Abstract
To test the hypothesis that cell cycle regulatory gene abnormalities are determinants of clinical outcome in adult acute lymphoblastic leukemia (ALL), we screened lymphoblasts from patients on a Southwest Oncology Group protocol for abnormalities of the genes, retinoblastoma (Rb), p53, p15(INK4B), and p16(INK4A). Aberrant expression occurred in 33 (85%) patients in the following frequencies: Rb, 51%; p16(INK4A), 41%; p53, 26%. Thirteen patients (33%) had abnormalities in 2 or more genes. Outcomes were compared in patients with 0 to 1 abnormality versus patients with multiple abnormalities. The 2 groups did not differ in a large number of clinical and laboratory characteristics. The CR rates for patients with 0 to 1 and multiple abnormalities were similar (69% and 54%, respectively). Patients with 0 to 1 abnormality had a median survival time of 25 months (n = 26; 95% CI, 13-46 months) versus 8 months (n = 13; 95% CI, 4-12 months) for those with multiple abnormalities (P < .01). Stem cells (CD34+lin−) were isolated from adult ALL bone marrows and tested for p16(INK4A) expression by immunocytochemistry. In 3 of 5 patients lymphoblasts and sorted stem cells lacked p16(INK4A) expression. In 2 other patients only 50% of sorted stem cells expressed p16(INK4A). By contrast, p16 expression was present in the CD34+ lin− compartment in 95% (median) of 9 patients whose lymphoblasts expressed p16(INK4A). Therefore, cell cycle regulatory gene abnormalities are frequently present in adult ALL lymphoblasts, and they may be important determinants of disease outcome. The presence of these abnormalities in the stem compartment suggests that they contribute to leukemogenesis. Eradication of the stem cell subset harboring these abnormalities may be important to achieve cure.
Substantial incremental success in the treatment and management of childhood acute lymphoblastic leukemia (ALL) over the past 3 decades has not impacted significantly on the prognoses of adults with ALL. Whereas 70% to 75% of children with ALL now can expect to be cured of their disease, the same expectation pertains to no more than 30% to 35% of adults.1,2 Compared to children, adults with ALL are more refractory to initial chemotherapy and more likely to develop resistance to ongoing treatment. These observations suggest that different etiologic events contribute to the pathogenesis of childhood and adult ALL. Although differences have been described in morphologic and immunologic characteristics and, more important, in the distribution of molecular genetic abnormalities, no single feature among these variables appears to account for the marked differences in clinical and biologic behaviors. For adult patients with ALL, age, white blood cells (WBC), and cytogenetics are well-established prognostic factors. However, many adult patients do not have any of these distinguishing features. Therefore, there is a need to identify other factors that may aid in assessing prognosis in this difficult group of patients. Recently many investigators have reported abnormalities of tumor suppressor or cell cycle regulatory genes in patients with ALL.3 4 Among the genes in this category most frequently altered in adult ALL are p16(INK4A), p15(INK4B), the retinoblastoma tumor suppressor gene (Rb), and p53. Increasing evidence indicates that abnormalities of these genes are common, perhaps universal, in adult ALL and that substantial numbers of patients have abnormalities of more than 1 gene in this category.
Although single tumor suppressor gene abnormalities have not generally correlated with prognosis, we hypothesized that multiple abnormalities of cell cycle regulatory genes may strongly influence clinical outcome in adult ALL. Furthermore, we suggest that the acquisition of these abnormalities by clonal neoplastic bone marrow precursor cells in the stem cell compartment is an important prerequisite of the leukemogenic process. These hypotheses are based on observations that progression is often associated with acquisition by the neoplastic clone of multiple genetic aberrations prevalent in leukemias with poor prognoses. To test these concepts we analyzed expression of a group of cell cycle regulatory genes, Rb, p53, p16(INK4A), and p15(INK4B), in a cohort of adult patients with ALL uniformly treated on a Southwest Oncology Group (SWOG) protocol with mature follow-up. In addition, to determine whether p16(INK4A) abnormalities were present in stem cells, we assessed alterations of p16(INK4A) in adult ALL bone marrow sorted into stem, early, and late lineage committed precursor compartments.
Materials and methods
Patient specimens and clinical protocols
Samples were analyzed from 42 patients with de novo adult ALL registered on treatment study S8417/19: Evaluation of 2 Consolidation Regimens in the Treatment of Adult Acute Lymphoblastic Leukemia.5 This protocol was open to patient accrual from June 14, 1985 to November 15, 1991. Eligibility criteria included age 15 years or older, absolute infiltration of the bone marrow (determined by % blasts × cellularity) with 50% lymphoblasts or 30% to 40% lymphoblasts with evidence of progressive disease, and no prior leukemia treatment. The diagnosis of ALL was confirmed by central review of morphology and cytochemistry according to the previously described SWOG modification of the French-American-British (FAB) criteria.6 The treatment included 5-drug induction with an L10M regimen,7 followed by randomization to consolidation therapy with either the L10M regimen or alternative chemotherapy with daunorubicin, cytosine arabinoside, 6-thioguanine, L-asparaginase, and methotrexate. After consolidation, all patients received uniform, multi-drug L10M maintenance therapy. No difference in outcomes was observed for patients registered to the 2 different consolidation arms.5 Central immunophenotype determinations were performed on pretreatment specimens at the SWOG Lymphoid Leukemia Reference Laboratory at the University of Texas Health Science Center at San Antonio by methods previously reported.8 Specimens were sent to the central laboratory by overnight courier. Samples in transit for more than 24 hours or samples with viability less than 90% were considered inevaluable. Cytogenetic evaluations were not required for patients registered to protocol S8417. Cytogenetic results performed by patients' local institutions were reviewed when possible by the SWOG Cytogenetics Committee. As a result, data were available for 16 subjects in this analysis. Furthermore, the presence or absence of the p190 or p210 BCR-ABL fusion genes was assessed by reverse transcription–polymerase chain reaction (RT-PCR) in 34 of these subjects.9
The stem cell sorting experiments were performed using 16 specimens from patients registered on protocol S9400: Treatment of Adult Acute Lymphoblastic Leukemia: A Phase II Trial of an Induction Regimen Including PEG-L-Asparaginase, in Previously Untreated Patients, Followed by Allogeneic Bone Marrow Transplantation or Further Chemotherapy in First Complete Remission. This ongoing protocol was open to patient accrual on July 15, 1995. Eligibility, diagnostic criteria, and immunophenotyping were performed as described above.
Immunodetection of Rb and p53 proteins
Assessments of lymphoblast expression of pRb and p53 were performed as previously described.10 Immunoblotting was used to analyze lymphoblast lysates for Rb or p53 protein expression. Limiting dilution experiments indicated that the technique was sufficiently sensitive to permit detection of pRB or p53 in as little as 1 μg lysate protein from cryopreserved normal activated peripheral blood lymphocytes (data not shown). All patient samples that lacked pRB or p53 expression by this technique were subsequently subjected to additional analyses by electrophoresis and silver staining to ensure integrity of the protein composition and to exclude the possibility that absence of pRb or p53 expression represented sample degradation. In addition, a nonisotopic RNase cleavage assay based on techniques originally described by Myers et al11 and Winter et al12 was used to analyze p53 overexpressing specimens for point mutations in exons 5 to 9 of the p53 gene. (Mismatch Detect Point Mutation Screening Kit; Ambion, Austin, TX). Point mutations were confirmed in 61% of patients with aberrant p53 expression identified by immunoblotting.10
Analyses for p16(INK4A) abnormalities
Two methods were used to analyze samples for p16(INK4A) abnormalities. The first was Southern blot analysis of p15, p16 abnormalities. DNA was extracted using standard methods.13Approximately 10 μg extracted DNA from blood and bone marrow samples was enzymatically digested with HindIII (New England Biolabs; Beverly, MA). Digests were electrophoresed on standard agarose gels and transferred to a nylon membrane (Gene-Screen Plus; Dupont/NEN, Wilmington, DE). Southern blot analysis was performed by hybridization of digests to a 1 kb Xbal/Xhol cDNA probe that recognizes exon 2 of p15 and exons 1 to 3 of p16. All blots were hybridized sequentially to a genomic probe (2.2 × X) from theTAL1 gene, which served as a control for DNA integrity.14 Blots were washed to a final stringency of 0.1 × saturated sodium citrate (SSC) and 1% sodium dodecyl sulfate at 65°C before autoradiography. Blots were exposed to film for 3 to 8 days.
The second method used to analyze samples for p16(INK4A) abnormalities was PCR. In some cases, PCR analysis was carried out to determine specific deletions of the various exons of the p16 gene as described previously for exons 1 and 215 and for exon 3.16 The PCR-amplified products were fractionated on a 2% agarose gel and visualized by ethidium bromide staining.
Flow cytometric analyses and sorting of ALL marrows and blood specimens
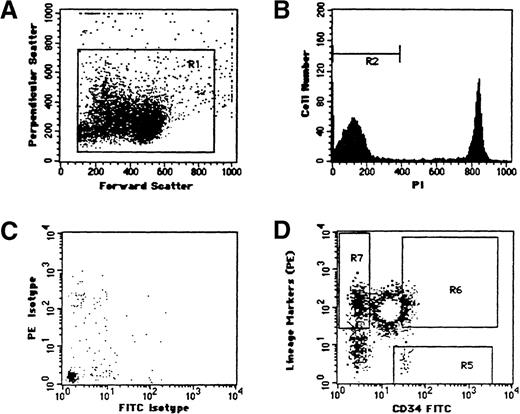
Cryopreserved specimens were sent to the Cancer Center at the University of California, San Francisco, for immunostaining and cell sorting. Specimens were sorted into 3 differentiation compartments as previously described17 18 (Figure 1).
Multivariate flow cytometric analysis of specimen 15.
(A) Bivariate light scatter distribution. R1 is the gate used to discriminate nucleated cells. (B) PI fluorescence intensity of R1 gated cells. R2 defines viable cells contained within the R1 gate. (C) Isotype FITC- and PE-labeled control and (D) CD34-linked immunofluorescence (abscissa) versus lineage markers (ordinate). Sort gates: R5 (CD34+lin−), R6 (CD34+lin+), R7 (CD34-lin+).
Multivariate flow cytometric analysis of specimen 15.
(A) Bivariate light scatter distribution. R1 is the gate used to discriminate nucleated cells. (B) PI fluorescence intensity of R1 gated cells. R2 defines viable cells contained within the R1 gate. (C) Isotype FITC- and PE-labeled control and (D) CD34-linked immunofluorescence (abscissa) versus lineage markers (ordinate). Sort gates: R5 (CD34+lin−), R6 (CD34+lin+), R7 (CD34-lin+).
Flow cytometric analysis and sorting were performed on a FACStar Plus Cell Sorter (Becton Dickinson, Mountain View, CA) equipped with 2 argon-ion lasers (Coherent, Palo Alto, CA) tuned to 488' nm (primary laser) and to 351-364 nm (secondary laser). Small debris and cell aggregates were excluded by forward and side light scatter measurements, and doublets were excluded using forward scatter pulse width and area measurements. Fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-linked immunofluorescence emissions from the primary laser excitation were collected through 530 nm (± 30 nm) and 575 nm (± 26 nm) bandpass filters, respectively. Propidium iodide (PI) emissions from secondary laser excitation were collected through a 620-nm long-pass filter.
The frequency of discriminated subpopulations was estimated as described previously.17 The number of PI-negative cells within the light scatter gate (see Figures 1A and 1B) in each specimen constituted the denominator value used for subpopulation estimation. The average frequency of viable cells in the 24 specimens approximated 85% (range 50% to 99%). Quadrant markers were set at the first decade of the FITC and PE fluorescence intensities, and photo multiplier tube voltages were adjusted so that gated specific regions contained less than 0.01% of viable nucleated cells from the isotype control sample. Fluorescence compensation was performed using single fluorochrome-positive controls. Rectangular regions within the positive quadrant boundaries were used to sort 3 compartments: CD34−33+38+19+(CD34−lin+), CD34+33+38+19+(CD34+lin+) and CD34+33−38−19−(CD34+lin−). The stringent sort regions, set 5% to 15% away from the quadrant boundaries, were used to minimize contamination from adjacent cell subpopulations during the sort. Cells from these regions were sorted into wells on Teflon-coated glass microscope slides (DuPont, Wilmington, DE) using a 2-drop packet in Normal-C mode to ensure maximal purity (ie, more than 98%), and cells fixed with Carnoy's (methanol/glacial acetic acid; 3/1, vol/vol). The sorted cells were dried on a slide warmer at 37°C for 30 minutes then fixed again with fresh Carnoy's.
Immunocytochemical determination of p16(INK4A)
Expression of p16(INK4A) in the sorted bone marrow compartments was assessed by immunocytochemistry. Briefly, slides containing sorted cells were incubated overnight with p16(INK4A) monoclonal mouse antibody 1:100 (Neomarkers p16-Ab-1; Neomarkers, Fremont, CA) and then incubated with biotinylated antimouse antibody 1:100 (Vector Laboratories, Burlingame, CA). Bound antibody was detected with the Vectastain ABC kit (Vector Laboratories). Slides were washed, incubated with osmium, fixed, and mounted for viewing by light microscopy.
Statistical analysis
Collection and quality control of patient pretreatment and outcome data were performed according to standard SWOG procedures. Pvalues for comparisons of continuous variables between groups of patients were 2-tailed and based on the Wilcoxon rank sum test.19,P values for dichotomous variables were based on the Fisher exact test20,21 using direct calculation to avoid large sample approximations. The remainingP values were based on the Pearson chi-squared test22 using direct calculation of P values to avoid large sample approximations. Overall survival was measured from the day of registration in the study until death from any cause and was censored only for patients known to be alive at last contact. Disease-free survival (DFS) was measured from the day that complete response (CR) was established until either relapse of ALL or death without relapse, and it was censored only for patients who were alive without evidence of relapse at the last follow-up. Distributions of overall survival and DFS curves were estimated by the method of Kaplan and Meier.23 Comparisons of overall survival or DFS between groups were based on the log-rank test.24 Comparisons adjusted for significant prognostic factors were based on Cox regression models23 for CR rates and on proportional hazards regression models24 for survival and DFS.
Results
Patient specimens
Cryopreserved bone marrow or peripheral blood specimens (greater than or equal to 80% lymphoblasts) were used to analyze 42 de novo adult patients with ALL from protocol S8417 for abnormalities of the cell cycle regulatory genes, Rb, p53, p15(INK4B), and p16(INK4A). These 42 patients were all those remaining in the S8417 repository with sufficient biologic material for the cell cycle regulatory gene analyses. Sufficient cells for assessment of all genes were available from 39 patients and for 2 genes from 3 patients. Characteristics of these patients and leukemic specimens are given in Table1. The patients in the current study are similar to the entire population registered to S8417 for FAB classification, blast cell lineage, co-expression of myeloid antigens, and blast cell expression of the CD34 stem cell antigen. In the current study, 27 of 42 (64%) patients achieved CR, which is similar to the CR frequency (62%) of all patients enrolled in the S8417 study.5 However, in previous analyses of S8417, age and WBC or peripheral lymphocyte count were prognostic for overall and relapse-free survival.5 The values of these parameters indicate that these 42 patients in the current study represent a poor prognostic subset.
Study population (protocol S8417)
| N = 42 . | |
|---|---|
| Age (y) | 34 (16-73)* |
| Sex (male/female) | 26/16 |
| Race (white/nonwhite) | 36/6 |
| Marrow blasts (%) | 91 (40-99) |
| Marrow lymphs (%) | 3 (0-40) |
| WBC ×103 | 89.9 (2.5-956) |
| Peripheral blasts (%) | 76.5 (0-99) |
| Peripheral blasts ×103 | 48.2 (0-850.8) |
| Peripheral lymphs (%) | 11 (0-80) |
| Peripheral lymphs ×103 | 7.4 (0-86.0) |
| Hemoglobin (g/dL) | 9.4 (4.4-16.3) |
| Platelets ×103 | 48.5 (5-184) |
| p16(INK4A) | |
| Normal | 25 (60%) |
| Abnormal | 17 (41%) |
| Rb | |
| Normal | 20 (49%) |
| Abnormal | 21 (51%) |
| P53 | |
| Normal | 29 (74%) |
| Abnormal | 10 (26%) |
| FAB classification | |
| L1 | 25 (60%) |
| L2 | 14 (33%) |
| L3 | 3 (7%) |
| Blast lineage | |
| B | 23 (55%) |
| T | 6 (14%) |
| Atypical | 3 (7%) |
| Calla + only | 1 (2%) |
| Null | 2 (5%) |
| Unavailable/inevaluable | 7 (17%) |
| Myeloid markers | |
| Negative | 20 (74%) |
| Positive | 7 (26%) |
| CD34 (<20% considered negative) | |
| Negative | 24 (65%) |
| Positive | 13 (35%) |
| Best response | |
| CR | 27 (64%) |
| No CR | 15 (36%) |
| N = 42 . | |
|---|---|
| Age (y) | 34 (16-73)* |
| Sex (male/female) | 26/16 |
| Race (white/nonwhite) | 36/6 |
| Marrow blasts (%) | 91 (40-99) |
| Marrow lymphs (%) | 3 (0-40) |
| WBC ×103 | 89.9 (2.5-956) |
| Peripheral blasts (%) | 76.5 (0-99) |
| Peripheral blasts ×103 | 48.2 (0-850.8) |
| Peripheral lymphs (%) | 11 (0-80) |
| Peripheral lymphs ×103 | 7.4 (0-86.0) |
| Hemoglobin (g/dL) | 9.4 (4.4-16.3) |
| Platelets ×103 | 48.5 (5-184) |
| p16(INK4A) | |
| Normal | 25 (60%) |
| Abnormal | 17 (41%) |
| Rb | |
| Normal | 20 (49%) |
| Abnormal | 21 (51%) |
| P53 | |
| Normal | 29 (74%) |
| Abnormal | 10 (26%) |
| FAB classification | |
| L1 | 25 (60%) |
| L2 | 14 (33%) |
| L3 | 3 (7%) |
| Blast lineage | |
| B | 23 (55%) |
| T | 6 (14%) |
| Atypical | 3 (7%) |
| Calla + only | 1 (2%) |
| Null | 2 (5%) |
| Unavailable/inevaluable | 7 (17%) |
| Myeloid markers | |
| Negative | 20 (74%) |
| Positive | 7 (26%) |
| CD34 (<20% considered negative) | |
| Negative | 24 (65%) |
| Positive | 13 (35%) |
| Best response | |
| CR | 27 (64%) |
| No CR | 15 (36%) |
Median (range).
Incidence of cell cycle regulatory gene abnormalities
The incidence of cell cycle regulatory gene abnormalities in the study cohort was high (Table 1). Aberrant expression of Rb, p16(INK4A), or p53 was identified in 21 of 41 (51%), 17 of 42 (41%), and 10 of 39 (26%), respectively. Twenty-nine specimens analyzed by Southern blotting were evaluable for p15(INK4B) deletions or rearrangements. Deletions were identified in 9 of 29 (31%) patients. Because in each of these 9 patients p15 was co-deleted with p16(INK4A), aberrant p15(INK4B) expression has not been analyzed separately. The incidences of Rb and p53 abnormalities are similar to those we reported previously in a larger subset of S8417 patients.9 The frequency of specimens with 1 or more abnormal cell cycle regulatory gene in the 39 patients who were evaluated for the 3 genes is shown in Table2. At least 1 abnormality of either Rb, p53, or p16(INK4A) occurred in 33 of 39 (85%) patients. Of these 33 patients, 20 (61%) had abnormalities of only 1 cell cycle gene. Twelve patients had abnormalities of 2 cell cycle genes, and 1 had abnormalities of Rb, p53, and p16. Thus 33% of all patients had abnormalities of multiple cell cycle regulatory genes.
Distribution of abnormalities in cell cycle regulatory gene expression in the S8417 study cohort
| Abnormality . | Frequency (%) . | . |
|---|---|---|
| None | 6 (15) | |
| Rb only | 10 (26) | |
| p16 only | 7 (18) | |
| p53 only | 3 (8) | |
| Rb + p16 | 6 (15) | |
| p53 + p16 | 3 (8) | |
| Rb + p53 | 3 (8) | 33% with multiple |
| Three abnormalities | 1 (3) | abnormalities |
| Abnormality . | Frequency (%) . | . |
|---|---|---|
| None | 6 (15) | |
| Rb only | 10 (26) | |
| p16 only | 7 (18) | |
| p53 only | 3 (8) | |
| Rb + p16 | 6 (15) | |
| p53 + p16 | 3 (8) | |
| Rb + p53 | 3 (8) | 33% with multiple |
| Three abnormalities | 1 (3) | abnormalities |
We used Southern blotting, PCR, or both to test for p16(INK4A) gene deletions or rearrangements. In the 39 patients analyzed for this study, both Southern blotting and PCR were preformed in 14, Southern blotting alone in 15, and PCR alone in 10. Sample availability precluded performing both assays in every patient. Overall, abnormalities of p16(INK4A) expression were detected in 17 patients (Table 3). Gene deletions/rearrangements were identified in 13 of 29 (45%) patients by Southern blotting and in 13 of 24 (54%) patients by PCR (data not shown).
p16(INK4A) Southern and PCR results for 17 adult patients with ALL
| Patient . | Southern analyses (deletion [D] or rearrangement [R]) . | PCR analyses . |
|---|---|---|
| 1 | D3-150 | Absent (exon 1 only) |
| 2 | D | Absent (exons 1 + 2) |
| 3 | D | Absent (exons 1 + 2) |
| 4 | D | Absent (exons 1 + 2) |
| 5 | D | Absent (exons 1 + 2) |
| 6 | D | Absent (exons 1 + 2) |
| 7 | R | Absent (exon 2 only) |
| 8 | R | Absent (exons 1 + 2) |
| 9 | D | Absent (exons 1 + 2) |
| 10 | R | Present |
| 11 | D | Present |
| 12 | D | N/A |
| 13 | D | N/A |
| 14 | N/A | Absent (exons 1 + 2) |
| 15 | N/A | Absent (exons 1 + 2) |
| 16 | N/A | Absent (exons 1 + 2) |
| 17 | N/A | Absent (exons 1 + 2) |
| Patient . | Southern analyses (deletion [D] or rearrangement [R]) . | PCR analyses . |
|---|---|---|
| 1 | D3-150 | Absent (exon 1 only) |
| 2 | D | Absent (exons 1 + 2) |
| 3 | D | Absent (exons 1 + 2) |
| 4 | D | Absent (exons 1 + 2) |
| 5 | D | Absent (exons 1 + 2) |
| 6 | D | Absent (exons 1 + 2) |
| 7 | R | Absent (exon 2 only) |
| 8 | R | Absent (exons 1 + 2) |
| 9 | D | Absent (exons 1 + 2) |
| 10 | R | Present |
| 11 | D | Present |
| 12 | D | N/A |
| 13 | D | N/A |
| 14 | N/A | Absent (exons 1 + 2) |
| 15 | N/A | Absent (exons 1 + 2) |
| 16 | N/A | Absent (exons 1 + 2) |
| 17 | N/A | Absent (exons 1 + 2) |
Results of Southern analyses and PCR for detection of p16(INK4A) in specimens from each of 17 adult patients with ALL with p16 aberrations.
In every patient except patient 1, p16(INK4A) deletions also involved the p15(INK4B) gene.
N/A = not available.
In patients analyzed by both Southern blotting and PCR, there was good concordance between results. Concordant results were observed in 12 of 14 patients. In 3 patients Southern analyses indicated an intact p16(INK4A) gene and PCR detected the appropriate exons 1 and 2 (not shown). Table 3 gives results of Southern blotting and PCR analyses in the 17 patients with aberrant p16(INK4A) expression. In 9 patients (patients 1 to 9) Southern analyses showed p16(INK4A) gene deletion or rearrangement corresponding to the loss of 1 or both exons 1 and 2 detected by PCR analysis. In 1 patient (patient 10) a rearranged p16(INK4A) gene was amplified by both the exon 1 and exon 2 primers. In another patient (patient 11), a large deletion involving both p16(INK4A) and p15(INK4B) genes was detected by Southern analysis, yet p16 remained detectable by PCR analysis. Nonetheless, overall there was excellent correspondence between the results given by the 2 techniques.
Southern blotting identified p16(INK4A) gene deletions in 10 patients and gene rearrangements in 3 patients (Table 3). Among the 13 patients in whom abnormalities were detected by PCR, neither exons 1 nor 2 of p16 were detected in 11 patients (85%). The p15(INK4B) gene was co-deleted with p16(INK4A) in 9 of 10 patients (data not shown). Rearrangements of p15(INK4B) were not identified. In 1 case of a p16(INK4A) deletion detected by Southern blotting (patient 1), exon 1 was absent but exon 2 was detected by PCR. Because the p15(INK4B) gene also was detected, it is likely that a micro-deletion occurred in this patient. In a second patient (patient 7) a p16(INK4A) gene rearrangement was associated with loss of exon 2 but retention of exon 1 by PCR analysis.
Outcome analyses
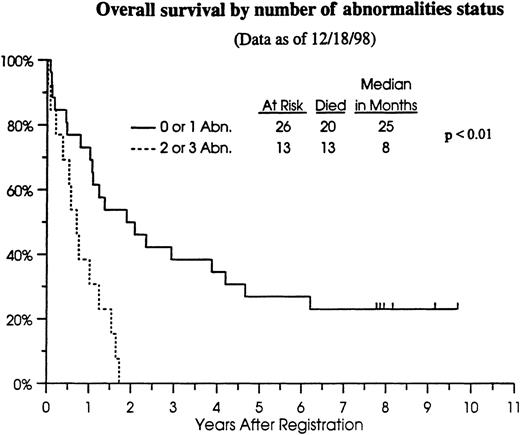
We previously have found no association between occurrence of any single gene abnormality (Rb or p53 only) and CR, overall, or relapse-free survival.10 However, preliminary analyses suggested a worse outcome for patients with aberrant expression of both Rb and p53.10 Therefore, we analyzed outcome data for the patients in this study in relation to the number of genetic aberrations (or hits) in the cell cycle regulatory genes (Table4). Patients with 0 to 1 abnormality in cell cycle regulatory genes had a CR rate of 69%, whereas those with more than 1 abnormality had a CR rate of 54% (not statistically significantly different). However, the significance of these comparisons is unknown because of the small sample size. Because 33 of 39 (85%) patients in this cohort have died (Figure2), relapse-free survival provided no additional information compared to overall survival. Patients with 0 to 1 abnormality in cell cycle regulatory genes had a median overall survival of 25 months (n = 26; 95% CI, 13 to 46 months) compared to 8 months (n = 13; 95% CI, 4 to 12 months) for those with 2 or 3 abnormalities (P < .01) (Figure 2).
CR by number of gene abnormalities4-150
| Abnormality . | CR . | 95% Confidence Interval . |
|---|---|---|
| 0 or 1 abnormality | 18/26 (69%) | 48%-86% |
| 2 or 3 abnormalities | 7/13 (54%) | 25%-81% |
| Abnormality . | CR . | 95% Confidence Interval . |
|---|---|---|
| 0 or 1 abnormality | 18/26 (69%) | 48%-86% |
| 2 or 3 abnormalities | 7/13 (54%) | 25%-81% |
39 patients with adult ALL registered to protocol S8417 were tested for abnormalities of Rb, p53, and p16(INK4A) expression in lymphoblasts. Patients were grouped according to either 0-1 or 2-3 genetic abnormalities.
Overall survival of cohort patients
by number of cell cycle regulatory gene abnormalities. — 0 to1 abnormality; ---2 to 3 abnormalities.
Overall survival of cohort patients
by number of cell cycle regulatory gene abnormalities. — 0 to1 abnormality; ---2 to 3 abnormalities.
Clinical (age, sex, race, marrow and peripheral blasts, WBC, peripheral lymphocyte counts, hemoglobin, platelets) and laboratory (FAB classification, blast lineage, myeloid antigen, and CD34 expression) features did not differ significantly between these 2 patient groups (data not shown). In addition, limited cytogenetic and molecular genetic data available for these patients permitted an estimate of the distribution of poor-risk cytogenetic characteristics between the 2 patient groups. Among the 26 patients with 0 to 1 regulatory gene abnormality, 3 had both the Ph1 chromosome and BCR-ABL detected by RT-PCR (2 subjects with p190 and 1 with p210); 1 had the p210 BCR-ABL by RT-PCR; 2 had t4;11, 1 had t8;14, and 1 was hypodiploid by cytogenetic analysis. Among the 13 patients with 2 or more regulatory gene abnormalities, 1 had the p210 BCR-ABL fusion gene by RT-PCR; 2 had t4;11, and 2 were hypodiploid by cytogenetic analysis. These data suggest that poor-risk cytogenetics was similarly distributed between the 2 groups defined by incidence of cell cycle regulatory gene abnormalities. Therefore, taken together, the similar clinical characteristics in the 2 groups indicate that abnormalities of cell cycle regulatory genes may impact clinical outcomes.
Stem cell sorting in adult ALL
CD34 analyses and stem cell sorting were performed on a group of 16 adult patients with ALL registered on protocol S9400. The cellularity, bone marrow blast count, frequency of CD34+ blasts, and p16(INK4A) abnormalities are shown in Table 5. Bone marrow cellularity ranged from 90% to 100%, and bone marrow blasts ranged from 55% to 98%. The CD34+ blast frequency ranged from 1% to 96%, and 7 of 16 of these specimens had p16(INK4A) abnormalities by immunocytochemistry. In preliminary experiments, we confirmed that p16(INK4A) deletions detected by immunocytochemistry were highly concordant (more than 90%) with our Southern blotting and RT-PCR data (Tsai T, unpublished observations).
Molecular and cellular features of samples used for stem cell sorting
| Pt. . | BM cellul (%) . | BM blast (%) . | Lymphoblast CD34 (%) . | p16(INK4A) . |
|---|---|---|---|---|
| 01 | 95 | 93 | 78 | Nl5-150 |
| 02 | 95 | 55 | 49 | Nl |
| 03 | N/A5-150 | N/A | 6 | Nl |
| 04 | 98 | 98 | 2 | Nl |
| 05 | N/A | N/A | 44 | Nl |
| 06 | N/A | N/A | 75 | Nl |
| 07 | N/A | N/A | 80 | Nl |
| 08 | 90 | 93 | 95 | Nl |
| 09 | 95 | 80 | 89 | Nl |
| 10 | 100 | 72 | 17 | Abn5-150 |
| 11 | 100 | N/A | 1 | Abn |
| 12 | 100 | 85 | 12 | Abn |
| 13 | 90 | 80 | N/A | Abn |
| 14 | 100 | 84 | 70 | Abn |
| 15 | 90 | 95 | 96 | Abn |
| 16 | 95 | 95 | 3 | Abn |
| Pt. . | BM cellul (%) . | BM blast (%) . | Lymphoblast CD34 (%) . | p16(INK4A) . |
|---|---|---|---|---|
| 01 | 95 | 93 | 78 | Nl5-150 |
| 02 | 95 | 55 | 49 | Nl |
| 03 | N/A5-150 | N/A | 6 | Nl |
| 04 | 98 | 98 | 2 | Nl |
| 05 | N/A | N/A | 44 | Nl |
| 06 | N/A | N/A | 75 | Nl |
| 07 | N/A | N/A | 80 | Nl |
| 08 | 90 | 93 | 95 | Nl |
| 09 | 95 | 80 | 89 | Nl |
| 10 | 100 | 72 | 17 | Abn5-150 |
| 11 | 100 | N/A | 1 | Abn |
| 12 | 100 | 85 | 12 | Abn |
| 13 | 90 | 80 | N/A | Abn |
| 14 | 100 | 84 | 70 | Abn |
| 15 | 90 | 95 | 96 | Abn |
| 16 | 95 | 95 | 3 | Abn |
N/A, not available; Nl, p16(INK4A) normal by Southern blotting and/or RT-PCR; Abn, p16(INK4A) abnormal by Southern blotting or RT-PCR.
The differentiation stage at which p16(INK4A) genetic hits were acquired was investigated by sorting bone marrow or blood cells from de novo adult patients with ALL using multivariate flow cytometry with lineage markers CD19, CD33, CD34, and CD38. Three subpopulations were discriminated: stem (CD34+lin−), committed progenitor (CD34+lin+), and (CD34−lin+).
Representative flow cytometric analysis of a leukemic specimen (patient 15) is shown in Figure 1. A light scatter gate (R1) (Figure 1A) was used to discriminate nucleated cells from debris. PI-positive cells were excluded from the analyses (Figure 1B). CD34+lin− (R5), CD34+lin+ (R6) and CD34−lin+ cells (R7) (Figure 1D) were identified on the basis of immunofluorescence that exceeded the isotype control (Figure 1C) and were sorted onto microscope slides. CD34+lin− cells, CD34+lin+, and CD34−lin+ cells comprised between 0.01% to 6.33%, 0.06% to 61.92%, and 1.04% to 80.43%, respectively (data not shown).
p16(INK4A) expression in sorted cell subpopulations and unsorted lymphoblasts from a subset of patients with or without p16 abnormalities was measured using immunocytochemistry (Table6). p16 expression was present in the CD34+lin− compartment in 95% (median) of patients whose lymphoblasts expressed p16(INK4A) (Table 6, Figure3). In contrast, p16 expression was observed in only 10% (median) of CD34+lin− compartments from patients with lymphoblasts displaying p16(INK4A) abnormalities. Approximately 20% (median) of specimens from patients with p16-aberrant lymphoblasts contained CD34+lin+ cells that expressed p16.
p16 Expression in three differentiation compartments6-150
| Group . | CD34+lin− . | CD34+lin+ . | CD34−lin+ . |
|---|---|---|---|
| p16 Normal | 95% (50-100) | 83% (30-100) | 100% (30-100) |
| (n = 9) | (n = 4) | (n = 3) | |
| p16 Abnormal | 10% (0-50) | 20% (0-50) | 65% (20-100) |
| (n = 5) | (n = 7) | (n = 3) |
| Group . | CD34+lin− . | CD34+lin+ . | CD34−lin+ . |
|---|---|---|---|
| p16 Normal | 95% (50-100) | 83% (30-100) | 100% (30-100) |
| (n = 9) | (n = 4) | (n = 3) | |
| p16 Abnormal | 10% (0-50) | 20% (0-50) | 65% (20-100) |
| (n = 5) | (n = 7) | (n = 3) |
Bone marrow cells from 16 patients were sorted into three differentiation compartments as described in “Materials and methods.” Cells in each compartment were examined for p16(INK4A) expression by immunocytochemical staining. Results are median percentages of p16-positive cells in each compartment with ranges in parentheses.
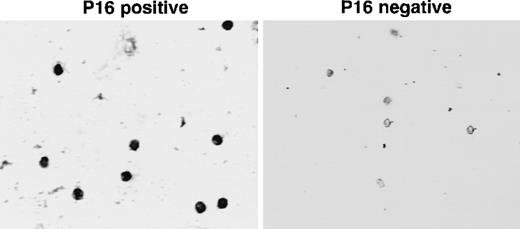
Immunocytochemical determination of p16(INK4A) expression in sorted bone marrow stem cells.
Adult ALL bone marrow was sorted as described in “Materials and methods.” Sorted stem cells (CD34+lin−) were collected on slides and stained for p16(INK4A) expression. The slide images were captured using an inverted microscope at 125× magnification. The images were transferred to a computer using a frame grabber board (Target+; Truevision) and stored in computer files using Java image analysis software (Jandel Scientific, Corte Madona, CA). Left panel: sorted CD34+lin− cells from a p16(INK4A)-positive ALL bone marrow. Note positive nuclear localization. Right panel: sorted CD34+lin− cells from a p16(INK4A)-negative ALL bone marrow. Nuclei are unstained.
Immunocytochemical determination of p16(INK4A) expression in sorted bone marrow stem cells.
Adult ALL bone marrow was sorted as described in “Materials and methods.” Sorted stem cells (CD34+lin−) were collected on slides and stained for p16(INK4A) expression. The slide images were captured using an inverted microscope at 125× magnification. The images were transferred to a computer using a frame grabber board (Target+; Truevision) and stored in computer files using Java image analysis software (Jandel Scientific, Corte Madona, CA). Left panel: sorted CD34+lin− cells from a p16(INK4A)-positive ALL bone marrow. Note positive nuclear localization. Right panel: sorted CD34+lin− cells from a p16(INK4A)-negative ALL bone marrow. Nuclei are unstained.
The frequency of p16-positive cells in CD34 subpopulations is shown in Table 7. In 3 of 5 evaluable patients (patients 11, 12, and 15), p16(INK4A) expression was effectively absent in the CD34+lin− compartment, whereas in 2 of 5 patients only 50% of cells expressed p16(INK4A). It is important to note that 3 of these patients (patients 11, 12, and 16) contained a low frequency (12% or less) of CD34+ lymphoblasts (Table 5), suggesting that even in CD34− ALL, cells in the CD34+lin− compartment contained cell cycle regulatory gene abnormalities.
p16(INK4A) Expression in sorted bone marrow compartments of patients with p16(INK4A) abnormalities7-150
| Patient . | CD34+lin− . | CD34+lin+ . | CD34−lin+ . |
|---|---|---|---|
| 10 | insuff. | 0 (5000) | N/A |
| 117-150 | 0 (100)7-151 | 50 (3000) | N/A |
| 127-150 | 0 (100) | 0 (2000) | N/A |
| 13 | 50 (50) | 50 (1000) | N/A |
| 14 | insuff. | 20 (1000) | 20 (130) |
| 15 | 10 (1000) | 50 (5600) | 65 (2000) |
| 167-150 | 50 (75) | 15 (3000) | 100 (190) |
| Patient . | CD34+lin− . | CD34+lin+ . | CD34−lin+ . |
|---|---|---|---|
| 10 | insuff. | 0 (5000) | N/A |
| 117-150 | 0 (100)7-151 | 50 (3000) | N/A |
| 127-150 | 0 (100) | 0 (2000) | N/A |
| 13 | 50 (50) | 50 (1000) | N/A |
| 14 | insuff. | 20 (1000) | 20 (130) |
| 15 | 10 (1000) | 50 (5600) | 65 (2000) |
| 167-150 | 50 (75) | 15 (3000) | 100 (190) |
Bone marrows were sorted into 3 differentiation compartments as described in “Materials and methods.” Cells in each compartment were examined for p16(INK4A) expression by immunocytochemical staining. Results are percentages of p16-positive cells detected in each compartment.
Patients in which lymphoblasts did not express CD34.
N/A, not available; insuff., insufficient cells sorted into the CD34+lin− compartment for analysis.
Numbers in parentheses indicate number of cells sorted per slide. On slides with less than 100 cells, all cells were counted.
Discussion
Abnormalities of cell cycle regulatory genes are being reported with increasing frequency in lymphoblasts of patients with adult ALL (reviewed in references 4 and 25). However, to date there have been few follow-up studies that systematically assessed the impact of these abnormalities on clinical outcomes in groups of patients treated uniformly. Tsai et al10 have examined influences of Rb and p53 gene abnormalities on outcome measures of patients registered to front-line and relapsed/refractory SWOG adult ALL protocols, and Stock et al26 have performed similar analyses for p16(INK4A) inactivation in adult patients enrolled in a front-line CALGB study. These studies involved analyses 89 SWOG de novo ALL patients, 68 CALGB de novo patients, and 26 refractory/relapsed patients (SWOG). In the SWOG study, immunoblotting was used to assess expression of Rb and p53. Loss of Rb expression occurred in 54 of 85 (64%) and p53 overexpression (indicative of p53 point mutations) in 16 of 75 (21%) de novo adult ALL patients. No significant correlations of Rb or p53 abnormalities with outcome measures were identified. De novo adult ALL patients with abnormalities of both Rb and p53 expression appeared to have an increased rate of early death, but the number of patients in this group was small.10 Likewise, adult patients with ALL who had relapses had a higher frequency of p53 overexpression than de novo patients (8 of 19 [42%] versus 16 of 75 [21%]), but the difference was not statistically significant (P = .09).10
In the CALGB study RT-PCR was used to detect p16(INK4A) mRNA, and Southern analyses were used to detect deletions or genomic rearrangements of the p16(INK4A) gene.26 Deletions or genomic rearrangements were detected in 19 of 68 (28%) of specimens. In 49 patients with germline p16(INK4A), 13 (27%) lacked p16 mRNA. Overall, 46% of patients had inactivation of p16. Analyses of overall survival and remission duration did not differ between patients with or without p16 inactivation.26
We hypothesized that simultaneous occurrence of multiple tumor suppressor gene abnormalities in adult ALL masks the prognostic effects of any single gene abnormality.10 We examined the relationship between multiple cell cycle gene abnormalities and outcome in adult patients with ALL treated uniformly on a cooperative group protocol. Multiple aberrations of these genes were present in 13 of 39 patients (Table 2). These include Rb and p16 abnormalities (6 patients), p53 and p16 abnormalities (3 patients), Rb and p53 abnormalities (3 patients), and abnormalities of all 3 genes, Rb, p16, and p53 (1 patient).
Sherr27 has described 2 major regulatory pathways of cell cycling. In 1, the “Rb pathway,” the CDK4 and CDK6 inhibitory proteins, so-called INK4 proteins, p16(INK4A), p15(INK4B), p18(INK4C), and p19(INK4D), regulate passage through the G1/S transition. In the second pathway p53-dependent checkpoints involving the p53, p21(WAF1/CIP1), p27(KIP1), and p57(KIP2) proteins regulate G1/S transition. The transcription activator, E2F, which binds Rb, is central to both pathways. Hyperphosphorylation of Rb by CDKs releases E2F, thereby enhancing transcription of target genes and cell cycling. The cross-talk between these 2 pathways may compensate for single gene abnormalities. For example, in situations with abnormal p16(INK4A), Rb hyperphosphorylation can be blocked by the p53 pathway.28,29 Similarly, in patients with p53 alterations, the INK4 pathway would remain operative. Thus, defects of more than 1 cell cycle regulatory gene involved in both the INK4 and p53-dependent pathways may be required to produce unregulated cell cycling and thereby predispose subjects to worse clinical outcomes. Absence of Rb protein, the most common defect documented in our study, would appear to make any additional gene defects in either of these pathways redundant. On that basis, it is not evident that acquisition of a second “hit” by a lymphoblast already lacking Rb would confer any cell cycling advantage. However, 6 of 9 patients had Rb abnormalities with p16(INK4A) and 3 of 9 displayed both Rb and p53 abnormalities. One patient had abnormalities of p16(INK4A), Rb, and p53. Hangaishi et al30 reported 4 patients with lymphoid malignancies (230 specimens) with inactivations of both Rb and p16(INK4A). These authors suggested that sequential acquisition of p16(INK4A) and Rb abnormalities might have occurred in these patients. If p16 abnormalities were to precede Rb inactivations, subsequent acquisition of Rb abnormalities could provide a growth advantage by abrogating inhibitory cross-talk of other cyclin dependent kinase inhibitors (CDKIs). This in turn would explain why patients with lymphoblasts having 2 or more “hits” might do less well than those with only 1 “hit.” We are unaware of studies in adult patients with ALL that support or refute this hypothesis.
In our analysis, patients with 0 to 1 gene abnormality had a better overall survival rate than those with more than 1 abnormality (25 months versus 8 months; P < .01) (Figure 2). Because these 2 patient groups did not differ with respect to other prognostic factors analyzed, these data support the hypothesis that multiple gene abnormalities abolish cross-talk.
One potential limitation of this study is the small number of patients with specimens suitable for analysis. With this as a consideration, it is especially noteworthy that survival in the 2 groups defined by the number of genetic hits differed significantly. Conversely, whereas the difference in CR rates between these groups was not statistically significant, this does not comprise strong evidence for the lack of effect resulting from inadequate statistical power. A second potential limitation is that our study group differed from the overall S8417 population by higher WBC, blast, and lymphocyte counts, features associated with a poorer outcome in a previous analysis of this study.5 Because cytogenetic analyses were not available for all patients, we cannot exclude definitively the possibility that the 2 groups defined by regulatory gene abnormalities may have differed also by poor-risk cytogenetic features. (Data that are available suggest that this possibility may not be likely.) These factors may limit the generalizability of the findings. However, these considerations provide persuasive arguments for studying the hypothesis in a larger patient population.
Leukemias in which the neoplastic clone originates in a stem cell compartment are likely to be more resistant to chemotherapy than those originating at a later stage of differentiation. Stem cells tend to be noncycling, express high MDR1, and are relatively radioresistant.31-33 One would further expect that this resistance might influence CR rate, disease-free survival, or overall survival. The phenotype CD34+lin− has been used to define hematopoietic stem cells.34-37 In 3 of 5 adult patients with ALL with abnormal p16 expression in lymphoblasts, CD34+lin− cells also lacked p16(INK4A) expression, and in 2 of 5 patients only 50% of stem cells expressed p16(INK4A) (Table 7). In contrast, sorted stem cells from patients with normal p16(INK4A) expression in lymphoblasts also expressed p16(INK4A) (Table 6). Both stem cells and lymphoblasts lacked p16(INK4A) expression in 3 of 5 patients. The observation that more cells in the CD34+lin+ intermediate and the CD34−lin+ differentiated compartments expressed p16(INK4A), than in the CD34+lin− stem compartments suggests that the nonclonal, nonneoplastic fraction of progenitor cells retains potential for normal differentiation.
The finding that p16(INK4A) silencing occurs in the stem (CD34+ lin−) compartment strongly suggests that cell cycle gene abnormalities are important etiologic factors in adult ALL leukemogenesis. We do not know whether the other cell cycle regulatory gene (Rb and p53) abnormalities also occur in the stem cell compartment in adult ALL. However, the observation that p16(INK4A) alterations occur at this stage provides a potential biologic basis for understanding how multiple gene abnormalities may contribute to clinical disease and therapeutic resistance. Thus, elimination of the CD34+lin− stem cell subset harboring abnormalities of p16(INK4A) or other cell cycle regulatory genes may be important to achieve durable CRs in adult ALL.
Acknowledgments
We thank Cheryl Muzzi Adams for secretarial assistance and Sheryl Dorsey, Sridevi Davalath, and Phyllis Eagan for technical assistance.
Supported in part by DHHS National Institutes of Health grants CA32102 and CA6032 to the SWOG Leukemia Biology and Cytogenetics Programs, American Cancer Society Career Development Award CDA-96-85 (W.S.), and American Cancer Society Clinical Fellowship Award 4497 (C.G.). M.L.S. is a member of the City of Hope Cancer Center Program and is supported by National Institutes of Health grants CA3333572 and CA30206.
Reprints:D. H. Boldt, Department of Medicine/Division of Hematology, Mail Code 7880, University of Texas Health Science Center, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900; e-mail: BOLDT@UTHSCSA.EDU.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal