Abstract
Marginal zone B-cell lymphoma (MZL) is a recently individualized lymphoma that encompasses mucosa-associated lymphoid tissue (MALT) lymphoma, splenic lymphoma with or without villous lymphocytes, and nodal lymphoma with or without monocytoid B-cells. If the clinical description and outcome of MALT lymphoma is well known, this is not the case for the other subtypes. We reviewed 124 patients presenting non-MALT MZL treated in our department to describe the morphologic and clinical presentation and the outcome of these lymphomas. Four clinical subtypes were observed: splenic, 59 patients; nodal, 37 patients; disseminated (splenic and nodal), 20 patients; and leukemic (not splenic nor nodal), 8 patients. These lymphomas were usually CD5-, CD10-, CD23-, and CD43-, but the detection of one or, rarely, two of these antigens may be observed. Bone marrow and blood infiltrations were frequent, except in the nodal subtype, but these locations were not associated with a poorer outcome. Splenic and leukemic subtypes were associated with a median time to progression (TTP) longer than 5 years, even in the absence of treatment or of complete response to therapy. Nodal and disseminated subtypes were associated with a median TTP of 1 year. However, in all these subtypes, survival was good with a median survival of 9 years, allowing these lymphomas to be classified as indolent. Because of the retrospective nature of this analysis, no conclusion may be drawn on the therapeutic aspects, but conservative treatments seem recommended for leukemic and splenic subtypes.
Among the new entities listed in the Revised European-American Lymphoma (REAL) classification from the International Lymphoma Study Group,1 most of the marginal zone B-cell lymphomas (MZL) were categorized as a provisional entity, including splenic lymphoma and nodal monocytoid B-cell lymphoma, but not mucosa-associated lymphoid tissue (MALT) lymphoma that was recognized and accepted years before.2 Even if the forthcoming World Heath Organization (WHO) classification of neoplastic diseases of the hematopoietic and lymphoid systems will consider them as full-individualized lymphomas,3,4 some pathologists have difficulties in recognizing them and in considering them as a true entity. In the WHO classification, MZL is described with three subtypes: extranodal MALT lymphoma,2,5 splenic MZL with/without villous lymphocytes,6,7 and nodal MZL with/without monocytoid B-cells.8,9 If these B-cell lymphomas have only recently been recognized, they were previously diagnosed under the names of other subtypes in the Working Formulation10 or the Kiel classification.11 In our preceding review of nonfollicular small-cell lymphomas,12 the non-MALT MZL lymphomas were classified among the lymphoplasmacytoid lymphomas, but some cases may be found in nearly all the other subtypes.13
The MZL term came from the supposed but controversial common origin of the lymphoma cells with possibly different mechanisms of lymphoma triggering. These lymphomas involve the marginal B-cell compartment of lymphoid tissue outside the follicle mantle zone with a peculiar growth pattern reminiscent of the marginal zone. They may also secondarily involve the normal germinal centers, a pattern described as follicular colonization.14 The cellular composition of these lymphomas shows considerable variations: mostly clear cells with a relatively abundant pale cytoplasm called monocytoid B cells or centrocytic-like cells with a small percentage of larger cells and plasmacytic cells. These cells have a virtually identical immunophenotype, with the presence of surface immunoglobulin (Ig), mainly IgM subtype, presence of B-cell markers (CD19+, CD20+, CD22+), and absence of CD5, CD10, and CD23 antigens. There is no rearrangement of bcl-1 or bcl-2 loci, but common cytogenetic abnormalities have been described, such as trisomy 3, trisomy X, trisomy 18, or translocation (1;14).15-17
If the clinical characteristics and outcome of patients with MALT lymphoma have been very well described,18 19 some interrogations persist concerning the clinical characteristics, prognostic parameters, and outcome of patients with non-MALT MZL. To determine the clinical presentation and natural history of these patients, we reviewed all our cases of MZL. We present here the description of these patients with four different groups of patients, according to their main sites of involvement.
Patients and methods
Patients
Between October 1987 and July 1998, 2152 lymphoma patients were treated in our department, and 281 of these patients had a MZL with 157 of them having a MALT lymphoma. Patients with an extranodal location of the lymphoma and either splenic, nodal, or bone marrow localizations were deemed to have a disseminated MALT lymphoma, and they were not included in this analysis.20 Thus, 124 cases of MZL were included in this analysis. All of them have been reviewed and confirmed by two of us (FB and PF) on morphologic aspect and immunophenotype. To be included in this analysis, a patient must fulfill the histological characteristics defined in the REAL classification1 and the proposed WHO classification.4 21
We planned to group patients according to the two identified categories in the REAL and WHO classifications, splenic and nodal MZL,4,7 9 but we rapidly found that some patients had both splenic and nodal involvement, thus the creation of the disseminated subtype. Finally, some patients did not have any of these localizations but only bone marrow and blood involvement, thus the creation of the leukemic subtype. Four clinical subtypes were defined, depending on the involved sites at diagnosis (Table 1): 59 (48%) patients had a spleen enlargement without peripheral lymph nodes (splenic subtype); 37 (30%) patients had enlarged peripheral lymph nodes without splenomegaly (nodal subtype); 20 (16%) patients had a disseminated disease with splenomegaly and peripheral lymph nodes (disseminated subtype); and 8 (6%) patients had bone marrow involvement without splenomegaly or peripheral lymph node (leukemic subtype).
Initial clinical, morphologic, and biologic characteristics of the 124 patients with MZL included in this analysis
| . | No. Patients . | Patients Not Evaluable . | Spleen Subtype . | Leukemic Subtype . | Nodal Subtype . | Disseminated Subtype . | P Value . |
|---|---|---|---|---|---|---|---|
| N | 124 | 59 | 8 | 37 | 20 | ||
| Sex: Male | 57 (46%) | — | 26 (44%) | 3 (37.5%) | 16 (43%) | 12 (60%) | |
| Age >60 y | 67 (54%) | — | 37 (63%) | 7 (87.5%) | 13 (35%) | 10 (50%) | .013 |
| Poor performance status | 21 (18%) | 9 | 10 (17%) | 0 | 4 (12.5%) | 7 (39%) | |
| B symptoms | 26 (21%) | — | 15 (25%) | 0 | 5 (13.5%) | 6 (30%) | |
| Stage III or IV | 106 (86%) | — | 54 (91.5%) | 8 (100%) | 25 (68%) | 19 (95%) | .003 |
| Peripheral lymph nodes | 55 (44%) | — | 0 | 0 | 35 (95%) | 20 (100%) | |
| Abdominal/thoracic lymph nodes | 49 (48%) | — | 20 (34%) | 0 | 18 (49%) | 11 (55%) | <.05 |
| Bone marrow + | 90 (73%) | — | 51 (86%) | 8 (100%) | 15 (43%) | 16 (80%) | <.0001 |
| Splenomegaly | 79 (64%) | — | 59 (100%) | 0 | 0 | 19 (95%) | |
| Liver involvement | 20 (16%) | — | 14 (24%) | 0 | 2 (5%) | 4 (20%) | |
| Pleura localization | 14 (11%) | — | 3 (5%) | 2 (25%) | 1 (3%) | 8 (40%) | <.0001 |
| Head and neck localization | 4 (3%) | — | 0 | 0 | 4 (11%) | 0 | <.05 |
| Extranodal sites >1 | 68 (56%) | 2 | 40 (68%) | 5 (62.5%) | 9 (25%) | 14 (74%) | .0002 |
| Bulky tumor | 45 (39%) | 10 | 28 (50%) | 0 | 6 (17%) | 11 (65%) | .0004 |
| Hemoglobin <12 g/dL | 54 (52%) | 20 | 34 (64%) | 1 (14%) | 8 (31%) | 11 (61%) | <.01 |
| Blood involvement | 53 (43%) | 2 | 34 (57%) | 6 (75%) | 4 (11%) | 9 (45%) | <.0001 |
| Serum albumin <35 g/L | 21 (25%) | 39 | 12 (25.5%) | 2 (40%) | 2 (10%) | 5 (38.5%) | |
| LDH > normal values | 43 (43%) | 24 | 20 (43%) | 1 (20%) | 12 (40%) | 10 (56%) | |
| β2-microglobulin >3 mg/L | 52 (59%) | 36 | 30 (65%) | 3 (75%) | 8 (33%) | 11 (79%) | .02 |
| M component | 19 (19%) | 22 | 14 (24%) | 0 | 3 (8%) | 2 (12%) | |
| International Prognostic Index | 26 | <.0001 | |||||
| Low risk | 15 (13%) | 5 (10%) | 1 (20%) | 9 (31%) | 0 | ||
| Low-intermediate risk | 33 (34%) | 15 (32%) | 4 (80%) | 8 (28%) | 5 (29.5%) | ||
| High-intermediate risk | 33 (34%) | 20 (43%) | 0 | 7 (24%) | 5 (29.5%) | ||
| High risk | 19 (19%) | 7 (15%) | 0 | 5 (17%) | 7 (41%) |
| . | No. Patients . | Patients Not Evaluable . | Spleen Subtype . | Leukemic Subtype . | Nodal Subtype . | Disseminated Subtype . | P Value . |
|---|---|---|---|---|---|---|---|
| N | 124 | 59 | 8 | 37 | 20 | ||
| Sex: Male | 57 (46%) | — | 26 (44%) | 3 (37.5%) | 16 (43%) | 12 (60%) | |
| Age >60 y | 67 (54%) | — | 37 (63%) | 7 (87.5%) | 13 (35%) | 10 (50%) | .013 |
| Poor performance status | 21 (18%) | 9 | 10 (17%) | 0 | 4 (12.5%) | 7 (39%) | |
| B symptoms | 26 (21%) | — | 15 (25%) | 0 | 5 (13.5%) | 6 (30%) | |
| Stage III or IV | 106 (86%) | — | 54 (91.5%) | 8 (100%) | 25 (68%) | 19 (95%) | .003 |
| Peripheral lymph nodes | 55 (44%) | — | 0 | 0 | 35 (95%) | 20 (100%) | |
| Abdominal/thoracic lymph nodes | 49 (48%) | — | 20 (34%) | 0 | 18 (49%) | 11 (55%) | <.05 |
| Bone marrow + | 90 (73%) | — | 51 (86%) | 8 (100%) | 15 (43%) | 16 (80%) | <.0001 |
| Splenomegaly | 79 (64%) | — | 59 (100%) | 0 | 0 | 19 (95%) | |
| Liver involvement | 20 (16%) | — | 14 (24%) | 0 | 2 (5%) | 4 (20%) | |
| Pleura localization | 14 (11%) | — | 3 (5%) | 2 (25%) | 1 (3%) | 8 (40%) | <.0001 |
| Head and neck localization | 4 (3%) | — | 0 | 0 | 4 (11%) | 0 | <.05 |
| Extranodal sites >1 | 68 (56%) | 2 | 40 (68%) | 5 (62.5%) | 9 (25%) | 14 (74%) | .0002 |
| Bulky tumor | 45 (39%) | 10 | 28 (50%) | 0 | 6 (17%) | 11 (65%) | .0004 |
| Hemoglobin <12 g/dL | 54 (52%) | 20 | 34 (64%) | 1 (14%) | 8 (31%) | 11 (61%) | <.01 |
| Blood involvement | 53 (43%) | 2 | 34 (57%) | 6 (75%) | 4 (11%) | 9 (45%) | <.0001 |
| Serum albumin <35 g/L | 21 (25%) | 39 | 12 (25.5%) | 2 (40%) | 2 (10%) | 5 (38.5%) | |
| LDH > normal values | 43 (43%) | 24 | 20 (43%) | 1 (20%) | 12 (40%) | 10 (56%) | |
| β2-microglobulin >3 mg/L | 52 (59%) | 36 | 30 (65%) | 3 (75%) | 8 (33%) | 11 (79%) | .02 |
| M component | 19 (19%) | 22 | 14 (24%) | 0 | 3 (8%) | 2 (12%) | |
| International Prognostic Index | 26 | <.0001 | |||||
| Low risk | 15 (13%) | 5 (10%) | 1 (20%) | 9 (31%) | 0 | ||
| Low-intermediate risk | 33 (34%) | 15 (32%) | 4 (80%) | 8 (28%) | 5 (29.5%) | ||
| High-intermediate risk | 33 (34%) | 20 (43%) | 0 | 7 (24%) | 5 (29.5%) | ||
| High risk | 19 (19%) | 7 (15%) | 0 | 5 (17%) | 7 (41%) |
LDH = lactic dehydrogenase; MZL = marginal zone B-cell lymphoma.
Morphologic features and immunophenotype
The available pathologic and cytological specimens were reviewed without knowledge of the clinical course and patients' outcome. All slides obtained at diagnosis and from subsequent biopsies performed during relapse or progression, whether they originated from nodal, spleen, bone marrow, or blood locations, were reviewed by histological, cytological, and immunologic methods. Immunologic characterization was performed on paraffin sections in 70% of the cases, on frozen sections and/or cell suspensions in 73% using a flow cytometer. CD5, CD10, CD23, and CD43 were prospectively evaluated on CD19+ cells by double staining.
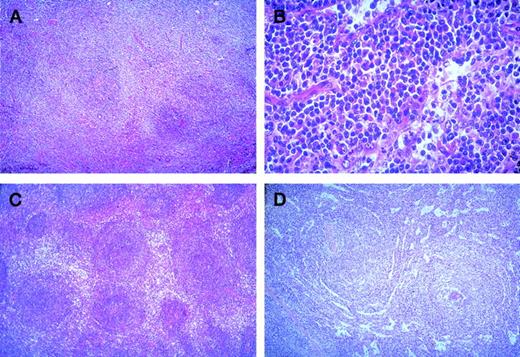
The histological pattern of infiltration in lymph nodes was peri- or interfollicular (Figure 1A), sometimes perisinusoidal or nodular (Figure 1B), and rarely follicular by colonization of germinal centers. In the spleen, it was either a perifollicular infiltration, surrounding the residual white pulp follicles, with more than a marginal-zone aspect (Figure 1C), or a nodular infiltration by colonization of white pulp and involvement of red pulp associated with a diffuse invasion of the sinuses (Figure 1D); rarely, it was an exclusive diffuse infiltration of congestive red pulp with normal or atrophic white pulp. This last aspect was mostly observed in splenic MZL with villous lymphocytes. In bone marrow, the aspect was variable with paratrabecular, nodular, or interstitial infiltration, sometimes limited to intrasinusoidal infiltration (mostly in splenic MZL with villous lymphocytes). Cytological aspects were very heterogeneous with several cell types usually associated in varying proportions: cells resembling small round lymphocytes, small cells with irregular nuclei (centrocyte-like cells), small cells with more regular nuclei and clear cytoplasm (monocytoid B-cells), small cells with plasmacytoid differentiation (Figure 1 B), plasma cells, and variable content of medium to large cells (centroblast- or immunoblast-like cells). In typical splenic white pulp involvement, central small round cells were surrounded by medium cells with clear cytoplasm, interspersed with variable numbers of large cells. In peripheral blood, splenic lymphoma with villous lymphocyte (SLVL) was defined by the presence of at least 20% of typical villous lymphocytes, showing a clumped chromatin and a basophilic cytoplasm with polar projections. In contrast to the usual homogeneous picture of SLVL, the cytological spectrum of other MZL varied from slightly atypical lymphoid cells, sometimes looking like chronic lymphocytic leukemia cells, to heterogeneous lymphoid populations, composed of plasmacytoid cells, prolymphocytes, centrocyte-like cells, villous lymphocytes (always <20%), and large atypical cells (sometimes with a prominent single nucleolus) in varying numbers. It is of note that villous lymphocytes were generally not typical, with shorter and thinner projections than in SLVL, and that circulating monocytoid B cells were rarely observed.
Marginal zone B-cell lymphoma.
(A) Nodal peri- and interfollicular infiltration by monocytoid B-cells with clear cytoplasm (HE × 60). (B) Nodal perisinusoidal infiltration by small tumoral B-cells with plasmacytic differentiation (HE × 400). (C) Splenic perifollicular infiltration surrounding the residual white pulp follicles, with typical “marginal-zone” pattern (HE × 60). (D) Splenic nodular infiltration with colonization of the white pulp by the tumoral cells associated with a diffuse invasion of the sinuses in the red pulp (HE × 60).
Marginal zone B-cell lymphoma.
(A) Nodal peri- and interfollicular infiltration by monocytoid B-cells with clear cytoplasm (HE × 60). (B) Nodal perisinusoidal infiltration by small tumoral B-cells with plasmacytic differentiation (HE × 400). (C) Splenic perifollicular infiltration surrounding the residual white pulp follicles, with typical “marginal-zone” pattern (HE × 60). (D) Splenic nodular infiltration with colonization of the white pulp by the tumoral cells associated with a diffuse invasion of the sinuses in the red pulp (HE × 60).
Patients were split into three subgroups according to morphologic features: common MZL (78 patients, 63%) without circulating villous lymphocytes and with less than 50% large cells, splenic MZL with circulating villous lymphocytes (SLVL, 12 patients, 10%),5and large cell-rich variant in cases with more than 50% of large cells or sheets of large B-cells (34 patients, 27%). These last cases may be considered as having a “transformed” lymphoma at diagnosis because these criteria were those retained to define transformation in relapsing patients or a composite lymphoma with MZL aspect and features of diffuse large B-cell lymphoma. Usually, large-cell proliferation was observed in lymph nodes and small-cell proliferation was observed in bone marrow and blood.
Cytogenetic analyses
Cytogenetic studies were performed on peripheral blood, lymph nodes, or spleen samples, as previously described.22 Chromosomal analyses were carried out on RHG-banded metaphases and evaluated according to the ISCN (1995) recommendations. Fluorescent in situ hybridization experiments were performed, using a panel of probes (paints, centromeric, and telomeric probes) specific for chromosomes X, 1, 3, 7, 8, 12, and 18.
Staging procedure
Initial staging procedures included complete physical examination, thorax and abdominal computerized tomography, endoscopic examinations in case of gastrointestinal symptoms, bone marrow biopsy, and dosage of blood levels of lactic dehydrogenase (LDH), β2-microglobulin, and serum albumin. Patients were staged according to the Ann Arbor system.
Treatment
This was not a prospective study. Thus, patients were treated according to disease stage and disease location with the therapeutic options in usage at the time of diagnosis. Table2 presents the initial treatment for the different clinical subtypes. No pattern was observed during this time period, but usually elderly patients were not initially treated except in cases of clinically aggressive disease; patients with large splenomegaly had a splenectomy, alone or followed by chlorambucil; disseminated lymphoma patients were treated with chemotherapy, either single agent (chlorambucil or fludarabine) or multidrug regimens (CHOP combining cyclophosphamide, doxorubicin, vincristine, and prednisone), CHOP-like regimens, or high-dose CHOP (ACVB regimen23). Only 40% of the patients were treated with CHOP or high-dose CHOP regimens, usually because of disseminated disease with adverse prognostic factors or a high percentage of large cells. Therapeutic options were very heterogeneous because diagnosis of MZL was often not originally done in ancient cases.
Initial treatment of the 124 patients with MZL included in this analysis
| . | N . | Splenic Subtype . | Leukemic Subtype . | Nodal Subtype . | Disseminated Subtype . | P Value . |
|---|---|---|---|---|---|---|
| No. patients | 124 | 59 | 8 | 37 | 20 | |
| Initial treatment: | .0001 | |||||
| No treatment | 22 | 12 (20%) | 7 (87.5%) | 2 (5%) | 1 (5%) | |
| Surgery alone | 17 | 13 (22%) | 0 | 4 (11%) | 0 | |
| Single agent | 35 | 16 (27%) | 1 (12.5%) | 8 (22%) | 10 (50%) | |
| CHOP-like regimen | 28 | 11 (19%) | 0 | 11 (30%) | 6 (30%) | |
| High-dose therapy | 22 | 7 (12%) | 0 | 12 (32%) | 3 (15%) | |
| Complete response | 43 (35%) | 15 (28%) | 0 | 24 (69%) | 4 (25%) | .0001 |
| Progression | 57 (46%) | 21 (36%) | 3 (37.5%) | 24 (65%) | 9 (45%) | <.05 |
| Median TTP (y) | 6.9 | 5.6 | 1.3 | 1.1 | .02 | |
| Death | 34 (27%) | 15 (25%) | 1 (12.5%) | 14 (38%) | 4 (20%) | |
| Median survival (y) | 9.1 | 6.8 | 5.5 | 14.7 |
| . | N . | Splenic Subtype . | Leukemic Subtype . | Nodal Subtype . | Disseminated Subtype . | P Value . |
|---|---|---|---|---|---|---|
| No. patients | 124 | 59 | 8 | 37 | 20 | |
| Initial treatment: | .0001 | |||||
| No treatment | 22 | 12 (20%) | 7 (87.5%) | 2 (5%) | 1 (5%) | |
| Surgery alone | 17 | 13 (22%) | 0 | 4 (11%) | 0 | |
| Single agent | 35 | 16 (27%) | 1 (12.5%) | 8 (22%) | 10 (50%) | |
| CHOP-like regimen | 28 | 11 (19%) | 0 | 11 (30%) | 6 (30%) | |
| High-dose therapy | 22 | 7 (12%) | 0 | 12 (32%) | 3 (15%) | |
| Complete response | 43 (35%) | 15 (28%) | 0 | 24 (69%) | 4 (25%) | .0001 |
| Progression | 57 (46%) | 21 (36%) | 3 (37.5%) | 24 (65%) | 9 (45%) | <.05 |
| Median TTP (y) | 6.9 | 5.6 | 1.3 | 1.1 | .02 | |
| Death | 34 (27%) | 15 (25%) | 1 (12.5%) | 14 (38%) | 4 (20%) | |
| Median survival (y) | 9.1 | 6.8 | 5.5 | 14.7 |
CHOP = combination of cyclophosphamide, doxorubicin, vincristine, and prednisone; MZL = marginal zone B-cell lymphoma; TTP = time to progression.
Statistical analyses
Overall survival was defined as the time from diagnosis (first biopsy) to death or last follow-up. Time-to-progression (TTP) survival was defined as the time from onset of treatment to the date of first progression or last follow-up. For patients who did not receive any specific therapy, TTP was defined as the date of the decision not to treat this patient to first progression or last follow-up. Complete remission (CR) was defined as the disappearance of all clinical evidence of the lymphoma. Partial response was defined as a >50% regression of lymphoma masses. Survival was analyzed according to the method of Kaplan and Meier.24 Differences between survival curves were evaluated with the log-rank test.25Multivariate analyses were performed, using a Cox stepwise proportional hazard model to identify factors that might be of independent significance influencing survival.26 Patient characteristics analyzed for possibly influencing survival were histological subtype, sex, age, performance status, presence of B symptoms, stage, peripheral and thoracic or abdominal lymph node enlargement, bone marrow involvement, spleen involvement, initial localizations, number of extranodal sites, tumor bulkiness (>10 cm), anemia defined as hemoglobin level <12 g/dl, serum albumin <35 g/L, LDH level above normal value, or β2-microglobulin level >3 mg/L.
Results
The identification of three clinical subtypes, splenic, nodal, or disseminated, was defined before the analysis because two of them were described in the REAL classification and because we observed that some patients had a disease too disseminated to enter into the other definitions of the initial clinical picture. The leukemic cases were defined secondarily because these cases did not fit into one of the previous categories. Disseminated disease patients had splenic, nodal, extranodal, and bone marrow involvement, and leukemic cases only had bone marrow and blood locations as described in Table 1.
Morphological and immunologic aspects
There was a direct correlation between the clinical subtypes and the three histological variants (Table 3). Among the SLVL patients, all except one were of the splenic subtype. The large cell-rich variant was more frequent in the nodal subtype but was seen in 20% of the cases of splenic and disseminated subtypes.
Correlations between the four clinical subtypes and the three morphologic variants (P = .0003)
| . | n . | Splenic Subtype . | Leukemic Subtype . | Nodal Subtype . | Disseminated Subtype . |
|---|---|---|---|---|---|
| No. patients | 124 | 59 | 8 | 37 | 20 |
| SLVL | 12 | 11 (19%) | — | — | 1 (5%) |
| Common | 78 | 38 (64%) | 8 (100%) | 18 (49%) | 14 (70%) |
| Large cell-rich variant | 34 | 10 (17%) | — | 19 (51%) | 5 (25%) |
| . | n . | Splenic Subtype . | Leukemic Subtype . | Nodal Subtype . | Disseminated Subtype . |
|---|---|---|---|---|---|
| No. patients | 124 | 59 | 8 | 37 | 20 |
| SLVL | 12 | 11 (19%) | — | — | 1 (5%) |
| Common | 78 | 38 (64%) | 8 (100%) | 18 (49%) | 14 (70%) |
| Large cell-rich variant | 34 | 10 (17%) | — | 19 (51%) | 5 (25%) |
SLVL = splenic lymphoma with villous lymphocyte.
MZL was described as a CD5-, CD23-, CD10-, and CD43-proliferation, but, in spite of the classical morphologic aspect, we found some cases with the presence of one or two of these antigens. Only two cases showed a positive reaction for CD10 antigen but 15, 13, and 13 cases were positive for CD5, CD23, and CD43 antigens, respectively. It is of note that CD43 positivity when evaluated by flow cytometry was faint and/or partial in most cases. Among them, five cases showed a double positivity for CD5 and CD43 or CD10 and CD23 antigens. One case showed a positivity for CD5, CD23, and CD43 antigens.
Cytogenetic abnormalities
Among these 124 patients, 56 had a cytogenetic analysis done, and 12 had a normal karyotype. Forty-four patients had one or several cytogenetic abnormalities: addition or deletion of some part of any chromosomes, trisomy, or translocations. In 16 cases, a single cytogenetic abnormality existed, and, in 28 cases, multiple abnormalities were present. An abnormality of chromosome 1 was present in 17 cases; of chromosome 3, mostly trisomy 3, in 17 cases; of chromosome 11 in 2 cases; and of chromosome 18 in 14 cases. A translocation that involved chromosome 1 was present in three cases, chromosome 3 in four cases, chromosome 11 in nine cases, and chromosome 18 in two cases. The rearrangement of bcl-2 gene was observed in none of these patients, but some patients having a translocation involving chromosome 11 or chromosome 14 had a bcl-1 rearrangement (manuscript in preparation).
Clinical presentation
Among the 124 cases, the sex ratio was 1:1 and median age was 60 years, which is not different from other lymphoma entities. However, the median age was younger in the nodal subtype and older in the leukemic subtype (.5 = 10.8, P < .05). By definition, patients with splenic involvement were observed in the splenic and disseminated subtypes, and patients with peripheral lymph nodes were observed in the nodal and disseminated subtypes. Thoracic and/or abdominal lymph nodes were present in 34%, 49%, and 55% of the splenic, nodal, and disseminated subtypes, respectively. They were absent in the leukemic subtype. Liver involvement was present in 26% of the cases, more frequently in splenic and disseminated subtypes (P = .06). Pleura involvement was present in 11% of the cases, more often in disseminated subtypes (P < .001). Bone marrow involvement was present in 72% of the cases, less frequently in the nodal subtype (43% compared with 80%-100% in other subtypes,P < .0001). Blood involvement was defined by the presence of abnormal lymphocytes or a lymphocyte count more than 5 × 109/L. It was present in 43% of the cases but was rare (11%) in nodal subtype, present in half of the cases with splenic or disseminated subtypes, and nearly constant in the leukemic subtype. The two patients without spleen or lymph node enlargement and without blood involvement had a bone marrow examination because of cytopenia. Only 14.5% of the patients had a localized disease, most of them in the nodal subtype (Table 1). Only 21% of the patients had B symptoms, and 18% had a poor performance status, most of them in the disseminated subtype. A bulky tumor (>10 cm) was observed in 39.5% of the patients, absent in leukemic subtype, and less frequent in nodal subtype (P < .001). More than one extranodal site of involvement was observed in 56% of the patients, less frequently in nodal subtype (P < 001), these extranodal sites being bone marrow and blood in the majority of the patients.
Forty-three percent of the patients had a high LDH level without difference among the subtypes. A β2-microglobulin level above 3 mg/L was observed in 59% of the patients, less frequently in nodal subtype (P < .05. Anemia (hemoglobin level <12 g/dL) was observed in 52% of the patients, mostly in splenic and disseminated subtypes (P < .01). Thrombocytopenia was observed in 17% of the cases, essentially in splenic subtype. A low serum albumin level (<35 g/L) was observed in 25% of the patients, less frequently in nodal subtype. At diagnosis, 19 (15%) patients had an M component, all in blood and three in urine. This M component was an IgM in 15 cases, IgG in 1 case, and IgA in 3 cases with an equal repartition for λ and κ chains. Median level of the M component was 8 g/L (1-51 g/L). Fourteen (11%) patients had a positive Coombs test, 12 of them with anemia.
According to the International Prognostic Index,27 15% of our patients had low risk, 33% low-intermediate risk, 33% high-intermediate risk, and 19% high risk, but the repartition was clearly different in the splenic, leukemic, nodal, or disseminated subtypes, with nodal subtype patients having more of a lower risk and disseminated subtype patients a higher risk (Table 1).
The differences observed in the clinical presentation between histological variants are shown in Table 4. Most of the observed differences are explained by the fact that SLVL was mainly observed in the splenic subtype and large cell-rich variant in the nodal and disseminated subtypes.
Clinical, biologic, and immunologic characteristics of the 124 MZL patients according to the morphologic variants
| . | SLVL . | Common . | Large Cell-Rich . | P Value . |
|---|---|---|---|---|
| Age >60 y | 11 (92%) | 42 (54%) | 14 (41%) | .01 |
| Performance status ≥2 | — | 14 (20%) | 7 (22%) | |
| Peripheral lymph nodes | 1 (8%) | 30 (38.5%) | 24 (71%) | .0002 |
| Profound lymph nodes | — | 35 (45%) | 14 (41%) | .01 |
| Large spleen | 12 (100%) | 52 (67%) | 15 (44%) | .002 |
| Localized stage | 1 (8%) | 9 (11.5%) | 8 (23.5%) | |
| Bone marrow infiltration | 10 (83%) | 63 (81%) | 17 (50%) | .002 |
| High LDH | 3 (30%) | 26 (42%) | 14 (50%) | |
| High β2-microglobulin | 8 (89%) | 36 (64%) | 8 (35%) | <.01 |
| Blood infiltration | 10 (83%) | 39 (51%) | 4 (12%) | <.0001 |
| High/intermediate-high risk IPI | 4 (40%) | 34 (57%) | 13 (46%) |
| . | SLVL . | Common . | Large Cell-Rich . | P Value . |
|---|---|---|---|---|
| Age >60 y | 11 (92%) | 42 (54%) | 14 (41%) | .01 |
| Performance status ≥2 | — | 14 (20%) | 7 (22%) | |
| Peripheral lymph nodes | 1 (8%) | 30 (38.5%) | 24 (71%) | .0002 |
| Profound lymph nodes | — | 35 (45%) | 14 (41%) | .01 |
| Large spleen | 12 (100%) | 52 (67%) | 15 (44%) | .002 |
| Localized stage | 1 (8%) | 9 (11.5%) | 8 (23.5%) | |
| Bone marrow infiltration | 10 (83%) | 63 (81%) | 17 (50%) | .002 |
| High LDH | 3 (30%) | 26 (42%) | 14 (50%) | |
| High β2-microglobulin | 8 (89%) | 36 (64%) | 8 (35%) | <.01 |
| Blood infiltration | 10 (83%) | 39 (51%) | 4 (12%) | <.0001 |
| High/intermediate-high risk IPI | 4 (40%) | 34 (57%) | 13 (46%) |
IPI = International Prognostic Index; LDH = lactic dehydrogenase; MZL = marginal zone B-cell lymphoma; SLVL = splenic lymphoma with villous lymphocyte.
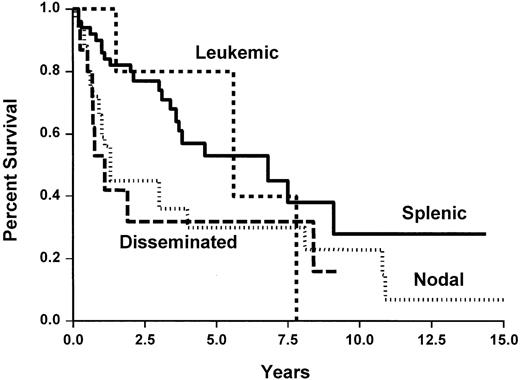
TTP and survival
Median survival and TTP for the whole group of patients was 9.1 years and 3.8 years, respectively, (Figure2) without any plateau and a nearly constant event rate. Figures 3 and4 show TTP and survival for the four clinical subtypes. Median TTP was 6.9 years and 5.6 years in splenic and leukemic subtypes compared with 1.3 years and 1.1 years in nodal and disseminated subtypes, respectively. If TTP was slightly longer in SLVL cases (not reached) and common (4 years) subtypes than in large-cell subtype (3 years), this was not statistically different.
Overall survival and time to progression of the 124 patients with marginal zone lymphoma.
Overall survival and time to progression of the 124 patients with marginal zone lymphoma.
Time to progression of the 124 patients with marginal zone B-cell lymphoma, according to the clinical subtypes.
Time to progression of the 124 patients with marginal zone B-cell lymphoma, according to the clinical subtypes.
Overall survival of the 124 patients with marginal zone B-cell lymphoma, according to the clinical subtypes.
Overall survival of the 124 patients with marginal zone B-cell lymphoma, according to the clinical subtypes.
Response to treatment varied in the different subtypes, and a CR was reached in 69% of nodal subtype compared with <30% in the other subtypes, but a partial response was the rule in these cases and truly refractory patients were rare (Table 2). A high CR rate was not associated with a longer TTP. Most of the patients with bone marrow involvement at time of diagnosis had a persisting involvement at the end of the treatment. However, TTP in these partial response patients was not worse than the TTP of patients who reached a CR.
Transformation
Twenty patients presented a histological transformation during the course of the disease. This diagnosis was made when a patient progressed with a high component of large cells or sheets of large cells in the new biopsy. This transformation was observed in first or subsequent progression and occurred with a median time of 4.5 years after the diagnosis (extreme: 1 year and 22 years). The clinical picture of the disease at time of transformation was not different of what has been described in the transformation of other indolent lymphoma: presence of B symptoms (8 patients), poor performance status (7 patients), bulky tumor (5 patients), extranodal locations other than bone marrow (13 patients), and high LDH level (14 patients). Response to treatment was poor, and survival after transformation was usually short.
Parameters associated with a good outcome
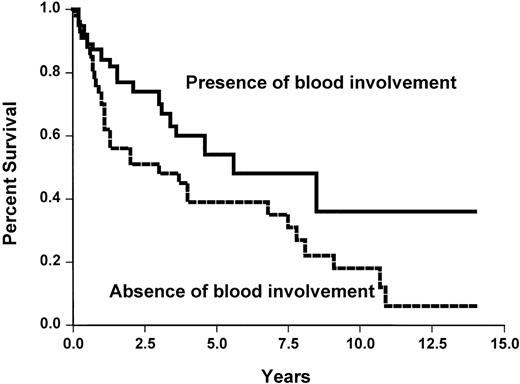
None of the studied initial parameters (Table5) was associated with a poorer survival. This may be related to the fact that overall survival is good in our short series. None of the classical prognostic parameters, such as those of the International Prognostic Index, was associated with a shorter TTP. However, the absence of peripheral lymph nodes and the presence of spleen involvement or blood involvement were associated with a longer TTP (Table 5 and Figure 5). No difference was observed between the three histological subtypes.
Parameters associated with a longer time to progression and a longer survival in the 124 patients with non-MALT MZL
| . | Time to Progression . | Survival . |
|---|---|---|
| Age (y) (<60 vs ≥60) | NS | NS |
| Performance status (0-1 vs ≥2) | NS | NS |
| Peripheral lymph nodes (yes/no) | P = .001 | NS |
| Abdominal or thoracic lymph nodes (yes/no) | NS | NS |
| Spleen involvement (yes/no) | P = .05 | NS |
| Stage (I-II vs III-IV) | NS | NS |
| Number of extranodal sites (0-1 vs ≥2) | NS | NS |
| Bulky tumor (yes/no) | NS | NS |
| β2-microglobulin level (<3 vs ≥3 mg/L) | NS | NS |
| LDH level (normal/abnormal) | NS | NS |
| Hemoglobin level (<12 vs ≥12 g/dL) | NS | NS |
| Blood involvement (yes/no) | P < .05 | NS |
| International Prognostic Index (0-1 vs 2 vs 3 vs 4-5) | NS | NS |
| . | Time to Progression . | Survival . |
|---|---|---|
| Age (y) (<60 vs ≥60) | NS | NS |
| Performance status (0-1 vs ≥2) | NS | NS |
| Peripheral lymph nodes (yes/no) | P = .001 | NS |
| Abdominal or thoracic lymph nodes (yes/no) | NS | NS |
| Spleen involvement (yes/no) | P = .05 | NS |
| Stage (I-II vs III-IV) | NS | NS |
| Number of extranodal sites (0-1 vs ≥2) | NS | NS |
| Bulky tumor (yes/no) | NS | NS |
| β2-microglobulin level (<3 vs ≥3 mg/L) | NS | NS |
| LDH level (normal/abnormal) | NS | NS |
| Hemoglobin level (<12 vs ≥12 g/dL) | NS | NS |
| Blood involvement (yes/no) | P < .05 | NS |
| International Prognostic Index (0-1 vs 2 vs 3 vs 4-5) | NS | NS |
LDH = lactic dehydrogenase; MALT = mucosa-associated lymphoid tissue; MZL = marginal zone B-cell lymphoma; NS = not significant.
Time to progression survival according to the presence or absence of blood involvement (X2 = 4.5,P < .05).
Blood involvement was defined by either an excess of blood lymphocytes (> 5 × 109/L) or presence of abnormal cells.
Time to progression survival according to the presence or absence of blood involvement (X2 = 4.5,P < .05).
Blood involvement was defined by either an excess of blood lymphocytes (> 5 × 109/L) or presence of abnormal cells.
Discussion
In this review, we have collected all patients with lymphoma who did not fit well into the new lymphoma classifications and reclassified them. We were able to find 124 cases of marginal non-MALT lymphoma with a satisfactory morphologic and clinical description. However, their treatment was quite heterogeneous, and, if this study may add to the knowledge of this newly described lymphoma, we are not able to describe the best therapeutic options for these patients. We were able to recognize without much difficulty the different morphologic aspects: splenic lymphoma with or without villous lymphocytes, nodal lymphoma with monocytoid B-cells, and cases with plasmacytic differentiation being in the past classified as immunocytoma (Figure 1B). However, we found histological aspects that did not fit very well in the proposed classifications, particularly cases with a high component of large cells at diagnosis, sometimes considered as a composite lymphoma at diagnosis. Because of the same phenotype observed in small and large cells, we prefer the name of large-cell variant to the name “composite lymphoma” that may suggest two origins of the lymphoid proliferation. This description does not mean that these patients must be treated as having an indolent lymphoma: The treatment has to be decided on the presence of clinical aggressiveness. Clearly, we need more work on a larger group of patients to propose a definitive classification for these lymphomas.
We described four clinical subtypes corresponding to the primary clinical aspects, two of them, splenic and nodal subtypes, being the subtypes proposed in the REAL and the WHO classifications. The splenic lymphomas are characterized by a predominantly enlarged spleen with frequent bone marrow and blood involvement. In some patients, abdominal lymph nodes may be observed. Contrarily, the nodal subtype is characterized by localized or disseminated lymph nodes with rare blood involvement. Disseminated cases represent cases with a disease too disseminated to identify the site of origin or the initial (or primary) site of the disease. These cases represent probably a late stage of the disease for either nodal or splenic subtypes. Contrarily, leukemic cases may represent early diagnosis of a splenic subtype with a small splenomegaly or no enlargement of the spleen at all. This last hypothesis is comforted by the fact that these patients have a disease that shares the same characteristics as those of splenic cases, and they have a very good outcome.
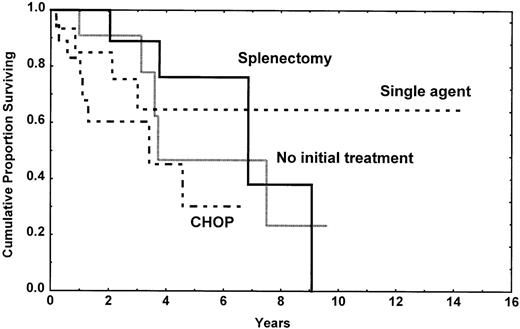
If the survival of these patients was truly good, defining these lymphomas as indolent lymphomas, TTP is clearly different between the splenic subtype and nodal or disseminated subtypes. Splenic MZL, with or without villous lymphocytes, is obviously an indolent lymphoma with difficulty to reach a CR whatever the treatment is, but with long TTP and survival. Splenectomy was done in our patients without adverse parameters, and it was associated with a very long TTP (Figure6). Contrarily, nodal MZL may be a localized disease, but the disease progressed very rapidly with the therapy we have used. Even if TTP was short, survival is longer, and this seems linked to the responses observed with salvage therapy. Disseminated MZL is a more aggressive disease, often with large-cell component and poor prognosis parameters, and it probably represents the end stage of the other subtypes.
Time to progression in the splenic subtype patients according to the type of initial treatment. It is of note that the initial treatment was decided according to the clinical presentation at time of diagnosis and that CHOP chemotherapy was always realized in patients with a more aggressive picture.
Time to progression in the splenic subtype patients according to the type of initial treatment. It is of note that the initial treatment was decided according to the clinical presentation at time of diagnosis and that CHOP chemotherapy was always realized in patients with a more aggressive picture.
The therapeutic strategy may not be defined after this retrospective analysis, but we may give some recommendations for future prospective studies or for the day-to-day care of these patients. First, a correct diagnosis needs in many cases at least immunophenotyping and often cytogenetic analysis, with nevertheless some persisting borderline cases. A review by a panel of hematopathologists seems necessary in prospective trials and to foresee a cytogenetic examination is recommended. Second, splenic and leukemic MZLs are clearly indolent lymphomas, and a conservative treatment is probably the best choice, particularly for old patients. Splenectomy may be done when the increase in spleen volume is too important or when cytopenia occurs. Finally, nodal and disseminated subtypes, particularly those with a component of large cells or composite lymphoma, are more aggressive, and CHOP may be considered as a first-line treatment.
Acknowledgments
We would like to thank the Programme National de Recherche Clinique (PNRC Lyon 97-062), the Comitès Dèpartementaux de la Ligue Nationale contre le Cancer du Rhùne, de l'Ardëche et de la Saùne et Loire for funding this study.
Reprints:B. Coiffier, Service d'Hèmatologie, Centre Hospitalier Lyon-Sud, 69495 Pierre-Bènite Cedex, France; e-mail:bertrand.coiffier@chu-lyon1.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal