To the editor:
Alloantigen-induced anti-HIV activity occurs prior to reverse transcription and can be generated by leukocytes from HIV-infected individuals
Immunization against alloantigens has been proposed as a possible prophylactic strategy against HIV.1,2 We have previously reported in Blood that alloantigen-stimulated cell lines derived from HIV-seronegative donors and their supernatants inhibit HIV-1 replication of a wide spectrum of isolates by a mechanism that is independent of IFN-γ and of the β-chemokines MIP-1α, MIP-1β, and RANTES.3 Here we provide further information on the characterization of allogeneic leukocyte-stimulated anti-HIV activity that could be relevant for the development of successful complementary immune-based strategies against HIV infection. We have found that alloantigen-stimulated anti-HIV activity (1) occurs prior to reverse transcription, (2) is generated by leukocytes from HIV-infected individuals, independently of CD4+ T-cell counts, and (3) circumvents the need for an intact CD28/B7 costimulatory pathway.
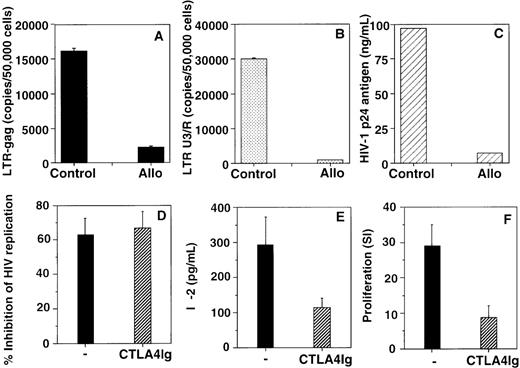
To define the molecular mechanism of inhibition of viral replication, we investigated the effect of anti-HIV supernatants derived from an alloantigen-stimulated cell line3 on HIV-1 reverse transcript levels in T-cell blasts infected with HIV-1BZ167. Levels of long terminal repeat-gag (LTR-gag) and LTR U3/R reverse transcripts were measured by quantitative, real-time DNA polymerase chain reaction (PCR) using primers and probe sequences previously described.4,5Both late (LTR-gag) (Figure, panel A) and early (LTR U3/R) (Figure, panel B) reverse transcripts were significantly decreased following incubation with the alloantigen-stimulated supernatant (86% and 97% inhibition, respectively), demonstrating that alloantigen-stimulated anti-HIV activity occurs prior to reverse transcription. Figure panel C shows that the alloantigen-stimulated supernatant used in these experiments had a strong inhibitory effect on HIV-1 replication (93% inhibition), measured by p24 antigen production. These data suggest that the antiviral activity mediated by alloantigen-stimulated supernatants is distinct from the antiviral activity produced by CD8 antiviral factors (CAF), since CAF does not affect the levels of early or late reverse transcripts.5
Alloantigen-stimulated anti-HIV activity occurs prior to reverse transcription (panels A, B, and C) and is generated in the absence of an intact CD28/B7 costimulatory pathway (panels D, E, and F).
PHA blasts were infected with HIV-1BZ167 (172 TCID50/105 cells) and cultured in the absence (control) or presence of an HIV-suppressive supernatant derived from an alloantigen-stimulated cell line (Allo).3 Levels of LTR-gag (A) and LTR U3/R HIV-1 reverse transcripts (B) in HIV-1BZ167-infected PHA blasts were determined by quantitative, real-time DNA PCR.7,8 Results in panels A and B represent means (number of copies/50 000 ± 10 000 cells) ± standard deviation of 2 independent experiments. HIV-1 p24 antigen production by HIV-1BZ167-infected PHA blasts (C) was determined by ELISA. Alloantigen-stimulated supernatants from cultures performed in the absence (−) or presence of CTLA4Ig fusion protein (5 μg/mL) added at the beginning of 7-day cultures7 were assayed for effect on HIV-1BZ167 replication in T-cell blasts (D) and IL-2 production (E) measured by ELISA. Alloantigen-specific proliferation (F) was measured 7 days after primary stimulation. Results are expressed as mean ± SEM of 3 (D), 5 (E), and 6 (F) independent experiments.
Alloantigen-stimulated anti-HIV activity occurs prior to reverse transcription (panels A, B, and C) and is generated in the absence of an intact CD28/B7 costimulatory pathway (panels D, E, and F).
PHA blasts were infected with HIV-1BZ167 (172 TCID50/105 cells) and cultured in the absence (control) or presence of an HIV-suppressive supernatant derived from an alloantigen-stimulated cell line (Allo).3 Levels of LTR-gag (A) and LTR U3/R HIV-1 reverse transcripts (B) in HIV-1BZ167-infected PHA blasts were determined by quantitative, real-time DNA PCR.7,8 Results in panels A and B represent means (number of copies/50 000 ± 10 000 cells) ± standard deviation of 2 independent experiments. HIV-1 p24 antigen production by HIV-1BZ167-infected PHA blasts (C) was determined by ELISA. Alloantigen-stimulated supernatants from cultures performed in the absence (−) or presence of CTLA4Ig fusion protein (5 μg/mL) added at the beginning of 7-day cultures7 were assayed for effect on HIV-1BZ167 replication in T-cell blasts (D) and IL-2 production (E) measured by ELISA. Alloantigen-specific proliferation (F) was measured 7 days after primary stimulation. Results are expressed as mean ± SEM of 3 (D), 5 (E), and 6 (F) independent experiments.
β-Chemokines, CAF, and other unidentified soluble factors, released either by alloantigen-stimulated cell lines or by primary alloantigenic-stimulated peripheral blood mononuclear cells (PBMC) from healthy HIV-uninfected individuals immunized in vivo with allogeneic PBMC have been reported to inhibit HIV-1 infection.2,3 6 To determine whether leukocytes from HIV-infected individuals have the potential to generate anti-HIV activity after primary alloantigenic stimulation, we analyzed the effect of supernatants obtained from alloantigen-stimulated PBMC of HIV-infected patients on HIV-1 replication. The supernatants from the patients' alloantigen-stimulated cultures inhibited HIV-1BZ167 and HIV-1Ba-L replication in T-cell blasts to an extent similar to that by supernatants of alloantigen-stimulated PBMC from healthy individuals (Table). Furthermore, the fraction of individuals whose culture supernatants inhibited viral replication greater than 50% was similar in patient and control cultures. Alloantigen-stimulated supernatants from an HIV-infected individual and healthy control also inhibited HIV-1Ba-L infection in monocyte-derived macrophages (94% and 64% inhibition of HIV-1 replication). These results demonstrate that alloantigen-mediated anti-HIV activity acts both in infected T cells and macrophages. Patient and control alloantigen-stimulated cultures generated similar amounts of RANTES but undetectable amounts of IFN-α. Although these control cultures produced more IL-2, IFN-γ, and IL-10 than the patient cultures, the differences in cytokine production were not statistically significant (p > .05, Student's t test). Furthermore, there was no correlation between the levels of these cytokines and the inhibitory effect on viral replication (r < 0.5). No significant correlation was observed between patients' CD4 T-cell counts (range 195-787 cells/μL) and ability of the patients' leukocytes to generate alloantigen-stimulated HIV-suppressive activity.
Inhibition of HIV-1 replication by alloantigen-stimulated supernatants derived from PBMC cultures of healthy blood bank donors and HIV-1– infected individuals
| Donors . | Inhibition* . | Cytokine Content (pg/mL) . | |||||
|---|---|---|---|---|---|---|---|
| HIV-1BZ167 . | HIV-1Ba-L . | IL-2 . | IFN-γ . | IL-10 . | IFN-α . | RANTES . | |
| Control | 6/12 (0-86%) | 4/9 (0-91%) | 87 ± 25 | 172 ± 72 | 27 ± 11 | <20 | 1125 ± 669 |
| HIV+ 1-153 | 5/13 (0-87%) | 6/12 (0-89%) | 27 ± 15 | 96 ± 30 | 12 ± 4 | <20 | 1025 ± 333 |
| Donors . | Inhibition* . | Cytokine Content (pg/mL) . | |||||
|---|---|---|---|---|---|---|---|
| HIV-1BZ167 . | HIV-1Ba-L . | IL-2 . | IFN-γ . | IL-10 . | IFN-α . | RANTES . | |
| Control | 6/12 (0-86%) | 4/9 (0-91%) | 87 ± 25 | 172 ± 72 | 27 ± 11 | <20 | 1125 ± 669 |
| HIV+ 1-153 | 5/13 (0-87%) | 6/12 (0-89%) | 27 ± 15 | 96 ± 30 | 12 ± 4 | <20 | 1025 ± 333 |
Fraction of individuals with supernatants that inhibited HIV-1 replication greater than 50% (range of inhibition of viral replication).
Median CD4 T-cell counts = 662 cells/μL (range, 195-787).
The role of costimulatory requirements in alloantigen-mediated anti-HIV activity has not been previously addressed. Here we demonstrate that generation of primary alloantigen-stimulated anti-HIV activity is not affected by inhibition of CD28/B7 interaction using the CTLA4Ig fusion protein (Figure, panel D), under conditions in which alloantigen-specific IL-2 production (Figure, panel E) and proliferation (Figure, panel F) are significantly inhibited (p < .05).7 These findings suggest that an intact CD28/B7 costimulatory pathway is not essential for the induction of alloantigen-stimulated HIV-suppressive activity and could explain the generation of this activity by alloantigen-stimulated cells from HIV-infected patients, which have been reported to exhibit immune costimulatory defects.8 9
These findings contribute to characterization of the molecular mechanisms and costimulatory requirements for alloantigen-stimulated anti-HIV activity that might be important for the development and application of immune-based strategies against HIV.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal