Abstract
Over a period of 8.5 years (February 1988 to October 1996), 1423 patients with chronic myelogenous leukemia (CML) underwent unrelated donor (URD) bone marrow transplants (BMTs) facilitated by the National Marrow Donor Program (NMDP) at 85 transplant centers. One hundred thirty-seven evaluable (9.9%) patients failed to engraft, and an additional 83 (6.6%) evaluable patients experienced late graft failure. Grade III/IV acute graft-versus-host disease (GVHD) developed in 33% of patients (95% confidence interval [CI], 30%-36%). The incidence of extensive chronic GVHD was 60% (95% CI, 56%-63%) at 2 years. Only 5.7% of patients (95% CI, 3.6%-7.8%) transplanted in chronic phase developed hematologic relapse at 3 years. Several factors were independently associated with improved disease-free survival (DFS), including transplant in chronic phase, transplant within 1 year of diagnosis, younger recipient age, a cytomegalovirus seronegative recipient, and development of no or mild acute GVHD. The combined effect of these factors on outcome is manifest in a subset (n = 157) of young (less than 35 years), chronic phase patients transplanted within 1 year of diagnosis using HLA-matched donors who had 63% (95% CI, 53%-73%) DFS at 3 years. URD BMT therapy for CML is both feasible and effective with more frequent and more rapid identification of suitable donors. Early URD transplant during chronic phase yields good results and should be considered in CML patients otherwise eligible for transplant but without a suitable related donor.
Despite promising advances in the use of interferon-based therapy1,2 and autologous transplantation,3 allogeneic marrow transplantation remains the only proven curative therapy for chronic myelogenous leukemia (CML).4,5 Transplant from an HLA-matched related donor is the primary technique for allogeneic transplantation; however, only 40% of otherwise eligible patients have a suitably histocompatible or closely HLA-matched related donor.6-9 Recently, unrelated donors (URDs) have been used successfully in marrow transplant therapy for a variety of pediatric and adult diseases, including aplastic anemia, metabolic diseases, inborn errors of metabolism, acute leukemias, and CML.10-12 The National Marrow Donor Program (NMDP) has facilitated and standardized the URD identification and marrow procurement processes and has established a system for data collection and analysis.11 12 Here we report a detailed analysis of 1423 patients with CML receiving URD transplants at 85 NMDP-affiliated centers. This report represents the largest and longest followed series of URD BMT for CML. We postulate that careful scrutiny of patients with CML receiving URD transplants will provide prognostic information and identify areas needing directed future research. Our data suggest that transplant in early chronic phase using a matched URD can improve the survival of patients with CML and should be promptly considered in patients with CML otherwise eligible for related donor transplant, but without a suitable related donor.
Patients and methods
Role of NMDP
URDs were identified through the NMDP, based in Minneapolis, MN. Marrow procurement procedures were performed through 94 NMDP-approved donor and collection centers, and transplants were performed at 85 transplant centers affiliated with the NMDP. The NMDP also has developed reciprocal arrangements for URD marrow procurement and collection with a number of non-U.S. registries. Donor identification, marrow procurement and transport, marrow transplantation, baseline donor and recipient data gathering, scheduled follow-up recipient data gathering, data storage, analysis, and patient and donor safety monitoring are coordinated and overseen by the NMDP.12 13
Marrow donors
Characteristics of unrelated marrow donors and recipients are provided in Table 1. All URDs were adults. They were evaluated by medical history and physical examination and counseled in detail by donor center personnel. All donors signed written statements of informed consent prior to donation. Anticoagulated marrow was transported by courier from NMDP collection centers to NMDP marrow transplant centers using standardized procedures.13
Patient characteristics: URD BMT recipients with CML
| . | N . | (%) . |
|---|---|---|
| Recipient age* | % | |
| 20 years or younger | 162 | (11) |
| 21-30 | 286 | (20) |
| 31-40 | 477 | (34) |
| 41-50 | 418 | (29) |
| 51+ | 80 | (6) |
| Gender (donor:recipient)† | ||
| M:F | 288 | (20) |
| M:M | 523 | (37) |
| F:F | 289 | (20) |
| F:M | 322 | (23) |
| Female donor (parous) | 345 | (57) |
| Female donor (nulliparous) | 266 | (43) |
| BMT within 12 months of diagnosis‡ | 452 | (32%) |
| Stage at BMT | ||
| CP1 | 916 | (65) |
| CP2+ | 97 | (7) |
| AP | 301 | (21) |
| BC | 101 | (7) |
| Missing | 8 | |
| Pre-BMT conditioning | ||
| TBI + Cy ± other | 1222 | (86) |
| Chemotherapy only | 201 | (14) |
| CMV serology pre-BMT | ||
| Recipient+; Donor+ or − | 659 | (49) |
| R−; D+ | 231 | (17) |
| R−; D− | 466 | (34) |
| GVHD prophylaxis | ||
| CSA/other | 957 | (68) |
| T-depletion (ex vivo) | 329 | (23) |
| Other | 127 | (9) |
| HLA matching | ||
| HLA-A, B, DR matched | 1150 | (81) |
| 1 A-locus mismatch; B, DR matched | 123 | (9) |
| 1 B-locus mismatch; A, DR matched | 107 | (8) |
| 1 DR-locus mismatch; A, B matched | 40 | (3) |
| . | N . | (%) . |
|---|---|---|
| Recipient age* | % | |
| 20 years or younger | 162 | (11) |
| 21-30 | 286 | (20) |
| 31-40 | 477 | (34) |
| 41-50 | 418 | (29) |
| 51+ | 80 | (6) |
| Gender (donor:recipient)† | ||
| M:F | 288 | (20) |
| M:M | 523 | (37) |
| F:F | 289 | (20) |
| F:M | 322 | (23) |
| Female donor (parous) | 345 | (57) |
| Female donor (nulliparous) | 266 | (43) |
| BMT within 12 months of diagnosis‡ | 452 | (32%) |
| Stage at BMT | ||
| CP1 | 916 | (65) |
| CP2+ | 97 | (7) |
| AP | 301 | (21) |
| BC | 101 | (7) |
| Missing | 8 | |
| Pre-BMT conditioning | ||
| TBI + Cy ± other | 1222 | (86) |
| Chemotherapy only | 201 | (14) |
| CMV serology pre-BMT | ||
| Recipient+; Donor+ or − | 659 | (49) |
| R−; D+ | 231 | (17) |
| R−; D− | 466 | (34) |
| GVHD prophylaxis | ||
| CSA/other | 957 | (68) |
| T-depletion (ex vivo) | 329 | (23) |
| Other | 127 | (9) |
| HLA matching | ||
| HLA-A, B, DR matched | 1150 | (81) |
| 1 A-locus mismatch; B, DR matched | 123 | (9) |
| 1 B-locus mismatch; A, DR matched | 107 | (8) |
| 1 DR-locus mismatch; A, B matched | 40 | (3) |
AP = accelerated phase; BC = blast crisis; BMT = bone marrow transplant; CML = chronic myelogenous leukemia; CMV = cytomegalovirus; CP = chronic phase; CSA = cyclosporine A; Cy = cyclophosphamide; GVHD = graft-versus-host disease; TBI = total body irradiation; URD = unrelated donor.
Median recipient age, 35 y; range, 1.9-59.
Median donor age, 38 y; range, 18-57.
Median interval from diagnosis to BMT, 18 mo; range, 2.9-325 mo.
Transplant recipients
One thousand four hundred twenty-three patients with CML transplanted with URD marrow at 85 U.S. centers between February 1988 and October 1996 were studied. During this time period, an additional 388 URD transplants for CML were performed; however, incomplete baseline and follow-up data reporting to the NMDP led to the exclusion of 222 cases. Data are also unavailable for 111 transplants performed through cooperating registries and 55 from international centers that do not submit data to the NMDP. Data reporting for analysis was updated as of August 1998, and the median (range) follow-up of the surviving patients was 4.0 years (0.4-10.3). Three hundred forty, 156, 62, and 12 patients survive at 3, 5, 7, and 9 years, respectively. Marrow metaphases from all patients examined prior to transplant contained the t(9;22) chromosome containing the “Philadelphia chromosome” (Ph) and/or variant translocations or molecular rearrangements (BCR-ABL) considered characteristic of CML. All recipients or their guardians signed informed consents for transplantation and for submission of data to the NMDP. Informed consents were approved by the local transplant center institutional review board. Previously described definitions for disease staging at transplant (chronic phase, accelerated phase, blast crisis, and second chronic phase) were reported prospectively on baseline forms and used subsequently for analyses.14 15
Donor-recipient HLA matching
Donor and recipient serologic HLA-A, -B, and -DR loci matching are presented in Table 1.16 Molecular analysis employing allele level typing performed either prior to transplant or retrospectively using pretransplant samples was available in only 535 (37%) cases. This analysis revealed donor/recipient nonidentity at the DRB1 region in 18% of cases identified as HLA-A, -B, and -DR matched by serologic testing.17 18
Transplant conditioning
In 1222 (86%) cases, patients received pretransplant fractionated or single-dose total body irradiation (TBI) plus cyclophosphamide alone or in combination with other chemotherapeutic agents. In 201 (14%) cases, patients received only chemotherapy, usually busulfan and cyclophosphamide. Most patients (68%) received in vivo graft-versus-host disease (GVHD) prophylaxis with cyclosporine or antithymocyte globulin along with either methotrexate or prednisone. Three hundred twenty-nine (23%) patients received marrow treated ex vivo to deplete T lymphocytes, usually along with additional in vivo GVHD prophylaxis.19 20
Data gathering and statistical analysis
NMDP data collection methods have been described.10,12 13 Briefly, all data in this study were obtained using forms prospectively designed and frequently updated by the NMDP. Baseline data on all donors and the harvest procedure were forwarded to the NMDP coordinating center for data entry, storage, and subsequent retrieval. Recipient baseline information and follow-up reporting were submitted at 100 days, 6 months, 1 year, and then annually posttransplant. Patient outcome was analyzed to date of last reported follow-up or to date of death. Elements of the data collection process are audited periodically by the NMDP, and all elements of the data set for approximately 50% of patients in this series were externally audited in 1995.
Disease-free survival (DFS) was defined as survival without morphologic evidence of recurrent leukemia in either the marrow or blood.15 In this report, persistence or recurrence of either the Ph chromosome or the BCR-ABL molecular abnormality in either marrow or blood was not considered relapse because of uneven sampling habits among centers leading to a potential reporting bias. Furthermore, the clinical consequences of such observations following URD transplant are not fully understood.14,18-24 Survival and DFS curves were calculated by the method of Kaplan and Meier.25 The time to event was defined as time from first transplant to time of hematologic relapse, death, or last contact in remission. Patients who were never free of disease after transplant (n = 5) were excluded from analyses of relapse. For the analysis of relapse or time to engraftment, surviving patients were censored at the time of a second transplant (n = 107). In univariate analyses, the log rank statistic was employed to test differences of outcomes among various groups.26 The 3-year DFS rates and their 95% CI (calculated from the standard errors) are cited unless otherwise specified. Posttransplant donor lymphocyte infusions were administered to 39 patients. These infusions were administered as therapy for hematologic (n = 15) or cytogenetic (n = 6) relapse and as added prophylaxis against relapse for the remainder.
In evaluation of engraftment, patients who died before day +22 without engraftment were considered not evaluable and censored at time of death. Patients who died after day +21 without engraftment were considered as graft failures and for analysis of engraftment were censored at death or at day +42, whichever came first. For surviving patients without events, survival time was censored at the last follow-up contact (or last scheduled submission of NMDP follow-up forms). Patients who failed to engraft were excluded from analyses of relapse as well as acute and chronic GVHD.
In multiple regression analyses of engraftment, acute and chronic GVHD, hematologic relapse, survival, and DFS, the proportional hazards model27 was employed with the following covariates: donor and recipient age, gender, cytomegalovirus (CMV) serology; disease stage; interval from diagnosis to transplant; donor-recipient HLA match using serological methods; TBI containing preparative regimens (yes/no); acute GVHD prophylaxis with ex vivo T-lymphocyte depletion (yes/no); year of transplant and transplant center. As specified, additional variables in some analyses included locus of donor-recipient HLA mismatch by serological methods, donor parity, and nucleated cell dose.
Results
Success of donor searches
From 1988 to 1996, NMDP donor searches have been performed on behalf of 7752 patients with CML; only 23% received an URD BMT during this time period. The proportion of patients for whom one or more candidate donors matched at the HLA-A, -B, and -DR locus by serologic testing were located has increased from 35.9% in 1988-1991 to 63.3% in 1992-1996, and the proportion for whom one or more donors matched or mismatched at only one HLA-A, -B, or -DR locus has also increased from 85.2% in 1988-1991 to 98.3% in 1992-1996. The median time from submission of an initial search request to identification of a donor for patients with CML was shortened significantly over the years of study. For 304 searches performed from 1988 to 1990, it took a median of 6.9 months (4.9-14.5 months; 25th-75th percentile range) to identify a donor, whereas in 1991-1993, 6.4 months (n = 568; 4.7-13.1 [25th-75th percentile]) and in 1994-1996 only 5.5 months (n = 848; 3.7-12.5 months [25th-75th percentile]) (P < .0001).
Engraftment and late graft failure
Engraftment was defined as the achievement of a peripheral blood absolute neutrophil count of more than 500/μL for three consecutive values. Prompt engraftment occurred in 1252 of 1417 (88.4%) evaluable URD recipients. The actuarial incidence of initial engraftment by day +42 was 92% (90%-94%, 95% CI), and the median time to engraftment was 20 days (range, 8-42 days). Thirty-five of 328 (10.7%) evaluable recipients receiving T-lymphocyte depleted marrow and 102 (9.7%) of 1050 recipients receiving unmanipulated URD marrow failed to engraft. In multiple regression analysis, use of TBI-containing preparative regimens, transplant in chronic phase, an HLA-matched donor, transplant in 1991-1993, and a nucleated cell dose of more than 2.1 × 108 cells/kg were independently associated with lower risks of graft failure (Table2).
Outcome following URD BMT for CML: multivariate analysis
| A. Graft failure (n = 137) . | B. Grade III/IV acute GVHD (n = 566) . | ||||
|---|---|---|---|---|---|
| . | RR (95% CI) . | P . | . | RR (95% CI) . | P . |
| Chronic phase TBI conditioning Younger recipient* Unmanipulated graft HLA-match Year of BMT 1988-90 1991-93 1994-96 Marrow cell dose† 2.0 2.1-3.99 4.0 | 0.63 (0.44-0.92) 0.30 (0.20-0.46) 0.89 (0.61-1.29) 1.18 (.71-1.92) 0.68 (.43-1.08) 1.0 0.79 (0.45-1.37) 0.78 (0.44-1.37) 1.0 0.63 (0.40-1.0) 0.50 (0.26-0.94) | .015 .0001 .54 .52 .01 .04 .38 .05 .03 | Chronic phase TBI conditioning Younger recipient* T-depletion HLA-match Early BMT‡ Donor parity | 0.68 (0.55-0.83) 0.96 (0.68-1.34) 0.91 (0.74-1.11) 0.58 (0.42-0.81) 0.64 (0.50-0.81) 0.86 (0.68-1.09) 0.97 (0.71-1.33) | .0002 .80 .34 .0012 .0003 .21 .85 |
| C. Chronic GVHD (extensive) (n = 510) | D. Leukemia relapse (n = 123) | ||||
| RR (95% CI) | P | RR (95% CI) | P | ||
| Chronic phase TBI conditioning Younger recipient* T-depletion HLA-match Early BMT‡ | 0.70 (0.56-0.86) 0.79 (0.57-1.10) 0.94 (0.78-1.13) 0.64 (0.46-0.89) 0.91 (0.70-1.16) 0.88 (0.72-1.07) | .0004 .16 .51 .008 .45 .19 | Chronic phase TBI conditioning Younger recipient* Unmanipulated graft HLA-match Early BMT‡ Grade 0-II acute GVHD Chronic GVHD | 0.14 (0.09-0.23) 0.78 (0.34-1.75) 0.82 (0.53-1.25) 0.41 (0.22-0.78) 0.80 (0.47-1.35) 1.11 (0.70-1.76) 1.25 (0.76-2.08) 0.91 (0.54-1.51) | .0001 .54 .35 .005 .41 .67 .37 .70 |
| A. Graft failure (n = 137) . | B. Grade III/IV acute GVHD (n = 566) . | ||||
|---|---|---|---|---|---|
| . | RR (95% CI) . | P . | . | RR (95% CI) . | P . |
| Chronic phase TBI conditioning Younger recipient* Unmanipulated graft HLA-match Year of BMT 1988-90 1991-93 1994-96 Marrow cell dose† 2.0 2.1-3.99 4.0 | 0.63 (0.44-0.92) 0.30 (0.20-0.46) 0.89 (0.61-1.29) 1.18 (.71-1.92) 0.68 (.43-1.08) 1.0 0.79 (0.45-1.37) 0.78 (0.44-1.37) 1.0 0.63 (0.40-1.0) 0.50 (0.26-0.94) | .015 .0001 .54 .52 .01 .04 .38 .05 .03 | Chronic phase TBI conditioning Younger recipient* T-depletion HLA-match Early BMT‡ Donor parity | 0.68 (0.55-0.83) 0.96 (0.68-1.34) 0.91 (0.74-1.11) 0.58 (0.42-0.81) 0.64 (0.50-0.81) 0.86 (0.68-1.09) 0.97 (0.71-1.33) | .0002 .80 .34 .0012 .0003 .21 .85 |
| C. Chronic GVHD (extensive) (n = 510) | D. Leukemia relapse (n = 123) | ||||
| RR (95% CI) | P | RR (95% CI) | P | ||
| Chronic phase TBI conditioning Younger recipient* T-depletion HLA-match Early BMT‡ | 0.70 (0.56-0.86) 0.79 (0.57-1.10) 0.94 (0.78-1.13) 0.64 (0.46-0.89) 0.91 (0.70-1.16) 0.88 (0.72-1.07) | .0004 .16 .51 .008 .45 .19 | Chronic phase TBI conditioning Younger recipient* Unmanipulated graft HLA-match Early BMT‡ Grade 0-II acute GVHD Chronic GVHD | 0.14 (0.09-0.23) 0.78 (0.34-1.75) 0.82 (0.53-1.25) 0.41 (0.22-0.78) 0.80 (0.47-1.35) 1.11 (0.70-1.76) 1.25 (0.76-2.08) 0.91 (0.54-1.51) | .0001 .54 .35 .005 .41 .67 .37 .70 |
BMT = bone marrow transplant; CML = chronic myelogenous leukemia; GVHD = graft-versus-host disease; RR = relative risk; TBI = total body irradiation; URD = unrelated donor.
Cox multivariate regression analysis demonstrating the relative risk (RR) (95% confidence interval [CI]) of clinical end points shown. Favorable factors associated with protection against the stated endpoint are shown. In panel C, prior acute or chronic GVHD were entered as time-dependent variables. Only hematologic relapse was considered in panel D.
No older than 35 years.
×108 nucleated cells/kg recipient weight.
<12 months from diagnosis.
Late graft failure was defined as development of sustained severe neutropenia in patients who had previously engrafted.14Late graft failure occurred in 83 (6.6%) of 1252 engrafted URD recipients, and thus approximately 15% (15.5%) of patients experienced either early or late graft failure. In multiple regression analysis, transplant in chronic phase was associated with a lower risk of late graft failure. No significant association with TBI preparation, donor-recipient HLA matching, T depletion, marrow cell dose, CMV serology, and recipient or donor age was identified (allP > .25).
Acute GVHD
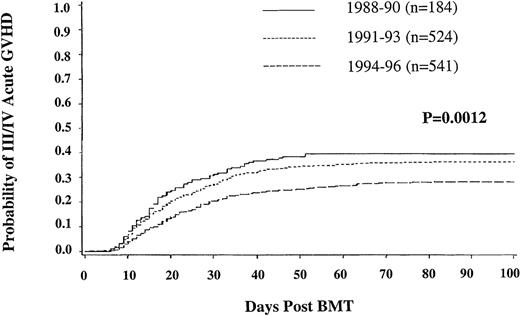
The incidence at 100 days of grades II-IV acute GVHD was 43% (40%-46%; 95% CI); grade III/IV (33%, 30%-36%). In univariate analysis, grade III/IV acute GVHD was less frequent during 1994-1996 (28%, 95% CI, 24%-32%) than during 1991-1993 (36%, 32%-40%) and 1988-1990 (40%, 32%-47%) (P = .0012) (Figure1). Multiple regression analysis revealed an independent, significantly lower risk of grade III/IV acute GVHD following transplants in chronic phase (P = .0002), use of T-lymphocyte depleted marrow (P = .0012), and use of an HLA-matched URD donor (P = .0003). Neither older recipient age nor donor alloimmunization through previous pregnancy (for either recipient gender) led to more frequent acute GVHD.
Incidence of grade III-IV acute graft-versus-host disease in unrelated donor bone marrow transplant recipients.
Incidence of grade III-IV acute graft-versus-host disease in unrelated donor bone marrow transplant recipients.
Chronic GVHD
The overall incidence of chronic GVHD was 73% (70%-77%; 95% CI) at 2 years, while the extensive chronic GVHD was 60% (95% CI, 56%-63%). In univariate analysis, the incidence of extensive chronic GVHD at 2 years was lower in more recent years (1994-1996, 48% [95% CI, 43%-53%]; 1991-1993, 72% [67%-77%]; 1988-1990, 63% [54%-72%]; P = .0001). In multiple regression analysis as shown (Table 2), transplant in chronic phase (P = .0004) and T-cell depletion (P = .008) led to lower risks of chronic GVHD. If added to this regression model as a time-dependent covariate, prior grade III/IV acute GVHD was independently associated with a 5.94 increased risk of developing chronic GVHD (P = .0001).
Hematologic relapse of leukemia
Only 123 (8.6%) of the 1423 patients transplanted developed hematologic relapse of leukemia. At 3 years, the incidence of hematologic relapse in chronic phase URD recipients was 5.7% (95% CI, 3.6%-7.8%), compared with accelerated phase patients 25.3% (18%-33%), second chronic phase patients 27% (11%-42%), and blast crisis patients 56% (38%-73%) (P = .0001). In chronic phase patients, the 3-year relapse incidence was remarkably low using unmanipulated marrow 3.4% (95% CI, 1.6%-5.1%) compared with T-depleted marrow 16% (7.8%-24.2%) (P = .0001) (Figure2). In multiple regression analysis (Table2), relapse was found to be less frequent following transplant in chronic phase (P = .0001) or with the use of non–T-depleted marrow (P = .005). Neither the development of acute or chronic GVHD nor the use of TBI-containing preparative regimens was significantly associated with a lower risk of relapse.
Incidence of hematologic relapse in chronic phase recipients of T-lymphocyte depleted or unmanipulated unrelated donor bone marrow transplant.
Incidence of hematologic relapse in chronic phase recipients of T-lymphocyte depleted or unmanipulated unrelated donor bone marrow transplant.
Disease-free survival
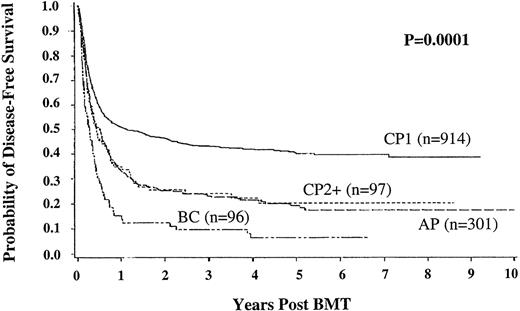
DFS was defined as survival without morphologic evidence of relapse in blood or marrow. The important advantage of transplant in chronic phase (43%; 95% CI, 40%-47% DFS at 3 years) compared with other disease stages is demonstrated in Figure 3. Multiple regression analysis indicates that DFS in the URD group is significantly better following transplant in chronic phase (relative risk [RR] 0.65 [0.57-0.75], P = .0001), transplant within 1 year from diagnosis (RR 0.74 [0.63-0.86], P = .0001), for CMV seronegative recipients (RR 0.81 [0.71-0.93],P = .002), and for younger recipients (RR 0.71 [0.62-0.81], P = .0001). DFS was also significantly better in patients without grade III/IV acute GVHD (RR 0.39 [0.34-0.45], P = .0001). At 3, 5, 7, and 9 years follow-up 321, 151, 59, and 11 patients, respectively, are alive without relapse.
Disease-free survival in unrelated donor bone marrow transplant recipients according to disease stage at time of transplant.
Disease-free survival in unrelated donor bone marrow transplant recipients according to disease stage at time of transplant.
Selection of clinical cohorts with several favorable factors resulted in demonstrable improvements in DFS. In the subset of younger (35 years of age or less) BMT recipients in early chronic phase (less than 1 year from diagnosis) with a serologically HLA-matched donor (n = 157), DFS at 5 years was 64% (56%-72%, 95% CI); recipients more than 35 years old with similar good prognosis features have a DFS of 47% (38%-56%) at 5 years (n = 137, P = .005).
Survival
Of these 1423 patients, 497 are alive 0.4 to 10.3 years following transplantation (37.5% at 3 years; 95% CI, 34.8-40.1). Multiple regression analysis (Table 3) showed significantly better survival in younger patients, in those transplanted in chronic phase, within 1 year from diagnosis, and without grade III/IV acute GVHD.
Survival following URD BMT for CML3-150
| Relapse or Death . | RR (95% CI) . | P . |
|---|---|---|
| Chronic phase | 0.67 (0.59-0.77) | .0001 |
| Early BMT (≤12 mo) | 0.72 (0.61-0.84) | .0001 |
| Younger recipient (≤35 y) | 0.69 (0.60-0.80) | .0001 |
| Younger donor (≤35 y) | 0.91 (0.79-1.05) | .20 |
| HLA-match | 0.91 (0.79-1.05) | .32 |
| CMV seronegative recipient | 0.80 (0.70-0.92) | .002 |
| TBI | 0.97 (0.78-1.22) | .82 |
| Unmanipulated graft | 0.90 (0.74-1.10) | .31 |
| Grade 0/II acute GVHD | 0.36 (0.31-0.41) | .0001 |
| Relapse or Death . | RR (95% CI) . | P . |
|---|---|---|
| Chronic phase | 0.67 (0.59-0.77) | .0001 |
| Early BMT (≤12 mo) | 0.72 (0.61-0.84) | .0001 |
| Younger recipient (≤35 y) | 0.69 (0.60-0.80) | .0001 |
| Younger donor (≤35 y) | 0.91 (0.79-1.05) | .20 |
| HLA-match | 0.91 (0.79-1.05) | .32 |
| CMV seronegative recipient | 0.80 (0.70-0.92) | .002 |
| TBI | 0.97 (0.78-1.22) | .82 |
| Unmanipulated graft | 0.90 (0.74-1.10) | .31 |
| Grade 0/II acute GVHD | 0.36 (0.31-0.41) | .0001 |
BMT = bone marrow transplant; CI = confidence interval; CML = chronic myelogenous leukemia; CMV = cytomegalovirus; GVHD = graft-versus-host disease; RR = relative risk; TBI = total body irradiation; URD = unrelated donor.
Favorable factors associated with a lower relative risk of death are shown. Acute GVHD was considered as a time-dependent co-variate.
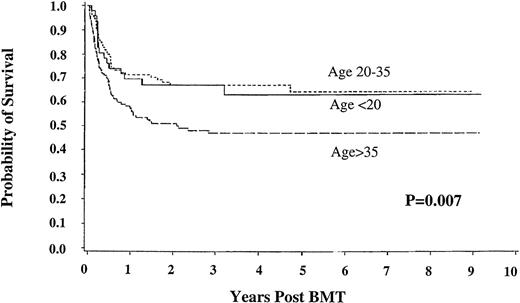
Among the subset of early chronic phase recipients of HLA-matched donor marrow, 5-year survival was similar for patients under 20 years of age and between 20 and 35 (63.2 [95% CI, 48.3-78.1] and 67.2% [58.1-76.3], respectively), but only 47% (38.3%-55.8%) for those over age 35 (P = .007) (Figure4).
Survival in good risk chronic phase patients with chronic myelogenous leukemia. Shown are results for patients under 20 years of age (n = 49), between 20 and 35 years old (n = 108), and older than 35 (n = 137); with HLA-matched unrelated donors; bone marrow transplant less than 12 months from diagnosis.
Survival in good risk chronic phase patients with chronic myelogenous leukemia. Shown are results for patients under 20 years of age (n = 49), between 20 and 35 years old (n = 108), and older than 35 (n = 137); with HLA-matched unrelated donors; bone marrow transplant less than 12 months from diagnosis.
Donor-recipient matching
In chronic-phase URD transplants, after adjusting for other variables, DFS was worse using marrow from either an HLA-A mismatched donor (RR of relapse or death was 1.5; 95% CI,1-2.1;P = .03) or an HLA-B mismatched donor (RR 1.7; 95% CI, 1.2-2.4; P < .01). No significant adverse association with HLA-DR mismatch (RR 0.79; 0.54-1.16; P = .22) was observed, although modest patient numbers in these cohorts limited the statistical power of these analyses. Additional analysis addressing the significance of high resolution histocompatibility testing in a larger subset of these recipients with CML is in progress.28
Causes of death
Clinical investigators were asked to report, in order of importance, the clinical conditions contributing to death. Following URD BMT, the most common clinical problems contributing to death included respiratory failure (14%), acute and chronic GVHD (18%), and infections (28%) (Table 4).
Cause of death following URD BMT for CML4-150
| . | % . |
|---|---|
| Respiratory failure | 14 |
| Acute GVHD | 11 |
| Fungal infections | 11 |
| Recurrent leukemia | 11 |
| Viral infections | 8 |
| Hemorrhage | 7 |
| Graft failure | 7 |
| Bacterial infections | 6 |
| Chronic GVHD | 6 |
| Hepatic toxicity | 5 |
| Other infections | 3 |
| Second cancer | 2 |
| Other | 9 |
| . | % . |
|---|---|
| Respiratory failure | 14 |
| Acute GVHD | 11 |
| Fungal infections | 11 |
| Recurrent leukemia | 11 |
| Viral infections | 8 |
| Hemorrhage | 7 |
| Graft failure | 7 |
| Bacterial infections | 6 |
| Chronic GVHD | 6 |
| Hepatic toxicity | 5 |
| Other infections | 3 |
| Second cancer | 2 |
| Other | 9 |
BMT = bone marrow transplant; CML = chronic myelogenous leukemia; GVHD = graft-versus-host disease; URD = unrelated donor.
Percentage of patients with listed primary cause of death.
Second malignancies
Second cancers occurred in 41 recipients of URDs. Thirty cases of B-cell lymphoproliferative disorder were detected. This disorder is often ascribed to Epstein-Barr virus infection of B lymphocytes in the setting of profound immunoincompetence.29 30 In this series, these B-cell lymphoproliferative disorders occurred at a median of 2.9 months (range 1.9-14.7 months) post-BMT and were more frequent following TBI (30 of 30) or use of T-lymphocyte depleted marrow (25 of 30). B-cell lymphoproliferative disorder was a contributing cause of death in 24 of 30 cases. Eleven other cancers (3 breast cancers, 2 squamous cell cancers, 4 sarcomas, 1 uncharacterized other carcinoma, and 1 uncharacterized central nervous system leukemia) developed following URD BMT. Multiple regression analysis demonstrated higher risks for second cancers in patients receiving T-depleted marrow (RR 7.86; 4.09-15.11; P = .0001) and HLA-mismatched donor marrow (RR 2.13; 0.86-5.3; P = .11) but not with use of TBI (RR 2.26; 0.53-9.58; P = .27).
Activity assessment of surviving patients
The performance status observed in 496 patients surviving at 2 years following URD transplantation was measured using the Karnofsky activity score.31 At 2 years following transplant, 75% of URD recipients (79% age < 35 years, n = 222; 70% age ≥ 35 years, n = 151) had normal or near normal activity scores (Karnofsky assessment = 90%-100%); however, 24% had significant ongoing limitations (score 50%-80%). Logistic regression analyses identified poorer performance status in recipients older than 35 years at transplant (P = .0001) and those with extensive chronic GVHD (P = .0001).
Discussion
CML is the most common indication for URD transplant, accounting for 35% of all NMDP transplants. URD transplant recipients with CML represent a relatively homogeneous group, usually stable enough to allow the necessary time for donor selection. The disease also represents a prototype of hematopoietic malignancies such that lessons learned from the patient group with CML may be directly applicable to clinical transplantation for other diseases.
In this large series of NMDP transplants for CML, 8% of URD transplant recipients had not engrafted by day 42 and nearly 7% of engrafting patients experienced late graft failure. Of interest, use of TBI rather than busulfan-containing preparative regimens had a significant favorable effect on engraftment. Previous reports are mixed regarding enhanced engraftment after conditioning with TBI-containing regimens.32-34 Some authors have speculated that the relatively low exposure of patients with CML to chemotherapy prior to transplantation may increase their risk of graft failure following transplantation, perhaps because of incomplete ablation of host alloreactive cells.35 This risk might be further exacerbated in the URD setting using preparative regimens not containing TBI. Additionally, the efficacy of busulfan as an ablative agent pre-BMT may be dependent on drug exposure (plasma area under the curve).32 URD recipients at higher risk of graft failure may need adjusted dose busulfan to enhance the effectiveness of this drug.
In this series, a borderline but significant correlation of HLA mismatch with graft failure could be identified. Other investigators20,34-40 have described an increased risk of graft failure with mismatching at the HLA-A, HLA-B, or HLA-C locus. The available serologic techniques used for donor selection limit this analysis of graft failure. Recent single-institution reports17-19,38,39,41,42 suggest that close attention to HLA donor/recipient matching using a variety of molecular techniques is associated with a low incidence of graft failure. Nonetheless, if graft failure occurs, willingness to resort to early second transplant43 or to infusion of stored, autologous “back up” marrow or blood mononuclear cells44 45 may be prudent.
The incidence of clinically significant (grade II-IV) acute GVHD was 43%, whereas 33% of patients experienced severe (grade III/IV) acute GVHD. Not surprisingly, use of a serologically HLA-A and HLA-B matched donor/recipient pair was independently associated with a decreased incidence of severe acute GVHD. This observation has been made in several previous reports.14,15,17,20,40 The marked decrease in the incidence of grade III-IV acute GVHD observed from 40% (1988-1990) to 28% (1994-1996) might be attributable to use of molecular-based techniques to avoid class II allele level disparity,17,18,40-42 but this finding may also reflect the application of URD BMT to better risk patients in more recent years. Recent reports highlighting the importance of HLA class I allele matching may further modify the strategies for selecting the best donor.38 39
Previous investigators14,19,20 21 have speculated that use of an URD may impart an added graft-versus-leukemia effect in donor transplant for CML. Indeed, in this series we observed only a 6% incidence of hematologic relapse at 3 years in chronic phase recipients. Disappointingly, a higher incidence of relapse was observed in chronic phase patients receiving T-lymphocyte depleted grafts. A more potent graft-versus-leukemia effect accompanying the URD graft may still be ablated by T depletion, thus leading to excessive relapse. The high incidence of relapse of patients in accelerated phase and blast crisis suggests that even URD BMT does not create a graft-versus-leukemia effect with sufficient potency to protect patients with advanced CML.
Improved survival and DFS could be predicted by patient variables determined at the time of transplant, especially early transplantation.21,46-48 The critical prognostic variables, including transplant in chronic phase, transplant within 1 year of diagnosis, younger recipient age, and CMV seronegative recipient status, have been identified both in the related and the URD setting.4,18-21 The combined beneficial effects of these favorable prognostic characteristics yielded a 64% 5-year DFS in the group of young, good risk, early chronic phase recipients; notably better than the older, good risk patients or than the entire chronic phase group. This experience must be compared with the favorable outcomes observed using α-interferon alone1,2 or in combination with cytarabine as initial therapy for CML.48The postulated hazard of extended pretransplant therapy with interferon,49 which could not be evaluated in this multicenter analysis, must also be acknowledged in clinical decision making about the timing of URD BMT. Careful selection of transplant candidates may contribute to improved outcomes reported from single institutions19,21 42 that might appear superior compared with the aggregate, multicenter results presented herein.
This retrospective analysis of data obtained from a large number of donor and transplant centers has inherent limitations requiring a cautious interpretation. As described, 388 patients with CML transplanted during the study interval were excluded because of incomplete baseline or follow-up information. Inclusion criteria for donor-recipient matching and ablative conditioning protocols differed among centers. Finally, although this analysis reflects results of URD transplant for CML in a large patient group observed systematically for nearly a decade, median follow-up of survivors is only 4 years. Long-term follow-up of this patient cohort may uncover late events that prompt reinterpretation of the role of URD transplant in CML.
The NMDP experience demonstrates that URD therapy for CML is feasible and effective. Over the past decade, improvements in donor procurement have increased the likelihood of finding a suitable URD, and improvements in transplant methodology have reduced the incidence and severity of transplant complications. A number of prognostic factors have been identified that should be useful in counseling patients, and the important beneficial effect of early transplant has been verified. Implementation of molecular typing and further improvements in preparative regimens, GVHD prophylaxis, and supportive care should lead to even better outcome over the next decade.
Supported in part by grants from the National Cancer Institute CA30206, CA33572, and CA65493.
Reprints:Philip McGlave, Box 480, University of Minnesota Medical School, 420 Delaware Street SE, MN 55455.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal