Abstract
The effects of thrombopoietic stimulation on megakaryocytopoiesis, platelet production, and platelet viability and function were examined in normal volunteers randomized to receive single bolus subcutaneous injections of 3 μg/kg pegylated recombinant megakaryocyte growth and development factor (PEG-rHuMGDF) or placebo in a 3:1 ratio. PEG-rHuMGDF transiently doubled circulating platelet counts, from 237 ± 41 × 103/μL to 522 ± 90 × 103/μL (P< .0001), peaking on day 12. Baseline and day-12 samples showed no differences in responsiveness of platelets to adenosine diphosphate or thrombin receptor agonist peptide (P > .4 in all cases); expression of platelet ligand-induced binding sites or annexin V binding sites (P > .6 in both cases); or density of platelet TPO-receptors (P > .5). Platelet counts normalized by day 28. The life span of autologous 111In-labeled platelets increased from 205 ± 18 hours (baseline) to 226 ± 22 hours (P < .01) on day 8. Platelet life span decreased from 226 ± 22 hours (day 8) to 178 ± 53 hours (P < .05) on day 18. The theoretical basis for senescent changes in mean platelet life span was illustrated by biomathematical modeling. Platelet turnover increased from 43.9 ± 11.9 × 103 platelets/μL/d (baseline) to 101 ± 27.6 × 103 platelets/μL/d (P = .0009), and marrow megakaryocyte mass expanded from 37.4 ± 18.5 fL/kg to 62 ± 17 × 1010 fL/kg (P = .015). Although PEG-rHuMGDF initially increased megakaryocyte volume and ploidy, subsequently ploidy showed a transient reciprocal decrease when the platelet counts exceeded placebo values. In healthy human volunteers PEG-rHuMGDF transiently increases megakaryocytopoiesis 2-fold. Additionally, peripheral platelets expand correspondingly and exhibit normal function and viability during the ensuing 10 days. The induced perturbation in steady state thrombopoiesis resolves by 4 weeks.
Endogenous thrombopoietin (TPO) initiates receptor-dependent signaling in TPO-receptor–bearing hematopoietic precursors and megakaryocyte lineage cells that induces differentiation, proliferation and endoreduplication.1-9TPO is produced constitutively by the liver and kidneys, and circulates in blood as unbound thrombopoietically active TPO and TPO bound to receptors on peripheral platelets and developing marrow megakaryocytes, in competitive equilibrium.7,10,11 Two recombinant thrombopoietic growth factors have been developed and evaluated in patients: a polyethylene glycol-conjugated, recombinant truncated polypeptide, PEG-rHuMGDF12-14; and the full-length, glycosylated recombinant protein, rHuTPO.2 15 In nonhuman primates, the subcutaneous administration of TPO or PEG-rHuMGDF produce molar-equivalent, log-linear increases in the concentrations of peripheral platelets. The purpose of this study is to examine the effects of single-dose thrombopoietic stimulation on megakaryocytopoiesis, platelet production, platelet life span, and platelet function in healthy human volunteers. Patients and methods
Subjects and study design
This placebo-controlled, blinded study, approved by the Institutional Review Board, involved 16 healthy human volunteers recruited from the Emory campus community. Informed consent was obtained from each participant on admission to the General Clinical Research Center (GCRC) at Emory University, Atlanta, GA, and detailed clinical history, physical examination, complete profile of blood counts, routine serum chemistries, and urinalysis were obtained. The subjects were between the ages of 19 and 50 years, asymptomatic without known disease, and not taking any medications. The blood cell counts and routine blood chemistries were within the normal range for these laboratories.
Volunteers in each of the two 8-subject cohorts were randomized to receive either a single subcutaneous injection of 3 μg/kg PEG-rHuMGDF, or placebo in a 3:1 ratio on day 1. In the first group of 8 subjects (6 receiving active drug and 2 placebo), baseline measurements of platelet life span, platelet function, and megakaryocyte measurements were compared with the results obtained on day 12, the time when the concentration of peripheral platelets was maximum. In the second cohort of 8 subjects (6 receiving active drug and 2 placebo), platelet life span, platelet function, and megakaryocyte measurements were performed on days 8 and 18, times when the rates at which new platelets would enter and leave the circulation were calculated to be maximal, respectively. Thus, baseline (day 1) results with active drug were compared with cohorts of 6 subjects on day 8, day 12, day 18, and 4 placebo subjects (2 from each cohort).
Megakaryocyte growth factor
rHu-MGDF is a nonglycosylated polypeptide produced inEscherichia coli transfected with cDNA encoding the 1 to 163 aminoterminal residues of human thrombopoietin.12 After extraction, refolding, and purification, this truncated protein was derivitized with poly-(ethylene glycol). The resulting pegylated monomeric molecular species was prepared in aqueous buffer, sterilized by filtration, and provided as a gift from Amgen Inc (Thousand Oaks, CA). Endotoxin in the final product was less than 2.5 EU/mL.
Serum levels of pegylated recombinant megakaryocyte growth and development factor (PEG-rHuMGDF) and endogenous thrombopoietin
Daily serum levels of megakaryocyte growth factor were measured immunometrically for 4 weeks using antibody-sandwich enzyme-linked immunosorbant assays (ELISAs). The assay system used a polyclonal antibody raised in rabbits against recombinant human MGDF as the capture antibody. The same antibody conjugated to horseradish peroxidase (HRP) was used as the signal antibody. The sensitivity of the assay was 30 pg/mL.16 17
Determination of thrombopoietin receptors on platelets
Mean TPO receptor numbers per platelet were estimated from platelet-binding isotherms of 125I-labeled recombinant human (rHu) TPO according to Scatchard analysis.18 19 In brief, TPO receptors on platelets were determined using purified rHu-TPO radiolabeled by Iodo-beads iodination reagent (Pierce, Rockford, IL), and incubating rHu-TPO with 50 mmol/L sodium phosphate buffer, pH 7.2, and 125I with Iodo-beads for 15 minutes. Platelets were obtained from blood drawn in acid-citrate-dextrose (ACD) anticoagulant (1:7 v/v), pelleting platelets from platelet-rich plasma by centrifuging at 500 × g for 15 minutes, and resuspending in Tyrode's buffer containing ACD (1:7 v/v), pH 6.2, 1% bovine serum albumin (BSA), and 0.01% Tween. Binding isotherms were obtained by incubating platelets in plasma-free Tyrode's buffer, ACD (1:7 v/v), 1% BSA, 0.01% Tween, pH 6.2, and various amounts of125I-rHuTPO (40 to 640 ng/mL final concentrations) for 1 hour at room temperature. Nonspecific binding was assessed by comparing the effects of 100-fold excess unlabeled rHu-TPO 30 minutes before the addition of 125I-rHuTPO. Nonspecific binding ranged from 10% to 20%. Binding isotherms were analyzed using the Biosoft Ligand Program (Cambridge, UK) to determine the number of binding classes, the number of molecules bound/platelet, and the dissociation constant, Kd. TPO receptors on peripheral platelets were measured before, and 8, 12, and 18 days after initiating rHuPEG-MGDF therapy. rHuTPO, a glycosylated protein produced in Chinese hamster ovary cells transfected with the cDNA encoding for human TPO, was provided as a gift from Kirin Pharmaceutical Laboratories, Takasaka, Japan. The purified molecular species was formulated in an aqueous buffer, and sterilized by filtration. Endotoxin levels in the final product were less than 2.5 EU/mL.
Measurements of platelet function
Platelet aggregation was determined within 1 hour of drawing blood in 1/10 volume 3.2% citrate anticoagulant using a Chrono-Log aggregometer (Havertown, PA) by recording the increase in light transmission through a stirred suspension of platelet-rich plasma (PRP) maintained at 37°C. PRPs and platelet-poor plasmas (PPPs) were prepared by differential centrifugation, as previously described.20,21 The platelet count in the PRP was adjusted to 300 × 103/μL. ADP (Sigma, St Louis, MO), Horm collagen (Nycomed Arzenmittel, Munich, Germany), and TRAP1-6 (Peninsula Labs, Belmont, CA) were added at doses spanning the range of responsiveness. The results were plotted and expressed as the agonist concentration inducing half-maximal aggregation (AC50).22 23
The appearance of activated platelets in the peripheral blood was evaluated by flow cytometry using fluoresceinated monoclonal antibodies (MoAbs) against neoantigens expressed on membrane surfaces of activated platelets, including conformationally altered integrin IIb3ligand-induced binding sites (LIBS), a gift from Dr E. Plow (Cleveland, OH),21,24 and the secretory granule membrane, P-selectin, a gift from Biogen Inc, Cambridge, MA.21,25 In addition, enhanced binding to platelets by fluoresceinated annexin V, a gift from Dr T. Yokoyama, Tokyo, Japan, was also examined using flow cytometry.26-28 Annexin V, a member of the multigene family of calcium-dependent phospholipid binding proteins, exhibits high affinity for phosphatidylserine-rich, negatively charged, phospholipid platelet membrane binding sites promoting assembly of the macromolecular coagulation enzyme complexes.29-31 Flow cytometric platelet studies were performed on blood collected in 1/10 volume 3.8% sodium citrate. PRP was obtained by differential centrifugation; 5 μL PRP was diluted to 50 μL in phosphate buffered saline (PBS) pH 7.4 containing saturating concentrations of fluoresceinated marker protein and 1% bovine serum albumin (BSA), and incubated at 22°C. Annexin V was diluted in HEPES buffer (0.01 mol/L) in 0.15 mol/L sodium chloride and 2.5 mmol/L calcium chloride and 1% BSA pH 7.4. After 30 minutes incubation with the appropriate antibody or binding protein and buffer, the platelets were diluted 10-fold with the incubation buffer without albumin and placed on ice until analyzed by flow cytometry. The findings were standardized each day against calibrated fluorescent beads (Flow Cytometry Standards Corp, San Juan, PR).
Measurements of platelet production
Megakaryocyte number, size, and ploidy were measured by flow cytometry using a previously reported method for multiparameter correlative marrow analysis with a single-argon-ion-laser FACScan analyzer (Becton Dickinson, San Jose, CA).32-34 Cell DNA in aspirated marrow was stained with propidium iodide, and surface membrane receptors were analyzed with antibodies labeled with fluorescein. Megakaryocytes expressing IIb3 integrin (CD41/61) were enumerated in relation to the nucleated erythroid precursors expressing glycophorin A.34,35 Measurements of megakaryocyte diameters were based on the “time-of-flight” principle, ie, time required for a cell in suspension to pass through a focused light beam.36 Aspirated bone marrow (3 mL) obtained from the pelvic bones was collected into 10-mL plastic syringes containing equal volume acid-citrate-dextrose (ACD formula A), 2.5 mmol/L EDTA, and 2.2 μmol/L prostaglandin E1(PGE1; Sigma, St Louis, MO) final concentrations. The marrow was gently pipetted, passed through a 120-mm monofilament nylon filter, and diluted with cold Ca++- and Mg++-free PBS containing 13.6 mmol/L sodium citrate, 2.2 μmol/L PGE1, 1 mmol/L theophylline (Sigma), 3% BSA fraction V (Calbiochem, La Jolla, CA), 11 mmol/L glucose, and adjusted to a pH of 7.3 and an osmolarity of 290 mOsm/L. Megakaryocytes were analyzed in marrow aspirates fractionated with 1.06 density Percoll (Pharmacia Biotech Inc, Piscataway, NJ). The nucleated erythroid marrow cells were analyzed from marrow separated over 1.08 density Percoll (Pharmacia Biotech Inc). Megakaryocytes were selected on the basis of their distinct immunofluorescence at levels above that of control cells labeled with an unrelated MoAb. In each sample, 2000 to 3000 megakaryocytes were analyzed. Each subject had 2 bone marrow studies, ie, either baseline and at day 12, or day 8 and day 18.
Estimates of marrow megakaryocyte mass were used to represent the marrow substrate giving rise to circulating platelets, and were calculated as the product of megakaryocyte numbers and mean megakaryocyte volumes.37
Steady-state platelet mass turnover (platelet concentration multiplied by mean platelet volume and divided by platelet life span and by recovery of injected labeled platelets) was used to estimate the rate at which viable platelet mass was delivered to the peripheral blood.34,37 To measure platelet survival time, autologous platelets were labeled with 111In-oxine using the method described previously.38 Labeling efficiencies averaged 90%, and the labeled platelets functioned normally.20After reinjection, daily blood samples were collected and analyzed for111In-labeled platelet activity to determine the rate at which 111In-labeled platelets were cleared from the circulation. Platelet survival time, ie, the average time in circulation, was then calculated using computer least-squares fitting of the raw data to a gamma-function modeling program.38 The time course of 111In-labeled platelet activity in blood was modeled as:
where Ls(t) is the fraction of remaining111In-platelet activity at time, t, after injection of labeled platelets, and λ is the mean platelet life span.41 The parameter, na, is the number of exponentials in the gamma function, which modulates the variance of λ. The recovery of labeled platelets in the circulation at equilibrium was estimated by extrapolating the survival curve to time zero and estimating the blood volume (70 mL/kg) using the formula: recovery in circulation = total circulating platelet radioactivity/total platelet radioactivity injected.
Nonstationary thrombokinetic model
Peripheral platelet counts and 111In-labeled platelet activity curves were modeled simultaneously using a nonstationary gamma model.39 Modeling was conducted using SAAM II v. 1.1 (SAAM Institute, Seattle, WA). Megakaryocyte proliferation and maturation after stimulation by Mpl ligand were described by a system of nm differential equations as follows:
where nm is the number of catenary-linked megakaryocyte compartments (nm = 5), Mi is the mass in the ith megakaryocyte compartment, S0 is the Mpl ligand-independent platelet production rate, C(t) is the serum concentration of Mpl ligand at time t, kmpl is a proportionality constant relating mpl ligand serum concentration to Mpl ligand-dependent rate of thrombopoiesis, γ modulates steepness of the concentration-response curve, and τm is the mean transit time of cells within the megakaryocyte maturation compartments. C(t) was calculated by linear interpolation of the measured serum Mpl ligand concentrations. The initial condition for the ithmegakaryocyte compartment, Mi(0), was calculated as:
where C0 is the serum concentration of Mpl ligand at baseline.
Megakaryocytopoiesis was described in the model by a black-box process. Without modeling of the time course of megakaryocyte mass, the model parameters estimate the Mpl ligand-dependent and Mpl ligand-independent platelet production rate,
The model assumes that the rate of Mpl ligand-mediated platelet production is proportional to the serum concentration of Mpl ligand, C(t), raised to the power λ. It is recognized that the relationship between serum Mpl ligand concentration and platelet production rate described in Equation 6 is applicable only over the range of Mpl ligand concentrations achieved in this study. At higher Mpl ligand concentrations, the relationship between platelet production rate and serum Mpl ligand may be appropriately described by a sigmoidal concentration-response relationship, such as a logistic function that could describe full receptor occupancy. Subsequent to stimulation of megakaryocytopoiesis, changes in platelet production rate are reflected by changes in platelet count after a transit time delay, τm. Biologic parameters such as the rate of megakaryocytopoiesis, differential release rates of platelets from megakaryocytes of different ploidy, and megakaryocyte death rate, are not directly identifiable from observations of platelet kinetics in blood, per se. However, these biologic events affect the transit time, τm, and the variance of the transit time; hence, the thrombokinetic effects of these events are indirectly represented by the model.
Destruction of peripheral platelets was modeled by random and senescent processes. The kinetics of peripheral platelets were described by the following system of na differential equations:
where na is the number of catenary-linked platelet “age” compartments (na = 20), P is the total number of peripheral platelets, VP is the apparent dilution volume of peripheral platelets (including splenic pooling), Pj is the number of platelets in the jth platelet age compartment, kρ is a constant rate of random platelet utilization, and Λ is intrinsic platelet longevity. The initial condition for the jth platelet age compartment, Pj(0), was calculated as:
where P0 is the baseline peripheral platelet count. The peripheral platelet concentration at time t was calculated as the sum of the platelet numbers within na age compartments divided by the peripheral platelet dilution volume, Vp.
The 111In-labeled platelet activity curves were modeled simultaneously with peripheral platelet counts to account for the effects of platelet count and mean platelet age on the kinetics of111In-labeled platelet decay:
where P*j is the platelet activity (cpm) associated with the jth age compartment. The initial amount of radiolabel, P*j(t*), within the jth platelet age cohort upon injection of the tracer bolus at time t* was assumed to be proportional to the fractional number of platelets within the jth age compartment, ie, incorporation of label was assumed to be independent of platelet age:
where Pj(t*) is the modeled number of platelets within the jth age compartment at time t* and P(t*) is the modeled number of peripheral platelets at time t*, and D* is the dose (cpm) of111In-labeled platelets.
The mean platelet age at time t, A(t), was calculated from the weighted average of na platelet age compartments:
where jΛ/na is the average platelet age in the jthage compartment.
Numerical integration was conducted in SAAM II using the Rosenbrock method.56 Integration was conducted using an adjustable step size. A relative integration error of 0.001 (0.1%) was used.
Blood cell counts
Peripheral platelet counts, mean platelet volumes, red cell counts, and total white cell counts were determined in whole blood collected every day in K3EDTA (2 mg/mL) using Serono/Baker model 9000 whole blood analyzer (Allentown, PA).38 The absolute neutrophil counts (ANCs) were ascertained manually from white cell differential counts on Wright-Giemsa–stained peripheral blood films.
Statistical analysis
The data were analyzed using SIGMA STAT (Jandel Scientific Software, San Rafael, CA). Comparisons between 2 groups were performed using the 2-tailed Student t test, unless the data were not distributed randomly, in which case nonparametric analysis was performed. Analysis of variance (ANOVA) was used to compare value for a particular group at various time points.40 Unless otherwise stated, variance about the mean is given as ± 1 SD.
Results
Serum levels of megakaryocyte growth factor
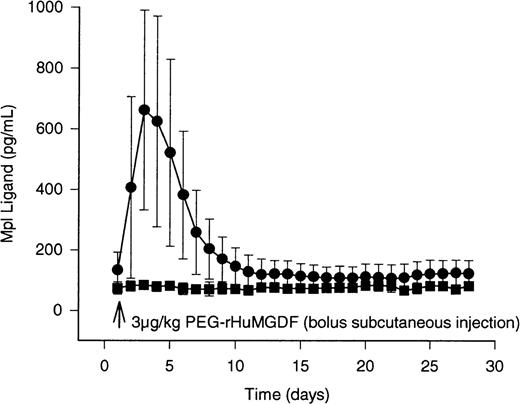
Serum levels of megakaryocyte growth factor were measured immunometrically each day for 4 weeks (Figure1, Table 1). In subjects receiving active agent, peak concentrations of Mpl ligand occurred 2 days after administering bolus subcutaneous injections of PEG-rHuMGDF, and averaged 500 ± 90 ng/mL (P < .001 compared with placebo). Compared with placebo subjects, significantly elevated concentrations were present in PEG-rHuMGDF–treated subjects for more than 5 days. The average time after injecting PEG-rHuMGDF to achieve half-maximal concentrations was 53 hours. The subjects receiving placebo exhibited serum encogenous TPO levels of 74 ± 20 ng/mL, which remained unchanged throughout the study period (Figure 1; Table 1;P > .6 in all cases).
Circulating levels of Mpl ligand in healthy human volunteers after bolus subcutaneous dosing of study drug. Serum concentrations of Mpl ligand were determined daily for 28 days in 16 normal human subjects using immunogenic ELISA assay after bolus subcutaneous injections of study drug. Subjects were randomized to receive PEG-rHuMGDF (3 μg/kg), or placebo in a 3:1 ratio, respectively. The results in 12 subjects receiving active drug are shown in the solid circles. The data for the 4 placebo subjects are represented by the solid squares. Peak values of 500 ng/mL developed on the second day after drug administration. The interval required for drug levels to decrease by 50% was 53 hours. The variance about the mean values represents ± 1 standard deviation (± 1 SD).
Circulating levels of Mpl ligand in healthy human volunteers after bolus subcutaneous dosing of study drug. Serum concentrations of Mpl ligand were determined daily for 28 days in 16 normal human subjects using immunogenic ELISA assay after bolus subcutaneous injections of study drug. Subjects were randomized to receive PEG-rHuMGDF (3 μg/kg), or placebo in a 3:1 ratio, respectively. The results in 12 subjects receiving active drug are shown in the solid circles. The data for the 4 placebo subjects are represented by the solid squares. Peak values of 500 ng/mL developed on the second day after drug administration. The interval required for drug levels to decrease by 50% was 53 hours. The variance about the mean values represents ± 1 standard deviation (± 1 SD).
Effect of thrombopoietic stimulation on platelet life span in healthy human volunteers
| . | Day 1 . | Day 8 . | Day 12 . | Day 18 . |
|---|---|---|---|---|
| Platelet concentration (×103/μL) | ||||
| Treated | 230 ± 43 | 354 ± 51 | 522 ± 90 | 420 ± 75 |
| Pvs day 1 | .0001 | .0001 | .0001 | |
| Placebo | 210 ± 47 | 228 ± 23 | 241 ± 28 | 230 ± 17 |
| MPV (fL) | ||||
| Treated | 8.0 ± 0.6 | 8.1 ± 0.6 | 7.7 ± 0.8 | 7.7 ± 0.6 |
| Pvs day 1 | .2 | .17 | ||
| Placebo | 8.1 ± 0.7 | 7.9 ± 0.3 | 8.0 ± 0.7 | 7.9 ± 0.4 |
| Platelet life span (hs) | ||||
| Treated | 205 ± 18 | 226 ± 22 | 212 ± 23 | 178 ± 53 |
| Pvs day 1 | .03 | .45 | .48 | |
| Pvs day 8 | .05 | |||
| Placebo | 193 ± 12 | 212 ± 9 | 200 ± 10 | 214 ± 30 |
| Platelet mass turnover (×105fL/μL/d) | ||||
| Treated | 3.6 ± 1.1 | 4.6 ± 0.8 | 6.3 ± 2.1 | 7.5 ± 2.1 |
| Pvs day 1 | .0008 | .0009 | ||
| Placebo | 3.95 ± 0.9 | 3.5 ± 0.1 | 4.4 ± 0.1 | 3.4 ± 0 |
| Mpl ligand level (pg/mL) | ||||
| Treated | 119 ± 63 | 150 ± 93 | 102 ± 59 | 98 ± 66 |
| Placebo | 74 ± 20 | 71 ± 23 | 76 ± 12 | 75 ± 13 |
| . | Day 1 . | Day 8 . | Day 12 . | Day 18 . |
|---|---|---|---|---|
| Platelet concentration (×103/μL) | ||||
| Treated | 230 ± 43 | 354 ± 51 | 522 ± 90 | 420 ± 75 |
| Pvs day 1 | .0001 | .0001 | .0001 | |
| Placebo | 210 ± 47 | 228 ± 23 | 241 ± 28 | 230 ± 17 |
| MPV (fL) | ||||
| Treated | 8.0 ± 0.6 | 8.1 ± 0.6 | 7.7 ± 0.8 | 7.7 ± 0.6 |
| Pvs day 1 | .2 | .17 | ||
| Placebo | 8.1 ± 0.7 | 7.9 ± 0.3 | 8.0 ± 0.7 | 7.9 ± 0.4 |
| Platelet life span (hs) | ||||
| Treated | 205 ± 18 | 226 ± 22 | 212 ± 23 | 178 ± 53 |
| Pvs day 1 | .03 | .45 | .48 | |
| Pvs day 8 | .05 | |||
| Placebo | 193 ± 12 | 212 ± 9 | 200 ± 10 | 214 ± 30 |
| Platelet mass turnover (×105fL/μL/d) | ||||
| Treated | 3.6 ± 1.1 | 4.6 ± 0.8 | 6.3 ± 2.1 | 7.5 ± 2.1 |
| Pvs day 1 | .0008 | .0009 | ||
| Placebo | 3.95 ± 0.9 | 3.5 ± 0.1 | 4.4 ± 0.1 | 3.4 ± 0 |
| Mpl ligand level (pg/mL) | ||||
| Treated | 119 ± 63 | 150 ± 93 | 102 ± 59 | 98 ± 66 |
| Placebo | 74 ± 20 | 71 ± 23 | 76 ± 12 | 75 ± 13 |
Peripheral platelet concentrations and volumes
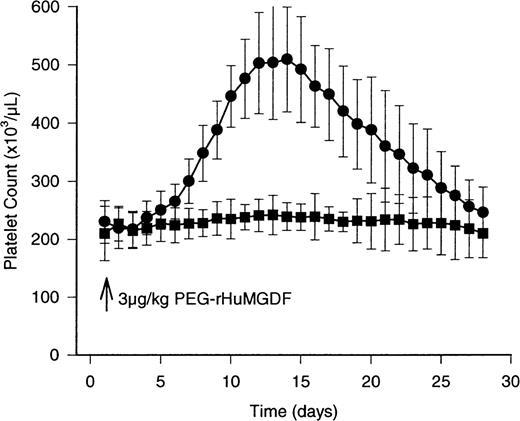
The concentration of peripheral platelets after the single bolus subcutaneous injection of PEG-rHuMGDF was detectably increased on day 6 (from baseline 230 ± 43 × 103/μL to 271 ± 35 × 103/μL; P = .01; Figure2), and doubled by day 12 (Figure 2; Table1; from baseline 230 ± 43 × 103/μL; P < .0001). The increase in peripheral platelet counts was associated with a small reduction in mean platelet volume (MPV) from 8.1 ± 0.6 fL on day 8 to 7.7 ± 0.8 fL; P < .05) on day 12. The peripheral platelet concentration and volume gradually normalized by day 28, ie, 243 ± 43 × 103/μL compared with placebo of 230 ± 43 × 103/μL (P > .6 compared with baseline), and 8.0 ± 0.6 fL to 8.0 ± 1.0 fL (P > .8 compared with baseline), respectively.
The effects of study drug on the peripheral concentration of platelets in healthy human volunteers . Mean peripheral platelet counts are depicted by the solid circles for the 12 subjects receiving active drug (3 μg/kg PEG-rHuMGDF) by bolus subcutaneous injection. The results in the 4 individuals receiving placebo are indicated by the solid squares. The peripheral platelet count peaks at 522 ± 90 × 103/μL on day 12, and normalizes by day 28. No significant changes occur in the placebo group. Variance about the mean values is ± 1 SD.
The effects of study drug on the peripheral concentration of platelets in healthy human volunteers . Mean peripheral platelet counts are depicted by the solid circles for the 12 subjects receiving active drug (3 μg/kg PEG-rHuMGDF) by bolus subcutaneous injection. The results in the 4 individuals receiving placebo are indicated by the solid squares. The peripheral platelet count peaks at 522 ± 90 × 103/μL on day 12, and normalizes by day 28. No significant changes occur in the placebo group. Variance about the mean values is ± 1 SD.
Platelet responsiveness to physiologic agonists, and expression of platelet receptors
The concentrations of ADP or TRAP inducing half-maximal platelet aggregation were not changed from baseline by administering PEG-rHuMGDF (Table 2; P > .4 in all cases). Similarly, administering PEG-rHuMGDF did not alter the expression of TPO receptors, LIBS, or annexin V binding sites during the study period (data not shown).
Platelet life span
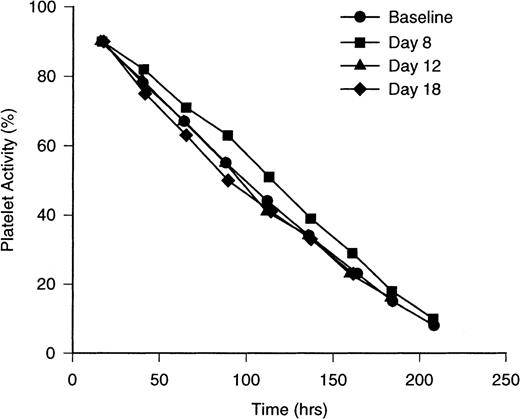
Life span measurements of autologous111In-labeled platelets were computed using gamma function routines, modified for nonsteady- state analysis.38,41 42The disappearance pattern of 111In-labeled platelets obtained at baseline from 8 normal subjects is nearly linear, but exhibits slight curvilinearity (Figure 3). The computed average time platelets remain in the circulation, ie, platelet life span (λ), was 205 ± 18 hours (Table 1). These results agreed closely with the estimates of platelet life span obtained in placebo subjects at 8, 12, and 18 days (P > .5 for each of the 4 estimates). In PEG-rHuMGDF–treated subjects, platelet life span was prolonged to 226 ± 22 hours on day 8 (Figure 3; P = .04), the time when the cohort of platelets produced in response to PEG-rHuMGDF was youngest.
Sequential 111In-labeled platelet disappearance curves in healthy human volunteers receiving PEG-rHuMGDF (3 μg/kg). Disappearance curves are depicted for111In-labeled platelets in 8 normal subjects at baseline (solid circles), in 6 subjects having received bolus subcutaneous injections of PEG-rHuMGDF (3 μg/kg) 8 days previously (solid squares), 12 days previously (solid triangles), and 18 days previously (solid diamonds). Platelet populations enriched with newly formed platelets on day 8 demonstrate upward bowing of the disappearance curve with a computed life span of 226 ± 22 hours. Conversely, platelet populations enriched with platelets approaching senescence on day 18 show accentuated downward bowing of the disappearance curve with a computed life span of 178 ± 53 hours. The differences are statistically significant between baseline and 8-day determinations and day 8 and day 18 values. The disappearance pattern of day 12 platelets resembles the baseline pattern. The placebo subjects yielded results consistent with baseline values. Variance about the mean values is ± 1 SD.
Sequential 111In-labeled platelet disappearance curves in healthy human volunteers receiving PEG-rHuMGDF (3 μg/kg). Disappearance curves are depicted for111In-labeled platelets in 8 normal subjects at baseline (solid circles), in 6 subjects having received bolus subcutaneous injections of PEG-rHuMGDF (3 μg/kg) 8 days previously (solid squares), 12 days previously (solid triangles), and 18 days previously (solid diamonds). Platelet populations enriched with newly formed platelets on day 8 demonstrate upward bowing of the disappearance curve with a computed life span of 226 ± 22 hours. Conversely, platelet populations enriched with platelets approaching senescence on day 18 show accentuated downward bowing of the disappearance curve with a computed life span of 178 ± 53 hours. The differences are statistically significant between baseline and 8-day determinations and day 8 and day 18 values. The disappearance pattern of day 12 platelets resembles the baseline pattern. The placebo subjects yielded results consistent with baseline values. Variance about the mean values is ± 1 SD.
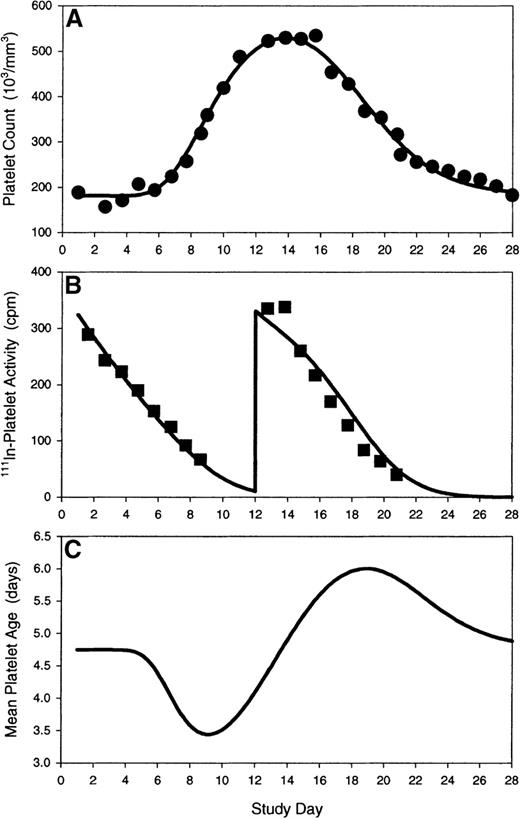
Peripheral platelet counts and 111In-labeled platelet kinetics were simultaneously modeled using a nonstationary thrombokinetic model (Figure 4). The model incorporated the effects of endogenous and exogenous Mpl ligand on megakaryopoiesis, elimination of platelets by senescence and endothelial use, and the effects of nonsteady-state alterations in mean platelet age on 111In-labeled platelet decay curves. Modeling of peripheral platelet counts and platelet tracer kinetics for a subject receiving autologous 111In-labeled platelets at baseline and on day 12 are illustrated in Figure5 (upper and middle plots). Data fitting yielded an intrinsic platelet longevity (Λ) estimate (platelet life span in absence of external hazard) of 9.7 days and a constant rate of platelet utilization (kp) of 8000 platelets per microliter per day. The apparent dilution volume (Vp) of platelets (including the splenic pool) was 7700 mL. The model predicted a linear platelet decay at baseline, due to a relatively uniform distribution of peripheral platelet ages. On day 12, the modeled tracer decay curve was slightly sigmoidal because of an increase in the proportion of younger platelets in circulation just before the platelet zenith. Modeling of peripheral platelet counts and platelet tracer kinetics for a subject receiving autologous 111In-labeled platelets on days 8 and 18 are illustrated in Figure 6 (upper and middle plots). In this subject, the intrinsic platelet longevity (Λ) was 10.3 days, platelet utilization (kp) was 7700 platelets per microliter per day, and the platelet dilution volume (Vp) was 6200 mL. A pronounced sigmoidicity in the platelet tracer decay curve on day 8 was noted, and was attributed to the high influx rate of new platelets into circulation. By contrast on day 18, the platelet tracer dose decayed rapidly and exponentially; the platelet tracer kinetics on day 18 were attributed to an increased proportion of old platelets in circulation with an associated increased destruction rate of the senescent cells.
Nonstationary gamma model of platelet production and destruction mediated by Mpl-ligand. The endogenous thrombopoietin and exogenous PEG-rHuMGDF serum concentration time course, C(t), stimulates megakaryopoiesis; de novo platelet production rate, S[S0, C(t)], stimulated by Mpl-ligand begins after a megakaryocyte maturation delay time, τm. Newly formed platelets emerge into blood and are diluted in a volume, Vp, which includes blood volume and splenic pooling. Platelets progress through a sequence of na age compartments until they are lost from circulation by processes of random destruction, ρ, or reach the limit of intrinsic platelet longevity, Λ.
Nonstationary gamma model of platelet production and destruction mediated by Mpl-ligand. The endogenous thrombopoietin and exogenous PEG-rHuMGDF serum concentration time course, C(t), stimulates megakaryopoiesis; de novo platelet production rate, S[S0, C(t)], stimulated by Mpl-ligand begins after a megakaryocyte maturation delay time, τm. Newly formed platelets emerge into blood and are diluted in a volume, Vp, which includes blood volume and splenic pooling. Platelets progress through a sequence of na age compartments until they are lost from circulation by processes of random destruction, ρ, or reach the limit of intrinsic platelet longevity, Λ.
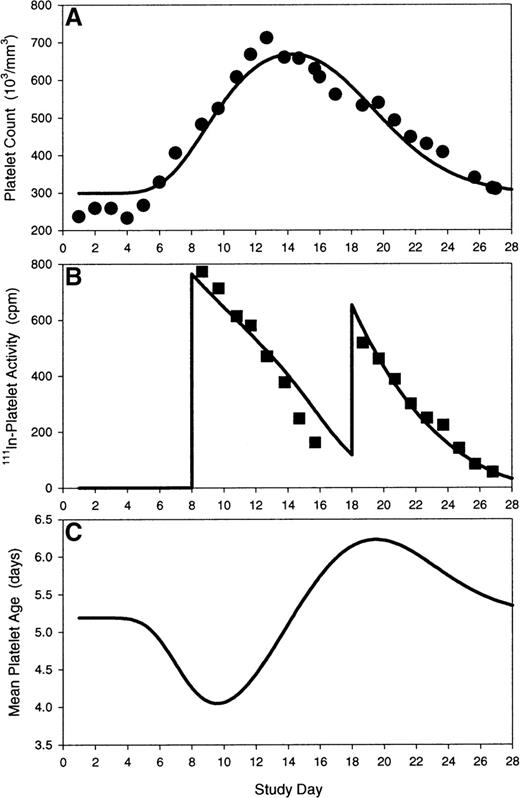
Modeling of platelet kinetics in a subject receiving bolus PEG-rHuMGDF on day 1 and autologous111In-labeled platelets at baseline and day 12. (A) Model prediction (solid line) of platelet response to PEG-rHuMGDF is overlaid on observed platelet counts (closed circles). (B) Model prediction (solid line) of platelet tracer kinetics during nonsteady state conditions are overlaid on observed111In-labeled platelet activities (closed squares). (C) Theoretical time course (solid line) of mean peripheral platelet age after transient stimulation of megakaryocytopoiesis explains the alteration in the platelet tracer profile on day 12 relative to baseline. Model parameters: S0, 53 100 platelets per day; kmpl, 956 000 platelets/d/(ng/mL); γ, 1.0; τm, 5.13 days; Vp, 7700 mL; Λ, 9.70 days; kρ, 8000 platelets/μL/d.
Modeling of platelet kinetics in a subject receiving bolus PEG-rHuMGDF on day 1 and autologous111In-labeled platelets at baseline and day 12. (A) Model prediction (solid line) of platelet response to PEG-rHuMGDF is overlaid on observed platelet counts (closed circles). (B) Model prediction (solid line) of platelet tracer kinetics during nonsteady state conditions are overlaid on observed111In-labeled platelet activities (closed squares). (C) Theoretical time course (solid line) of mean peripheral platelet age after transient stimulation of megakaryocytopoiesis explains the alteration in the platelet tracer profile on day 12 relative to baseline. Model parameters: S0, 53 100 platelets per day; kmpl, 956 000 platelets/d/(ng/mL); γ, 1.0; τm, 5.13 days; Vp, 7700 mL; Λ, 9.70 days; kρ, 8000 platelets/μL/d.
Modeling of platelet kinetics in a subject receiving bolus PEG-rHuMGDF on day 1 and autologous111In-labeled platelets on day 8 and day 18. (A) Model prediction (solid line) of platelet response to PEG-rHuMGDF is overlaid on observed platelet counts (closed circles). (B) Model prediction (solid line) of platelet tracer kinetics during nonsteady state conditions are overlaid on observed111In-labeled platelet activities (closed squares). (C) Theoretical time course (solid line) of mean peripheral platelet age after transient stimulation of megakaryocytopoiesis explains the alteration in the platelet tracer profile on day 18 relative to day 8. Model parameters: S0, 93 300 platelets per day; kmpl, 1 030 000 platelets/d/(ng/mL); γ, 1.0; τm, 5.16 days; Vp, 6200 mL; Λ, 10.3 days; kρ, 7700 platelets/μL/d.
Modeling of platelet kinetics in a subject receiving bolus PEG-rHuMGDF on day 1 and autologous111In-labeled platelets on day 8 and day 18. (A) Model prediction (solid line) of platelet response to PEG-rHuMGDF is overlaid on observed platelet counts (closed circles). (B) Model prediction (solid line) of platelet tracer kinetics during nonsteady state conditions are overlaid on observed111In-labeled platelet activities (closed squares). (C) Theoretical time course (solid line) of mean peripheral platelet age after transient stimulation of megakaryocytopoiesis explains the alteration in the platelet tracer profile on day 18 relative to day 8. Model parameters: S0, 93 300 platelets per day; kmpl, 1 030 000 platelets/d/(ng/mL); γ, 1.0; τm, 5.16 days; Vp, 6200 mL; Λ, 10.3 days; kρ, 7700 platelets/μL/d.
The theoretical time course of mean platelet age, A(t), after transient stimulation of megakaryocytopoiesis by a bolus dose of PEG-rHuMGDF was calculated (Equation 15) and is illustrated in Figures 5 and 6 (lower plot). The theoretical nadir in mean platelet age was predicted to be around day 9, and the theoretical zenith in mean platelet age was predicted to be around day 19. Modeling of the data calculated a difference in mean platelet age between the theoretical age zenith and nadir of approximately 2.0 to 2.5 days. The modeling projection corresponded closely to the observed platelet life span difference of 48 hours between days 8 and 18 (Table 1). After model correction for nonstationary alterations in the age distribution of peripheral platelets, the model estimates of intrinsic platelet longevity and endothelial use were similar to published steady state determinations (intrinsic platelet longevity, 10.5 days; endothelial utilization rate 7100 platelets per microliter per day) in absence of exogenous cytokine stimulation.52 53
Platelet production
Platelet production was assessed in 2 ways: the rate at which viable platelets appeared in the circulation, calculated as platelet turnover (the peripheral concentration of platelets divided by the mean platelet life span, and corrected for splenic pooling); and megakaryocyte cytoplasmic substrate destined to give rise to platelets, calculated as megakaryocyte mass (the product of megakaryocyte number and megakaryocyte volume). The administration of PEG-rHuMGDF doubled platelet turnover, platelet mass turnover, and marrow megkaryocyte mass (Table 2), ie, platelet turnover increased from baseline of 44 ± 12 × 103 platelets/μL to 101 ± 28 × 103 platelets/μL/d (P = .0009), platelet mass turnover (Table 1) increased from 3.6 ± 1.1 fL/μL/d to 7.5 ± 2.1 fL/μL/d (P = .0009), and marrow megakaryocyte mass (Table 3) expanded from 37 ± 19 × 1010 fL/kg to 62 ± 17 × 1010 fL/kg (P = .015).
Effects on Mpl ligand on platelet function
| . | Baseline . | Day 8 . | Day 12 . | Day 18 . |
|---|---|---|---|---|
| Aggregation* | ||||
| ADP (μmol/L) | 2.7 ± 1.5 | 2.1 ± 1.4 | 2.6 ± 1.9 | 2.5 ± 2.0 |
| TRAP (μmol/mL) | 2.2 ± 1.9 | 1.5 ± 1.2 | 1.6 ± 1.4 | 1.9 ± 1.8 |
| Activation epitopes† | ||||
| LIBS | 11 ± 7 | 12 ± 8 | 18 ± 15 | 16 ± 11 |
| . | Baseline . | Day 8 . | Day 12 . | Day 18 . |
|---|---|---|---|---|
| Aggregation* | ||||
| ADP (μmol/L) | 2.7 ± 1.5 | 2.1 ± 1.4 | 2.6 ± 1.9 | 2.5 ± 2.0 |
| TRAP (μmol/mL) | 2.2 ± 1.9 | 1.5 ± 1.2 | 1.6 ± 1.4 | 1.9 ± 1.8 |
| Activation epitopes† | ||||
| LIBS | 11 ± 7 | 12 ± 8 | 18 ± 15 | 16 ± 11 |
Agonist concentration producing half-maximal aggregation.
Binding sites × 103/platelet.
Megakaryocyte volume and ploidy
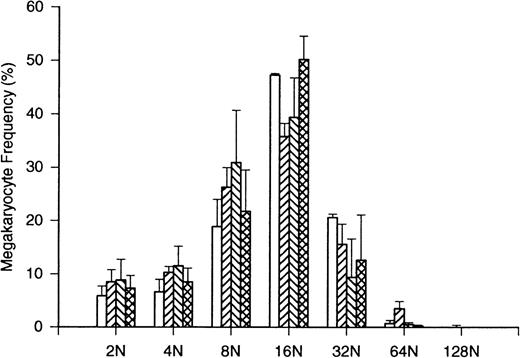
Megakaryocyte volume (Table 3) was significantly decreased on day 12 (from 30 ± 1.8 × 103fL to 24 ± 3.7 × 103 fL; P = .0003). This decline was reciprocally related to the increase in peripheral platelet counts beyond the placebo values. Corresponding changes were noted in megakaryocyte ploidy (Figure 7). The proportion of megakaryocytes with ploidy of 16N and 32N was decreased on days 8 and 12 (P < .05 in both cases) when peripheral platelet counts in treated volunteers significantly exceeded placebo controls (Figure 2; P < .05 in both cases).
Platelet production following single bolus injection of 3 μg/kg PEG-rHuMGDF
| . | Day 1 . | Day 8 . | Day 12 . | Day 18 . |
|---|---|---|---|---|
| Platelet turnover | ||||
| (×103plt/μL/d) | 44 ± 12 | 58.3 ± 9.8 | 78.8 ± 26.0 | 101 ± 28 |
| Pvs day 1 | .0008 | .0009 | ||
| Megakaryocyte number | ||||
| (×106/kg) | 13 ± 6.4 | 17 ± 7.5 | 27 ± 9.4 | 11 ± 7.2 |
| Pvs day 1 | .69 | .03 | .69 | |
| Megakaryocyte volume | ||||
| (×103fL) | 30 ± 1.8 | 28 ± 3.0 | 24 ± 3.7 | 26 ± 3.2 |
| Pvs day 1 | .0003 | |||
| Megakaryocyte mass | ||||
| (×1010fL/kg) | 37 ± 19 | 46 ± 19 | 62 ± 17 | 30 ± 18 |
| Pvs day 1 | .42 | .015 |
| . | Day 1 . | Day 8 . | Day 12 . | Day 18 . |
|---|---|---|---|---|
| Platelet turnover | ||||
| (×103plt/μL/d) | 44 ± 12 | 58.3 ± 9.8 | 78.8 ± 26.0 | 101 ± 28 |
| Pvs day 1 | .0008 | .0009 | ||
| Megakaryocyte number | ||||
| (×106/kg) | 13 ± 6.4 | 17 ± 7.5 | 27 ± 9.4 | 11 ± 7.2 |
| Pvs day 1 | .69 | .03 | .69 | |
| Megakaryocyte volume | ||||
| (×103fL) | 30 ± 1.8 | 28 ± 3.0 | 24 ± 3.7 | 26 ± 3.2 |
| Pvs day 1 | .0003 | |||
| Megakaryocyte mass | ||||
| (×1010fL/kg) | 37 ± 19 | 46 ± 19 | 62 ± 17 | 30 ± 18 |
| Pvs day 1 | .42 | .015 |
Effects of bolus PEG-rHuMGDF on megakaryocyte ploidy frequency distribution. The frequency distribution of ploidy classes from 2N to 128N is shown baseline for 8 normal subjects (open bars). After PEG-rHuMGDF administration, ploidy frequency distributions are compared in 6 normal subjects evaluated on day 8 (acute hatching), day 12 (grave hatching), and day 18 (double cross-hatching). Placebo subjects yielded data consistent with baseline distribution. The proportion of 16N cells was significantly reduced during the period of induced thrombocytosis on day 8 and day 12, consistent with the decreased megakaryocyte volumes reported in Table 3. Variance about the mean values is ± SD.
Effects of bolus PEG-rHuMGDF on megakaryocyte ploidy frequency distribution. The frequency distribution of ploidy classes from 2N to 128N is shown baseline for 8 normal subjects (open bars). After PEG-rHuMGDF administration, ploidy frequency distributions are compared in 6 normal subjects evaluated on day 8 (acute hatching), day 12 (grave hatching), and day 18 (double cross-hatching). Placebo subjects yielded data consistent with baseline distribution. The proportion of 16N cells was significantly reduced during the period of induced thrombocytosis on day 8 and day 12, consistent with the decreased megakaryocyte volumes reported in Table 3. Variance about the mean values is ± SD.
Discussion
This study demonstrates that administering single bolus subcutaneous PEG-rHuMGDF (3 μg/kg) to healthy human volunteers doubles the peripheral platelet concentration by stimulating megakaryocyte proliferation and endoreduplication, thereby doubling the mass of megakaryocyte cytoplasm destined for release as circulating platelets, without modifying platelet viability, platelet responsiveness to physiologic agonists, or platelet expression of activation epitopes.
Circulating concentrations of unbound endogenous TPO regulate physiologic platelet production to maintain constant peripheral platelet counts by compensating for changes in the peripheral demand for platelets.4,6,9,43 rHuTPO and PEG-rHuMGDF are 2 thrombopoietic growth factors that have been developed and evaluated in patients.8,9 These 2 agents produce molar-equivalent effects on megakaryocytopoiesis. In healthy human volunteers, exogenous stimulation of thrombocytopoiesis by PEG-rHuMGDF produces measurable increases in marrow megakaryocyte endoreduplication and cytoplasmic volume within 24 hours, and in marrow megakaryocyte number several days later (Table 3).44 Corresponding increases in circulating platelets are detectable after 5 to 6 days and peak by 10 to 14 days.45,46 Under steady-state conditions PEG-rHuMGDF produces comparable log-linear dose responses in megakaryocyte mass and platelet mass turnover.47 48 This study in volunteers underscores the reliability of megakaryocyte ploidy as a morphologic indicator of thrombopoietic stimulus, as shown by the transient decreases in megakaryocyte ploidy during induced thrombocytosis.
In healthy human volunteers, minimal reduction in MPV develops as the peripheral platelet counts are elevated (Table 1), consistent with previous reports in nonhuman primates regarding the reciprocal relationship between MPV and the peripheral concentration of platelets.48 Presumably, the blunted decrease in MPV observed in normal subjects (Table 1), compared with the greater reduction in nonhuman primates, is due to the striking difference in the relative total dose and duration of PEG-rHuMGDF administration.
Modulation of peripheral platelet counts in healthy human volunteers provides an opportunity to evaluate the effects of platelet concentration and age on the measurements of platelet life span under physiologic conditions. Current understanding of platelet survival time has evolved from the Mills-Dornhorst equation,41,49 which postulates an intrinsic 10-day time-dependent viability for circulating platelets in normal individuals (analogous to the 120-day life span for normal erythrocytes). However, superimposed on this 10-day life span is ongoing fixed random utilization that is required to maintain physiologic vascular integrity, a process requiring approximately 10% of the circulating platelet mass.38,50 Thus, in normal individuals the disappearance pattern of mixed population111In-labeled platelets is predominately linear, due to senescent removal, with slight curvilinearity resulting from fixed random vascular utilization, so-called “external hazard function” (Figure 3).50,51 Gamma function analysis of these disappearance curves provides good estimates of the average time that platelets survive in the circulation.38 51 As peripheral platelet counts decrease below 50 000/μL the relative importance of vascular utilization progressively increases, resulting in shortened platelet survival times secondary to thrombocytopenia, per se, apart from any other mechanism(s) of platelet destruction.
In this study, platelet life span was measured for platelet populations that were (1) enriched with young, recently formed platelets (day 8); (2) enriched with platelets approaching senescence (day 18); and (3) platelets at twice the baseline concentration (day 12). Gamma function analysis revealed prolonged platelet life span for platelets enriched with newly formed platelets (day 8; Figure 3; Table 1), shortened platelet life span for platelets approaching senescence (day 18; Figure3; Table 1), and minimally prolonged platelet life span at peak-induced thrombocytosis, compared with baseline (day 12; Figure 3; Table 1). These findings are concordant with the concepts that platelets are removed from the circulation predominantly by time-related senescent mechanism(s), with superimposed fixed vascular utilization that becomes proportionately less important when the peripheral concentration of platelets is increased. Clearly, the observed effects reported here would be accentuated by enhancing the degree of thrombocytosis induced by PEG-rHuMGDF or rHuTPO.
To provide a theoretical basis for the effects of changing platelet count and nonsteady-state alterations in mean peripheral platelet age, peripheral platelet counts, and 111In-labeled platelet decay curves were simultaneously modeled using a nonstationary model of platelet destruction and PEG-rHuMGDF-mediated megakaryocytopoiesis (Figure 4). The gamma function used for calculating platelet life span from platelet tracer curves (Table 1) was derived using steady state assumptions. Although the gamma function can approximate mean platelet life span under nonsteady-state conditions, the gamma function, per se, does not correct for nonstationary alterations in platelet age distributions or the effects of changing platelet count on endothelial platelet clearance during the time course of tracer decay. Therefore, to estimate intrinsic platelet longevity and endothelial platelet utilization rate after transient stimulation of megakaryocytopoiesis, a nonstationary thrombokinetic model was implemented. Using this model, excellent fits to peripheral platelet counts and tracer decay curves were attained (Figures 5 and 6). Model estimates of intrinsic platelet longevity and endothelial utilization rate were consistent with published values determined under steady-state conditions,52 53 thereby supporting the notion that the observed shifts in mean platelet life span on days 8, 12, and 18 relative to baseline were attributable to anticipated effects of changing platelet count and mean peripheral platelet age.
Because the Mpl ligands, PEG-rHuMGDF and rTPO, initiate receptor-dependent platelet signaling in vitro,52,53 and enhance platelet aggregative responsiveness to physiologic agonists in vitro and ex vivo,48 it has been postulated that their therapeutic use would increase the risk of developing vascular thrombosis.8,9 However, the negative results reported in Table 3 for normal subjects, and by others for cancer patients,15,54 support the conclusions resulting from studies in nonhuman primates,48 that therapy with Mpl ligands does not increase the risk of developing vascular thrombotic events, at least for the dosing and indications evaluated to date. However, in patients with underlying vascular disease, the degree of thrombocytosis complicating Mpl ligand administration may be of importance in the genesis of thrombotic events.28
The development of PEG-rHuMGDF has been suspended because of the development of neutralizing antibodies that inactivate endogenous TPO in some individuals after repeated injections.55 This observation represents a significant impediment to development of Mpl ligands. Recent descriptions of short peptide Mpl agonists with amino acid sequences unrelated to TPO indicate a possible means of circumventing this problem.55 The findings in this study support the rationale for using modest doses of nonimmunogenic Mpl ligands in volunteer donors undergoing platelet apheresis, to improve yields and efficiency.
In conclusion, 1 week after bolus thrombopoietic stimulation with 3 μg/kg PEG-rHuMGDF, megakaryocytopoiesis doubles, leading to corresponding doubling on day 12 of platelets that exhibit normal function and viability during the ensuing 10 days. All values return to baseline 4 weeks after bolus thrombopoietic stimulation.
Supported in part by Amgen Inc, and in part by PHS Grant MO1-RR00039 from the General Clinical Research Centers Program, National Institutes of Health, National Center for Research Resources.
Reprints:Laurence A. Harker, Blomeyer Professor and Director, Division of Hematology and Oncology, Emory University School of Medicine, 1639 Pierce Dr, Room 1003 WMB, Atlanta, GA 30322.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 4. Nonstationary gamma model of platelet production and destruction mediated by Mpl-ligand. The endogenous thrombopoietin and exogenous PEG-rHuMGDF serum concentration time course, C(t), stimulates megakaryopoiesis; de novo platelet production rate, S[S0, C(t)], stimulated by Mpl-ligand begins after a megakaryocyte maturation delay time, τm. Newly formed platelets emerge into blood and are diluted in a volume, Vp, which includes blood volume and splenic pooling. Platelets progress through a sequence of na age compartments until they are lost from circulation by processes of random destruction, ρ, or reach the limit of intrinsic platelet longevity, Λ.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2514/4/m_bloo00825004x.jpeg?Expires=1765886837&Signature=DGn~SXqy5-0kUbqpEhvC73cLKFvVQwc1bg8x4Lg16n-BQKDAIVQSYQo5QjU4-5zXuK6Ty4kAssu7Hsd663KEOZw1hinMBezenw3NvxQY5N45pj26-0R9GQnsV8dnssPlTmdiHP9hpOAKvYmLVzQRCo6meJkdYmado3btZk7jtHPL2FORFdV0P7OKERLpxf9ugW56ciOD9ibv~vT2Nd7w8eEPSMSggJ60M80~uaG5E7nINTdcJM8ravl6kJp5onQjpplCQWnSW1FlTRM9tuUi3yA8PGQ7emsNwZ~WDajhtZgd0OfbXKqLsjfVqUgx2iUD4VlutLJEBPjr1eEM6atPUQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal