Abstract
6-[3-adamantyl-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) is a novel compound that represents the prototype of a new class of synthetic retinoids with apoptogenic properties in acute promyelocytic leukemia (APL) and other types of leukemia. In this article, using SCID mice xenografted with APL-derived NB4 cells, we demonstrate that CD437 has significant antileukemic activity in vivo. In addition, we report on the isolation and characterization of an APL cell line (NB4.437r) resistant to CD437. The cell line retains expression of PML-RAR and is approximately 33-fold more resistant than the parental counterpart to the apoptogenic effects of the retinoid. Resistance is relatively specific to CD437 and structural congeners because the NB4.437r cell line is still sensitive to various types of apoptogenic compounds. The CD437-resistant cell line maintains sensitivity to the antiproliferative and apoptotic action of all-trans-retinoic acid, AM580, and fenretinide, though it shows partial resistance to the cytodifferentiating effects of the first 2 compounds. Resistance to CD437 lays upstream of the CD437-induced release of cytochrome c from the mitochondria and the activation of caspase-3, -7, -8, and -9. Furthermore, NB4.437r cells are deficient in the CD437-dependent activation of nuclear NFkb and AP1-binding activities and in the phosphorylation of the protein kinase Akt. In the case of AP1, deficient assembly of the complex is not caused by the lack of activation of the Jun N-terminal kinase (JNK) family of kinases. The novel cell line will be useful in the elucidation of the molecular mechanisms underlying the apoptogenic action of CD437 and structurally related retinoids.
6-[3-adamantyl-4-hydroxyphenyl]-2-naphthalene carboxylic acid (CD437) is the prototype of a new and unique class of synthetic retinoids1-10 that may find clinical application in the first- and second-line treatment of acute promyelocytic leukemia (APL) and other types of acute myelogenous leukemia. The retinoid is likely to exert its apoptogenic effects through the activation of intracellular pathways that are distinct from those stimulated by all-trans retinoic acid (ATRA) and many of the available chemotherapeutic agents.3,6 In vitro, we and others recently demonstrated that the compound induces programmed cell death (PCD)2 in ATRA-sensitive and ATRA-resistant APL and acute myelogenous leukemia cell lines3,6 and in freshly isolated leukemia cells.3 In vivo, though no data on preclinical models of leukemia are available, the retinoid is well tolerated and shows antitumor activity on other types of neoplasia.9 11
The mechanism underlying the apoptogenic action of CD437 is still obscure, but it does not entail activation of the nuclear retinoic acid receptor.3,6 In APL cells, the process of PCD does not require active protein synthesis. It is accompanied by the release of cytochrome c (cyt c) from the mitochondria and the activation of caspase-3 and caspase-7, which results in the degradation of many proteins, including the PML-RARα oncogene.3 Caspase activation is fundamental for the apoptogenic effect of CD437 because specific inhibitors block the entire process.3
In this article, we report on the in vivo antileukemic activity of CD437 and on the isolation and biochemical characterization of a novel APL cell line, NB4.437r, made resistant in vitro to the apoptogenic action of the retinoid.
Materials and methods
Reagents and cell lines
CD437, CD2325,12 and AM580 were synthesized by Galderma Research and Development (Sophia Antipolis, France). ATRA, staurosporine, cycloheximide, p-(trifluoromethoxy)phenylhydrazone (FCCP), phorbol myristyl acetate (PMA), arsenic trioxide (Arsenic), and wortmannin were from Sigma (St. Louis, MO). Doxorubicin (Pharmacia & Upjohn, Kalamazoo, MI), taxol (Bristol-Myers Squibb, Wallingford, CT) and cisplatin (Aldrich, Milwaukee, WI) were of the highest purity available. Fenretinide and arabinosylcytosine (AraC) were synthesized by the Chemical Branch of the National Cancer Institute (NCI, Baltimore, MD). The fluorogenic caspase substrates DEVD-amc (caspase-3 and caspase-7 substrates), VEID-amc (caspase-6 substrate), and IETD-amc (caspase-8 substrate) as well as the caspase inhibitor z-VAD-fmk (z-VAD) were from the Peptide Research Institute (Osaka, Japan). The APL-derived NB413 and the ATRA-resistant NB4-R114 cell lines were kind gifts of Dr Michel Lanotte (Hôpital St. Louis, Paris, France). The 2 cell lines were routinely cultured in RPMI-1640 containing 10% FCS.
In vivo experiments
SCID mice (National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD) were kept in barrier conditions in the central animal house facilities of the Istituto Mario Negri. For the survival experiments, 6 animals/experimental group were inoculated intraperitoneally with 2 × 106 NB4 cells and treated intraperitoneally with vehicle alone (carboxymethylcellulose 0.5% and 0.01% Tween 80 in water) or CD437 at the dosages indicated in “Results,” and survival was monitored daily.
To evaluate the mechanism of action underlying the in vivo antitumor activity of CD437, 2 × 106 cells were xenografted in SCID mice. Twenty days after inoculation, animals (4 mice/experimental group) were treated with a single dose of CD437 (30 mg/kg) or vehicle alone. Sixteen hours later, the ascitic fluid was withdrawn and leukemic blasts were counted and evaluated for viability after staining with erythrosin; signs of apoptosis using the annexin-V assay or the 4′-6-diamidine-2-phenylindole (DAPI) staining; proliferation using flow cytometry after staining with propidium iodide (PI) and fluoresceinated antiproliferating cell nuclear antigen (PCNA) antibodies; and granulocytic maturation after the determination of CD13, CD11b, and CD11c by flow cytometry or the measurement of nitrobluetetrazolium (NBT)-reducing activity. Procedures involving animals were conducted according to national and international standards (Italian Governing Law, Legislative decree 116, January 27, 1992; NIH Guide for the Care and Use of Laboratory Animals, 1996; and EU directives and guidelines). The in vivo experiments described in this article received the approval of the Animal Care and Use Committee of the Mario Negri Institute.
Isolation of the NB4.437r cell line
To isolate the CD437-resistant cell line, NB4 cells were cultured for approximately 6 months in medium containing increasing concentrations of CD437. The selection protocol was started at 10−9 mol/L CD437, and the concentration of the compound was progressively raised to 10−5 mol/L. At the end of the selection protocol, we isolated a cell line that survived in CD437 at 10−5 mol/L and was routinely passaged in medium containing the retinoid at 10−6mol/L. The CD437-resistant derivative line (NB4.437r) was cloned by limiting dilution and has been cultured for almost 2 years without phenotypic changes. Resistance to CD437 is stable; the line has been subcultured in the absence of the retinoid for up to 4 months without loss of its characteristic insensitivity to the retinoid. The number of chromosomes determined by karyotypic analysis of NB4.437r cells (66 < n < 92) is not significantly different from that of the parental counterparts (68 < n < 90). Before each experiment, the NB4.437r cell line was cultured for 24 hours in the absence of CD437.
Uptake and intracellular distribution of CD437
Uniformly 3H-labeled CD437 (specific activity, 50 Ci/mmol) was synthesized by Galderma Research and Development and used for the uptake and intracellular distribution experiments. For the uptake experiments, cells were incubated with 10−7mol/L 3H-CD437, washed once with phosphate-buffered saline and centrifuged, and the pellet was solubilized in Soluene (Packard, Meriden, CT) before liquid scintillation counting. For the intracellular distribution studies, cells were incubated in 10−7 mol/L 3H-CD437 for 1 hour and washed once with phosphate-buffered saline. Cytosolic, microsomal, mitochondrial, and nuclear fractions from NB4 and NB4.437r cells were obtained by ultracentrifugation according to standard procedures and subjected to liquid scintillation counting.
Cytodifferentiation of NB4 and NB4.437r cells
Cell viability, apoptosis, and determination of DEVD-amc, VEID-amc, and IETD-amc hydrolytic activity
Cell viability was determined by counting the percentages of red and white cells after staining with erythrosin.3 For the determination of the apoptotic index, cells were fixed with methanol and stained with DAPI as described.3 The annexin-V assay was performed by flow cytometry (FACSORT system; Becton Dickinson) with a commercially available kit (Annexin- V-FLUOS staining kit; Boheringer Mannheim, Mannheim, Germany). The level of expression of PCNA was evaluated by biparametric flow cytometry after permeabilization and staining with PI and anti-PCNA fluorescein-conjugated antibodies.16 Determination of DEVD-amc hydrolytic activity was performed on NB4 and NB4.437r cell extracts as previously reported3 after normalization for the protein content.17 An identical protocol was used for the determination of VEID-amc and IETD-amc hydrolytic activity.
Western blot analysis, cytochrome c intracellular redistribution assay, and determination of the mitochondrial membrane potential
In the case of polyADP ribose polymerase (PARP), caspase isozymes, and cyt c, Western blot analysis3 was performed on cytosolic extracts from NB4 or NB4.437r with the after antibodies: caspase-3 (CPP32 p20 [N-19]; Santa Cruz Biotechnology, Santa Cruz, CA); PARP (PARP [N-20]; Santa Cruz Biotechnology); caspase-8 (Biomedia GmbH, Baesweiler, Germany); and caspase-6, caspase-7, caspase-9, and cyt c (Pharmingen, San Diego, CA). In the case of Akt, Western blot analysis experiments were performed on total cellular extracts with antibodies specific for the phosphorylated form of the protein and control antibodies, using a commercially available kit (New England Biolabs, Beverly, MA) according to the instructions of the manufacturer. The antibodies and the protocol used for PML-RARα and actin detection have already been described.3Immunoreactive protein bands were visualized with the ECL detection kit (Amersham, Little Chalfont, UK). The transmembrane potential was assessed by flow cytometry after loading cells with the fluorescent dye 3,3′-dihexyloxadicarbocyanine iodide (DiOC6; Sigma).18
Jun N-terminal kinase activity and electrophoresis mobility shift assays
Jun N-terminal kinase (JNK) activity was determined on immunoprecipitates obtained after challenge of NB4 or NB4.437r cell extracts with agarose-immobilized anti-JNK antibodies (Santa Cruz Biotechnology). Immunoprecipitates were incubated with gst-Jun (Santa Cruz Biotechnology) as a substrate in the presence of γ32P-ATP (Amersham). The whole procedure was carried out as described by Lee et al.19 The reaction mixture was subjected to polyacrylamide gel electrophoresis and autoradiography. The amount of JNK protein present in the immunoprecipitates was determined by Western blot analysis using an anti–JNK-1 antibody (Santa Cruz Biotechnology).
Electrophoresis mobility shift assays (EMSAs) for the AP1, NFkb, and SP1 transcriptional complexes were performed on nuclear extracts, as already described.20 The double-stranded oligonucleotides used were AP1, 5′-GTGTGATGACTCAGGTTTCCGATC3′21; NFkb, 5′-TGACAGAGGGGACTTTCCGAGAGGATCA-3′22; and SP1, 5′-GATCGGGAGGCGTGGCCTGGGCGGGACTGGGGAGTGGCGAGATC-3′.23NFkb supershift experiments were performed with an anti-p65 antibody.24
Results
CD437 has antileukemic activity in SCID mice xenografted with NB4 cells and induces apoptosis in the ATRA-resistant NB4.R1 cell line in vitro
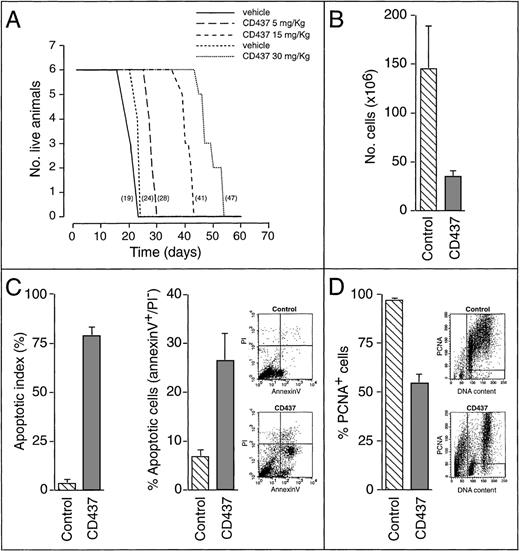
To establish whether CD437 has antileukemic activity in vivo, we inoculated SCID mice with NB4 cells and determined the survival of vehicle- and CD437-treated animals (Figure1A). CD437 administration caused a dose-dependent increase in the lifespan of NB4-xenografted animals, which was significant at the 15-mg/kg and the 30-mg/kg doses. This effect was primarily the consequence of the rapid and strong apoptogenic action of CD437. In fact, harvesting the ascitic fluid 18 hours after a single administration of the retinoid (30 mg/kg) demonstrated an approximately 5-fold decrease in the number of leukemic blasts compared with what was observed in vehicle-treated animals (Figure 1B). This was associated with a remarkable increase in the number of blasts showing signs of apoptosis, such as nuclear fragmentation (apoptotic index) (Figure 1C, left panel) and plasma membrane phosphatidyl serine externalization (annexin-V positivity) (Figure 1C, right panel). Treatment with CD437 caused arrest in the proliferation of the residual viable cells (Figure 1D), as demonstrated by the dramatic decrease in the level of expression of PCNA, a proliferation-associated nuclear protein.16 The decrease in PCNA was evident for the cells in the G1 phase and less dramatic for the cells in the G2/M phase of the cycle. In untreated animals, the majority of blasts in the S phase were PCNA+. Most of the cells in this phase of the cycle disappeared in CD437-treated animals, suggesting death or transit and arrest in the G2/M phase of the cycle. CD437-induced PCD and growth arrest were not the result of a granulocytic maturation effect because treatment of the animals with CD437 did not alter the level of expression of a number of myeloid markers. In fact, regardless of CD437 treatment, the majority of xenografted NB4 cells were CD13-positive (vehicle = 73.2% ± 4.1%, CD437 = 61.3% ± 3.0%; mean ± SD of 4 animals), CD11b-negative (vehicle = 6.5% ± 1.7%, CD437 = 7.7% ± 0.9%; mean ± SD of 4 animals), and CD11c-negative (vehicle = 4.3% ± 2.3%, CD437 = 6.7% ± 1.9%; mean ± SD of 4 animals) and were unable to reduce NBT after PMA stimulation (data not shown). Interestingly, the administration of CD437 (30 mg/kg) for 3 consecutive days resulted in a further decrease in the amount of leukemic cells from the ascitic fluid (8.0% ± 3.5% of those present in vehicle-treated animals; mean ± SD of 4 animals).
In vivo activity of CD437 in the SCID/NB4 model of acute promyelocytic leukemia. (A) SCID mice (6 animals/experimental group) were inoculated intraperitoneally with 2 × 106 NB4 cells. Two days later, treatment was started and continued for 3 weeks with 1 daily intraperitoneal injection of vehicle or CD437, as indicated. The number in parentheses represents the median survival time for each experimental group. Combined results from 2 separate experiments are presented. In the first experiment (solid line for the vehicle), animals were treated with 5 and 15 mg/kg CD437 or vehicle alone. In the second experiment (dashed line for the vehicle), animals were injected with 30 mg/kg CD437 or vehicle alone. Increases in survival time for the retinoid-treated groups were analyzed by the log-rank test: CD437 5 mg/kg (P < .05); CD437 15 mg/kg and CD437 30 mg/kg (P < .01). (B-D) SCID mice (4 animals/experimental group) were inoculated intraperitoneally with 2 × 106 NB4 cells. Twenty days later, animals were treated with a single intraperitoneal injection of vehicle (control) or CD437 (30 mg/kg). Sixteen hours after treatment, the ascitic fluid was withdrawn and the leukemic cells were subjected to various types of analysis. The results, summarized by the bar graph data, are the mean ± SD of 4 separate animals. In B the total number of cells was counted after staining with erythrosin to determine the level of viability (control, 98% ± 1%; CD437, 80% ± 2%; mean ± SD of 4 animals). In C the percentage of apoptotic cells showing signs of nuclear fragmentation, after staining with DAPI (left panel) or showing cytoplasmic membrane phosphatidylserine externalization (annexin-V positivity and PI negativity; right panel), was evaluated. For annexin-V, the cytofluorometric analysis of 1 representative vehicle-treated and 1 representative CD437-treated animal, from which the data summarized in graphic form were derived, is also shown. The lower right quadrant contains apoptotic cells (annexin-V+/PI−); the lower left quadrant contains viable cells (annexin-V−/PI−; control, 92% ± 1%; CD437, 68% ± 6%; mean ± SD of 4 animals). In this analysis, the upper right quadrant contains necrotic cells (annexin-V+/PI+; control, 1% ± 1%; CD437, 4 ± 2%; mean ± SD of 4 animals). In D the percentage of nonproliferating viable cells was evaluated by biparametric flow cytometry after staining with an anti-PCNA antibody and counterstaining with PI. The cytofluorometric analysis of 1 representative vehicle-treated and 1 representative CD437-treated animal, from which the data summarized in graphic form were derived, is also shown. The left quadrant contains apoptotic cells and cell debris and was not considered in the analysis. The 2 right quadrants contain viable PCNA+ or PCNA− cells in the G1, S, or G2/M phase of the cycle as indicated by the content of DNA determined after PI staining. The percentage of viable PCNA−cells is 3% ± 1% for control animals and 45% ± 4% for CD437-treated animals (mean ± SD; n = 4).
In vivo activity of CD437 in the SCID/NB4 model of acute promyelocytic leukemia. (A) SCID mice (6 animals/experimental group) were inoculated intraperitoneally with 2 × 106 NB4 cells. Two days later, treatment was started and continued for 3 weeks with 1 daily intraperitoneal injection of vehicle or CD437, as indicated. The number in parentheses represents the median survival time for each experimental group. Combined results from 2 separate experiments are presented. In the first experiment (solid line for the vehicle), animals were treated with 5 and 15 mg/kg CD437 or vehicle alone. In the second experiment (dashed line for the vehicle), animals were injected with 30 mg/kg CD437 or vehicle alone. Increases in survival time for the retinoid-treated groups were analyzed by the log-rank test: CD437 5 mg/kg (P < .05); CD437 15 mg/kg and CD437 30 mg/kg (P < .01). (B-D) SCID mice (4 animals/experimental group) were inoculated intraperitoneally with 2 × 106 NB4 cells. Twenty days later, animals were treated with a single intraperitoneal injection of vehicle (control) or CD437 (30 mg/kg). Sixteen hours after treatment, the ascitic fluid was withdrawn and the leukemic cells were subjected to various types of analysis. The results, summarized by the bar graph data, are the mean ± SD of 4 separate animals. In B the total number of cells was counted after staining with erythrosin to determine the level of viability (control, 98% ± 1%; CD437, 80% ± 2%; mean ± SD of 4 animals). In C the percentage of apoptotic cells showing signs of nuclear fragmentation, after staining with DAPI (left panel) or showing cytoplasmic membrane phosphatidylserine externalization (annexin-V positivity and PI negativity; right panel), was evaluated. For annexin-V, the cytofluorometric analysis of 1 representative vehicle-treated and 1 representative CD437-treated animal, from which the data summarized in graphic form were derived, is also shown. The lower right quadrant contains apoptotic cells (annexin-V+/PI−); the lower left quadrant contains viable cells (annexin-V−/PI−; control, 92% ± 1%; CD437, 68% ± 6%; mean ± SD of 4 animals). In this analysis, the upper right quadrant contains necrotic cells (annexin-V+/PI+; control, 1% ± 1%; CD437, 4 ± 2%; mean ± SD of 4 animals). In D the percentage of nonproliferating viable cells was evaluated by biparametric flow cytometry after staining with an anti-PCNA antibody and counterstaining with PI. The cytofluorometric analysis of 1 representative vehicle-treated and 1 representative CD437-treated animal, from which the data summarized in graphic form were derived, is also shown. The left quadrant contains apoptotic cells and cell debris and was not considered in the analysis. The 2 right quadrants contain viable PCNA+ or PCNA− cells in the G1, S, or G2/M phase of the cycle as indicated by the content of DNA determined after PI staining. The percentage of viable PCNA−cells is 3% ± 1% for control animals and 45% ± 4% for CD437-treated animals (mean ± SD; n = 4).
Because induced retinoic acid resistance is a major clinical problem in the management of APL, we examined the ability of CD437 to induce apoptosis in an NB4-derived cell line made resistant to ATRA (NB4-R1).14 The in vitro sensitivity of the ATRA-resistant cell line to the apoptogenic effects of CD437 was not different from that of the NB4 parental cell line. In fact, treatment of NB4 and NB4.R1 blasts for 6 hours with optimal concentrations of the retinoid caused apoptosis in 86% ± 11% (mean ± S.D, n = 3) and 86% ± 21% (mean ± SD, n = 3) of the cells, respectively. Furthermore, the calculated EC50 levels for the apoptogenic effect of ATRA in NB4 (0.3 ± 0.1μmol/L; mean ± SD, n = 3) and NB4.R1 (0.5 ± 0.1μmol/L; mean ± SD, n = 3) blasts were similar. This confirmed and extended the results obtained in the NB4.306 cell line,3 another APL model of induced ATRA resistance. The in vivo activity of CD437 in the NB4/SCID model and the in vitro apoptogenic effect of the retinoid on ATRA-resistant APL blasts encouraged us to study the mechanism(s) of the CD437 antileukemic action by developing an APL-derived cell line resistant to the compound.
NB4-derived NB4.437r cell line resistant to apoptosis induced by CD437
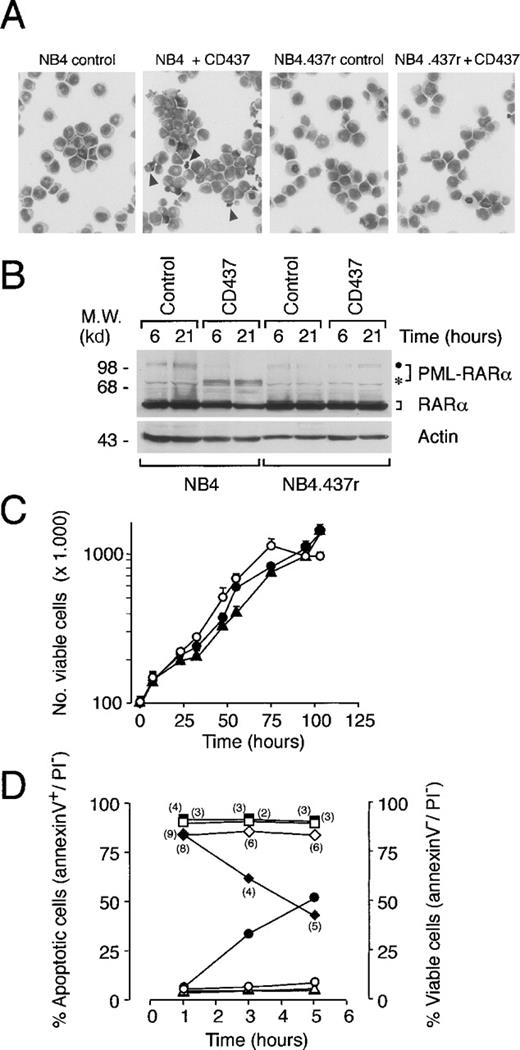
Challenge of NB4 promyelocytes with increasing concentrations of CD437 resulted in the isolation of the resistant cell line NB4.437r. As shown in Figure 2A, when cultured in standard conditions, the morphology of NB4.437r cells was promyelocytic and was not different from that of the parental cells. The NB4 parental cell line underwent rapid PCD in 10−6 mol/L CD437, whereas the resistant counterpart did not show any morphologic sign of toxicity. NB4.437r blasts have similar steady-state levels of the PML-RARα oncogene and the normal RARα protein relative to the original NB4 cell line (Figure 2B). In addition, the PML-RARα protein synthesized in NB4.437r cells was structurally identical to that expressed in the parental cell line, at least as far as its junctional breakpoint and RARα portions.25 In fact, sequence analysis after polymerase chain reaction (PCR) amplification of PML-RARα messenger RNA (mRNA) (from nucleotide 1546 of PML to nucleotide 2956 of the RARα) from NB4.437r did not demonstrate the presence of any nucleotide substitution relative to what has been reported for NB4 cells.25 As expected,3 on treatment with CD437, PML-RARα was degraded in NB4 blasts, but a similar effect was not observed in NB4.437r cells. The growth curves of NB4 and NB4.437r (either in the presence or in the absence of CD437) blasts were similar (Figure 2C). The saturation density of the NB4.437r cell line was slightly higher (1.2 × 106 cells/mL) than that of the parental counterpart (1.0 × 106 cells/mL).
Morphology, PML-RAR levels, growth curves, and apoptosis of NB4 and NB4.437r cells in the absence and presence of CD437. (A) NB4 or NB4.437r cells (1 × 105/mL) were treated for 6 hours with vehicle or with CD437 (10−6 mol/L) as indicated. Cells were stained with May–Grunwald–Giemsa and photographed (magnification, ×400). Arrowheads in microscopic fields corresponding to CD437-treated NB4 cultures indicate apoptotic cells or apoptotic bodies. (B) NB4 or NB4.437r cells were treated with CD437 (10−6 mol/L) for the indicated amounts of time. Cell extracts were subjected to Western blot analysis. The filter was sequentially challenged with an anti-RARα and an anti-actin antibody. The position of the intact PML-RARα protein and a specific degradation product are indicated with a dot and an asterisk, respectively, on the right. The positions of appropriate molecular weight markers are indicated on the left. (C) Growth curves of NB4 in control conditions (open circles) and NB4.437r cells in the absence (solid circles) or presence (solid triangles) of CD437 (10−6 mol/L). The results are the mean ± SD of 3 separate culture dishes. (D) Surface expression of phosphatidylserine in NB4 and NB4.437r cells after treatment with CD437. NB4 (circles and diamonds) or NB4.437r (triangles and squares) cells (5 × 105/mL) were treated for the indicated amounts of time with dimethyl sulfoxide as vehicle (open symbols) or CD437 (10−6 mol/L) (solid symbols). The number of viable (diamonds and squares), apoptotic (triangles and circles), and necrotic cells (numbers in parentheses) was determined by flow cytometry after staining with fluoresceinated annexin-V and PI. Viable cells are PI− and annexin-V−; apoptotic cells are PI− and annexin-V+(PI−/annexin-V+); the necrotic cells are PI+ and annexin-V+. Flow cytometric analysis was performed as in the inset of Figure 1. Data are representative of at least 2 independent experiments with identical results.
Morphology, PML-RAR levels, growth curves, and apoptosis of NB4 and NB4.437r cells in the absence and presence of CD437. (A) NB4 or NB4.437r cells (1 × 105/mL) were treated for 6 hours with vehicle or with CD437 (10−6 mol/L) as indicated. Cells were stained with May–Grunwald–Giemsa and photographed (magnification, ×400). Arrowheads in microscopic fields corresponding to CD437-treated NB4 cultures indicate apoptotic cells or apoptotic bodies. (B) NB4 or NB4.437r cells were treated with CD437 (10−6 mol/L) for the indicated amounts of time. Cell extracts were subjected to Western blot analysis. The filter was sequentially challenged with an anti-RARα and an anti-actin antibody. The position of the intact PML-RARα protein and a specific degradation product are indicated with a dot and an asterisk, respectively, on the right. The positions of appropriate molecular weight markers are indicated on the left. (C) Growth curves of NB4 in control conditions (open circles) and NB4.437r cells in the absence (solid circles) or presence (solid triangles) of CD437 (10−6 mol/L). The results are the mean ± SD of 3 separate culture dishes. (D) Surface expression of phosphatidylserine in NB4 and NB4.437r cells after treatment with CD437. NB4 (circles and diamonds) or NB4.437r (triangles and squares) cells (5 × 105/mL) were treated for the indicated amounts of time with dimethyl sulfoxide as vehicle (open symbols) or CD437 (10−6 mol/L) (solid symbols). The number of viable (diamonds and squares), apoptotic (triangles and circles), and necrotic cells (numbers in parentheses) was determined by flow cytometry after staining with fluoresceinated annexin-V and PI. Viable cells are PI− and annexin-V−; apoptotic cells are PI− and annexin-V+(PI−/annexin-V+); the necrotic cells are PI+ and annexin-V+. Flow cytometric analysis was performed as in the inset of Figure 1. Data are representative of at least 2 independent experiments with identical results.
To assess the level of apoptosis in CD437-treated NB4 and NB4.437r cells, the amount of phosphatidylserine on the outer aspect of the plasma membrane (Figure 2D) was determined with the use of biparametric flow cytometry after fluoresceinated–annexin-V and PI staining.26 Challenge of NB4 cells with CD437 for 1 hour did not result in an augmentation in the proportion of annexin-V+ cells compared with what was observed in control conditions. However, at 3 hours, this proportion rose and was further increased at 5 hours. This was accompanied by a concomitant decrease in the percentage of viable cells (annexin-V− and PI−). Regardless of the treatment applied, the percentage of NB4 necrotic cells (annexin-V+ and PI+) was low and did not vary significantly. Treatment of NB4.437r cells with the retinoid for 1, 3, or 5 hours did not cause the appearance of a number of annexin-V+ cells above control levels. Similarly, the percentage of viable NB4.437r cells was unaltered and that of necrotic cells was always negligible.
The results on the number of apoptotic cells assessed by annexin-V binding correlated well with those obtained by scoring nuclear fragmentation with DAPI (apoptotic index). Using this second method, we observed that challenge of NB4 promyelocytes with CD437 for 6 hours resulted in a dose-dependent increase in the number of apoptotic cells, which tended to plateau at approximately 1 μmol/L, with a calculated EC50 of 0.35 μmol/L (0.29-0.43 μmol/L; n = 3). By contrast, a concentration of CD437 of 11.9 μmol/L (10.5-13.6 μmol/L; n = 3) was necessary to cause an apoptogenic effect in 50% of the NB4.437r cells, and the plateau was reached at approximately 50 μmol/L.
NB4.437r cells maintain sensitivity to the growth-inhibitory and apoptogenic effects of ATRA, whereas retinoid-dependent cytodifferentiation is partially impaired
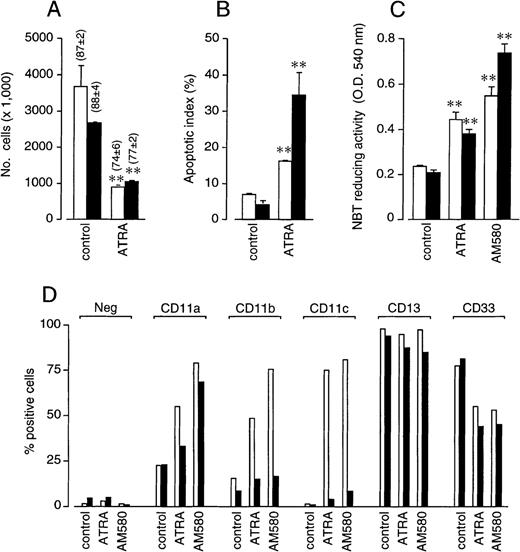
Treatment of NB4 or NB4.437r promyelocytes with ATRA (10−6 mol/L) resulted in an antiproliferative effect that was similar (Figure 3A). Furthermore, the retinoid induced the same level of apoptosis in both NB4 and NB4.437r cells (Figure 3B). The effect of ATRA on various markers of granulocytic differentiation was more complex (Figures 3C and 3D). The increase in NBT-reducing activity and the down-regulation of CD33 were equivalent in NB4 and NB4.437r cells. By contrast, the induction of CD11a, CD11b, and CD11c observed in the NB4 parental cell line was dramatically reduced in the CD437-resistant line. Surface expression of CD13 was high and left unaffected by ATRA, regardless of the cell line taken into consideration. Similar effects were observed with the synthetic retinoid AM580, which has been shown to activate specifically RARα, PML-RARα, or both in NB4 cells.27 As expected, AM580 at 10−8 mol/L was slightly more potent than ATRA at 10−6 mol/L in inducing NBT reducing activity (Figure 3C) and the surface expression of CD11a and CD11b (Figure 3D) in NB4 cells. By contrast, the compound was as active as the natural retinoid in up-regulating CD11c and in down-regulating CD33, respectively, and did not affect the level of expression of CD13 (Figure 3D).
In vitro growth inhibitory, apoptotic and cytodifferentiating activity of ATRA and AM580 in NB4 and NB4.437r cells. NB4 (open bars) or NB4.437r (solid bars) cells (2 × 105/mL) were treated with ATRA (10−6 mol/L), the powerful cytodifferentiating retinoid AM580 (10−8 mol/L) or vehicle (dimethyl sulfoxide, control) for 4 days. (A) The total number of cells was counted after staining with erythrosine (the percentage of viable cells is indicated in parentheses). (B) The proportion of apoptotic cells after staining with DAPI is shown. (C) NBT-reducing activity was measured spectrophotometrically after stimulation of cells with PMA for 30 minutes. Results are the mean ± SD of 3 separate culture dishes. (D) CD11a, CD11b, CD11c, CD13, and CD33 surface expression was measured by flow cytometry after staining with specific fluoresceinated antibodies. “Neg” indicates the level of background fluorescence observed in the different samples. Data are representative of at least 2 independent experiments with superimposable results. **Significantly higher or lower than the respective control values according to the Student t test (P < .01).
In vitro growth inhibitory, apoptotic and cytodifferentiating activity of ATRA and AM580 in NB4 and NB4.437r cells. NB4 (open bars) or NB4.437r (solid bars) cells (2 × 105/mL) were treated with ATRA (10−6 mol/L), the powerful cytodifferentiating retinoid AM580 (10−8 mol/L) or vehicle (dimethyl sulfoxide, control) for 4 days. (A) The total number of cells was counted after staining with erythrosine (the percentage of viable cells is indicated in parentheses). (B) The proportion of apoptotic cells after staining with DAPI is shown. (C) NBT-reducing activity was measured spectrophotometrically after stimulation of cells with PMA for 30 minutes. Results are the mean ± SD of 3 separate culture dishes. (D) CD11a, CD11b, CD11c, CD13, and CD33 surface expression was measured by flow cytometry after staining with specific fluoresceinated antibodies. “Neg” indicates the level of background fluorescence observed in the different samples. Data are representative of at least 2 independent experiments with superimposable results. **Significantly higher or lower than the respective control values according to the Student t test (P < .01).
NB4.437r cell line sensitive to various chemotherapeutic drugs and apoptogenic compounds
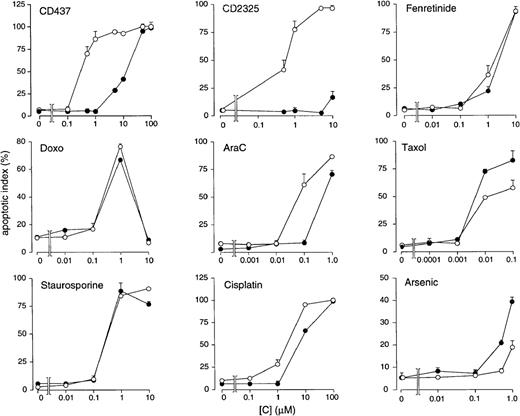
We investigated whether induced resistance in NB4.437r cells to CD437 is specific or is associated with an alteration in the apoptogenic response to other stimuli (Figure4). NB4.437r blasts show complete cross-resistance to CD2325, a CD437 structural analog.3 By contrast, the chemopreventive and antitumoral retinoid fenretinide28-30 is equally effective in causing apoptosis in the parental and the CD437-resistant cell line, suggesting that this compound and CD437 have different mechanisms of action. The 2 cell types are equally susceptible to challenge with doxorubicin, a drug inducing apoptosis through DNA intercalation and damage.31This is similar to what is observed for taxol, an active antitumor agent that disorganizes the cytoskeleton.32 Relative to the parental counterpart, the NB4.437r cell line shows limited cross-resistance to AraC, an antimetabolite contained in various polychemotherapeutic protocols,33 and to cisplatin, a widely used anti-neoplastic agent that damages DNA.34Staurosporine, a protein kinase C inhibitor35 and a strong apoptogenic agent,36 caused the same level of DNA fragmentation in both the NB4 and the NB4.437r cell lines. Arsenic, an antileukemic compound targeting the PML-RARα oncogene,37 38 was slightly more effective in the NB4.437r than in the parental NB4 cell line. Finally, in conditions in which tumor necrosis factor-α induces PCD in U937 cells, the cytokine was totally ineffective in killing both the parental and the CD437-resistant cells (data not shown).
In vitro apoptogenic effects of various compounds and chemotherapeutic agents in NB4 and NB4.437r cells. NB4 (open circles) or NB4.437r (closed circles) cells (5 × 105/mL) were treated for an optimal amount of time with the indicated compounds at the indicated concentrations. The number of apoptotic cells was determined after staining with DAPI. Results are the mean ± SD of 3 separate culture dishes. Cells were treated for these periods of time: CD437, 6 hours; CD2325, 21 hours; fenretinide, 24 hours; doxorubicin (Doxo), 13 hours; AraC, 24 hours; taxol, 24 hours; staurosporine, 8 hours; cisplatin, 24 hours; Arsenic, 24 hours.
In vitro apoptogenic effects of various compounds and chemotherapeutic agents in NB4 and NB4.437r cells. NB4 (open circles) or NB4.437r (closed circles) cells (5 × 105/mL) were treated for an optimal amount of time with the indicated compounds at the indicated concentrations. The number of apoptotic cells was determined after staining with DAPI. Results are the mean ± SD of 3 separate culture dishes. Cells were treated for these periods of time: CD437, 6 hours; CD2325, 21 hours; fenretinide, 24 hours; doxorubicin (Doxo), 13 hours; AraC, 24 hours; taxol, 24 hours; staurosporine, 8 hours; cisplatin, 24 hours; Arsenic, 24 hours.
NB4.437r resistance to CD437 is not explained by decreased uptake or altered subcellular localization of the compound
A widespread form of induced drug resistance (multidrug resistance [MDR]) is caused by activation of a membrane pump that effectively decreases the intracellular levels of compounds, such as doxorubicin and vincristine.39 To test possible differences in the intracellular levels of CD437 in NB4 and NB4.437r blasts, the rate of uptake and subcellular distribution of the 3H-labeled compound were determined. Both cell lines showed a rapid and similar uptake of 3H-CD437, which plateaued in less than 5 minutes and remained constant for at least 4 hours. At this last time point, the concentrations of 3H-CD437 in the NB4 and NB4.437r cell lines were 4.0 ± 0.4 and 3.6 ± 0.4 pmol/1 × 106 cells (mean ± SD of 3 culture dishes), respectively. The subcellular distribution of the radiolabeled compound was almost superimposable in NB4 and NB4.437r cells, and more than 90% of the intracellular radioactivity localized in the nuclei (1.1 ± 0.1 versus 1.2 ± 0.1 pmol/1 × 106 cells) and in the mitochondria (0.5 ± 0.1 versus 0.4 ± 0.1 pmol/1 × 106 cells). The residual amount of3H-CD437 localized in the microsomes (0.023 ± 0.002 versus 0.022 ± 0.001 pmol/1 × 106 cells) and cytosol (0.10 ± 0.02 versus 0.12 ± 0.06 pmol/1 × 106 cells). Most of the radioactivity that accumulated in the cells was caused by intact CD437; high-performance liquid chromatography analysis of cellular extracts demonstrated that the retinoid was not metabolized at a significant level (data not shown).
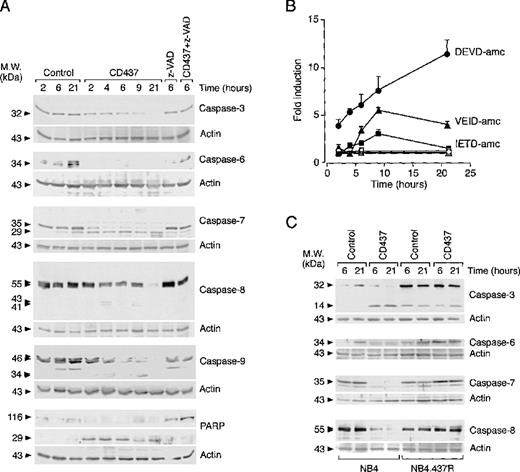
CD437 induces degradation and activation of various caspase isoenzymes in NB4 but not in NB4.437r blasts
In Figure 5A, the levels of the precursors and relative degradation products of caspase-3, -6, -7, -8, and -9 were determined in NB4 cells before and after treatment with CD437. Whereas the amounts of all pro-caspases were constant throughout the course of the experiment in untreated cells, a reduction in the levels of the caspase zymogens was already evident 2 hours after challenge with CD437. Pro-caspase-6, -7, and -9 disappeared rapidly, whereas pro-caspase-3 and -8 persisted for longer times. Disappearance of the zymogens was blocked by the caspase inhibitor z-VAD, indicating that proteolytic degradation is the consequence of an autocatalytic process or the result of a cascade activation of caspases. Specific degradation products were visible in the case of caspase-3 (Figure 5C), -7, -8, and -9, and the apparent molecular weights of these proteolytic bands were consistent with those of the respective active enzymes. To support further CD437-dependent activation of the various caspase isoforms, we determined the ability of NB4 cell extracts to hydrolize peptide fluorogenic substrates (Figure 5B). Stimulation of DEVD-amc (specific for caspase-3 and -7),40 IETD-amc (specific for caspase-8),41 and VEID-amc (specific for caspase-6)42 hydrolytic activity was evident in cytosolic extracts of CD437-treated cells relative to vehicle-treated extracts. Although DEVD-amc hydrolytic activity increased linearly between 2 and 21 hours of CD437 treatment, degradation of VEID-amc and IETD-amc peaked at 9 hours. Neither degradation of pro-caspase-3, -6, -7, and -8 (Figure 5C) nor stimulation of DEVD-amc, IETD-amc, or VEID-amc hydrolytic activity (data not shown) was observed in NB4.437r cells after treatment with CD437. This indicated that the molecular lesion(s) responsible for the CD437-resistance phenotype of NB4.437r cells lays upstream of caspase activation.
Effect of CD437 on the levels and state of activation of caspase isoenzymes in NB4 and NB4.437r cells. NB4 (A) and NB4 or NB4.437r (C) cells (5 × 105/mL) were treated with vehicle (control), CD437 (10−6 mol/L), the caspase inhibitor z-VAD (100 μmol/L), or CD437 + z-VAD for the indicated amounts of time. The levels of the indicated caspase proenzymes and polyADP ribose polymerase (PARP) as well as relative degradation products were analyzed by Western blot analysis using specific polyclonal antibodies. Western blot filters were subsequently challenged with an anti-actin antibody to confirm equal protein loading in each lane of the gel. (B) The state of caspase activation was measured in NB4 cell extracts with fluorogenic peptide substrates specific for caspase-3 and caspase-7 (DEVD-amc), caspase-6 (VEID-amc), and caspase-8 (IETD-amc) after treatment with vehicle (open symbols) and CD437 (closed symbols) for the indicated amounts of time. Results are the mean ± SD of 3 separate culture dishes. Data are representative of at least 2 independent experiments with similar results.
Effect of CD437 on the levels and state of activation of caspase isoenzymes in NB4 and NB4.437r cells. NB4 (A) and NB4 or NB4.437r (C) cells (5 × 105/mL) were treated with vehicle (control), CD437 (10−6 mol/L), the caspase inhibitor z-VAD (100 μmol/L), or CD437 + z-VAD for the indicated amounts of time. The levels of the indicated caspase proenzymes and polyADP ribose polymerase (PARP) as well as relative degradation products were analyzed by Western blot analysis using specific polyclonal antibodies. Western blot filters were subsequently challenged with an anti-actin antibody to confirm equal protein loading in each lane of the gel. (B) The state of caspase activation was measured in NB4 cell extracts with fluorogenic peptide substrates specific for caspase-3 and caspase-7 (DEVD-amc), caspase-6 (VEID-amc), and caspase-8 (IETD-amc) after treatment with vehicle (open symbols) and CD437 (closed symbols) for the indicated amounts of time. Results are the mean ± SD of 3 separate culture dishes. Data are representative of at least 2 independent experiments with similar results.
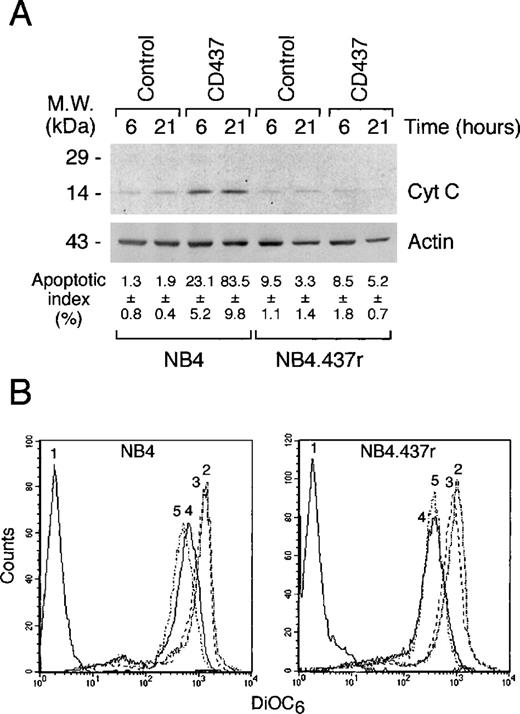
CD437-dependent release of cytochrome c into the cytosol is not dependent on changes in mitochondrial membrane potential in NB4 cells and is not observed in NB4.437r blasts
Cyt c relocalization from the mitochondria into the cytosol plays an important role in the activation of downstream caspases, such as caspase-3, -6, and -7, through the formation of a DISC complex with Apaf-1 and caspase-9.43 In basal conditions, no significant differences in the amounts of total or mitochondria-associated cyt c were observed between NB4 and NB4.437r cells (data not shown). As expected,3 treatment of NB4 cells with CD437 resulted in increased amounts of cyt c present in the cytosol compared with what was observed in control conditions; however, a similar effect was not observed in NB4.437r cells (Figure6A). In NB4 cells, the surge of cytosolic cyt c was associated with a dramatic augmentation in the level of apoptosis.
Effect of CD437 on the release of cytochrome c into the cytosol and the mitochondrial transmembrane potential of NB4 and NB4.437r cells. (A) The amount of cytochrome c released in the cytosol of NB4 and NB4.437r cells (5 × 105/mL) after challenge with vehicle (control) or CD437 (10−6mol/L) for the indicated amount of time was measured by Western blot analysis using a specific polyclonal antibody. The proportion of apoptotic cells was determined by DAPI staining on an aliquot of the cells used for the cytochrome c release assay. (B) NB4 or NB4.437r cells were preloaded with the fluorescent dye 3,3′-dihexiloxadicarbocyanine iodide (DiOC6), and treated with vehicle,2 CD437 (10−6mol/L),3 the proton translocator carbonyl cyanide p-(trifluorometoxy)phenylhydrazone (250 nM) FCCP,4 or CD437 + FCCP5 for 30 minutes. The transmembrane potential was assessed by flow cytometry. The fluorescence level in the absence of DiOC6 is indicated by tracing.1 Data are representative of at least 2 independent experiments with similar results.
Effect of CD437 on the release of cytochrome c into the cytosol and the mitochondrial transmembrane potential of NB4 and NB4.437r cells. (A) The amount of cytochrome c released in the cytosol of NB4 and NB4.437r cells (5 × 105/mL) after challenge with vehicle (control) or CD437 (10−6mol/L) for the indicated amount of time was measured by Western blot analysis using a specific polyclonal antibody. The proportion of apoptotic cells was determined by DAPI staining on an aliquot of the cells used for the cytochrome c release assay. (B) NB4 or NB4.437r cells were preloaded with the fluorescent dye 3,3′-dihexiloxadicarbocyanine iodide (DiOC6), and treated with vehicle,2 CD437 (10−6mol/L),3 the proton translocator carbonyl cyanide p-(trifluorometoxy)phenylhydrazone (250 nM) FCCP,4 or CD437 + FCCP5 for 30 minutes. The transmembrane potential was assessed by flow cytometry. The fluorescence level in the absence of DiOC6 is indicated by tracing.1 Data are representative of at least 2 independent experiments with similar results.
In certain forms of PCD, relocalization of cyt c is secondary to impairment of the mitochondrial membrane potential,44,45which can be monitored by the fluorescent intracellular sensor DiOC646 (Figure 6B). Although the proton translocator FCCP, used as a positive control, caused an evident shift to the left in the peak of DiOC6–cell-associated fluorescence, indicating disruption of the mitochondrial membrane potential, CD437 did not cause a similar effect. Contemporaneous treatment of NB4 cells with CD437 and FCCP did not result in a further and significant decrease in DiOC6 fluorescence. Similarly, in NB4.437r cells, only FCCP affected the mitochondrial transmembrane potential, and its effect was superimposable on that observed in NB4 cells. These data suggested that the transmembrane potential of the mitochondria does not play a role in the release of cyt c into the cytosol and that alterations in this parameter are not important for the CD437 resistance observed in NB4.437r cells.
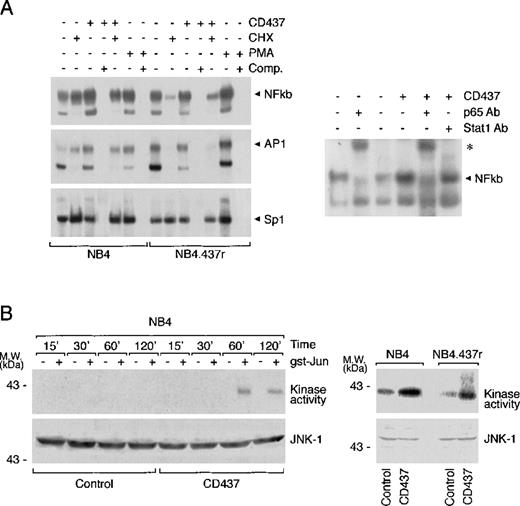
CD437-dependent induction of NFkb and AP1 nuclear complexes are observed in NB4 but not in NB4.437r cells
In NB4.437r cells, AP1 induction is dissociated from JNK activation. The transcriptional complex NFkb seems to play a role in the resistance to apoptosis observed in various types of tumors,47-49whereas activation of AP1 has been involved as a possible mediator of the CD437 apoptogenic action.5,9 As shown in Figure7A, in basal conditions NB4 cells contained detectable amounts of NFkb and AP1 nuclear complexes, which were increased by CD437 treatment. The effect was quantitatively similar to that observed in the presence of PMA used as a positive control. Induction of the 2 nuclear complexes was specific because CD437 did not affect the amounts of the SP1 complex observed in control conditions. Although 2 bands that can be competed away by cold oligonucleotides were visible in the EMSAs, corresponding to both NFkb and AP1 complexes, only the upper band was specific. As to NFkb, the upper band contained the p65 component of the p50/p65 heterodimer because it could be supershifted by antibodies recognizing this protein but not by anti-STAT1 antibodies used as a negative control. Similarly, the upper band of the EMSA corresponding to AP1 could be supershifted by anti-Jun antibodies (data not shown). In NB4.437r cells, the basal levels of active NFkb and AP1 transcription factors were higher than those in NB4 promyelocytes and were not modulated by the addition of CD437 to the growth medium. In NB4 cells, pretreatment with cycloheximide (CHX) had only marginal effects on the basal or CD437-induced levels of NFkb and AP1 transcriptional complexes. By contrast, in NB4.437r cells, CHX caused a substantial decrease of both complexes regardless of CD437 treatment. AP1 activity is often regulated by JNK-dependent phosphorylation of Jun.50 As demonstrated in Figure 7B, JNK-1 immunoprecipitates from NB4 cells treated for 1 and 2 hours with CD437 contained significant amounts of a kinase activity that phosphorylates Jun in vitro. After treatment with the retinoid, the levels of kinase activity were much higher than those observed in basal conditions (visible only on longer exposure of the autoradiograms) and were not explained by differences in the amounts of immunoprecipitated JNK protein. Interestingly, treatment of NB4.437r cells with the retinoid for 1 hour resulted in a similar activation of the JNK protein. This appeared to be the only marker of CD437-activity similarly regulated in the sensitive and resistant cell lines.
Effect of CD437 on NFkb and AP1 nuclear complexes as well as JNK kinase and protein levels in NB4 and NB4.437r cells.
(A) NB4 or NB4.437r cells (5 × 105/mL) were treated with vehicle, CD437 (10−6 mol/L), cycloheximide (CHX) (50 μM), PMA (1 μg/mL), or the indicated combinations of the compounds for 1 hour. Nuclear extracts were subjected to EMSA using radiolabeled oligonucleotide probes specific for NFkb, AP1, and SP1 transcription factors. Comp., cold oligonucleotide competitor. Supershift assay (right panel): nuclear extracts from cells treated with vehicle or CD437 (10−6 mol/L) for 1 hour were incubated with antibodies to the p65 component of the NFkb complex or with an irrelevant antibody (STAT1) of the same isotype before challenge with the radiolabeled oligonucleotide and subsequent EMSA. (B) NB4 or NB4.437r cells (5 × 105/mL) were treated with vehicle or CD437 (10−6 mol/L) for the indicated amounts of time. Cell extracts were prepared and JNK was immunoprecipitated with agarose-linked antibodies specific for the protein. Immunoprecipitates were incubated with γ32P-ATP in the absence (−) or the presence (+) of the JNK substrate gst-Jun. Equivalent amounts of the reaction mixtures were subjected to PAGE under denaturing conditions and subsequent autoradiography. To ensure that the same amount of JNK was present, Western blot analysis was performed on each immunoprecipitate using a second antibody specific to JNK-1. Data are representative of at least 2 independent experiments with similar results.
Effect of CD437 on NFkb and AP1 nuclear complexes as well as JNK kinase and protein levels in NB4 and NB4.437r cells.
(A) NB4 or NB4.437r cells (5 × 105/mL) were treated with vehicle, CD437 (10−6 mol/L), cycloheximide (CHX) (50 μM), PMA (1 μg/mL), or the indicated combinations of the compounds for 1 hour. Nuclear extracts were subjected to EMSA using radiolabeled oligonucleotide probes specific for NFkb, AP1, and SP1 transcription factors. Comp., cold oligonucleotide competitor. Supershift assay (right panel): nuclear extracts from cells treated with vehicle or CD437 (10−6 mol/L) for 1 hour were incubated with antibodies to the p65 component of the NFkb complex or with an irrelevant antibody (STAT1) of the same isotype before challenge with the radiolabeled oligonucleotide and subsequent EMSA. (B) NB4 or NB4.437r cells (5 × 105/mL) were treated with vehicle or CD437 (10−6 mol/L) for the indicated amounts of time. Cell extracts were prepared and JNK was immunoprecipitated with agarose-linked antibodies specific for the protein. Immunoprecipitates were incubated with γ32P-ATP in the absence (−) or the presence (+) of the JNK substrate gst-Jun. Equivalent amounts of the reaction mixtures were subjected to PAGE under denaturing conditions and subsequent autoradiography. To ensure that the same amount of JNK was present, Western blot analysis was performed on each immunoprecipitate using a second antibody specific to JNK-1. Data are representative of at least 2 independent experiments with similar results.
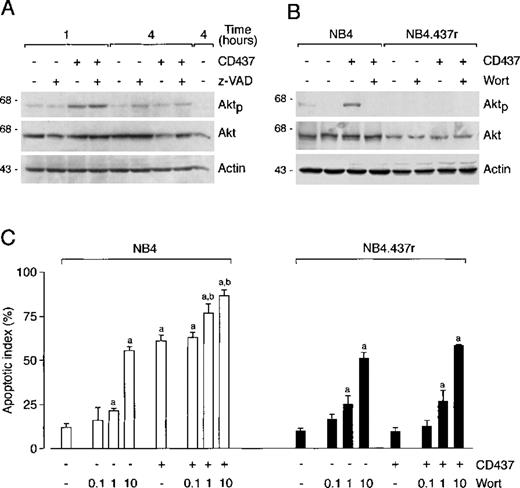
CD437 causes phosphorylation of Akt in NB4 but not in NB4.437r cells
Wortmannin inhibits phosphorylation of Akt without affecting sensitivity or resistance to CD437-triggered apoptosis. Akt kinase is activated by survival factors through phosphatidyl-inositol-3-kinase (PI3K)-dependent phosphorylation.51 Although NB4 and NB4.437r cells synthesized significant amounts of Akt in basal conditions (Figures 8A and 8B), the levels of this kinase were slightly lower in the CD437-resistant than in the CD437-sensitive cell line. Consistent with this, a background level of phosphorylation was evident in the NB4 cell line, whereas longer exposures of films were required to highlight a similar level of phosphorylation in the CD437-resistant counterpart (data not shown). Challenge of NB4 blasts with CD437 for 1 hour resulted in increased Akt phosphorylation. Phosphorylation was not secondary to CD437-triggered caspase activation because the phenomenon was not affected by the caspase inhibitor z-VAD. CD437-dependent Akt phosphorylation was transitory; it returned to baseline levels by 4 hours. This last effect was at least partially explained by a caspase-dependent cleavage of the Akt protein, which was observed at 4 hours and was blocked by z-VAD (Figure 8A). Treatment of NB4.437r cells with CD437 for 1 hour did not result in Akt phosphorylation or alteration in the total levels of the protein (Figure 8B).
Effect of CD437 on the phosphorylated form of Akt protein in NB4 and NB4.437r cells. Consequences of the inhibition of Akt phosphorylation by wortmannin on CD437-induced apoptosis. (A) NB4 cells (5 × 105/mL) were treated with vehicle, CD437 (10−6 mol/L), the caspase inhibitor z-VAD (100 μmol/L), or the indicated combinations of the compounds for 1 hour or 4 hours. (B and C) NB4 or NB4.437r cells (5 × 105/mL) were preincubated with vehicle or the PI3K inhibitor wortmannin (Wort, in B) for 1 hour. Subsequently cells were treated with vehicle, CD437 (10−6 mol/L), or the indicated combinations of the compounds for 1 hour. In A and B, the levels of the phosphorylated form of Akt (Aktp) protein or of total Akt protein were determined by Western blot analysis using aliquots of the same cellular extracts and specific antibodies. Western blot filters were subsequently challenged with an anti-actin antibody to confirm equal protein loading in each lane of the gel. In C, the proportion of apoptotic cells was determined after staining of nuclei with DAPI. Results are the mean ± SD of 3 separate culture dishes. a, significantly higher than the relative control cultures (CD437−, Wort−) according to the Student t test (P < .01); b, significantly higher than the relative CD437-treated cultures (CD437+, Wort−) according to the Student t test (P < .01). Data are representative of 2 independent experiments with similar results.
Effect of CD437 on the phosphorylated form of Akt protein in NB4 and NB4.437r cells. Consequences of the inhibition of Akt phosphorylation by wortmannin on CD437-induced apoptosis. (A) NB4 cells (5 × 105/mL) were treated with vehicle, CD437 (10−6 mol/L), the caspase inhibitor z-VAD (100 μmol/L), or the indicated combinations of the compounds for 1 hour or 4 hours. (B and C) NB4 or NB4.437r cells (5 × 105/mL) were preincubated with vehicle or the PI3K inhibitor wortmannin (Wort, in B) for 1 hour. Subsequently cells were treated with vehicle, CD437 (10−6 mol/L), or the indicated combinations of the compounds for 1 hour. In A and B, the levels of the phosphorylated form of Akt (Aktp) protein or of total Akt protein were determined by Western blot analysis using aliquots of the same cellular extracts and specific antibodies. Western blot filters were subsequently challenged with an anti-actin antibody to confirm equal protein loading in each lane of the gel. In C, the proportion of apoptotic cells was determined after staining of nuclei with DAPI. Results are the mean ± SD of 3 separate culture dishes. a, significantly higher than the relative control cultures (CD437−, Wort−) according to the Student t test (P < .01); b, significantly higher than the relative CD437-treated cultures (CD437+, Wort−) according to the Student t test (P < .01). Data are representative of 2 independent experiments with similar results.
Wortmannin inhibited Akt phosphorylation in NB4 (Figure 8B) and NB4.437r (visible only on longer exposure of the film) cells either in the presence or in the absence of CD437. The PI3K inhibitor had no effect on the amounts of Akt protein in our experimental conditions. Treatment of NB4 or NB4.437r blasts with wortmannin caused a similar and dose-dependent apoptogenic action (Figure 8C), indicating that in basal conditions Akt has a role in the survival of both cell types. In NB4 cells, the contemporaneous presence of wortmannin and the retinoid had an additive or less-than-additive effect relative to what was observed after treatment with each compound separately. In NB4.437r cells, the compound caused a similar level of apoptosis regardless of the presence of CD437 in the medium.
Discussion
The major findings of this study are the demonstration of the in vivo activity of CD437 using a preclinical model of APL and the development and biochemical characterization of a novel APL cell line made resistant to the retinoid.
As to the first point, the in vivo antileukemic effect of CD437 is predominantly the result of a rapid and massive apoptogenic action and is not the consequence of a cytodifferentiating phenomenon. CD437 apoptosis is accompanied by a cytostatic action that causes accumulation of cells in the G2/M phase of the cell cycle. This may also contribute to the antileukemic action of the compound in vivo. The data obtained on the SCID mouse xenografted with NB4 leukemic cells are promising, given the aggressive nature of this model and relative resistance to treatment with standard therapeutic agents such as ATRA52 and doxorubicin (Garattini E, unpublished results). The results are particularly interesting in consideration of the fact that, in the experiments presented, the treatment schedule was tailored so as to discontinue the administration of CD437 just before death of the control animals. Thus, it is likely that longer treatments result in greater increases in survival because the chronic administration of CD437, albeit at the 2 lower doses, is well tolerated and is not associated with weight loss or other signs of overt toxicity such as liver damage.
As to the second point, we developed an APL-derived cell line, NB4.437r, which is highly resistant to all the aspects of the apoptotic process induced by CD437. The CD437-resistance phenotype seems to be relatively stable because it is not reverted by a variety of compounds that include tyrosine kinase inhibitors such as genistein and herbstatin, glutathione-depleting agents such as buthionine sulfoxide, or differentiating compounds such as butyrate or ATRA. Cross-resistance experiments performed with a panel of apoptogenic compounds indicate that the observed resistance is relatively selective for CD437 and structural congeners, such as CD2325. Lack of cross-resistance to doxorubicin is of clinical interest because anthracyclines are used in the management of APL and other types of acute myelogenous leukemia. It is also reassuring that NB4.437r blasts are sensitive to arsenic, a compound that is gaining popularity in the second-line treatment of APL.37 At present, the significance of the slight level of cross-resistance of NB4.437r cells to the antimetabolite AraC and the alkylating agent cisplatin is uncertain. Regardless of this, our data indicate that CD437 is an apoptogenic agent that acts through a specific and novel mechanism of action substantially different from that of most of the other known cytotoxic agents. In addition, they suggest that the specific defect(s) responsible for CD437-resistance in NB4.437r cells does not involve primary alterations in the function or structure of a general pro-apoptotic or anti-apoptotic factor(s) but rather modifications in the “private” phase of the CD437-triggered apoptogenic pathway.
Given the chemical structure of CD437, it is of interest that NB4.437r cells are fully sensitive to the apoptogenic action of ATRA. The observation is in line with the fact that CD437 induces apoptosis in ATRA-resistant cellular contexts,3 6 and it indicates that the 2 retinoids exert their apoptogenic action through distinct intracellular signaling pathways. Sensitivity of NB4.437r cells to the pharmacologic action of ATRA is not limited to the induction of PCD but extends to growth arrest and, albeit incompletely, to cytodifferentiation. Regarding this last aspect, a selective deficit in the ATRA- and AM580-dependent expression of CD11b and CD11c is observed in the NB4.437r cell line compared with the normal counterpart. We do not know whether this phenomenon relates to the primitive molecular alteration(s) responsible for the resistance to CD437 or is a feature linked to clonal variation. Nevertheless, it is possible that there is a certain degree of cross talk between some of the intracellular pathways set in motion by CD437 and ATRA as well as other synthetic retinoids with cytodifferentiating properties.
Definition of the mechanism by which the CD437-dependent apoptogenic process is disrupted in the NB4.437r cell line is likely to give insight into the determinants of the sensitivity/resistance to the retinoid. Unlike what is observed for some of the ATRA-resistant NB4–derived cell lines, resistance to CD437 is not caused by alteration in the level of expression53 or in the structure of the ligand-binding site of PML-RARα.54 This is consistent with the fact that the apoptogenic action of CD437 in APL3 and other types of leukemic cells6 is independent of retinoic acid receptor activation. In addition, resistance is not caused by differences in pharmacokinetics because CD437 accumulation is similar in the NB4.437r and the parental cell lines, and this is the result of a similar intracellular distribution of the retinoid localized predominantly in the nuclear and mitochondrial fraction. Similarly, differences in metabolism are unlikely to underlie resistance because, in both cell lines, the majority of CD437 is present in its unmetabolized form. Furthermore, lack of sensitivity to CD437 does not seem to be caused by a multidrug-resistant (MDR) phenotype. In fact, pretreatment with verapamil, a drug known to revert MDR,50 does not sensitize NB4.437r cells to CD437 (Garattini E, unpublished results), and there is no difference in the levels of the MDR gene product between the CD437-resistant and parental cell lines (Garattini E, unpublished results). Finally, resistance is apparently not the result of differences in the level of expression of the nuclear orphan receptor protein Nur-77 (Garattini E, unpublished results), recently implicated as a mediator of CD437 toxicity in lung carcinoma cells.5
CD437-triggered PCD in NB4 cells is accompanied by the degradation and activation of many caspase isoforms. At present it is impossible to establish the order of activation of the various caspases on the basis of kinetic experiments because degradation of these proteins and the appearance of the corresponding enzymatic activities are rapid and almost synchronous. Nevertheless, activation of caspase-9 is consistent with cyt c relocalization and the formation of a tripartite complex with caspase-9 and APAF-1,43 which is likely to be, at least partially, responsible for the activation of caspase-3, -6, and -7. Furthermore, in our system, it is equally possible that caspase-8 is directly activated by CD437 through an as yet unknown pathway or is secondarily activated by other caspase isoenzymes, causing amplification of the apoptogenic response. Regardless of the relative importance that each member of the caspase family has in the process of PCD caused by CD437, our results suggest that these proteases are activated in a cascade fashion and are instrumental in effecting apoptosis through the cleavage of relevant substrates that may include the PML-RARα oncogene. In fact, apoptosis and degradation/activation of caspase -3, -6, -7, -8, and -9 are blocked by z-VAD in NB4 cells and are not observed in the CD437-resistant cell line. In NB4.437r cells, lack of caspase activation is accompanied by a lack of cyt c release from the mitochondria, a process that, in the case of CD437, seems to be independent of an alteration in the mitochondrial membrane potential. All these data indicate that resistance to CD437 in NB4.437r cells lays upstream of caspase activation and relocalization of cyt c into the cytosol. Furthermore, they suggest a central role for cyt c in the activation of caspases by CD437.
The NB4.437r cell line shows alterations in the levels and in the regulation of some molecular targets (NFkb, AP1, JNK, and Akt) activated by CD437 in the parental counterpart. All these molecular targets may mediate the apoptogenic action of CD437 in NB4 cells and may be involved in resistance to the retinoid in NB4.437r cells. In basal conditions, CD437-resistant blasts contain larger amounts of NFkb- and AP1-active complexes than CD437-sensitive cells. In addition, these transcriptional complexes are regulated by CHX in a different fashion in the 2 cell lines. Furthermore, in NB4.437r cells, CD437 does not increase AP1-binding activity, whereas it is still capable of inducing phosphorylation of JNK, a kinase that phosphorylates and activates the Jun proto-oncogene.50 This suggests that CD437 induction of the AP1 complex is either independent of JNK activation or that the process is impaired in NB4.437r cells downstream of the kinase. Finally, in the NB4 cell line, we observed a transitory rise in the levels of the phosphorylated form of the PI3K-dependent Akt kinase,54 which is completely blunted in the NB4.437r counterpart.
In the case of NFkb and AP1, our results can be interpreted in at least 2 ways. On one hand, NFkb is generally considered an anti-apoptotic determinant,47-49 and AP1 is often activated by growth/survival factors.55 Thus, it is possible that increased basal levels of the 2 complexes in NB4.437r cells have an anti-apoptotic function and determine, albeit incompletely, resistance to CD437. On the other hand, NFkb is activated by many apoptogenic stimuli56,57 and has been recently suggested to serve a pro-apoptotic function,58,59 whereas AP1 mediates CD437-dependent apoptosis in non–small-cell lung carcinoma and melanoma.5,9 Hence, activation of the 2 complexes in NB4 cells, but not in NB4.437r cells, may have pro-apoptotic significance. However, in the NB4 setting, assignment of a pro-apoptotic role to NFkb, AP1, or any other transcription factor must take into account the fact that CD437-induced cell death does not require de novo protein synthesis because it is CHX insensitive.3 6 CHX insensitivity is compatible with a pro-apoptotic function of NFkb and AP1 only if the 2 act as transcriptional repressors. In that case, increased gene expression and de novo protein synthesis would not be involved.
JNK is the only biochemical marker that is turned on by CD437 in both NB4 and NB4.437r cells and, thus, is unlikely to be involved in the mechanisms of resistance to the retinoid. Furthermore, JNK activation in both cell lines indicates that either the kinase does not play a significant role in CD437-dependent apoptosis or that NB4.437r cells are blocked in their apoptotic program downstream of this step. In this last event, impairment of an AP1-dependent pathway leading to apoptosis cannot be excluded. With reference to Akt, the kinase transduces survival signals inside the cell,51 and it is likely that its phosphorylation and activation by CD437 is the result of a simple adaptive response to the retinoid. In fact, the PI3K inhibitor wortmannin, which inhibits Akt, causes apoptosis and does not block CD437 action in NB4 cells. Moreover, it does not affect resistance to the retinoid in NB4.437r blasts.
In conclusion, the demonstration of CD437 in vivo antileukemic activity in the SCID/NB4 model of APL leukemia is a fundamental first step toward the introduction of CD437 into clinical practice. In addition, the novel CD437-resistant cell line described in this article is an important tool for the dissection of the molecular mechanisms underlying the apoptogenic action of the retinoid. In fact, NB4.437r cells show specific resistance to CD437 and its analog CD2325, suggesting that a fundamental determinant(s) of the apoptogenic program set in motion by this class of compounds has been inactivated. Although this determinant(s) has not yet been identified and further studies are necessary to define this point, the NB4 and the NB4.437r cell couple will certainly be useful in screening programs aimed at identifying congeners of CD437.
Acknowledgments
We thank Prof S. Garattini and Dr M. Salmona for critical reading of the article.
Supported by a grant from the Associazione Italiana and Fondazione Italiana per la Ricerca contro il Cancro (R.G. and E.G.) and by a grant from the Istituto Superiore di Sanità. I.P. is the recipient of a fellowship from La Via di Natale.
I.P. and M.G. contributed equally to the results presented in this article.
Reprints:Enrico Garattini, Laboratory of Molecular Biology, Istituto di Ricerche Farmacologiche Mario Negri, via Eritrea, 62, 20157 Milano, Italy; e-mail: egarattini@irfmn.mnegri.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal