Abstract
The importance of angiogenesis for the progressive growth and viability of solid tumors is well established. In contrast, only few data are available for hematologic neoplasms. To investigate the role of angiogenesis in acute myeloid leukemia (AML), bone marrow biopsies from 62 adults with newly diagnosed, untreated AML (day 0) were evaluated. Further studies were done after the completion of remission induction chemotherapy (day 16 of induction chemotherapy, n = 21; complete remission, n = 20). Microvessels were scored in at least 3 areas (×500 field, 0.126 mm2) of the highest microvessel density in representative sections of each bone marrow specimen using immunohistochemistry for von Willebrand factor and thrombomodulin. Microvessel counts were significantly higher in patients with AML (n = 62) compared with control patients (n = 22): median (interquartile range) 24.0 (21.0-27.8)/×500 field vs 11.2 (10.0-12.0)/×500 field, respectively (P < .001). On day 16 of induction chemotherapy, microvessel density was reduced by 60% (44-66) (P < .001) in hypoplastic marrows without residual blasts, in contrast to only 17% (0-37) reduction in hypoplastic marrows with ≥ 5% residual blasts (P < .001 for the difference between both groups). Bone marrow biopsies taken at the time of complete remission displayed a microvessel density in the same range as the controls. In conclusion, there is evidence of increased microvessel density in the bone marrow of patients with AML, which supports the hypothesis of an important role of angiogenesis in AML. Furthermore, these findings suggest that antiangiogenic therapy might constitute a novel strategy for the treatment of AML.
Formation of new blood vessels (angiogenesis) is an absolute requirement for the viability and growth of solid tumors.1,2 This neovascularization is mediated by angiogenic molecules released by tumor cells themselves and by accessory host cells such as macrophages, mast cells, and lymphocytes. In turn, the newly formed endothelial cells of the tumor can stimulate tumor growth in a paracrine fashion.3-5Furthermore, angiogenesis is important for the development of a malignant phenotype,6,7 and numerous studies8-17 have demonstrated that the vascular density of a tumor directly correlates with metastasis and patient outcome.
In contrast to solid tumors, few data are available regarding angiogenesis in hematologic malignancies. In multiple myeloma, bone marrow neovascularization correlates with disease activity,18,19 and in B-cell non-Hodgkin's lymphomas, a correlation of the degree of angiogenesis with the stage of the lymphoma was reported.20 Recently, an increased microvessel density has been demonstrated in the bone marrow of children with acute lymphoblastic leukemia (ALL).21
Until now, information concerning bone marrow neovascularization in acute myeloid leukemia (AML) has been limited to 2 recent abstract reports22 23 with conflicting results. The present study was undertaken to investigate the extent of angiogenesis in the bone marrow of adult patients with newly diagnosed, untreated AML. In addition, we evaluated the degree of neovascularization in the bone marrow of patients with AML following induction chemotherapy.
Materials and methods
Materials
Bone marrow specimens from 62 adult patients with newly diagnosed, untreated AML were studied. Diagnosis and classification of AML according to the criteria of the French-American-British (FAB) Cooperative Group24 were confirmed by centralized review of bone marrow morphology, cytochemistry, and immunophenotyping within the AML Cooperative Group (AMLCG).25 Patient characteristics, including AML FAB-subtypes, are depicted in Table1. A bone marrow core biopsy (iliac crest) for histological diagnosis was obtained from all patients at presentation (day 0). Additional biopsies were available from 30 patients for the studies following induction chemotherapy (day 16 of induction chemotherapy, n = 21; complete remission, n = 20). In 27 patients of this group, a biopsy had been performed at presentation so that comparisons of microvessel counts with specimens at day 0 could be performed. To establish controls, we studied bone marrow biopsies obtained at diagnosis, ie, before any chemo- or radiotherapeutic treatment, from 22 adult patients with various diseases but with normal bone marrow morphology. In case of non-Hodgkin's lymphomas, Hodgkin's disease, and solid tumors, the bone marrow was histologically not involved by the underlying disease (Table 1). After every core biopsy, a bone marrow aspiration was obtained through a separate puncture for cytological analyses.
Patient characteristics
| . | AML Patients (n = 62) . | Control Patients (n = 22) . |
|---|---|---|
| Age (y)* | 64 (17-84) | 60 (17-82) |
| Sex (males/females) | 35/27 | 15/7 |
| FAB distribution† | 2 M0, 8 M1, 21 M2, 2 M3, 10 M4, 14 M5, 4 M6, 1 M7 | — |
| Percentage of leukemic blasts* (bone marrow) | 80 (30-99) | — |
| Disease | — | 1 Hodgkin's disease |
| 6 Non-Hodgkin's lymphomas | ||
| 2 Solid tumors | ||
| 13 Nonmalignant disorders |
| . | AML Patients (n = 62) . | Control Patients (n = 22) . |
|---|---|---|
| Age (y)* | 64 (17-84) | 60 (17-82) |
| Sex (males/females) | 35/27 | 15/7 |
| FAB distribution† | 2 M0, 8 M1, 21 M2, 2 M3, 10 M4, 14 M5, 4 M6, 1 M7 | — |
| Percentage of leukemic blasts* (bone marrow) | 80 (30-99) | — |
| Disease | — | 1 Hodgkin's disease |
| 6 Non-Hodgkin's lymphomas | ||
| 2 Solid tumors | ||
| 13 Nonmalignant disorders |
Immunohistochemical staining
Bone marrow specimens were fixed in paraformaldehyde, embedded in paraffin, and decalcified with EDTA. Serial sections (4-μm–thick) of each sample were processed for immunohistochemical identification of microvascular endothelial cells with antihuman von Willebrand factor (vWF monoclonal antibody [MoAb], clone F8/86, Dako, Glostrup, Denmark; working dilution 1:25) and antihuman thrombomodulin antibodies (TM MoAb, clone 1009, Dako; working dilution 1:50). Anti-vWF antibodies are commonly used for highlighting endothelial cells,26,27although on paraffin sections, anti-CD31 antibodies have been suggested as first option on the basis of sensitivity/specificity.27,28 However, immunohistochemistry employing anti-CD31 (clone JC/70A, Dako) as well as anti-CD34 antibodies (clone QBEND 10, Immunotech, Marseille, France) was not further pursued because of the frequently observable strong staining of leukemic blasts in our study (data not shown). Because TM is constitutively expressed in high density by a restricted number of cells, including endothelial and mesothelial cells,29 we used TM as an endothelial marker. By applying anti-TM antibodies, we observed low background staining and a highly specific and intense labeling of endothelial cells. Controls for immunostaining using nonimmune mouse immunoglobulin G (IgG) (20 μg/mL; Sigma Chemical, St Louis, MO) in substitution for the specific first antibodies were consistently negative (data not shown).
Immunohistochemical localization was performed by the alkaline phosphatase/anti-alkaline phosphatase double bridge technique (Dako-APAAP Kit; Dako). Before staining, tissue sections were deparaffinized in xylene, rehydrated in a graded ethanol series, and permeabilized by treatment with 0.23% (w/v) pepsin (Sigma Chemical) for 6 minutes at 37°C. The primary antibodies were applied overnight at 4°C. Subsequent steps were performed according to the manufacturer's instructions. The fast red substrate (Dako) supplemented with 0.1% (w/v) levamisole was employed for revelation of phosphatase activity (30 minutes at room temperature). Sections were counterstained with 0.1% (w/v) erythrocin solution.
Microvessel counting
The degree of angiogenesis was determined by the microvessel density in defined areas of bone marrow sections according to the method of Weidner et al8 and an international consensus report.27 Microvessel counting was simultaneously assessed by 2 independent experienced investigators using light microscopy. The investigators were not aware of the diagnosis and clinical characteristics of the patient before performing the microvessel counting. The entire bone marrow section was systematically scanned, ie, field per field, at ×100 magnification to find the areas showing the most intense vascularization. The decision to start counting individual microvessels was based on observing restricted areas within a field at ×100 magnification with an impression of a higher density of vWF or TM antigen-positive cells and cell clusters relative to adjacent areas of the same field and to areas of the previous fields. The magnification was then changed to ×250, or to ×500, and the investigators were allowed to reposition the slide until the highest number of microvessels were within the ×500 field. This area was defined as a hot spot after achievement of a consensus between both investigators, thus reducing the interobserver error of microvessel counting.30,31 These vascular hot spots were, of course, only suitable for analysis provided they were within cellular areas of the marrow, because the noncellular areas (bone lamellae, fat and connective tissue areas, necrotic foci) are devoid of microvessels and hamper comparison between sections.18 Areas of vascularization adjacent to bone or dense connective tissue were also excluded, because vascularization is not representative of neoangiogenesis in these areas. In each hot spot, both investigators performed individual microvessel counting in a ×500 field (0.126 mm2 field area). In a slight modification of the method described by Weidner et al,8 any red staining endothelial cell or endothelial cell cluster, with or without a lumen, that was clearly separated from adjacent microvessels, blasts, and other bone marrow cells was considered as a single, countable microvessel. In addition to the endothelial cells, megakaryocytes were also strongly stained with anti-vWF and at lower intensity with anti-TM antibodies. However, these megakaryocytes were easily recognized by their characteristic size and morphology. Other TM- or vWF-positive staining cells were rarely found in the bone marrow samples and were also easily differentiated from endothelial cell positivity on the basis of morphological differences. In each biopsy sample, microvessels were counted in at least 3 independent hot spots per section (range 3-5) and in 2 to 3 sections stained with vWF as well as TM antibodies. The microvessel density of a bone marrow specimen was calculated as the mean value of all independent readings and recorded as the number of microvessels per ×500 field. The variability between the investigators for the microvessel counts was < 4.0% (± 3.5%). Analysis of serial marrow sections (n = 6) from a single biopsy sample revealed an intra-individual variability of less than 10% in the microvessel counts using anti-TM as well as anti-vWF antibodies (median [range], TM 4.0% [0.6%-7.4%]; vWF 4.1% [1.1%-9.6%]). Moreover, microvessel counts of 3 hot spots from the same bone marrow sections had a median coefficient of variation of 9.4% for vWF (range: 1.8%-36.7%) and 9.8% for TM (range:1.0%-33.0%) staining.
To confirm that the counting technique is truly representative of the total degree of angiogenesis in the bone marrow samples, we performed additional analyses in randomly selected AML (n = 20) and control (n = 10) patients. Three representative intertrabecular spaces covering cellular areas with intense vascularization were chosen in each bone marrow section at ×250 magnification comprising an area of 0.50 mm2/field. Subsequently, microvessels were counted over the complete field at ×500 magnification with the help of an ocular grid. Angiogenesis was expressed as the number of vessels counted in the total area of 1.50 mm2. This area comprised 30%-80% of the total cellular area in the bone marrow sections. Thus, the hot-spot area evaluated, according to the definition mentioned above, represented 7.5%-20% of the total cellular bone marrow cross-sectional area.
Quantification of leukemic blast infiltration and criteria for response to chemotherapy
Quantitative analysis of leukemic blast infiltration was performed in bone marrow aspirates by routine cytological analysis as described by the FAB Cooperative Group.24 A complete remission was defined as a bone marrow with normal hematopoiesis of all cell lines, less than 5% blast cells, and a peripheral blood count with at least 1500 neutrophils/μL, and 100 000 platelets/μL.32
Statistics
Data are presented as individual data plots or as medians, interquartile ranges (low quartile-high quartile [LQ-HQ]), and ranges. Differences in microvessel density between AML and control groups were analyzed by the Mann-Whitney rank sum test for independent groups. Statistical significance of overall differences between more than 2 groups was analyzed by the Kruskal-Wallis one-way analysis of variance. The Wilcoxon matched-pair signed rank test was used to compare the number of microvessels/×500 field in the bone marrow of individual patients between day 0, day 16 following induction chemotherapy, and complete remission. Correlation between variables was assessed by the Pearson's coefficient (r). Two-sided Pvalues of .05 or less were considered significant.
Results
At presentation (day 0), areas with intense neovascularization (hot spots) were widely distributed in cellular regions of the bone marrow from AML patients. Endothelial cell sprouts and microvessels without visible lumina prevailed in these samples, contrasting with the bigger and well-shaped microvessels in the controls (Figure1). When counting the number of vessels in these hot spots (area of 0.125 mm2), the bone marrow microvessel density was significantly increased in AML patients (n = 62) compared with control patients (n = 22): median (LQ-HQ) values for TM as well as vWF staining were 25.5 (22.1-29.3) and 22.9 (18.1-26.2) microvessels/×500 field in AML versus 13.2 (11.4-14.8) and 9.8 (7.7-10.5) microvessels/×500 field in the controls (P < .0001; Figure 2). The significant difference in the degree of bone marrow angiogenesis was confirmed when microvessel counts were obtained from a 12-fold larger cellular area of the bone marrow sections (1.50 mm2) in a subset of AML patients (n = 20) and control patients (n = 10): median (LQ-HQ) values for TM staining were 233 (192-294) microvessels/1.5 mm2 compared with 138 (131-147) microvessels/1.5 mm2 (P = .0001; Figure3). Furthermore, microvessel counts obtained by both methods strongly correlated with each other (r = .962, P < .0001; Figure 3 insert).
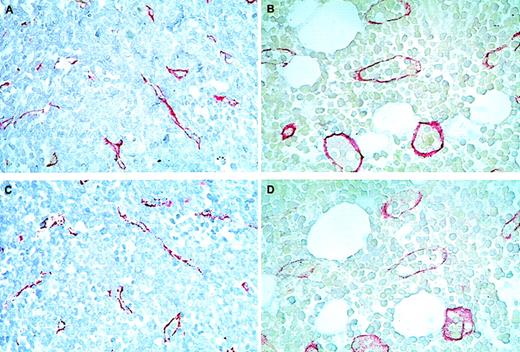
Immunohistochemical staining of bone marrow sections from patients with acute myeloid leukemia (AML) at presentation (A and C) and control patients (B and D) using antibodies against thrombomodulin (TM) (A and B) and von Willebrand factor (vWF) (C and D).
Note that endothelial cell sprouts and small microvessels without visible lumina were prevailing in AML sections (red staining in A and C), contrasting with big and well-shaped vessels in the controls (B and D). Original magnification ×500.
Immunohistochemical staining of bone marrow sections from patients with acute myeloid leukemia (AML) at presentation (A and C) and control patients (B and D) using antibodies against thrombomodulin (TM) (A and B) and von Willebrand factor (vWF) (C and D).
Note that endothelial cell sprouts and small microvessels without visible lumina were prevailing in AML sections (red staining in A and C), contrasting with big and well-shaped vessels in the controls (B and D). Original magnification ×500.
Microvessel density in the bone marrow from 62 patients with acute myeloid leukemia (AML) at presentation and from 22 control patients.
Microvessel quantification was performed in adjacent sections of each bone marrow biopsy stained in parallel for thrombomodulin (TM) (A) and von Willebrand factor (vWF) (B) as described in “Materials and methods.” Data are presented as individual values (open circles) and interquartile ranges (boxes). The difference in microvessel counts between the 2 groups was statistically significant (P < .0001 for TM as well as for vWF; Mann-Whitney rank sum test for independent groups).
Microvessel density in the bone marrow from 62 patients with acute myeloid leukemia (AML) at presentation and from 22 control patients.
Microvessel quantification was performed in adjacent sections of each bone marrow biopsy stained in parallel for thrombomodulin (TM) (A) and von Willebrand factor (vWF) (B) as described in “Materials and methods.” Data are presented as individual values (open circles) and interquartile ranges (boxes). The difference in microvessel counts between the 2 groups was statistically significant (P < .0001 for TM as well as for vWF; Mann-Whitney rank sum test for independent groups).
Microvessel counts of the bone marrow from 20 patients with acute myeloid leukemia (AML) and from 10 control patients expressed as the sum of microvessels in an area of 1.50 mm2[thrombomodulin (TM) staining].
The difference between groups was statistically significant (P = .0001; Mann-Whitney rank sum test for independent groups). The insert denotes the regression line (r = 0.960,P < .0001) for microvessel counts obtained using hot spots (mean value of three 0.125 mm2 fields) and using a larger area of 1.5 mm2 from the same bone marrow sections.
Microvessel counts of the bone marrow from 20 patients with acute myeloid leukemia (AML) and from 10 control patients expressed as the sum of microvessels in an area of 1.50 mm2[thrombomodulin (TM) staining].
The difference between groups was statistically significant (P = .0001; Mann-Whitney rank sum test for independent groups). The insert denotes the regression line (r = 0.960,P < .0001) for microvessel counts obtained using hot spots (mean value of three 0.125 mm2 fields) and using a larger area of 1.5 mm2 from the same bone marrow sections.
The pattern of angiogenesis in the bone marrow specimens was consistently similar when sections were stained with anti-TM or anti-vWF antibodies. Indeed, there was a strong correlation between microvessel counts in adjacent marrow sections stained with these endothelial cell markers (r = 0.875, P < .0001; Figure 4). Therefore, to enhance the validity of the scores, microvessel density in each sample was subsequently expressed as the mean value of microvessel counts obtained with both endothelial markers. Accordingly, the median (LQ-HQ) values for microvessel density in the bone marrow were 24.0 (21.0-27.8)/×500 field in the AML group and 11.2 (10.0-12.0)/×500 field in the control group (P < .001).
Relationship between microvessel counts per ×500 field in adjacent sections of the bone marrow stained for thrombomodulin (TM) or von Willebrand factor (vWF).
For each sample (62 patients with acute myeloid leukemia [AML] and 22 control patients), microvessel density measured by vWF staining was plotted against the microvessel density obtained with TM staining. Significance of the regression analysis was calculated by the Pearson test (r = 0.875, P < .0001).
Relationship between microvessel counts per ×500 field in adjacent sections of the bone marrow stained for thrombomodulin (TM) or von Willebrand factor (vWF).
For each sample (62 patients with acute myeloid leukemia [AML] and 22 control patients), microvessel density measured by vWF staining was plotted against the microvessel density obtained with TM staining. Significance of the regression analysis was calculated by the Pearson test (r = 0.875, P < .0001).
Microvessel counts were not related to age or sex of the patients in the AML group and in the control group (AML patients: < 64 vs ≥ 64 years, 22.9 vs 25.5; males vs females, 23.9 vs 23.8; control patients: < 60 vs ≥ 60 years, 9.5 vs 10.1; males vs females, 10.1 vs 9.1). Furthermore, statistical analysis did not reveal any significant differences in microvessel density between the AML FAB-subtypes (Kruskal-Wallis test: P = .139; Table2).
Bone marrow microvessel density according to the AML subtype
| AML Subtype (FAB Classification) . | Microvessel Counts* (×500 Field) . |
|---|---|
| M0 (n = 2) | 22.6 [9.7-35.4] |
| M1 (n = 8) | 23.9 [21.0-28.4] |
| M2 (n = 21) | 23.4 [21.5-27.0] |
| M3 (n = 2) | 18.0 [18.5-18.6] |
| M4 (n = 10) | 23.2 [20.2-24.2] |
| M5 (n = 14) | 25.9 [23.5-31.2] |
| M6 (n = 4) | 27.5 [26.5-28.2] |
| M7 (n = 1) | 29.2 |
| AML Subtype (FAB Classification) . | Microvessel Counts* (×500 Field) . |
|---|---|
| M0 (n = 2) | 22.6 [9.7-35.4] |
| M1 (n = 8) | 23.9 [21.0-28.4] |
| M2 (n = 21) | 23.4 [21.5-27.0] |
| M3 (n = 2) | 18.0 [18.5-18.6] |
| M4 (n = 10) | 23.2 [20.2-24.2] |
| M5 (n = 14) | 25.9 [23.5-31.2] |
| M6 (n = 4) | 27.5 [26.5-28.2] |
| M7 (n = 1) | 29.2 |
AML = acute myeloid leukemia; FAB = French-American-British.
Values represent medians and interquartile ranges. No significant differences between the groups were observed by Kruskal-Wallis analysis.
Bone marrow in the AML patients studied was usually highly infiltrated by blast cells. The median (LQ-HQ) percentage of blasts was 80% [50-90]. However, microvessel density in the biopsies of AML patients did not correlate with the percentage of blasts found in the marrow aspirates (r = −0.161; P = .212).
From 62 patients with AML enrolled in the study, a subgroup of 45 patients was chosen to investigate whether bone marrow angiogenesis at diagnosis may predict response to induction chemotherapy in terms of achieving a complete remission. This group was selected because these patients did not have secondary AML (ie, history of myelodysplasia, other antecedent hematologic disorder, previous exposure to cytostatic drugs or radiotherapy), did not die during treatment-induced bone marrow hypoplasia, and received standard induction chemotherapy according to the AMLCG protocol.25 Median (LQ-HQ) microvessel counts in bone marrow biopsies at presentation (day 0) were slightly higher in specimens from patients not achieving a complete remission after induction chemotherapy (25.5 [22.5-27.6]/×500 field; n = 12) compared with those achieving a complete remission (22.8 [19.3-25.0]/×500 field; n = 33). However, the difference was not statistically significant (Mann-Whitney test,P = .147).
To study the effect of chemotherapy on angiogenesis, follow-up biopsies from 30 patients with AML were available for the study on day 16 of induction chemotherapy (hypoplastic bone marrow; n = 21) and at the time of complete remission (n = 20). Bone marrow microvessel density significantly decreased from 24.0/×500 field on day 0 to a median of 10.8/×500 field on day 16 of induction chemotherapy using the TAD-protocol25 (standard-dose cytarabine, daunorubicin, and 6-thioguanine) (P < .001; Figure 5). Similarly, bone marrow biopsies obtained from patients after having achieved a complete remission displayed a significantly lower microvessel count (median: 12.4/×500 field) compared with initial diagnosis (P < .001; Figure 5). This decrease in microvessel density was observed regardless of whether bone marrow sections were stained with anti-TM or anti-vWF antibodies (data not shown). Apart from quantitative changes in the microvessel counts, changes in the morphology of microvessels were observed after chemotherapy as well. On day 16 of induction chemotherapy, microvessels were of big size with clearly discernible lumina. Endothelial sprouts, normally present on day 0, were completely absent after chemotherapy (Figure6). Microvessels in bone marrow biopsies taken at complete remission showed a morphology similar to those in the controls (not shown).
Effects of induction chemotherapy on microvessel density in the bone marrow of patients with acute myeloid leukemia (AML).
Microvessel counts are depicted for day 0 (presentation), day 16 of induction chemotherapy, the time of complete remission, and the control patients. Values are given as individual data points (mean values of microvessel counts for thrombomodulin [TM] and von Willebrand factor [vWF] staining). The difference of microvessel density for day 16 and complete remission was statistically significant compared with day 0 (P < .0001; Mann-Whitney test). On day 16, patients with at least 5% residual blast infiltration (closed circles) had higher microvessel counts than patients without blast infiltration (open circles; P < .001, Mann-Whitney test).
Effects of induction chemotherapy on microvessel density in the bone marrow of patients with acute myeloid leukemia (AML).
Microvessel counts are depicted for day 0 (presentation), day 16 of induction chemotherapy, the time of complete remission, and the control patients. Values are given as individual data points (mean values of microvessel counts for thrombomodulin [TM] and von Willebrand factor [vWF] staining). The difference of microvessel density for day 16 and complete remission was statistically significant compared with day 0 (P < .0001; Mann-Whitney test). On day 16, patients with at least 5% residual blast infiltration (closed circles) had higher microvessel counts than patients without blast infiltration (open circles; P < .001, Mann-Whitney test).
Immunohistochemical staining of marrows with anti-thrombomodulin (TM) antibodies on day 16 of induction chemotherapy.
(A) Section from a bone marrow without residual blast infiltration. (B) Section from a bone marrow with 10% residual leukemic blast infiltration. Note the bigger size and sinusoidal appearance of the microvessels compared with those on day 0 (Figure 1A and 1C). Original magnification ×500.
Immunohistochemical staining of marrows with anti-thrombomodulin (TM) antibodies on day 16 of induction chemotherapy.
(A) Section from a bone marrow without residual blast infiltration. (B) Section from a bone marrow with 10% residual leukemic blast infiltration. Note the bigger size and sinusoidal appearance of the microvessels compared with those on day 0 (Figure 1A and 1C). Original magnification ×500.
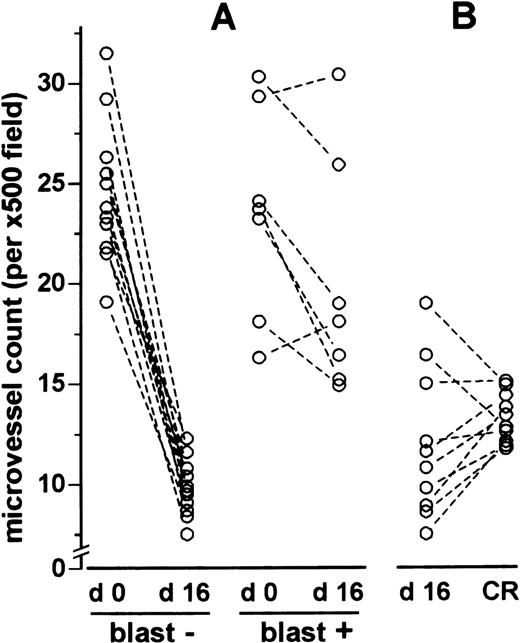
Bone marrows from patients with at least 5% residual leukemic blasts (median [range]: 10% [10-90]) on day 16 of induction chemotherapy showed higher microvessel counts than marrows without a significant blast infiltration (median [LQ-HQ]: 18.1 [15.7-22.4] vs 9.9 [9.8-11.6], P < .001; Figures 5 and 6). Individual comparisons between biopsy specimens taken at diagnosis (day 0) and on day 16 of induction chemotherapy revealed a 60% (44-66) reduction in microvessel density in hypoplastic marrows without residual leukemic blasts (n = 10; P < .001), in contrast to only 17% (0-37) reduction in hypoplastic marrows with at least 5% residual leukemic blasts (n = 7; P = .052; Figure7).
Microvessel density on day 0 compared with day 16 and day 16 compared with complete remission.
(A) Microvessel density on day 16 (d 16) of induction chemotherapy compared with presentation (d 0). (B) Pair comparisons of microvessel density between day 16 (d 16) of induction chemotherapy and the time of complete remission (CR). In (A) “blast –” refers to patients without residual leukemic blast infiltration and “blast +” to patients with at least 5% residual leukemic blast infiltration. In A and B, each pair of dots linked by a line represents the values from an individual patient. The difference in microvessel counts between day 0 and day 16 in the “blast –” group was statistically significant (P < .001, Wilcoxon test), in contrast to the “blast +” group (P = .052). No differences were found by Wilcoxon test (P = .185) between day 16 and the time of complete remission.
Microvessel density on day 0 compared with day 16 and day 16 compared with complete remission.
(A) Microvessel density on day 16 (d 16) of induction chemotherapy compared with presentation (d 0). (B) Pair comparisons of microvessel density between day 16 (d 16) of induction chemotherapy and the time of complete remission (CR). In (A) “blast –” refers to patients without residual leukemic blast infiltration and “blast +” to patients with at least 5% residual leukemic blast infiltration. In A and B, each pair of dots linked by a line represents the values from an individual patient. The difference in microvessel counts between day 0 and day 16 in the “blast –” group was statistically significant (P < .001, Wilcoxon test), in contrast to the “blast +” group (P = .052). No differences were found by Wilcoxon test (P = .185) between day 16 and the time of complete remission.
Microvessel counts in bone marrow specimens on day 16 of induction chemotherapy did not differ significantly from microvessel counts in bone marrow specimens at the time of complete remission. This result was obtained when differences were analyzed by the Mann-Whitney test for independent groups (day 16: n = 21, complete remission: n = 20;P = .441; Figure 5) as well as by individual pair comparisons, in case biopsy specimens from individual patients were available for both time points (n = 10;P = .185, Figure 7). However, a significantly higher microvessel density in the bone marrow at the time of complete remission was revealed when only patients without residual leukemic blast infiltration on day 16 were considered (n = 7;P = .014, data not shown).
Discussion
The present investigation has unequivocally demonstrated a significant increase of bone marrow microvessel density in patients with newly diagnosed, untreated AML compared with control patients. Indeed, the bone marrow of 75% of the patients with AML showed a 2- to 3-fold higher microvessel count than the median of the control group. This difference was independent of the field size in which microvessel counts were performed, thus underscoring that microvessel counting using hot spots is truly representative of the total degree of angiogenesis in the bone marrow samples. Together, these findings suggest that bone marrow angiogenesis might play an important role in the pathogenesis of AML.
Our results are in line with the reports of increased bone marrow microvessel density in pediatric patients with ALL21 and in adult patients with multiple myeloma18 compared with control subjects. Moreover, more intense vascularization has been described in B-cell non-Hodgkin's lymphoma compared with benign lymphadenopathies20 and in colorectal cancers compared with adjacent unaffected mucosa.33
In contrast to anti-vWF antibodies, immunostaining with anti-TM antibodies has not been described for quantification of angiogenesis up to now. Our study shows a strong correlation between microvessel counts obtained by anti-vWF and anti-TM antibodies, 2 highly specific endothelial cell markers. This correlation underscores the validity of our findings. Furthermore, staining with the anti-TM antibody displayed a slightly better sensitivity and a higher reproducibility for quantifying angiogenesis. Other groups have also reported that vWF, although highly specific for the vasculature, was partly absent in the capillary endothelium of tumor tissues.33 34 This finding and the higher focal background frequently observed with the vWF antibody (probably because of plasma vWF) may explain the lower microvessel counts found in bone marrow sections stained with this endothelial marker. We, therefore, suggest TM staining as a reliable tool for quantification of angiogenesis in paraffin-embedded AML bone marrow samples. Immunohistochemistry with anti-CD31 and anti-CD34 antibodies is not useful, because of the frequently observable strong staining of myeloid leukemic blasts.
The degree of angiogenesis found in our study with a median of 190 vessels/mm2 for AML patients and 89 vessels/mm2for control patients is in accordance with a recent report35 in multiple myeloma patients (mean: 294/mm2 vs 93/mm2 in the controls). However, these counts were approximately 2- to 3-fold higher than those obtained in pediatric ALL patients.21 The reasons for this apparent discrepancy may be the differences in age (adults vs children), the different sensitivities of the antibodies, or the magnification at which microvessel counting was performed. Indeed, a 2-fold higher microvessel density has been reported when the magnification was increased from ×200 to ×400 in CD31 stained breast cancer sections, provided microvessel density was expressed in microvessels/mm2.30
Of course, it cannot be excluded that the marked increase in microvessel counts in the bone marrow of patients with AML is related to reactivation of dormant marrow sinusoids that do not react with the anti-vWF antibody because of low-level vWF expression. This nonreactivity to the vWF antigen has been reported in normal hepatic sinusoids.36-40 These hepatic sinusoids become reactive to the vWF antibody in certain disorders of the liver, including chronic hepatitis, alcohol liver disease, and nodular regeneration.36-40 However, such a phenomenon has not been described for the TM antigen. Furthermore, the highly variable morphology of the microvessels with arborizing branching, the presence of endothelial sprouts without discernible lumina, the presence of hot spots, and the high microvessel density all suggest a truly neoangiogenic phenomenon. This pattern of angiogenesis is in accordance with the observations reported in pediatric patients with ALL.21
The mechanisms by which AML blasts induce angiogenesis, however, remain to be elucidated. Among the large number of identified angiogenic factors produced by tumor cells themselves and by accessory host cells, attention has recently focused on members of the fibroblast growth factor and vascular endothelial growth factor families as the most common angiogenic factors in tumors.41-43 These cytokines stimulate migration and proliferation of endothelial cells. Indeed, vascular endothelial growth factor expression by leukemic blasts has recently been demonstrated in patients with AML.44 An excess of urokinase plasminogen activator, a key enzyme for regulation of pericellular proteolysis and degradation of matrix proteins,45 has been found in the bone marrow of patients with AML.46 Furthermore, increased urinary basic fibroblast growth factor levels were reported in children with ALL.21Together, these data support the hypothesis of an important role of angiogenesis in acute leukemias.
The lack of correlation between microvessel density and the bone marrow blast count at diagnosis (day 0) may be due to the low variation of the percentage of blast infiltration in our study population (median: 80; HQ-LQ: 50-90) or to interindividual differences in expression of pro- and antiangiogenic factors by leukemic blasts. This observation is in line with the findings in multiple myeloma in which the degree of bone marrow angiogenesis did not correlate with the percentage of plasma cell infiltration.18
Numerous studies8-17 have demonstrated that the vascular density of a solid tumor directly correlates with metastasis and patient outcome. In multiple myeloma, microvessel density has been suggested as a new prognostic factor.18 In contrast, no differences in microvessel density at diagnosis have been found between relapsed and nonrelapsed leukemia in follow-up periods of more than 10 years in children with ALL.21 Similarly, our results suggest that bone marrow microvessel density at diagnosis cannot be used to predict the clinical outcome in terms of achieving a complete remission after induction chemotherapy. However, because of the relatively short follow-up periods in our patient population (range 5-24 months), we have not yet analyzed the prognostic value of microvessel density at presentation (day 0) for event-free and overall survival.
Following induction chemotherapy, we found a significant decrease in microvessel density on day 16 compared with day 0. This observation is in contrast to the data in pediatric patients with ALL. In this group, no significant change in microvessel density was observed in biopsies taken after 3 days of single-agent antileukemic treatment.21 This apparent contrast, however, may be explained by the greater intensity of the induction chemotherapy regimen employed for adult AML,25,47 as well as the longer interval between both bone marrow evaluations in our study (day 16 vs day 3). Nevertheless, as described in childhood ALL,21microvessels appeared dilated, empty, and with a sinusoidal form on day 16 of induction chemotherapy. This shape of the vessels might be attributed solely to reduced interstitial hypertension because of a reduction in cell density after intense cytoreductive chemotherapy. Although it has so far not been investigated in acute leukemias, interstitial hypertension is a well-known phenomenon in solid tumors.48 On the one hand, the appearance and the decrease in microvessel density could be the result of a direct or indirect toxic effect of chemotherapy on endothelial cells. Indeed, a direct toxic effect of chemotherapy on endothelial cells is conceivable. In response to a certain angiogenic stimulus, endothelial cells can leave their quiescent state and proliferate as fast as hematopoietic cells from the bone marrow or epithelial cells from the mucosa of the gastrointestinal tract.49,50 Thus, the observed decrease in microvessel density on day 16 of induction chemotherapy could be partially due to a direct cytotoxic effect on the fast proliferating endothelial cells. On the other hand, the observed higher microvessel counts at the time of complete remission compared with the hypoplastic bone marrow without residual blasts on day 16 of induction chemotherapy may be due to the recovery of endothelial cells from cytotoxic chemotherapy. Alternatively, the decrease in microvessel density on day 16 may indirectly be mediated by a loss of survival signals from leukemic blasts undergoing chemotherapy-induced apoptosis. Of course, both mechanisms could enhance each other since paracrine growth signal exchange between AML blasts and endothelial cells has been proposed.44 Therefore, antiangiogenic mechanisms of cytostatic chemotherapy may contribute to the eradication of leukemic cells. Indeed, direct toxic effects on endothelial cells as well as real antiangiogenic effects have been described in in vitro and in vivo models for different cytostatic agents (anthracyclines, vinca alkaloids, paclitaxel, bleomycin, and titanocene).51-55Microvessel counts of the bone marrow taken from patients at the time of complete remission remained low and were in the same range as the controls. This and the data obtained on day 16 of induction chemotherapy emphasize the strong association between the degree of angiogenesis and clinically overt AML disease. Furthermore, this is in accordance with the data from patients with ALL in which a mild, although statistically insignificant, decrease in microvessel counts after 1 month of chemotherapy has been reported.21
The marked difference in microvessel density on day 16 of induction chemotherapy between patients with and without residual leukemic blast infiltration underscores the strong correlation of angiogenesis with the persistence of AML disease. The higher degree of neovascularization in patients with persistent leukemia might reflect only continuous angiogenic stimulation of endothelial cells by residual leukemic blasts. Moreover, the higher number of endothelial cells could be explained by a lower individual susceptibility for the “antiangiogenic” effect of chemotherapy. Thus, the endothelial cells could, in turn, stimulate the growth of leukemic blasts in a paracrine manner. This growth might favor induction chemotherapy failures in these patients. Such a suggested paracrine interaction has indeed been shown in vitro. For example, porcine brain microvascular endothelial cells are able to promote the expansion of human hematopoietic progenitor cells in concert with hematopoietic growth factors.56 In addition, paracrine mechanisms between leukemic blasts and endothelial cells have been described for interleukin-1 and tumor necrosis factor,57 as well as for vascular endothelial growth factor and granulocyte-macrophage colony- stimulating factor.44
In summary, we have demonstrated that AML is associated with increased bone marrow microvessel density. Microvessel counts significantly decreased on day 16 of induction chemotherapy and remained in the same range as the controls at the time of complete remission. These results, together with the persistence of increased microvessel counts in patients with residual leukemic blast infiltration on day 16 of induction chemotherapy, strongly support the hypothesis of an important role of angiogenesis in AML. Furthermore, these findings suggest that antiangiogenic therapy could constitute a novel strategy for the treatment of AML.
Reprints:Rolf M. Mesters, Department of Medicine/Hematology and Oncology, University of Muenster, Albert-Schweitzer Str-33, D-48129 Muenster, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Fig. 3. Microvessel counts of the bone marrow from 20 patients with acute myeloid leukemia (AML) and from 10 control patients expressed as the sum of microvessels in an area of 1.50 mm2[thrombomodulin (TM) staining]. / The difference between groups was statistically significant (P = .0001; Mann-Whitney rank sum test for independent groups). The insert denotes the regression line (r = 0.960,P < .0001) for microvessel counts obtained using hot spots (mean value of three 0.125 mm2 fields) and using a larger area of 1.5 mm2 from the same bone marrow sections.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2637/4/m_bloo00807003x.jpeg?Expires=1764991430&Signature=xNUcuPiDzXHzshJVWkjkQ9WjlB~EN6cLSiwR9WcELG9ebi7BtdRNupNSRNLyJbkCol~XyghQn4acMb0K0ScNWHdkyO2JoI-~4gN1zvJKG54KHL5xgcKz6rPDSvUdGFPYLIFTGOA7JMd7fFqvAIcGf5ZDEDY8NAKSzREnPhFS112SoXdxfhvy33jbtGNs9xhFQHeoEr4DCXr383~ryzLeisXbcT2e2lT89JDW08Bvn8oUl2~SSY-nta-soxtYwtAWFP~8zeIHKLvJqawzM84-uv1~IU39Xg2xMtEEczVb16YjGMeWrv2up3BdLRwLM6ajXV79HTlzXL7362TVIo0P3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Relationship between microvessel counts per ×500 field in adjacent sections of the bone marrow stained for thrombomodulin (TM) or von Willebrand factor (vWF). / For each sample (62 patients with acute myeloid leukemia [AML] and 22 control patients), microvessel density measured by vWF staining was plotted against the microvessel density obtained with TM staining. Significance of the regression analysis was calculated by the Pearson test (r = 0.875, P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2637/4/m_bloo00807004x.jpeg?Expires=1764991430&Signature=OctI6OjsZCq3R9O4n00gWOyWNN~ShTSDXe-S6-3m-tBlZ0nc7dZzAcP9nAkdEhCg~Pobt-Q2zly9z0jHbRo17Rw~tCb54RXlw0h4hwxCVYK5EeyRtv-iprkMdW0KIybUVWAVfBENjgjRUEX5fb-2GLG9wd~C8K2QkwKfmOnH7c-QHmI1KKwIM-mPfnazX-OSb9aOLj37fYZZTjqD5F8JM2tgvFZ4PT3xICTa6p63kKRbs8SKcQcylXBMDpoZzVy93W4mgOWjICdNZgx9w11izVI3aeDzB3uoX0gCo2y4l7XC3Byh5wak6ZifO1myk-tJr5py2FfeMFIwjq99wPbKmg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effects of induction chemotherapy on microvessel density in the bone marrow of patients with acute myeloid leukemia (AML). / Microvessel counts are depicted for day 0 (presentation), day 16 of induction chemotherapy, the time of complete remission, and the control patients. Values are given as individual data points (mean values of microvessel counts for thrombomodulin [TM] and von Willebrand factor [vWF] staining). The difference of microvessel density for day 16 and complete remission was statistically significant compared with day 0 (P < .0001; Mann-Whitney test). On day 16, patients with at least 5% residual blast infiltration (closed circles) had higher microvessel counts than patients without blast infiltration (open circles; P < .001, Mann-Whitney test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2637/4/m_bloo00807005x.jpeg?Expires=1764991430&Signature=EV3iRu9A3uXeTHyrya41Qb6yNp7MuZrkMj3~HIcYOGuzWz653k4~55mjUAhexihJaV6Lq~VPLwLZjG96JMzCG5p3so686CB~pv83bc4xTMo4hQnOqTnpWNBPnLtxpssbIM0Hqroy3jmDZWanwlFtY-TOzJqqQuoNniMqL3bC~fv33VlXG79FcZxnxHgm98ulvfLQk4RXZmT3wmsDHaZKs75tsyPluaoT61WhsWBAzl~P9030OXiw8VFbe-MDPKzI~SYZyeAhc3u8o1YiCILxlWmJpUjb~bYVaptZaT1muQixSSc6HqFvGolm29~r6rLvQuSMuoIKYSXgqUPXP~QECA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal