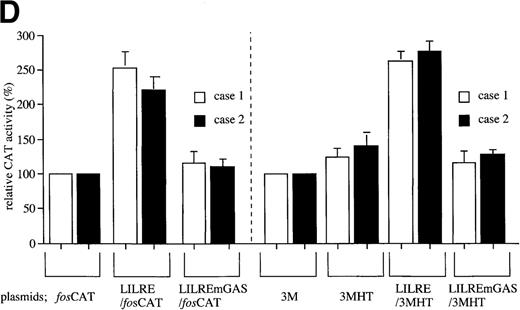
Constitutive activation of LIL-Stat in leukemic cells of patients with ATL.
LILRE was used as a radiolabeled probe. Nuclear extracts were prepared from ATL cells (A, B, and lanes 3 to 10 of C) and LPS-stimulated THP-1 monocytic leukemia cells (lanes 1 and 2 of C). The double-stranded oligonucleotides used in this study were described in “Materials and methods.” Unlabeled competitor oligonucleotides were used at a 50-fold molar excess over radiolabeled LILRE probe. D shows the enhancer activity of LILRE in leukemic cells of 2 patients with ATL. Transfection of CAT reporters into ATL cells and CAT assays were carried out as described in “Materials and methods.” The CAT data were normalized to the average activity elicited by eitherfos/CAT or 3M. Error bars represent SDs from triplicate cultures.
Constitutive activation of LIL-Stat in leukemic cells of patients with ATL.
LILRE was used as a radiolabeled probe. Nuclear extracts were prepared from ATL cells (A, B, and lanes 3 to 10 of C) and LPS-stimulated THP-1 monocytic leukemia cells (lanes 1 and 2 of C). The double-stranded oligonucleotides used in this study were described in “Materials and methods.” Unlabeled competitor oligonucleotides were used at a 50-fold molar excess over radiolabeled LILRE probe. D shows the enhancer activity of LILRE in leukemic cells of 2 patients with ATL. Transfection of CAT reporters into ATL cells and CAT assays were carried out as described in “Materials and methods.” The CAT data were normalized to the average activity elicited by eitherfos/CAT or 3M. Error bars represent SDs from triplicate cultures.
The LILRE is located within the previously identified 406-base pair (bp) enhancer of the human IL1B gene. Therefore, we further examined the enhancer activity of the LILRE in transiently transfected leukemic cells obtained from 2 patients with ATL (Figure 1D). A single copy of the LILRE (LILRE/fosCAT) was inserted into an enhancer-dependent CAT construct (fos/CAT) by using the minimal murine c-fos promoter. As shown in Figure 1D, the presence of a single copy of LILRE (LILRE/fosCAT) resulted in approximately 2-fold increase in CAT activity in both cases. The transcriptional activity of the LILRE was significantly inhibited by a single point mutation of the LILRE (LILREmGAS/fosCAT). Furthermore, a 3MHT CAT vector containing the IL1B promoter sequence (HT fragment; −131 to +12) was used in this study. Constructs LILRE/3MHT and LILREmGAS/3MHT were generated by inserting LILRE and LILRE/mGAS, respectively into the 3MHT. As shown in Figure 1D, although the presence of HT IL1B promoter sequence caused only a little increase in CAT activity, LILRE/3MHT containing a single copy of LILRE upstream of the IL1B gene promoter sequence generated significantly higher CAT activity than did 3MHT in both cases. A single point mutation of the LILRE (LILREmGAS/3MHT) caused almost complete loss of the transcriptional activity. Thus, the results obtained from this study argue that LIL-Stat in ATL cells can function as a transcriptional activator through binding to the LILRE in theIL1B gene.
In a previous study, we showed that IL-1 induced DNA binding of LIL-Stat in murine EL4 thymoma cells.6 However, in contrast to the results obtained from ATL cells, no spontaneous DNA binding of LIL-Stat was detected in EL4 cells.6 On the other hand, Tuyt et al7 observed neither spontaneous nor inducible LIL-Stat DNA binding in fully differentiated monocytes and granulocytes. In this study, we carried out EMSA studies by using nuclear extracts of unstimulated peripheral lymphocytes obtained from 5 healthy blood donors to elucidate the presence and activation status of LIL-Stat in normal human peripheral lymphocytes. As shown in Figure2A (lanes 2 to 6), unstimulated normal lymphocytes did not show LIL-Stat DNA binding activity. Moreover, in contrast to ATL cells in which LIL-Stat was spontaneously bound to LILRE, DNA binding of LIL-Stat was detected only after stimulation with IL-1 in normal peripheral lymphocytes (Figure 2B).
LIL-Stat, which specifically recognizes the LILRE sequence, is not constitutively activated in normal lymphocytes.
A, LILRE was used as a radiolabeled probe. Nuclear extracts prepared from unstimulated lymphocytes of 5 healthy donors were used in lanes 2 to 6. As a control, an ATL cell nuclear extract was used in lane 1. B, LILRE was used as a radiolabeled probe. Nuclear extracts were obtained from untreated (lanes 1 and 3) and IL-1–treated (lanes 2 and 4) lymphocytes of 2 healthy donors. C, An ATL cell nuclear extract and radiolabeled hSIE (lanes 1 to 5) and SIE (lanes 7 to 11) probes were used. As a control study (lane 6), the ATL cell nuclear extract was incubated with a radiolabeled LILRE probe. D, LILRE (lanes 1 and 2) and hSIE (lanes 3 and 4) were used as radiolabeled probes. Lymphocytes preactivated by PHA treatment were not stimulated (lanes 1 and 3) or were stimulated with IL-2 (lanes 2 and 4). EMSAs were conducted as described in Figure 1.
LIL-Stat, which specifically recognizes the LILRE sequence, is not constitutively activated in normal lymphocytes.
A, LILRE was used as a radiolabeled probe. Nuclear extracts prepared from unstimulated lymphocytes of 5 healthy donors were used in lanes 2 to 6. As a control, an ATL cell nuclear extract was used in lane 1. B, LILRE was used as a radiolabeled probe. Nuclear extracts were obtained from untreated (lanes 1 and 3) and IL-1–treated (lanes 2 and 4) lymphocytes of 2 healthy donors. C, An ATL cell nuclear extract and radiolabeled hSIE (lanes 1 to 5) and SIE (lanes 7 to 11) probes were used. As a control study (lane 6), the ATL cell nuclear extract was incubated with a radiolabeled LILRE probe. D, LILRE (lanes 1 and 2) and hSIE (lanes 3 and 4) were used as radiolabeled probes. Lymphocytes preactivated by PHA treatment were not stimulated (lanes 1 and 3) or were stimulated with IL-2 (lanes 2 and 4). EMSAs were conducted as described in Figure 1.
Takemoto et al5 observed constitutive activation of Stat1 in ATL cells by EMSAs using an Ab raised against the N terminus of Stat1 and a radiolabeled hSIE probe. In this study, we examined the abilities of hSIE and SIE to bind LIL-Stat by EMSAs using radiolabeled hSIE and SIE probes and an ATL cell nuclear extract (Figure 2C). This nuclear extract showed spontaneous LIL-Stat binding to LILRE (lane 6 of Figure 2C). As shown in lanes 1 to 3, incubation of the ATL cell nuclear extract with a radiolabeled hSIE probe generated a DNA/protein complex (arrow) recognized by both anti-Stat1C and anti-Stat1N Abs. Moreover, the complex was not competed for by LILRE (lane 4). A similar protein/DNA complex recognized by both anti-Stat1C and anti-Stat1N Abs (arrow; lanes 7 to 11 of Figure 2C) was observed by using a radiolabeled SIE probe. The SIE/protein complex was not competed for by LILRE (lane 10). These results showed that LIL-Stat does not bind to either hSIE or SIE. This argument is further supported by our previous report, which has demonstrated that mutation of a G residue to any other residue at position 8 of the LILRE (TTCCTGAGA) significantly reduced affinity of the LILRE for LIL-Stat.6In this regard, neither the hSIE (TTCCCGTAA) nor the SIE (TTCCCGTCA) contains a G residue at position 8. On the other hand, when ATL cell nuclear extracts and a radiolabeled LILRE probe were used, LILRE did not bind a protein recognized by anti-Stat1C Ab (lane 3 of Figure 1A and lane 3 of Figure 1B). These results show that LILRE does not efficiently bind Stat1, and further suggest that the LIL-Stat/LILRE complex does not contain Stat1. In agreement with this argument, we have previously reported that Stat1 derived from IFN-γ–treated U937 monocytic cells cannot efficiently bind to LILRE.6 However, because it is possible that LIL-Stat heterodimerizes with a protein(s) that binds to LILRE, further studies are required for characterization of the LIL-Stat.
IL-2 signaling requires ligand-induced heterodimerization of the IL-2 receptor (IL-2R) β and γ chain cytoplasmic domains.11The β and γ chains of IL-2R are used in common by IL-15 and IL-2.12 Several studies have reported that IL-2/IL-15 signaling pathway plays an important role in proliferation of ATL cells and HTLV-I–transformed T cells.13-17 Migone et al3 have shown that the IL-2R γ chain is constitutively associated with Jak3 in HTLV-I–transformed MT-2 T cells. They further observed constitutive association of the IL-2R β chain with both the γ chain and Jak3 in MT-2 cells. In addition, granulocyte-colony stimulating factor (G-CSF)18 and IL-419 have also been shown to induce proliferation of leukemic cells in some patients with ATL. We have previously demonstrated that IL-4 does not activate LIL-Stat.6 Moreover, our EMSA using lymphocytes showed that IL-2 did not activate LIL-Stat, but induced formation of a protein/hSIE complex (Figure 2D; arrow). However, it remains unknown whether G-CSF activates LIL-Stat. On the other hand, elevated serum levels of IL-620 and the IL-6 signal transducer gp13021 have been reported in ATL patients. The fact that LIL-Stat is activated by IL-6 as well as IL-16 may suggest the possibility that increased production of these factors causes constitutive activation of LIL-Stat in ATL cells.
On the basis of our results obtained from this study, we conclude that LIL-Stat is constitutively activated in ATL cells. SpontaneousIL1B gene expression has been observed in leukemic cells of a considerable number of patients with ATL.22 Recently, constitutive activation of LIL-Stat was detected in AML cells.7 AML cells have also been reported to spontaneously express the IL1B gene.23 Moreover, our transient transfection studies argue that LIL-Stat in ATL cells acts as a transcriptional activator through binding to the LILRE in theIL1B gene.
Reprints:Junichi Tsukada, First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu, 807-8555 Japan; e-mail; jtsukada@med.uoeh-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal