Abstract
X inactivation makes females mosaics for 2 cell populations, usually with an approximate 1:1 distribution. Skewing of this distribution in peripheral blood cells is more common among elderly women.1–3 The depletion of hematopoietic stem cells followed by random differentiation may explain the acquired skewing with age.4 However, an animal model suggests that selection processes based on X-linked genetic factors are involved.5 We studied peripheral blood cells from 71 monozygotic twin pairs aged 73 to 93 years and from 33 centenarians, and we found that with age, 1 of the cell populations becomes predominant for most women. We also observed a strong tendency for the same cell line to become predominant in 2 co-twins. This suggests that X-linked genetic factors influence human hematopoietic stem cell kinetics. The fact that females have 2 cell lines with different potentials could be one of the reasons women live longer than men.
Skewed X inactivation (greater than 3:1) occurs in the myeloid blood cell lineages in one third to one half of women older than 60 years.1-3 The reason for this is unclear. Data from autologous marrow transplantation studies of female Safari cats heterozygous for glucose 6-phosphate dehydrogenase mutation has been the basis for mathematical modeling showing that clonal dominance can in fact occur simply by chance when the number of hematopoietic stem cells is small.4 Chance events could act on many levels, such as stem cell replication, apoptosis, and the initiation of differentiation or maturation. Depletion of hematopoietic stem cells and random differentiation of the few residual hematopoietic stem cells is a possible explanation.1,2,4 Theoretically, the increased frequency of skewed X-inactivation patterns with age could be caused by (emerging) neoplastic clones, but the rarity of myelodysplasia makes this explanation unlikely.1,4 A recent follow-up of 11 heterozygous female Safari cats showed evidence that excessive age-related skewing could result from hemizygous selection, that is, a growth advantage conferred by 1 of the parental X chromosomes.5
Genetically identical persons provide a unique research resource. It has been suggested that monozygotic twinning and the X-inactivation process might interact.6,7 However, a recent study8 showed no difference in the degree of X-inactivation skewing between young monozygotic twins and young singletons, either in blood cells or in buccal mucosa. If the often observed predominance of 1 of the 2 cell lines in peripheral blood in elderly women was determined by a stochastic process with no selection, little correlation in the X-inactivation patterns between monozygotic co-twins would be expected. A selection process based on X-linked genetic factors, on the other hand, would create a tendency for the same cell line to become predominant in monozygotic co-twins. Furthermore, a selection process based on X-linked genetic factors would lead to an increased prevalence of skewed X inactivation among centenarians compared to, for example, octogenarians.
Study design
Subjects
The Longitudinal Study of Aging Danish Twins comprises twins aged 73 years and older in the nationwide Danish Twin Registry. In 1997, 2172 individuals (79% of the twins) were interviewed, regardless of whether the co-twin was alive.9 Seventy-one monozygotic female twin pairs were available for X-inactivation analysis. This sample was unselected insofar as no one was excluded because of disease. The Danish Centenarian Study is a nationwide epidemiologic survey of all persons living in Denmark who celebrated their 100th birthdays during a 14-month period from 1995 to 1996. A year and a half later, the surviving participants were revisited; the women from this follow-up survey were included in the current study. In the twin and the centenarian studies, after the nature and the possible consequences of the study were explained, informed consent was obtained from the participants. DNA was extracted from peripheral blood using standard procedures.
X-inactivation analysis and zygosity determination
The X-chromosome inactivation pattern was determined by polymerase chain reaction (PCR) analysis of a polymorphic CAG repeat in exon 1 of the androgen receptor gene.10 Cleavage by the methylation-sensitive enzyme HpaII gave a PCR product from the inactive X chromosome only. The PCR products were separated on an ABI 373A automated sequencer and analyzed by GeneScan software (Biosystems, Oslo, Norway). Each sample was analyzed twice and for twins with no knowledge of the result for the co-twin. The X-inactivation pattern was given as a ratio between the PCR products, with the smallest allele indicated first. Zygosity was determined using a PCR analysis of the 5 highly polymorphic markers D7S482, ApoB 3′VNTR, D19S191, LIPE, and the CAG repeat in the androgen receptor. Identity in all markers had greater than 0.998 probability of monozygosity.
Results and discussion
Table 1 shows that the elderly monozygotic twins did not differ from the elderly singletons in terms of overall X-inactivation patterns (P > .5) and that, as expected, elderly twins and singletons had patterns that were significantly more skewed than in younger persons (P < .01).
Distribution of X-inactivation patterns in Scandinavian twins and singletons
| . | Blood donors11 . | Elderly singletons12 . | Elderly twins . | Centenarians . |
|---|---|---|---|---|
| N | 148 | 43 | 142 | 33 |
| Age range | 19-65 | 83-101 | 73-93 | 101 |
| X-inactivation pattern (% of persons) | ||||
| Random (most common X ∈ [50%; 80%[) | 93 | 65 | 65 | 33 |
| Skewed (most common X ∈ [80%; 95%[) | 7 | 26 | 27 | 49 |
| Extremely skewed (most common X ∈ [95%; 100%]) | 0 | 9 | 8 | 18 |
| . | Blood donors11 . | Elderly singletons12 . | Elderly twins . | Centenarians . |
|---|---|---|---|---|
| N | 148 | 43 | 142 | 33 |
| Age range | 19-65 | 83-101 | 73-93 | 101 |
| X-inactivation pattern (% of persons) | ||||
| Random (most common X ∈ [50%; 80%[) | 93 | 65 | 65 | 33 |
| Skewed (most common X ∈ [80%; 95%[) | 7 | 26 | 27 | 49 |
| Extremely skewed (most common X ∈ [95%; 100%]) | 0 | 9 | 8 | 18 |
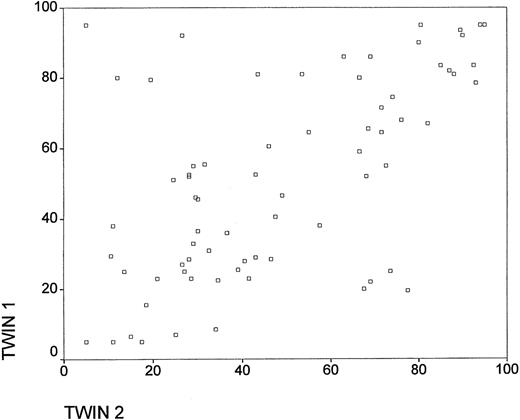
The intraclass correlation within monozygotic twin pairs for percentage of inactivation of an X chromosome (percentage of smaller allele) was 0.57 (P < .01). When the 8 outliers (see Figure1) were excluded from the analyses, the intraclass correlation rose to 0.84 (P < .01). Some of these outliers most likely resulted from non-concordant X inactivation. Monteiro et al8 found that 13% (3 of 23) of monozygotic twin pairs aged 0 to 24 years had large absolute differences in X-inactivation patterns. If there is a similar selection for the 2 cell lines in such pairs, they can remain discordant for the X-inactivation pattern throughout life. Another possibility is the presence of myelodysplasia in 1 twin that could result in outliers. Finally, somatic recombination of the X chromosome (between a stem cell kinetic gene and the androgen receptor gene) could lead to apparently discordant X inactivation patterns in monozygotic twin pairs.
X inactivation patterns in female monozygotic twins aged 73 to 93 years.
Percentage of inactivation of an X chromosome (percentage of smaller allele). The measurements are truncated at 5% and 95%.
X inactivation patterns in female monozygotic twins aged 73 to 93 years.
Percentage of inactivation of an X chromosome (percentage of smaller allele). The measurements are truncated at 5% and 95%.
The strong tendency for the same blood cell line to become predominant in elderly female monozygotic co-twins provides evidence that human stem cell kinetics is influenced by X-linked genetic factors. Based on studies of younger persons, some evidence of linkage between skewed X inactivation and loci on the X chromosome has been found in large healthy families selected for the presence of skewed X inactivation in several females.13 14 Our finding of an increased prevalence of skewed X inactivation among centenarians compared with 73- to 93-year-old women (P < .01, Table 1) is compatible with a selection process that depends on X-linked genetic factors.
It seems likely that having 2 cell lines in all organs provides a health advantage, which is clearly the case for X-linked diseases. In Wiskott–Aldrich syndrome, Lesch–Nyhan syndrome, and some of the immunodeficiency syndromes, tissue-specific, nonrandom, X-chromosome inactivation occurs with better survival of the nonmutated X.15-17 Similarly, in heterozygotes for glucose 6- phosphate dehydrogenase mutations causing severe enzyme deficiency, there is a significant excess of normal blood cells.18 19 It seems plausible, not only in such X-linked diseases but also for mutations with more subtle effects, that having 2 cell lines offers an advantage to females. This advantage tends to be greatest in mitotically active tissues such as blood cells or mucosa cells. Furthermore, the predominance could be tissue specific, with 1 cell line predominant in 1 organ because of better survival, whereas the other cell line could be more frequent in another organ. If the X-linked factors that lead to better survival for a blood cell line can be determined, this will have major implications for marrow transplantation and gene therapy on hematopoietic stem cells.
The selective survival of 1 cell line may be beneficial to a person. Our finding that predominance of 1 cell line is rare among young females and common among the oldest old is compatible with an X chromosome-dependent selection process. Furthermore, a trait that is rare among the young and common among the oldest old may be a factor that helps women survive to extreme ages.20 Hence, the ability to reach very old age may be influenced by X-linked genes. We speculate that having 2 cell lines with different potentials that may affect lifespan could contribute to the longer lifespan of women. This hypothesis has indirect support from animal life-span studies. In mammals the male is heterogametic (XY) and has a shorter life span than the female. In birds it is the females that are heterogametic (ZW), and the available data suggest that male birds tend to live longer than female birds.21
Supported by the United States National Institute on Aging (AG-08761), the Danish Research Councils, the Danish National Research Foundation, the Norwegian Cancer Society, the Research Council of Norway, and Ullevål University Hospital Research Forum.
Reprints:Kaare Christensen, Danish Twin Registry, University of Southern Denmark, Main Campus, Odense University, Winslowparken 17,1, DK-5000 Odense C, Denmark; e-mail:kchristensen@health.sdu.dk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal