Abstract
This study demonstrates that the human platelet F11 receptor (F11R) functions as an adhesion molecule, and this finding is confirmed by the structure of the protein as revealed by molecular cloning. The F11R is a 32-/35-kd protein duplex that serves as the binding site through which a stimulatory monoclonal antibody causes platelet aggregation and granule secretion. A physiological role for the F11R protein was demonstrated by its phosphorylation after the stimulation of platelets by thrombin and collagen. A pathophysiological role for the F11R was revealed by demonstrating the presence of F11R-antibodies in patients with thrombocytopenia. Adhesion of platelets through the F11R resulted in events characteristic of the action of cell adhesion molecules (CAMs). To determine the structure of this protein, we cloned the F11R cDNA from human platelets. The predicted amino acid sequence demonstrated that it is an integral membrane protein and an immunoglobulin superfamily member containing 2 extracellular C2-type domains. The structure of the F11R as a member of a CAM family of proteins and its activity in mediating adhesion confirm each another. We conclude that the F11R is a platelet-membrane protein involved in 2 distinct processes initiated on the platelet surface. The first is antibody-induced platelet aggregation and secretion that are dependent on both the FcγRII and the GPIIb/IIIa integrin and that may be involved in pathophysiological processes associated with certain thrombocytopenias. The second is an F11R-mediated platelet adhesion that is not dependent on either the FcγRII or the fibrinogen receptor and that appears to play a role in physiological processes associated with platelet adhesion and aggregation.
The pathophysiologic significance of circulating autoantibodies and alloantibodies directed against platelet membrane proteins has been observed in patients diagnosed with immune thrombocytopenia and posttransfusion purpura.1-11 These studies have pointed at the clinical importance of elucidating the mechanism of action of stimulatory antibodies that bind to the platelet surface and cause platelet activation. Among these are antibodies directed against the major platelet glycoproteins GPIIb/IIIa (CD42), CD9, CD69, and GPIV (CD36).12-20 Our studies have identified and characterized a monoclonal antibody (mAb), mAb.F11, that stimulates human platelets and acts as an agonist that induces platelet aggregation and granule secretion.21-25 The first step in the biochemical pathway by which mAb.F11 induces platelet aggregation and secretion was shown to be the recognition by mAb.F11 of a platelet surface protein duplex named the F11 receptor (F11R). The F11R was identified as 2 membrane proteins on the platelet surface with molecular masses of 32 kd and 35 kd.22 After the purification of the F11 receptor by ion exchange and affinity chromatography, it was found that N-deglycosylation of either the 32 kd or the 35 kd protein produces a core protein of 29 kd, recognizable by mAb.F11.23Subsequently, approximately one third the length of this core protein has been sequenced, including 26 amino acids of the N-terminus of this protein (SVTVHSSEPEVRIPENNPVKLSXAYS) and several fragments including 43 amino acids of an endoproteinase proteolytic fragment (WKFDQGDTTRLVEYNNKITASYEDRVTFLPTGITFKSVTRED)23 (NCB accession number S56749). Extensive database searches performed at that time determined that the F11 receptor represented a novel protein not reported previously.
In the process that induces platelet aggregation, the initial recognition of the F11R by mAb.F11 was shown to be coupled to the cross-linking of the antibody through the Fc domain to the platelet FcγRII receptor.23 This binding resulted in the activation of signal transduction pathways leading to a transient, reversible translocation of the protein kinase C (PKC) isozymes α and ζ from the cytoplasm to the membrane and a sustained, irreversible association of the PKC isoenzymes δ, β, n′, and θ with the plasma membrane.24 We have shown that this activation of the platelets' PKC isozymes by mAb.F11 is followed by increased intracellular phosphorylation of p47,22 which is the PKC substrate pleckstrin whose phosphorylation is required for platelet secretion.26 In addition, we have shown22 that platelet stimulation by mAb.F11 results in the increased phosphorylation of protein p20, the light chain of myosin that is a substrate for myosin light chain kinase that plays a role in platelet shape change.27 The ensuing aggregation of platelets induced by mAb.F11 was shown to involve activation of latent fibrinogen receptors, the GPIIb/IIIa integrin complex. This was demonstrated by the use of a functional anti-GPIIIa antibody that completely blocked the mAb.F11-induced platelet aggregation and secretion21 22and demonstrated for the first time a functional role for the GPIIb/IIIa integrin complex in a pathway that leads to the activation of granular secretion in platelets.
The elucidation of the biochemical pathways outlined above determined that the activation of platelets by mAb.F11 involved enzymatic mechanisms that are activated by physiological platelet agonists. However, by themselves, these studies did not reveal a physiological role for the F11 receptor, nor did they demonstrate directly the clinical significance of this platelet surface protein. The study reported here has been designed to provide information relevant to these important questions. Using mutually complementary approaches, functional studies of platelet activation, and molecular biologic techniques of gene cloning, we report here that the platelet F11 receptor is a cell surface adhesion molecule (CAM) of the immunoglobulin superfamily. The F11R appears to be involved in the activation of platelets by physiological agonists and by stimulatory antibodies; in addition, it participates in mechanisms underlying the adhesion of platelets and other hematopoietic cells. Finally, the finding in the plasma of patients with thrombocytopenia of antibodies that recognize the F11R points to the potential clinical importance of the investigation of this protein and suggests that peptides with sequences of critical sites in the F11R protein should be tested as novel therapeutic agents for the treatment of certain thrombocytopenias.
Materials and methods
Reagents
The reagents used for electrophoresis were purchased from Bio-Rad (Hercules, CA). The 5′- and 3′ Rapid amplification of complementary DNA ends (RACE) systems were purchased from GIBCO BRL (Rockville, MD), the AdvanTAge PCR Cloning kit was purchased from Clontech (Palo Alto, CA), the QIAprep Miniprep kit and the Rneasy Mini kit were purchased from Qiagen (Valencia, CA), and Taq DNA polymerase and restriction endonuclease EcoRI were purchased from Roche (Indianapolis, IN). Restriction endonucleasesKpnl, Sacl, and Smal, T4 DNA polymerase, T4 polynucleotide kinase, T4 ligase, and calf intestinal alkaline phosphatase were obtained from Promega (Madison, WI). Fetal bovine serum albumin (BSA) was obtained from HyClone (Logan, UT). Other reagents, of the highest grade, were obtained from Sigma (St Louis, MO).
Platelet isolation
Cell preparation and cell culture
HEL cells were purchased from the American Type Culture Collection (Rockville, MD). Megakaryocytic cell lines (CMK, CMK11-5, and CMK6) were the generous gift of Dr Hava Avraham (Beth Israel Deaconess Medical Center, Boston, MA). Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). Bovine aortic endothelial cells (BAEC) were obtained from Dr George Tuszynski (MCP Hahnemann University, Philadelphia, PA). The human endothelial hybrid cell line (Ea.hy 926) was obtained from Dr Cora-Jean S. Edgell (University of North Carolina, Chapel Hill, NC). The Eahy926 cells were cultured in DMEM containing 10% FBS and 100 μmol hypoxanthine, 0.4 μmol aminopterin, and 16 μmol thymidine. The K562 cell line was maintained in 15% FBS–RPMI medium.
Preparation of antibodies
mAb.F11 was identified and characterized as previously described.21,22 A polyclonal antibody directed to the 21-amino acid N–terminal sequence of the F11 receptor23(SVTVHSSEPEVRIPENNPVKL-Cys) was prepared in rabbits by Quality Controlled Biochemicals (Hopkinton, MA) and was subsequently purified by affinity chromatography.
Electrophoresis and immunoblotting
Samples were prepared for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) as follows: red blood cell ghosts were prepared as described.28 Cell extracts of HEL, K562, CMK, CMK6, and CMK11-5 cells were harvested, washed, and solubilized in Laemmli buffer. Eahy926, HUVEC, and BAEC cells were suspended in 10 mmol/L EDTA–phosphate-buffered saline (PBS), harvested, washed, suspended in Laemmli buffer, and processed for SDS-PAGE. Nitrocellulose membranes were incubated with the primary monoclonal (mAb.F11), rabbit polyclonal F11 receptor antibodies, or purified human antibodies, followed by incubation with secondary, rabbit or goat antimouse/human Ig–AP conjugates 1:4000, or donkey antirabbit antibodies conjugated with HRP 1:2000 (Amersham Pharmacia Biotech, Piscataway, NJ).
Labeling of human platelet with [32P]-orthophosphate
Washed platelets were labeled with [32P]-orthophosphate at 37°C for 60 minutes, then washed and resuspended in Tyrode's buffer as described previously.22
Flow cytometry
The CMK, CMK11-5, CMK6, HEL, and K562 cell lines were washed with PBS and resuspended in 0.1% BSA/PBS. HUVEC, Eahy926, and BAEC cells were treated with 10 mmol/L EDTA/PBS, washed, and resuspended in 0.1% BSA/PBS. To isolate the buffy coat fraction, whole blood was mixed with equal amounts of Hanks' balanced salt solution (HBS), overlaid on Ficoll, and centrifuged, and the buffy coat layer and RBC were collected separately. Cells were resuspended in 0.1% BSA/PBS (4 × 105 cells/sample) and incubated with mAb.F11 (5 μg/mL) or with an isotype-identical nonreactive IgG (control). After a 1-hour incubation at 22°C, the cells were washed with 0.1% BSA/PBS, treated with 50 μL 1/100 diluted goat antimouse Ig–FITC (GAM), incubated for 30 minutes on ice, washed, and resuspended in 0.1% BSA/PBS. The samples were analyzed using an Immunocytometry Systems flow cytometer (FAC Sort, Becton Dickinson, San Jose, CA). Flow cytometric analysis of isolated human platelets was performed in the presence of PGE1 (1 mg/mL).
Detection of platelet antibodies detecting the F11R in patients
Serum was obtained from a 64-year-old man with a history of hypertension after 2 separate cerebrovascular accidents within a 2-week period. Although workup revealed no obvious source of the infarcts (results of carotid Doppler imaging were normal, and no thrombus was detected), aspiration pneumonia developed and the patient was treated with antibiotics. After acute renal failure developed, dialysis was initiated. At this time, thrombocytopenia developed accompanied by a decrease in platelet count from an admission level of 266 000/mL to 50 000/mL. Bilateral gangrene then developed in both legs. Although the patient was administered heparin, it was only begun after the gangrene developed, thus excluding a diagnosis of heparin-induced thrombocytopenia. Severe thrombocytopenia continued, and the patient died 3 weeks after hospital admission because of overwhelming sepsis, respiratory failure, and cardiac failure. Another patient, a 40-year-old man with thrombocytopenia and end-stage renal disease secondary to hypertension, was examined for F11R antibodies in his circulatory system. He underwent cadaveric kidney transplantation and was administered the immunosuppressive drug tacrolimus (FK506). The patient is alive and has persistent thrombocytopenia.
RNA isolation and processing
Platelets were solubilized in lysis buffer (4 mol/L guanidine isothiocyanate, 25 mmol/L sodium citrate, 0.5% sodium sarcosine, and 100 mmol/L β-mercaptoethanol) dissolved in DEPC-treated water and processed according to standard purification techniques.29
Primers
The sequence of the degenerate primers was based on the published23 GLμ-C(4) fragment sequence: F11/f-6 (GARTAYAAYAAYAARATHAC), F11/r-5(A) (TTRAANGTDATNCCNGTAGG), F11/r-5(C) (TTRAANGTDATNCCNGTCGG), F11/r-5(G) (TTRAANGTDATNCCNGTGGG), and F11/r 5(T) (TTRAANGTDATNCCNGTTGG). Primers used for 3′ and 5′-RACE were GSP1 (AGCTTCCTATGAGGACCGGG), GSP2 (GTCACGGACTTGAAGGT), GSP3 (TTRAANGTDATNCCNGTTGG), and GSP4 (GGCAAGAAGGTCACCCGGTCC). The universal adaptor primer, the adaptor abridged universal amplification primer (AUAP), and the abridged anchor primer were provided with the RACE systems (GIBCO BRL).
All custom primers were synthesized by GIBCO BRL except for the F11/r-5 series (DNA International, Lake Oswego, OR) and the GSP1 (Ransom Hill Bioscience, Ramona, CA).
Reverse transcription–polymerase chain reaction with degenerate primers to obtain unique internal complementary DNA (cDNA) sequences
Reverse transcription (RT) was carried out using 1 μg total RNA and the oligo d(T)16 primers (Perkin Elmer [currently PE Biosystems], Foster City, CA) as detailed in the manufacturer's protocol. Polymerase chain reaction (PCR) was carried out using degenerate primer pairs derived from the published23partial amino acid sequence of the F11 receptor, termed the GLμ-C(4) fragment (final concentration of each primer, 2 pmol/μL). The dNTP and Mg2+ were present at 200 μmol/L and 2 mmol/L, respectively. The PCR mix was subjected to hot start at 99°C/10 minutes followed by addition of the Taq polymerase at 85°C. Thermocycling consisted of 5 cycles at 95°C/1 minute, 48°C/2 minutes, and 72°C/2 minutes, followed by 45 cycles at 94°C/1 minute, 48°C/2 minutes, and 72°C/2 minutes, followed by a final extension at 72°C/5 minutes.
DNA analysis by polyacrylamide gel electrophoresis
The DNA samples (PCR products or plasmid DNA) were separated by electrophoresis in 15% polyacrylamide gels and analyzed according to standard procedures.30
Purification and cloning of the amplified PCR products
After PAGE, the DNA fragment was eluted by the standard crush-and-soak method as described.30 The eluted DNA was digested with T4 DNA polymerase (Promega), and the resultant blunt-end product was phosphorylated at its 5′ ends using T4 polynucleotide kinase. The insert was then cloned into pBLUESCRIPT SK(+) (Stratagene, La Jolla, CA). The molar ratio of insert to vector was approximately 5:1. The subsequent construct was transformed into DH5a-competent cells (GIBCO BRL) as suggested by the manufacturer. The plasmid DNA was isolated using the Wizard Midipreps DNA purification system (Promega), and the presence of the cloned insert was confirmed by KpnI andSacI digestion of the purified plasmid DNA.
DNA sequencing
Isolated and purified plasmid DNA was sequenced by ACGT (Northbrook, IL). The fidelity of the data was confirmed by sequencing both strands using M13 universal forward and reverse primers.
3′ Rapid amplification of cDNA ends
3′-RACE was used for the amplification of nucleic acid sequences from an mRNA template between the internal site of the GLμ-C(4) fragment determined above and the 3′ end of the mRNA. Total platelet RNA (0.5 μg) was prepared as described above. RT was primed with the adaptor primer as described in the 3′-RACE system (GIBCO BRL). PCR was carried out using GSP1 and AUAP primers (35 cycles at 94°C/5 seconds, 58°C/15 seconds, and 72°C/3 minutes, followed by a final extension at 72°C/3 minutes). The PCR product was cloned into pT-Adv vector (Clontech) as described by the manufacturer. Two clones were sequenced in both directions, and the remaining 4 clones were sequenced on 1 strand.
5′ Rapid amplification of cDNA ends
Total RNA was isolated from freshly prepared human platelets using a RNeasy Mini Kit (Qiagen) as described in the manufacturer's RNeasy Mini Handbook. The 5′-RACE system for rapid amplification of cDNA ends (GIBCO BRL) was used to amplify the 5′ end of the F11 cDNA following procedures detailed in the manufacturer's manual. In brief, the first-strand cDNA synthesis was primed using GSP2 antisense. The cDNA was then purified using a GlassMax DNA Isolation Spin Cartridge (GIBCO BRL, Rockville, MD). A homopolymeric C-tail was subsequently added to the 3′-cDNA end, and the cDNA was amplified by PCR using GSP3 and AUAP (45 cycles at 94°C/30 seconds, 48°C/1 minute, and 72°C/3 minutes, followed by a final extension at 72°C/3 minutes). The 100-fold diluted primary products were reamplified using a nested GSP4 primer and AUAP (35 cycles at 94°C/30 seconds, 55°C/1 minute, and 72°C/3 minutes, followed by a final extension at 72°C/3 minutes). The resultant PCR products were processed as described above for the 3′-RACE system. Two clones were sequenced in both directions, and 1 clone was sequenced on 1 strand.
Data analysis
The translated amino acid sequence of the platelet F11 receptor was analyzed using the following on the Internet: TMpred, FASTA, ScanProsite, PfamHMM, PSORT, SSPRED, ProfileScan, ProtScale, PatScan, pI/Mw, and Motif (available through Pedro's BioMolecular Research Tools athttp://www.public.iastate.edu/∼pedro/ rt_1.html), SignalP (http://www.cbs.dtu.dk/services/SignalP/output.html), Pfam (The Sanger Centre, http://www.sanger.ac.uk), and Simple Modular Architecture Research Tool (SMART) (http://coot.emblheidelberg.de/predict protein).
Results
F11R-mediated platelet adhesion and spreading
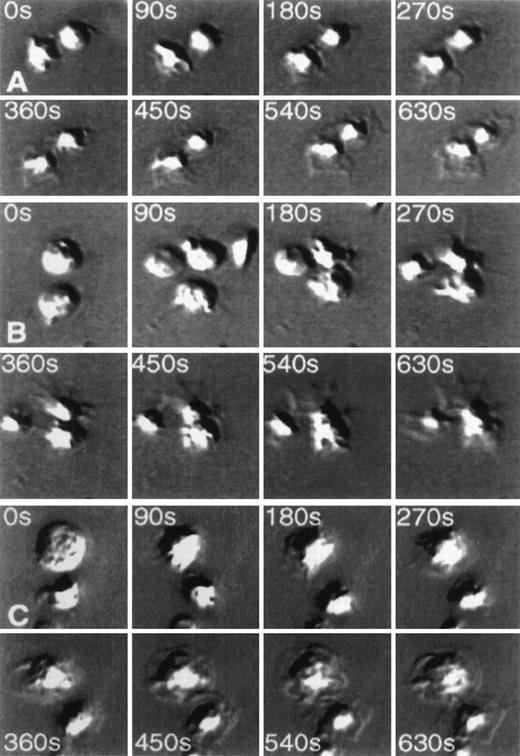
The dramatic change in the morphology of platelets after their adherence to an antibody mAb.F11-coated matrix is demonstrated in Figures 1A to 1C, showing video frames of the antibody-induced shape changes in a time-dependent manner under 3 different conditions. Figure 1A follows the adhesion of platelets to the antibody-coated surface in the absence of added inhibitors. It can be seen that after adhesion to the matrix, the platelets developed lamellipodia and filopodia, and the spreading of platelets through these structures could be observed within minutes. These morphologic changes were not inhibited by the fibrinogen receptor inhibitory peptide RGDS (Figure 1B) or by the presence of an antibody to the FcγRII receptor mAb.IV.3 (Figure 1C).
Time-lapse video photographs showing spreading of platelets on monoclonal antibody mAb.F11-coated glass coverslips.
Videos were taken every 5 seconds. Selected frames at 8 different time points are displayed from time 0 (at the instance of platelet application to mAb.F11-coated coverslips to 10.5 minutes later. (A, 0-630 seconds) Demonstrates the spreading of platelets on the mAb.F11-coated surface. (B, 0-630 seconds) Spreading of platelets on the mAb.F11-coated surface in the presence of 300 μmol/L RGDS. (C, 0-630 seconds) Demonstrates the ability of platelets to spread on mAb.F11-coated surfaces in the presence of mAb.IV.3. Note: in the absence of the coated mAb.F11 IgG, the platelets did not adhere onto the surface.
Time-lapse video photographs showing spreading of platelets on monoclonal antibody mAb.F11-coated glass coverslips.
Videos were taken every 5 seconds. Selected frames at 8 different time points are displayed from time 0 (at the instance of platelet application to mAb.F11-coated coverslips to 10.5 minutes later. (A, 0-630 seconds) Demonstrates the spreading of platelets on the mAb.F11-coated surface. (B, 0-630 seconds) Spreading of platelets on the mAb.F11-coated surface in the presence of 300 μmol/L RGDS. (C, 0-630 seconds) Demonstrates the ability of platelets to spread on mAb.F11-coated surfaces in the presence of mAb.IV.3. Note: in the absence of the coated mAb.F11 IgG, the platelets did not adhere onto the surface.
Platelet activation induces phosphorylation of the F11 receptor
Figure 2 demonstrates that together with the mAb.F11-induced platelet shape change and aggregation, stimulation of the F11 receptor resulted in phosphorylation of the receptor protein duplex, shown in Figure 2D. Moreover, not only stimulation by the F11 antibody but also platelet stimulation by the physiological agonists thrombin (Figure 2B) and collagen (Figure 2C) resulted in an increase in the phosphorylation state of the F11 receptor, with enhanced phosphorylation of both the 32 kd and the 35 kd glycosylated forms of the F11R protein.
Phosphorylation of the F11 receptor after activation of human platelets by thrombin, collagen, and mAb.F11.
Human platelets were incubated with [32P] for 1 hour as described in “Materials and methods” and as previously described.22 Washed platelet suspensions were activated by thrombin, collagen, or mAb.F11 and solubilized by the addition of NP-40 as described.22 Detergent extracts were immunoprecipitated with mAb.F11-coupled Sepharose (A to D) or with uncoupled Sepharose (E to H).22 The autoradiogram demonstrates the immunoprecipitates obtained from platelets treated under the following conditions: A and E, control, nonstimulated; B and F, mAb.F11-stimulated; C and G, thrombin-stimulated; D and H, collagen-stimulated platelets.
Phosphorylation of the F11 receptor after activation of human platelets by thrombin, collagen, and mAb.F11.
Human platelets were incubated with [32P] for 1 hour as described in “Materials and methods” and as previously described.22 Washed platelet suspensions were activated by thrombin, collagen, or mAb.F11 and solubilized by the addition of NP-40 as described.22 Detergent extracts were immunoprecipitated with mAb.F11-coupled Sepharose (A to D) or with uncoupled Sepharose (E to H).22 The autoradiogram demonstrates the immunoprecipitates obtained from platelets treated under the following conditions: A and E, control, nonstimulated; B and F, mAb.F11-stimulated; C and G, thrombin-stimulated; D and H, collagen-stimulated platelets.
Cloning of the platelet F11R cDNA
Based on the previously determined sequence of the F11R GLμ-C(4) fragment WKFDQGDTTRLVEYNNKITASYEDRVTFLPTGI- TFKSVTRED,23 4 degenerate oligonucleotide primers F11/r5(A), F11/r-5(C), F11/r5(G), and F11/r5(T) and the forward primer F11/f-6 were designed. The 3′ part of the GLμ-C(4) cDNA was obtained as described in “Materials and methods.” On analysis of its sequence, we determined that the translated amino acid sequence of the internal section of this product was identical to the internal amino acid sequence of the published23 GLμ-C(4) fragment, as detailed above.
To obtain the full F11 cDNA sequence, we used 5′- and 3′-RACE techniques. The 3′-RACE procedure used a 5′ primer based on the nondegenerate sequence determined by PCR based on the reverse translation of the middle portion of the GLμ-C(4) fragment. The sequence of the 5′ end of the 3′-RACE clones was consistent with the predicted (degenerate) sequence obtained by reverse translation of the C-terminal and the GLμ-C(4) fragment.
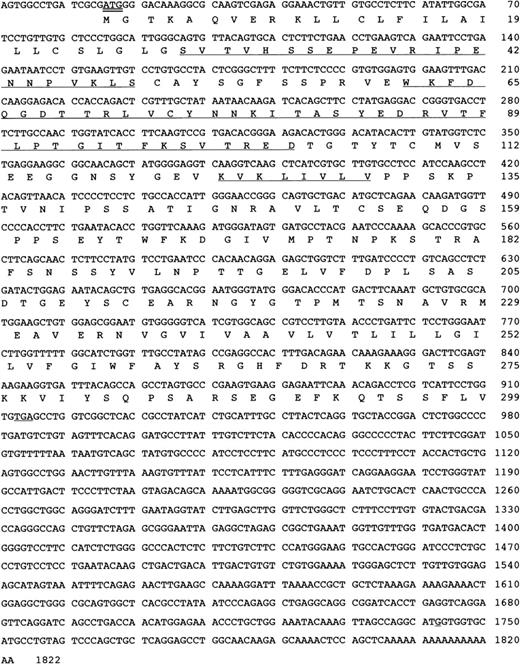
5′ RACE was carried out as described using nested primers, the sequences of which were directly derived from the 5′ end of the clone obtained in 3′-RACE. Analysis of the products revealed a reading frame containing the previously determined N-terminus of the F11R23 and the 26 N-terminal amino acids of the GLμ-C(4) fragment.23 The protein N-terminus was preceded by a signal sequence with a translation initiation codon having a consensus flanking sequence PuXXAUGG.31 32 The complete platelet F11R cDNA and the deduced amino acid sequences are shown in Figure3.
Human platelet F11 receptor cDNA sequence and deduced amino acid sequence.
The N-terminal amino acid sequence of the F11R (SVT … KLS) and internal sequences of the GLμ-C(4) fragment (WKF … TRE) and the GLμ-C(2) fragment (KVK …VLV) as reported by us23 are underlined. The start codon is indicated by a double line. The GenBank accession number for the nucleotide sequence of the human platelet F11 receptor isAF207907.
Human platelet F11 receptor cDNA sequence and deduced amino acid sequence.
The N-terminal amino acid sequence of the F11R (SVT … KLS) and internal sequences of the GLμ-C(4) fragment (WKF … TRE) and the GLμ-C(2) fragment (KVK …VLV) as reported by us23 are underlined. The start codon is indicated by a double line. The GenBank accession number for the nucleotide sequence of the human platelet F11 receptor isAF207907.
Analysis of the platelet F11R amino acid sequence
Features of the F11R apparent from analysis of the amino acid sequence are summarized in Table 1. The F11R expressed in human platelets is encoded by an mRNA of 1822 bases that on translation yielded a mature protein of 272 amino acids without its signal peptide. The estimated molecular mass of the mature protein is 29 682 d, and the estimated pI value equals 6.72.33 34
Properties of the platelet F11 receptor obtained by analysis of its amino acid composition deduced from the F11R cDNA sequence
| Properties . | Amino Acids . |
|---|---|
| Molecular weight | 29.682 kd |
| Isoelectric point | 6.72 |
| Transmembrane helices | 12-29 (inside → outside) |
| 239-258 (outside → inside) | |
| Cleavable N-term signal sequence | 1-27 |
| Cytoplasmic tail | 255-299 |
| N-glycosylation site | Asn-185 |
| cAMP- or cGMP-dependent protein kinase phosphorylation site | Thr-273 |
| PKC phosphorylation sites | Ser-57 Thr-269 |
| Thr-69 Ser-274 | |
| Thr-95 Ser-275 | |
| Ser-179 Ser-284 | |
| Casein kinase II phosphorylation sites | Ser-34 Ser-154 |
| Ser-82 Thr-193 | |
| Thr-100 Ser-203 | |
| Ser-118 Ser-287 | |
| Thr-152 | |
| Tyrosines in the cytoplasmic tail | Tyr-260 Tyr-279 |
| Properties . | Amino Acids . |
|---|---|
| Molecular weight | 29.682 kd |
| Isoelectric point | 6.72 |
| Transmembrane helices | 12-29 (inside → outside) |
| 239-258 (outside → inside) | |
| Cleavable N-term signal sequence | 1-27 |
| Cytoplasmic tail | 255-299 |
| N-glycosylation site | Asn-185 |
| cAMP- or cGMP-dependent protein kinase phosphorylation site | Thr-273 |
| PKC phosphorylation sites | Ser-57 Thr-269 |
| Thr-69 Ser-274 | |
| Thr-95 Ser-275 | |
| Ser-179 Ser-284 | |
| Casein kinase II phosphorylation sites | Ser-34 Ser-154 |
| Ser-82 Thr-193 | |
| Thr-100 Ser-203 | |
| Ser-118 Ser-287 | |
| Thr-152 | |
| Tyrosines in the cytoplasmic tail | Tyr-260 Tyr-279 |
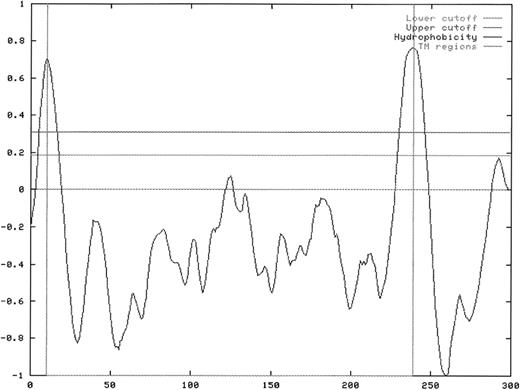
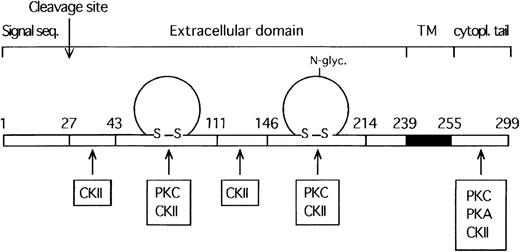
Further database searches of potential membrane localization and orientation sites have determined that the F11R is an integral membrane protein with a potential transmembrane domain in amino acids 239 to 258, a cytoplasmic-oriented C-terminus (amino acids 255 to 299), and an extracellular N-terminus (28 to 239), and it contains a cleavable signal sequence corresponding to amino acids 1 to 27.35,36In addition, these analyses indicated that the F11R contains 1 potential N-glycosylation site.37-39 The cytoplasmic tail of the F11R was shown to contain 2 phosphorylable tyrosine residues at positions 260 and 279 and putative intracellular and extracellular sites of phosphorylation by casein kinase II,40 protein kinase C,41,42 and cAMP- or cGMP-dependent protein kinases.43,44 The extracellular sites suggest that the F11R is a substrate for ecto-protein kinases found in platelets.45-47 Hydrophobicity analysis of the platelet F11R amino acid sequence, shown in Figure4, was performed using the ProtScale program following the method of Kyte and Doolittle.48 We identified the positions of the membrane-spanning domain and the signal peptide.
Hydrophobicity plot of the deduced amino acid sequence of the platelet F11 receptor.
The transmembrane domain of the F11R was found in the region spanning amino acids 239 to 258 (marked with a vertical line in the center of the peak). The signal peptide sequence is found at amino acids 1 to 27.
Hydrophobicity plot of the deduced amino acid sequence of the platelet F11 receptor.
The transmembrane domain of the F11R was found in the region spanning amino acids 239 to 258 (marked with a vertical line in the center of the peak). The signal peptide sequence is found at amino acids 1 to 27.
Platelet F11R is a member of the immunoglobulin superfamily
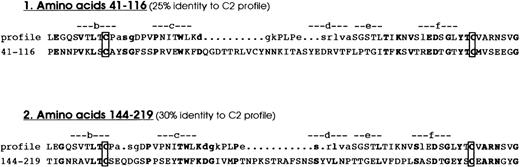
A combined literature and protein database search of sequences homologous to the F11R revealed that this protein exhibits 2 regions (amino acids 43 to 111 and 146 to 214) of high homology to members of the immunoglobulin superfamily (IgSF).49-54 These 2 amino acid sequences, representing putative Ig domains, were compared to the conserved patterns for Ig folds using the SMART program. Based on their length, sequence similarity, and secondary structure analysis, the F11R was found to be comprised of 2 C2-type immunoglobulin domains (amino acids 43 to 111 and 46 to 193), shown in Figure 5. Localization of the C2 Ig domains within the structure of the F11R is illustrated in the schematic model shown in Figure6.
The platelet F11 receptor is a member of the immunoglobulin family.
The platelet F11R contains 2 C2-type immunoglobulin domains. This figure shows the alignment of the C2-type immunoglobulin domain consensus sequence profiles with the F11R at amino acids 41 to 116 (profile 1) and with the F11R at amino acids 144 to 219 (profile 2). The conserved cysteine residues are boxed.
The platelet F11 receptor is a member of the immunoglobulin family.
The platelet F11R contains 2 C2-type immunoglobulin domains. This figure shows the alignment of the C2-type immunoglobulin domain consensus sequence profiles with the F11R at amino acids 41 to 116 (profile 1) and with the F11R at amino acids 144 to 219 (profile 2). The conserved cysteine residues are boxed.
Schematic model illustrating the main deduced structural features of the platelet F11 receptor.
TM indicates the transmembrane region of F11R (shaded region). Phosphorylation sites are indicated and further detailed in Table 1.
Schematic model illustrating the main deduced structural features of the platelet F11 receptor.
TM indicates the transmembrane region of F11R (shaded region). Phosphorylation sites are indicated and further detailed in Table 1.
Immunologic detection of the F11 receptor in platelet membranes using antibodies directed to the N-terminal sequence
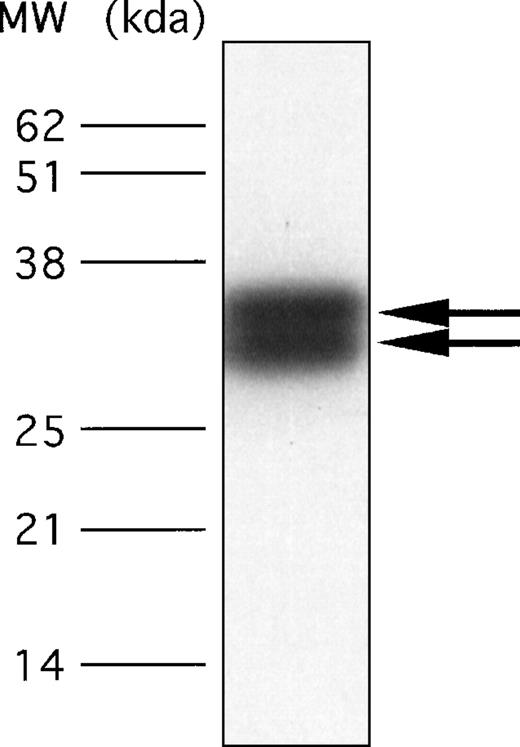
To confirm that a protein with the cloned sequence shown in Figure 3 is a constituent of the human platelet, we developed a polyclonal antibody against a synthetic peptide containing 21 amino acids with the sequence of the N-terminal portion. As shown in Figure 7, immunoblotting of platelet proteins with this polyclonal antibody revealed in platelet membranes the presence of 2 protein bands migrating with Mr of 32 kd and 35 kd These bands comigrated exactly with the 2 proteins immunostained by mAb.F11. In contrast to mAb.F11, which induces platelet aggregation and secretion, the polyclonal antibody to the N-terminal of the F11R did not induce platelet aggregation, suggesting that the stimulatory epitope recognized by mAb.F11 is not found in the N-terminal region of this molecule.
Detection of the F11 receptor in human platelets by use of a polyclonal antibody directed against the 21 amino acid sequence of the N-terminus of the purified F11R.
Total platelet proteins, solubilized from approximately 2 × 107 platelets, were resolved on 15% SDS-PAGE. For immunoblotting, an anti-F11R polyclonal antibody, developed against the N-terminal sequence as detailed in “Materials and methods,” was used at a concentration of 20 μg/mL. The arrows point to the 32-kd and 35-kd glycoproteins.
Detection of the F11 receptor in human platelets by use of a polyclonal antibody directed against the 21 amino acid sequence of the N-terminus of the purified F11R.
Total platelet proteins, solubilized from approximately 2 × 107 platelets, were resolved on 15% SDS-PAGE. For immunoblotting, an anti-F11R polyclonal antibody, developed against the N-terminal sequence as detailed in “Materials and methods,” was used at a concentration of 20 μg/mL. The arrows point to the 32-kd and 35-kd glycoproteins.
Cellular distribution of the platelet F11R
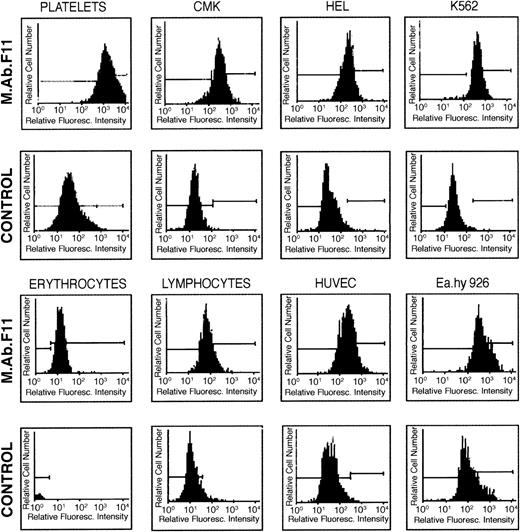
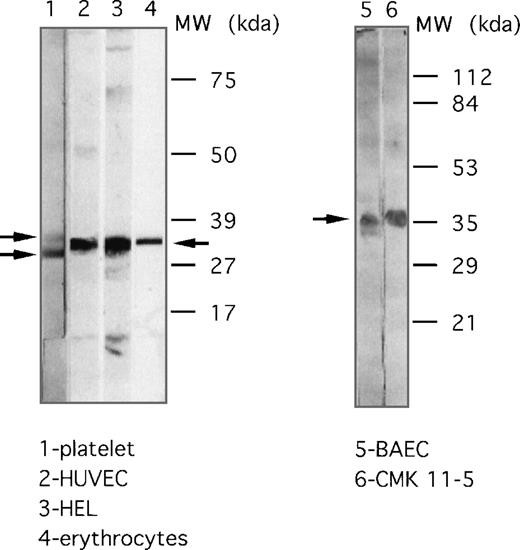
Fluorescence flow cytometry and immunoblotting procedures using mAb.F11 were used to examine the expression of the F11R in various hematopoietic cells and cell lines. As shown in Figure8, significant F11R was detected in parent CMK cells and subclones CMK6 and CMK11-5, HEL, K562, primary HUVEC, a human endothelial hybrid cell line (Ea.hy 926), erythrocytes, and lymphocytes. Consistent with these observations was the identification of several glycosylated forms of the F11R by immunoblotting. As shown in Figure 9, the F11R was detected in human platelets as 2 proteins of 32 kd and 35 kd, a single protein of 35 kd in red blood cells, and a broad protein band of 33 to 36 kd in HUVEC and bovine aortic endothelial cells, and K562 cells. In addition, mAb.F11 detected a broad 33- to 36-kd band in human lymphocytes (data not shown).
Cellular distribution of the F11 receptor as determined by flow cytometry.
Each cell type was incubated with mAb.F11 (5 mg/mL), followed by the addition of the secondary antibody GAM IgG–FITC. Cells were analyzed by FAC Sort flow cytometry. As controls, cells were incubated with nonimmune mouse IgG, followed by incubation with the secondary antibody. Cells considered positive for F11R exhibited a mean fluorescence intensity of 40 channels higher than control groups. Each figure is representative of 5 experiments.
Cellular distribution of the F11 receptor as determined by flow cytometry.
Each cell type was incubated with mAb.F11 (5 mg/mL), followed by the addition of the secondary antibody GAM IgG–FITC. Cells were analyzed by FAC Sort flow cytometry. As controls, cells were incubated with nonimmune mouse IgG, followed by incubation with the secondary antibody. Cells considered positive for F11R exhibited a mean fluorescence intensity of 40 channels higher than control groups. Each figure is representative of 5 experiments.
Cellular distribution of the F11 receptor as determined by immunoblotting.
Expression of the F11R in platelets (lane 1), endothelial cells HUVEC (lane 2), HEL cells (lane 3), human erythrocytes (lane 4), endothelial BAEC (lane 5), and CMK 11-5 cells (lane 6). Proteins were separated on 5% to 15% polyacrylamide gel (lanes 1 to 4) or by use of 12% SDS-PAGE (lanes 5, 6). Immunoblotting was performed using mAb.F11 (4 μg/mL).
Cellular distribution of the F11 receptor as determined by immunoblotting.
Expression of the F11R in platelets (lane 1), endothelial cells HUVEC (lane 2), HEL cells (lane 3), human erythrocytes (lane 4), endothelial BAEC (lane 5), and CMK 11-5 cells (lane 6). Proteins were separated on 5% to 15% polyacrylamide gel (lanes 1 to 4) or by use of 12% SDS-PAGE (lanes 5, 6). Immunoblotting was performed using mAb.F11 (4 μg/mL).
Identification of antibodies recognizing the F11R protein in the circulation of patients with thrombocytopenia
In a survey of antibodies recognizing platelet antigens in a patient population, the presence of F11R antibodies was detected in the circulation of 2 patients with thrombocytopenia. As shown in Figure 10, antibodies isolated from 2 patients with thrombocytopenia (lanes 3 and 4) detected a protein comigrating with the F11R. The position of both the 32-kd and the 35-kd proteins, as recognized by mAb.F11 antibody, is shown in lane 1. As control, isolated Ig fractions and plasmas obtained from healthy donors (lanes 5 to 7) did not contain F11R antibodies as evidenced by a complete lack of binding of these fractions to either the 32-kd or the 35-kd proteins or to any other platelet membrane protein.
Identification of F11 receptor antibodies in the circulation of patients.
The F11 receptor (5 μg applied/lane) was purified from platelet plasma membranes using the affinity chromatography procedures previously described1 2 and resolved by SDS-PAGE. Immunoblotting was performed using the following antibodies: lane 1, incubation with monoclonal antibody mAb.F11 (4 μg/mL); lane 2, control, incubation with only 2° antibody, rabbit antimouse immunoglobulin; lane 3, incubation with the purified immunoglobulin fraction obtained from the serum of a patient with thrombocytopenia as described in “Materials and methods” (25 μg/mL); lane 4, incubation with plasma from a patient with thrombocytopenia after kidney transplantation, as described in “Materials and methods”; lanes 5 and 6, incubation with plasma obtained from 2 healthy donors and used for immunoblotting; lane 7, control using 2° antibody, rabbit antihuman immunoglobulin.
Identification of F11 receptor antibodies in the circulation of patients.
The F11 receptor (5 μg applied/lane) was purified from platelet plasma membranes using the affinity chromatography procedures previously described1 2 and resolved by SDS-PAGE. Immunoblotting was performed using the following antibodies: lane 1, incubation with monoclonal antibody mAb.F11 (4 μg/mL); lane 2, control, incubation with only 2° antibody, rabbit antimouse immunoglobulin; lane 3, incubation with the purified immunoglobulin fraction obtained from the serum of a patient with thrombocytopenia as described in “Materials and methods” (25 μg/mL); lane 4, incubation with plasma from a patient with thrombocytopenia after kidney transplantation, as described in “Materials and methods”; lanes 5 and 6, incubation with plasma obtained from 2 healthy donors and used for immunoblotting; lane 7, control using 2° antibody, rabbit antihuman immunoglobulin.
Discussion
The main new finding of this report is that F11R, a protein studied extensively for its role in antibody-induced platelet aggregation,21-25 functions as a mediator of cell adhesion processes and is a member of a family CAMs. These conclusions were derived, respectively, from functional determinations and gene cloning studies that confirmed each another, as discussed below.
First, we demonstrated that the adhesion of platelets to an F11-antibody matrix involves changes in platelet morphology, including extensive platelet spreading that results from the development of filopodia and lamellipodial networks. Such morphologic changes are known consequences of adhesion mediated by CAMs and produced by CAM-induced signal transduction processes.55-57 The adhesion of platelets to the F11 matrix and the ensuing morphologic changes were not blocked by the presence of the fibrinogen receptor inhibitory peptide, RGDS, nor by antibody IV.3. The lack of inhibition by RGDS indicated that the fibrinogen receptor or other RGDS-dependent integrins are not involved in this adhesion/spreading process. This finding also substantiates our previous report showing that platelets of a patient with thrombasthenia exhibited secretion induced by mAb.F11 without the involvement of the fibrinogen receptor.21 Furthermore, whereas platelet aggregation induced by mAb.F11 was found to be dependent on the binding of the antibody to both its specific antigen (F11R) and to the platelet FcγR11 receptor,23 the platelet adhesion and spreading mediated by the F11 receptor were not blocked by an FcγRII antibody. Thus, the F11R appeared to be involved in 2 distinct processes initiated on the platelet surface—an antibody-induced platelet aggregation dependent on the involvement of both FcγRII and the GPIIb/IIIa integrin and an F11R-mediated platelet adhesion not dependent on either the FcγRII or on the fibrinogen receptor.
The finding that the F11R can function as a CAM but is not an integrin suggests that this protein may be a cadherin-type CAM or is a member of the immunoglobulin superfamily of adhesion molecules. We resolved this issue by direct determination of the structure of the human platelet F11R. For this purpose, we used sequences of proteolytic fragments of purified F11R for cloning the complete F11R cDNA from human platelets. In these experiments, a 1.8-kb F11R cDNA was obtained with an open reading frame encoding a mature protein of 272 amino acids. The predicted size of F11R, as calculated from the deduced amino acid sequence, was determined to be 29 kd. This is identical to the size of the core protein generated previously by N-deglycosylation of the purified F11R protein.23 Furthermore, the sequence of the platelet F11R cDNA was found to contain the sequences of the proteolytically derived internal peptide fragments of the purified F11R protein and the NH2-terminal sequence.23Accordingly, we conclude that the nucleotide sequence of the F11R cDNA reported here encodes for the platelet F11R protein studied previously.21-25 Confirmation that the sequence of the F11R (Figure 3) belongs to both proteins of the 32-/35-kd duplex was obtained by immunoblotting (Figure 7), which verified that both proteins have the same N-terminal sequence.
The previously published partial amino acid sequences of the purified F11R protein comprise approximately one third of the whole molecule.23 Comparison of the deduced amino acid sequence of the cloned F11R cDNA (Figure 3) to the partial amino acid sequences of the F11R protein23 demonstrates that they are identical. Current literature and database searches revealed homology of the F11R to immunoglobulin superfamily members based on the amino acid sequences found within 2 regions of this molecule: 2 amino acid stretches from 43 to 111 and from 146 to 214 were identified as immunoglobulin folds and classified as C2-type, similar to other immunoglobulin superfamily members involved in cell–cell adhesion such as NCAM, ICAM, VCAM-1, MAdCAM-1, and PECAM-1.49-54 Other platelet proteins that have been reported as members of the immunoglobulin superfamily include the PDGF receptor and PECAM-1.58 59
The determination that the F11R is a member of the immunoglobulin superfamily of CAMs is consistent with the functional findings shown in Figure 1, that this platelet surface protein can mediate adhesion-dependent processes. The conclusion that the F11R can function as a CAM is also confirmed by the high degree of sequence similarity between the F11R and previously reported proteins of the same size shown to mediate cell adhesion. The platelet F11R shares sequence homology with the recently reported murine junctional adhesion molecule (JAM).60 Comparison of the murine JAM sequence to our previously published,23 proteolytically generated sequences of the platelet F11 molecule revealed 31% identity to the N-terminus of the F11R molecule and 70% identity to the GLμ-C(3), GLμ-C(4) fragments, and the trypsin digestion fragment. The 2 immunoglobulin folds of the platelet F11R represent the C-2 type of structure, whereas the 2 immunoglobulin folds of JAM were described by the authors as V-type. However, our analysis of the murine JAM sequence using the SMART program has determined that JAM contains 2 immunoglobulin domains of the C-2 type, similar to the F11R. Thus, the F11R appears to be the human homologue of murine JAM, which was shown to be localized at intercellular junctions of mouse endothelial and epithelial cells.60 In confirmation of this conclusion, we have recently observed the presence of the F11R at junctions between cultured HUVEC (Muller WA, Kornecki E, et al, manuscript in preparation). Another protein related to the F11R appears to be the A33 antigen, which is a transmembrane glycoprotein expressed in gastrointestinal epithelium and in 95% of human colon cancers.61-63 Finally, a very recent article reports human and bovine homologues of JAM.64 The reported sequence of the human homologue of JAM is identical to the sequence of the human platelet F11R as shown here and as reported previously.23It is unclear to us why Martin-Padura et al60 and Ozaki et al64 did not refer in their publications to the previously published sequences of the platelet F11R23 that are identical to their findings. These sequences have been easily retrievable from the databanks since 1995 (NCB accession number S56 749) and are reported to be present in platelets.21-25
Every megakaryoblastic (HEL, K562) and megakaryocytic (CMK, CMK-6, CMK 11-5) cell line examined in the current study was found to express the stimulatory epitope of platelet F11R as recognized by mAb.F11 (Figures8 and 9). Thus, the platelet protein named F11R appears to be expressed in the early stages of megakaryoblast development and continues to be expressed in mature megakaryocytes and platelets. In addition, red blood cells, lymphocytes, and endothelial cells also were found to express a surface protein that is recognized by mAb.F11 and that comigrates with the F11R (Figures 8 and 9). The wide distribution of the F11R on a variety of hematopoietic cells is indicative of the potential roles it can play in the adhesion of these cells. These findings also suggest a new functional role for the F11R in platelets as a CAM contributing to the initial adhesion of platelets to the walls of injured blood vessels, followed by the activation of platelets leading to secretion and platelet aggregation.
The cross-linking of the F11R with the FcγRII receptor was found to be a required step in the process by which mAb.F11 induces platelet aggregation and secretion.23 However, the F11R can also mediate platelet activation by mechanisms independent of the FcγRII receptor. These include platelet shape change, adhesion, and spreading (Figure 1) and the potentiation of platelet aggregation by physiologic agonists (Sobocka MB et al, manuscript in preparation). Relevant to the latter observation, we report here that platelet stimulation by the physiologic agonists thrombin and collagen results in increased phosphorylation of the F11R, even when antibody is not added. The mechanism by which the F11R contributes to the process of platelet aggregation induced by physiological agonists may involve its activity as an adhesion protein. In addition, another main function of the F11R may be in the role it plays in the adhesion of platelets to other cells, including endothelial cells. The presence of the F11R on the surface of endothelial cells shown in Figures 8 and 9 demonstrates that such homologous interactions between F11R molecules on the surface of different cells can be a physiologic process.
The presence of autoantibodies and alloantibodies directed against platelet surface proteins in the circulating plasma is thought to play an important role in the pathophysiology of idiopathic thrombocytopenia purpura1-9 and in thrombocytopenia associated with human immunodeficiency virus infections.10 11 In the current study, we detected antibodies that recognize the F11R in the circulation of 2 patients with thrombocytopenia. Thus, this report opens a new direction for the investigation of both basic and clinical aspects of platelet function: the role of F11R-mediated adhesion in platelet activation and platelet-endothelial cell interactions and the role of anti-F11R antibodies in the etiology of thrombocytopenias. Accordingly, we suggest that peptides with sequences that can inhibit F11R-mediated platelet activation have potential therapeutic action in the treatment of certain thrombocytopenias.
Supported by grants from the American Heart Association/New York City Affiliate/Heritage Foundation (E.K.), and from the Center for Biotechnology, New York State Center for Advanced Technology, Grant #X318Q, Stony Brook, NY; and by a Research Career Development Award (E.K.) from the NHLBI of the National Institutes of Health.
Reprints:Elizabeth Kornecki, Department of Anatomy and Cell Biology, Downstate, State University of New York, Box 5, 450 Clarkson Avenue, Brooklyn, NY 11203; e-mail:ekornecki@netmail.hscbklyn.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Phosphorylation of the F11 receptor after activation of human platelets by thrombin, collagen, and mAb.F11. / Human platelets were incubated with [32P] for 1 hour as described in “Materials and methods” and as previously described.22 Washed platelet suspensions were activated by thrombin, collagen, or mAb.F11 and solubilized by the addition of NP-40 as described.22 Detergent extracts were immunoprecipitated with mAb.F11-coupled Sepharose (A to D) or with uncoupled Sepharose (E to H).22 The autoradiogram demonstrates the immunoprecipitates obtained from platelets treated under the following conditions: A and E, control, nonstimulated; B and F, mAb.F11-stimulated; C and G, thrombin-stimulated; D and H, collagen-stimulated platelets.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2600/4/m_bloo00828002w.jpeg?Expires=1767703610&Signature=qbVWNaY7Tfi1zJQE5AloVGMYUijT16xx8Kno682O3FRVRue7gM3yc-~8iFpUeMbpnqjw47GlFiPA5-KAhlLJIKe3MdgMZTW81JElvfgdVpqUmK5QiTrQVyajAyJoqph9-vur630oVPV1UK4zrQhYamR9hb9c2UoxX9UC~fBxmquLcG3kYl5pCxNQzBAUBfGM5hzNJYz4YwOGjkde~ZxAs~lu5gE2lhY0sBrxkbHNf2jQrOtDve5zHtR3NL~RzptyBM7JWKnIfeyYcawrL4uJKGwmlncv-efKJsbhPnmVfUIqWfzvdpkSuz3GmiI0vs~qRFzOnlDxVJXo0NX0sXghWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal