Mice lacking the erythroid Kruppel-like factor (EKLF) die in utero at embryonic day 15 (E15) from severe anemia. EKLF−/− embryos display a marked deficit in β-globin gene expression. To test whether β-globin deficiency was solely responsible for the anemia and intrauterine death, we corrected the globin chain imbalance in EKLF−/− embryos by breeding with a strain of mice that express high levels of human γ-globin. Despite efficient production of hybrid m2-hγ2 hemoglobin in the fetal livers of EKLF−/− animals, hemolysis was not corrected and survival was not prolonged. We concluded that deficiency of nonglobin EKLF target genes is a major contributor to the definitive red blood cell abnormalities and prenatal death in EKLF−/−embryos. These results suggest that strategies designed to antagonize EKLF function in adults with hemoglobinopathy, in an attempt to reactivate γ-globin gene expression, may adversely affect other essential aspects of red blood cell physiology.
In adult red blood cells, oxygen is efficiently shuttled between tissues and the lungs by the hemoglogin A molecule (HbA), a tetramer of 2 α-globin chains and 2 β-globin chains. The α- and the β-globin genes are members of separate multigene families. The gene order of each cluster correlates with the developmental sequence of gene expression. In the human β-globin locus 5 genes are arranged 5′-ε-Aγ-Gγ-δ-β-3′ and are sequentially expressed in the yolk sac (ε), fetal liver (Aγ and Gγ), and bone marrow (δ and β). The murine β-globin locus also contains 5 functional genes that are arranged 5′-ε-βh0-βh1-βmaj-βmin-3′, but none of them is expressed uniquely at the fetal liver stage of development. Instead, the 2 adult murine globin genes, βmin and βmaj, are expressed in the fetal liver and in the bone marrow. On the other hand, the 2 murine genes most similar to human γ-globin in sequence and position in the locus, βh0 and βh1, are uniquely expressed in embryonic red cells.
Throughout ontogeny the production of α-like globin chains and β-like globin chains remains balanced through mechanisms that are incompletely understood. In humans, loss of β-globin production from gene mutation causes β-thalassemia, a disease in which unbalanced α-globin chain production results in the precipitation of globin, red blood cell damage, and shortened red blood cell survival.1The disease is accompanied by iron overload resulting from a combination of exogenous iron delivery in blood transfusions and an increased drive to intestinal iron absorption.
Erythroid Kruppel-like factor (EKLF) is a member of the Kruppel subfamily of transcription factors that are characterized by the presence of 3 C2H2-type zinc finger motifs at the C-terminus.2 Conservation of critical DNA-interacting amino acids with the related zinc finger protein, Zif 268, and the crystal structure of the latter bound to DNA suggest EKLF binds to DNA sequences that fall within an NCNCNCCCN consensus (where N is any nucleotide).3 Thus, EKLF can bind the β-globin (CCACACCCT) but not to the γ-globin (CTCCACCCA) promoter CACC box element.4 The NCNCNCCCN consensus occurs in the promoters of many erythroid genes, including the proximal promoters of other globin genes, many heme synthesis enzymes, metabolic enzymes, transmembrane proteins, and transcription factors. However, it is not yet clear whether EKLF can bind efficiently to all these CACC sites or just to a subset of them.
EKLF is expressed specifically in erythroid cells,2 and its absence results in a severe defect in definitive erythropoiesis with fatal anemia at E15 of development.5,6EKLF−/− embryos display a severe deficit in β-globin gene expression in fetal liver erythroid cells, whereas α-globin gene expression is unaffected. EKLF−/− embryos also accumulate iron in the reticuloendothelial system, consistent with ineffective erythropoiesis or hemolysis.5 On the other hand, EKLF−/− embryos display no defect in embryonic erythropoiesis, a developmental time point when the β-globin gene is silent.
Although the stage specificity of the EKLF null phenotype reflects the stage specificity of β-globin gene expression, the abnormal erythroid morphology does not precisely mirror the changes that occur in human β-thalassemia. In particular, most of the fetal liver-derived circulating red cells from EKLF−/− embryos retain a nucleus,5 suggesting either the presence of greater hemolysis than commonly exists in β-thalassemia major or some additional red blood cell defect. Furthermore, gene targeting of both the βmin and the βmaj genes leads to perinatal anemia and death, with red cell morphologic abnormalities more like those found in human β-thalassemia major than those found in EKLF−/− embryos.7 Because many erythroid gene promoters harbor functionally critical CACC box elements,8,9 they may also be important EKLF target genes. Furthermore, defective expression of these putative target genes may contribute to the definitive red cell abnormalities in EKLF−/− embryos. We have previously examined the expression of some other potential target genes, including the erythropoietin receptor (EpoR), porphobilinogen deaminase (PBDG), and GATA-1, in EKLF−/− fetal liver cells, and we determined that they were EKLF independent.5 However, the expression of other as yet undetermined EKLF-dependent genes may be crucial for the viability of definitive erythroid cells.
To test directly the hypothesis that β-globin deficiency was the principal cause of hemolytic anemia in EKLF null embryos, we attempted to restore globin chain balance by the expression of β-globin–like chains in EKLF−/− fetal liver erythrocytes. We considered the use of transgenic mice that expressed the β-globin gene itself for this purpose but opted for an alternative approach because β-globin transgene expression was anticipated to be EKLF dependent.
The duplicated human γ-globin genes (Aγ andGγ) have an alternate CACC element sequence (CTCCACCCA) in their promoters that does not efficiently bind EKLF.4Moreover, γ-globin genes, as they exist in the context of the entire human β-globin locus, are not dependent on EKLF for expression.10 11 Furthermore, human HbF (α2γ2) markedly improves the severity of anemia in humans with β-thalassemia when expressed at 5% to 10% of adult HbA (α2β2) levels. Thus, the expression of γ-globin at reasonable levels in the fetal liver of EKLF−/− embryos was predicted to lead to a marked improvement in anemia and survival if the red cell defect were primarily the result of globin chain imbalance.
We report here that a deregulated human Aγ transgene (μLCR-201Aγ)12 is expressed at high levels in EKLF−/− embryos, with efficient production of hybrid mα2hγ2 hemoglobin molecules in fetal liver erythrocytes. Despite a significant improvement in globin chain balance, EKLF−/− fetal liver erythrocytes remained morphologically defective, and EKLF−/−Aγ+ embryos had no significant survival advantage over EKLF−/−Aγ− litter mates. We concluded that, in addition to its role in β-globin gene expression, EKLF must play an essential role in the expression of other genes whose protein products are required for the integrity of definitive red blood cells.
Materials and methods
Generation of mice expressing the human Aγ transgene
EKLF+/− mice were bred with mice containing a single copy of a human Aγ transgene linked to the micro locus control region, μLCR−201Aγ.12 These transgenic mice express Aγ globin at high levels during all 3 waves of hematopoiesis—in the yolk sac, the fetal liver, and the bone marrow. EKLF+/−, μLCR−201Aγ+ mice were identified by Southern blotting of HindIII-digested genomic tail DNA. Presence of the mutant and wild-type EKLF alleles was determined as described.5 Presence of the μLCR−201Aγ transgene was determined by hybridization with a 722-bp Asp718-HindIII human HS-2 probe derived from pUC19-HSII1.9β.13 In most cases, the presence of 1 versus 2 copies of the μLCR−201Aγ transgene could not be determined with certainty. EKLF+/−μLCR−201Aγ+ mice were interbred, and, in some cases, EKLF+/−μLCR−201Aγ+ mice were bred with EKLF+/−μLCR−201Aγ− mice. Staged litters were killed at E12 to E17 to examine definitive hematopoiesis. The morning of vaginal plug discovery was designated E0.
RNase protection analyses
Total RNA was prepared from fetal livers,14 and RNase protection analyses were performed as described.15 One microgram total RNA was hybridized simultaneously with murine α-globin and human γ-globin riboprobe, generated as described.15 The γ-globin probe was generated with 5-fold less cold rCTP than the α-globin probe, so that the specific activity, and therefore the signal, was 5-fold greater. The intensity of bands corresponding to the protected globin mRNA was quantitated using a Molecular Dynamics PhosphorImager and ImageQuant software (Amersham Pharmacia Biotech, Uppsala, Sweden).
Hemoglobin analysis by immunofluorescence, isoelectric focusing, and electrospray mass spectrometry
Embryos were carefully dissected from the uterus to maintain the integrity of the uterine and vitelline circulations. The umbilical and vitelline vessels were clamped, the yolk sac was punctured, and whole blood was immediately collected from embryos by direct cardiac puncture of the beating heart. Twenty-five microliters whole blood was diluted immediately into 75 μL acid-citrate-dextrose and analyzed on a Technicon H-3 automated blood analyzer.16Values for hematocrit and hemoglobin were multiplied by the dilution factor and reported as the mean ± SEM from embryos of equivalent genotype. Because Aγ+/− and Aγ+/+ could not be reliably distinguished, they have been reported together as Aγ+ animals.
Fetal livers were surgically resected, and single-cell suspensions were made in phosphate-buffered saline (PBS) by passage through a 21-gauge needle. Cells (1 × 105) were cytocentrifuged at 500g and fixed in methanol:acetone (1:1). Specimens were stained with a fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody specific for human γ-globin chains [9C3, a kind gift from Dr Thomas Campbell] as described.10 Specimens were simultaneously stained with 0.01% 4′-6-diamidino-2-phenylindole HCl (DAPI; Sigma, St. Louis, MO) to identify all cell nuclei in the field. Fresh fetal liver cells (105 cells in 100μL PBS) were stained for hemoglobin by incubation in 0.2% o-dianisidine (D-9143; Sigma) in 0.3% glacial acetic acid/3% H2O2 for 5 minutes. Cells were subsequently cytocentrifuged (as above) and counterstained in Harris' hematoxylin for 30 seconds.
To analyze the component hemoglobins in blood, circulating red cells were isolated by bleeding E14 to E17 embryos into 1.5 mL PBS. Hemolysates were prepared from the packed red cells by freeze-thawing in water. Hemoglobins were separated by isoelectric focusing and visualized after staining in o-dianisidine. Individual hemoglobin bands were excised, extracted with water, and subjected to electrospray mass spectroscopy to identify constituent globin chains according to their precise average molecular weights as described before.17Selected separated hemoglobin species were analyzed by analytical reverse-phase high-performance liquid chromatography (HPLC) using the system previously described.18 The elution gradient was based on a method of Shelton et al,19 and it was optimized to afford separation of murine adult and embryonic globins and human fetal globins. It consisted of 3 linear steps, from 58%A/42%B to 56%A/44%B in 20 minutes, then to 44% A in 60 minutes, and then to 15% A in 40 minutes, where A was 20% acetonitrile/0.1% trifluoroacetic acid and B was 60% acetonitrile/0.1% trifluoroacetic acid. Identity and N-terminal processing of the murine embryonic ζ-globin were confirmed by observing its isolation from hemolysate by analytical reverse-phase HPLC, tryptic digestion, and LC/MS analysis of proteolytic fragments.18
Results
High-level expression of the μLCR−201Aγ-globin transgene in the absence of EKLF
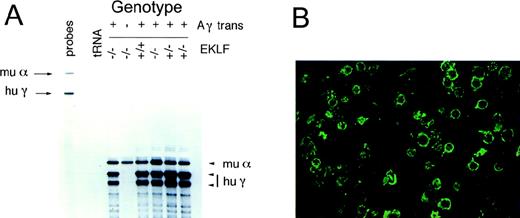
We previously suggested that the fatal anemia in EKLF−/− embryos is primarily caused by β-thalassemia.5 To improve globin chain balance in EKLF−/− fetal liver erythrocytes and thereby improve the anemia, EKLF+/− animals were bred with a mouse strain that contains a single copy of a μLCR−201Aγ-globin transgene.12EKLF ± μLCR−201Aγ+ mice were identified by Southern blotting of tail DNA (see “Materials and methods”) and interbred. We could not be certain whether embryos harbored 1 or 2Aγ transgene alleles by Southern blotting, so the genotype has been reported as + or − to reflect the presence (+/+ or +/−) or absence (−/−) of the Aγ transgene. Expression of the human Aγ transgene in the fetal livers of embryos was 30% or greater than that of the endogenous murine α-globin gene. This was determined by PhosphorImager quantitation of RNase protection analyses of the α-globin and γ-globin mRNA after a correction was made for the 5-fold greater specific activity of the γ-globin riboprobe (Figure1A). There was no alteration in γ-globin mRNA levels in the fetal livers of EKLF−/−versus EKLF+/− embryos. Thus, EKLF was not required for γ-globin promoter function or for LCR function in its capacity to interact with the γ-globin promoter. Significantly, our experimental objective, which was to generate high-level expression of β-like mRNA (in this case, Aγ-globin) in the fetal liver of EKLF−/− embryos, was achieved.
Expression of human γ-globin in μLCR-Aγ+, EKLF−/− embryos.
(A) Human γ-globin is highly expressed in the fetal livers of μLCR-Aγ+ transgenic animals. RNase protection for human γ-globin and murine α-globin transcripts in E15 fetal liver-derived erythroid cells. The presence of the transgene and the EKLF genotype, as determined by Southern blotting, is indicated above each lane. The specific activity of the human γ-globin probe was 10-fold greater than the murine α-globin probe. Migration of undigested murine α-globin and human γ-globin riboprobes is indicated by arrows. The protected mRNA species corresponding to murine α-globin and human γ-globin are indicated by arrowheads. (B) Human γ-globin protein was readily detectable by immunofluorescence in fetal liver cells of embryos harboring the μLCR-Aγ transgene. Cytocentrifuge preparations of E15 fetal liver cells from EKLF−/− Aγ+ embryos were stained with a FITC-conjugated monoclonal antibody raised against HbF (see “Materials and Methods”). There was no detectable green fluorescence in a control sample of EKLF−/−Aγ− fetal liver cells (not shown).
Expression of human γ-globin in μLCR-Aγ+, EKLF−/− embryos.
(A) Human γ-globin is highly expressed in the fetal livers of μLCR-Aγ+ transgenic animals. RNase protection for human γ-globin and murine α-globin transcripts in E15 fetal liver-derived erythroid cells. The presence of the transgene and the EKLF genotype, as determined by Southern blotting, is indicated above each lane. The specific activity of the human γ-globin probe was 10-fold greater than the murine α-globin probe. Migration of undigested murine α-globin and human γ-globin riboprobes is indicated by arrows. The protected mRNA species corresponding to murine α-globin and human γ-globin are indicated by arrowheads. (B) Human γ-globin protein was readily detectable by immunofluorescence in fetal liver cells of embryos harboring the μLCR-Aγ transgene. Cytocentrifuge preparations of E15 fetal liver cells from EKLF−/− Aγ+ embryos were stained with a FITC-conjugated monoclonal antibody raised against HbF (see “Materials and Methods”). There was no detectable green fluorescence in a control sample of EKLF−/−Aγ− fetal liver cells (not shown).
Amelioration of globin chain imbalance with production of mouse–human hybrid hemoglobin
To confirm that γ-globin was present in EKLF−/− Aγ+ fetal liver erythrocytes at the protein level, we performed immunofluorescence analysis for human γ-globin (see “Materials and methods”). Most EKLF−/− Aγ+ fetal liver cells expressed cytoplasmic human γ-globin (Figure 1B), whereas there was no detectable green fluorescence in EKLF−/−fetal liver cells that harbored no transgene (not shown).
E15 hemolysates contained 6 different hemoglobin bands (Hb), as determined by isoelectric focusing (labeled 1-6, from anode to cathode, in Figure 2A). EKLF−/− embryos contained less of hemoglobin bands 4 and 5 than EKLF+/− litter mates. These were isolated from control hemolysates and subjected to electrospray mass spectroscopy to confirm the identity of the component globin chains by determination of their precise molecular masses. They were murine α2βmaj2 and murine α2βmin2, respectively (data not shown). This confirmed that the murine βmin gene and the βmaj gene are EKLF dependent in vivo, as expected from the sequence similarity and the relative position of the CACC box elements within the 2 promoters.
Efficient generation of hybrid human-mouse hemoglobin in mice expressing the μLCR-Aγ transgene.
(A) Isoelectric focusing of hemolysates from E15 embryos revealed hybrid human-mouse hemoglobins. Presence (+) or absence (−) of the μLCR-Aγ transgene and the EKLF genotype (± or −/− ) is indicated above each lane. Six hemoglobin (Hb) bands were identifiable, labeled 1 to 6, according to migration from anode to cathode. Hemoglobins 1 and 3 were detectable only in mice that harbored the μLCR-Aγ transgene. They were equally prevalent in EKLF−/− (lane 4) and± embryos (lanes 1 and 3). Bands 4 and 5 represent murine β-major and β-minor hemoglobin, respectively; each was markedly and selectively reduced in EKLF−/− blood. The direction of the anode and cathode is indicated. (B, C) Electrospray mass spectroscopy on gel-purified bands 1 and 3. Hemoglobin band 1 (B) contained 2 proteins of 16 009 and 16 146 kd, which correspond to the predicted molecular weights of human Aγ-globin and murine ζ-globin, respectively. Hemoglobin band 3 (C) contained proteins whose molecular masses were consistent with murine α globins, α 1 (Mr 14 981.0), α 5 (Mr 14 995.0), and human Aγ globin (Mr 16 009.3). (D) Reverse-phase HPLC separation of globins expressed by animal 3 in A (genotype EKLF+/+ Aγ+). Peaks annotated with dots represent artefacts of sample storage (single dot, mixed disulfides of murine β-major and β-minor with either cysteine of glutathione; double dot, disulfide-linked murine β-globin dimers).
Efficient generation of hybrid human-mouse hemoglobin in mice expressing the μLCR-Aγ transgene.
(A) Isoelectric focusing of hemolysates from E15 embryos revealed hybrid human-mouse hemoglobins. Presence (+) or absence (−) of the μLCR-Aγ transgene and the EKLF genotype (± or −/− ) is indicated above each lane. Six hemoglobin (Hb) bands were identifiable, labeled 1 to 6, according to migration from anode to cathode. Hemoglobins 1 and 3 were detectable only in mice that harbored the μLCR-Aγ transgene. They were equally prevalent in EKLF−/− (lane 4) and± embryos (lanes 1 and 3). Bands 4 and 5 represent murine β-major and β-minor hemoglobin, respectively; each was markedly and selectively reduced in EKLF−/− blood. The direction of the anode and cathode is indicated. (B, C) Electrospray mass spectroscopy on gel-purified bands 1 and 3. Hemoglobin band 1 (B) contained 2 proteins of 16 009 and 16 146 kd, which correspond to the predicted molecular weights of human Aγ-globin and murine ζ-globin, respectively. Hemoglobin band 3 (C) contained proteins whose molecular masses were consistent with murine α globins, α 1 (Mr 14 981.0), α 5 (Mr 14 995.0), and human Aγ globin (Mr 16 009.3). (D) Reverse-phase HPLC separation of globins expressed by animal 3 in A (genotype EKLF+/+ Aγ+). Peaks annotated with dots represent artefacts of sample storage (single dot, mixed disulfides of murine β-major and β-minor with either cysteine of glutathione; double dot, disulfide-linked murine β-globin dimers).
Hb 2 contained predominant globin chains of molecular mass 16 006 and 16 146 kd. The MWt of the first, 16 006 kd, was very close to the mass expected for murine ε-y globin (MWt 16 005.5); this identification was further confirmed by analytical reverse-phase HPLC (data not shown). The MWt of the second, 16 146 kd, did not correspond to the size calculated for murine ζ-globin according to its cDNA-derived protein sequence. However, after peptide mapping and partial sequencing of the N-terminal peptide (Ac-Ser-Leu-Met-Lys, MWt 519.3 kd), this species was authenticated as the N-terminally processed murine ζ-globin (removal of initiator Met and acetylation of the N-terminus, MWt 16 145.9, data not shown). Hb 6 was murine α2ε2. Because murine ε-y is only expressed in the yolk sac, the presence of this band reflected the persistence of some circulating yolk sac–derived erythroid cells at E15. The presence of the Aγ transgene had no effect on the level of α2ε2 (Figure 2A).
Two novel hemoglobins were detectable in all Aγ+ embryos but not in Aγ− litter mates (bands 1 and 3 in Figure 2A). Hb 3 contained peaks corresponding to MWt 14 981 kd, 14 996 kd, and 16 009 kd, which identified them as murine α1, murine α520, and human Aγ chains (Figure2C). Thus, Hb 3 is a hybrid murine α2-humanAγ2 hemoglobin (mα2hAγ2). It was the predominant hemoglobin present in EKLF−/− Aγ+ embryos. The amount of Aγ chains normalized to murine α-globin chains in the hemolysate was 31% in the EKLF+/+ Aγ+ embryo (Figure2A, lane 3), as measured by reverse-phase HPLC (Figure 2D). Thus, the strategy to ameliorate the globin chain imbalance was successful.
Hb 1 contained a predominant MWt species of 16 009 kd, which identified it as human Aγ. Murine ζ-globin (see above), with a MWt of 16 146 kd (Figure 2B), was also detectable in isolated Hb 1 but only at 10% to 20% of Aγ chain levels. Again, Hb 1 was only present in embryos that were subsequently genotyped as Aγ+. Thus, Hb 1 consisted primarily ofAγ4 (HbBarts), with some comigration of a hybrid mζ2Aγ2 hemoglobin. HbBarts accounted for less than 15% of the total hemoglobin in E15 EKLF−/− Aγ+ embryos (see Figure2A, lane 4), suggesting that the interaction between humanAγ chains and murine α-chains within fetal liver erythrocytes was efficient but incomplete. Low-level amounts of embryonic βh1 globin was observed (by electrospray mass spectroscopy and reverse-phase HPLC) in E15 hemolysates of all embryos, but a mobility of the βh1-containing hemoglobin in isoelectric-focusing gels was not established.
Partial rescue of hemoglobinization of fetal liver–derived erythrocytes
The EKLF null phenotype is highly consistent. Embryos killed at E11 are indistinguishable from wild-type litter mates, embryos killed at E12 have slight pallor, and the severity of pallor increases until E15 when the embryos are severely anemic.5 We have never detected a living EKLF−/− embryo at E16. This time course correlated precisely with the progressive switch from circulating embryonic to fetal liver–derived red blood cells.
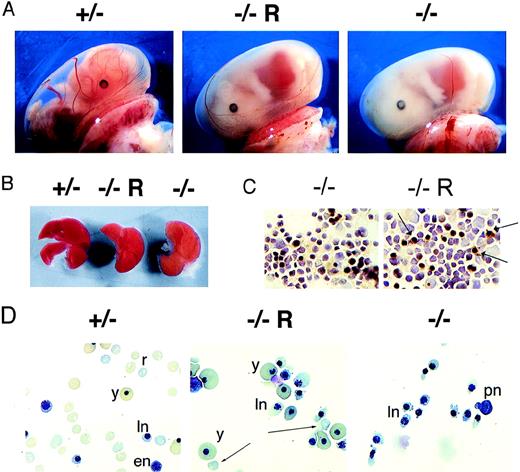
Surprisingly, there were no live-born EKLF−/−Aγ+ animals of the 98 live-born mice generated from an F2 cross of EKLF+/− Aγ+ animals. Therefore, litters were analyzed at E11 to E17 to determine whether there was any improvement in the severity of anemia in EKLF−/− embryos afforded by the Aγ transgene. At E15 there were 2 apparent degrees of pallor in the litters. One set of animals displayed pallor typical of EKLF−/−embryos, and the other set had slightly less pallor (Figure3A). Southern blot analysis of carcass DNA subsequently revealed that the pinker animals contained the Aγ transgene. The fetal livers of these EKLF−/−Aγ+ embryos were also slightly more crimson than those of the EKLF−/− Aγ− litter mates, but not as crimson as those of the wild-type embryos (Figure3B). Furthermore, o-dianisidine staining of fetal liver erythrocytes revealed a slight improvement in the presence of the Aγ transgene (Figure 3C). Taken together, these results indicated that there was slight improvement in the production of hemoglobin in EKLF−/− fetal liver erythrocytes that expressed high levels of γ-globin; this was consistent with the formation of hybrid mouse–human hemoglobin.
Improvement in hemoglobinization but persistent hemolysis in EKLF−/− embryos.
(A) Photographs of a litter of E15 embryos (magnification ×10). The EKLF genotype (± and −/− ) and the presence of the transgene (R) are indicated above each photograph. (B) Slight improvement in the crimson hue of an EKLF−/−fetal liver that harbors the μLCR− Aγ+transgene (−/−R) compared to 1 that does not (−/−). (C) Slight improvement in the benzidine staining of EKLF−/− fetal liver cells that harbor the transgene (−/−R) compared with those that do not (−/−). Arrows indicate benzidine-positive cells. (D) May-Gruenwald-Giemsa (MGG) stained cytocentrifuge specimens of the blood from the same 3 embryos depicted in A indicating some rescue in hemoglobinization of fetal liver-derived erythroid cells. However, morphologically abnormal nucleated erythrocytes persist. y, yolk sac derived nucleated red cells; PN, pronormoblast; en, early normoblast; ln, late normoblast; r, fetal liver-derived enucleate red cell. Arrows indicate occasional enucleate red cells in EKLF−/− , μLCR−Aγ + fetal liver samples.
Improvement in hemoglobinization but persistent hemolysis in EKLF−/− embryos.
(A) Photographs of a litter of E15 embryos (magnification ×10). The EKLF genotype (± and −/− ) and the presence of the transgene (R) are indicated above each photograph. (B) Slight improvement in the crimson hue of an EKLF−/−fetal liver that harbors the μLCR− Aγ+transgene (−/−R) compared to 1 that does not (−/−). (C) Slight improvement in the benzidine staining of EKLF−/− fetal liver cells that harbor the transgene (−/−R) compared with those that do not (−/−). Arrows indicate benzidine-positive cells. (D) May-Gruenwald-Giemsa (MGG) stained cytocentrifuge specimens of the blood from the same 3 embryos depicted in A indicating some rescue in hemoglobinization of fetal liver-derived erythroid cells. However, morphologically abnormal nucleated erythrocytes persist. y, yolk sac derived nucleated red cells; PN, pronormoblast; en, early normoblast; ln, late normoblast; r, fetal liver-derived enucleate red cell. Arrows indicate occasional enucleate red cells in EKLF−/− , μLCR−Aγ + fetal liver samples.
Persistent hemolysis in EKLF−/−embryos containing the Aγ transgene
Despite improvement in hemoglobinization, there was minimal, if any, improvement in the survival of EKLF−/−Aγ+ embryos compared with their EKLF−/− Aγ− litter mates. At E16, all EKLF−/− Aγ+ embryos (n = 2) and EKLF−/− Aγ−embryos (n = 3) were dead, whereas all (n = 13) EKLF+/+and EKLF+/− litter mates were alive irrespective of the presence or absence of the transgene (Table1).
Influence of the −201 Aγ transgene on the survival of EKLF−/− animals
| Litter age* . | Genotype† . | |||
|---|---|---|---|---|
| EKLF−/−Aγ− . | EKLF−/−Aγ+ . | |||
| Alive . | Dead . | Alive . | Dead . | |
| E14 | 1 | 0 | 0 | 0 |
| E14 | 1 | 0 | 2 | 0 |
| E15 | 0 | 1 | 1 | 0 |
| E15 | 1 | 0 | 2 | 0 |
| E15 | 1 | 0 | 1 | 0 |
| E15.5 | 0 | 2 | 1 | 0 |
| E15.5 | 0 | 0 | 0 | 1 |
| E16 | 0 | 2 | 0 | 1 |
| E16 | 0 | 1 | 0 | 1 |
| Litter age* . | Genotype† . | |||
|---|---|---|---|---|
| EKLF−/−Aγ− . | EKLF−/−Aγ+ . | |||
| Alive . | Dead . | Alive . | Dead . | |
| E14 | 1 | 0 | 0 | 0 |
| E14 | 1 | 0 | 2 | 0 |
| E15 | 0 | 1 | 1 | 0 |
| E15 | 1 | 0 | 2 | 0 |
| E15 | 1 | 0 | 1 | 0 |
| E15.5 | 0 | 2 | 1 | 0 |
| E15.5 | 0 | 0 | 0 | 1 |
| E16 | 0 | 2 | 0 | 1 |
| E16 | 0 | 1 | 0 | 1 |
The morning of vaginal plug discovery was designated E0.
Ag+/+ and Aγ+/− animals have been grouped together at Aγ+.
In addition, the peripheral blood of EKLF−/−Aγ+ embryos was similarly abnormal compared with that of EKLF−/− Aγ− litter mates. There were few enucleated fetal liver–derived erythrocytes in EKLF−/− Aγ+ embryos, whereas these account for more than 80% of circulating cells in EKLF+/− litter mates by E15.5 Rather, the circulating cells were predominantly nucleated with dyserythropoietic, poorly hemoglobinated cytoplasm, though a few cells had pinker cytoplasm than cells in EKLF−/−Aγ− litter mates (Figure 3D). Furthermore, there was still a marked increase in fetal liver iron deposition (data not shown), consistent with persistent red cell destruction or ineffective erythropoiesis in the fetal livers of EKLF−/−Aγ+ embryos.
Finally, there was no significant improvement in hemoglobin and hematocrit values of EKLF−/− Aγ+embryos compared with their EKLF−/−Aγ− litter mates (Table2). We conclude that there was no significant improvement in hemolysis or survival of EKLF−/− embryos afforded by the Aγ transgene. In view of the marked benefit even the moderate expression of γ-globin produced in humans with β-thalassemia, we propose that the reduced expression of nonglobin EKLF target genes must play a major role in the lethal EKLF−/− phenotype.
Hematology of litters killed at E14 and E15
| Genotype . | Hemoglobin (gm/L) . | Hematocrit (%) . |
|---|---|---|
| EKLF−/− Aγ+ (n = 6) | 1.9 ± 0.4* | 12 ± 3.2† |
| EKLF−/−Aγ− (n = 4) | 1.7 ± 0.4* | 11 ± 4.5† |
| EKLF+/− Aγ+ (n = 9) | 9.8 ± 0.8 | 48 ± 12.2 |
| EKLF+/− Aγ−(n = 10) | 9.4 ± 0.6 | 47 ± 9.2 |
| Genotype . | Hemoglobin (gm/L) . | Hematocrit (%) . |
|---|---|---|
| EKLF−/− Aγ+ (n = 6) | 1.9 ± 0.4* | 12 ± 3.2† |
| EKLF−/−Aγ− (n = 4) | 1.7 ± 0.4* | 11 ± 4.5† |
| EKLF+/− Aγ+ (n = 9) | 9.8 ± 0.8 | 48 ± 12.2 |
| EKLF+/− Aγ−(n = 10) | 9.4 ± 0.6 | 47 ± 9.2 |
The differences in hemoglobin (*) and hematocrit (
) between EKLF−/− Aγ+ and Aγ−embryos were not statistically significant (P > .1 by Student t test). Again, Aγ+/+ and Aγ+/− animals have been grouped together at Aγ.
Discussion
We found no significant improvement in red blood cell morphology, level of anemia, or survival in EKLF−/− mice in which the globin chain imbalance was significantly reversed by transgene-derived γ-globin chain synthesis. Thus, we concluded that EKLF target genes other than β-globin alone must play a crucial role in fetal liver erythropoiesis. At first glance, this conclusion appears to oppose that of Lim et al,21 who found that reintroduction of an LCR-γ-globin gene into EKLF−/− ES cells rescues contribution to circulating erythroid cells in chimeric animals. A close examination of the published data suggested that the level of EKLF−/− ES cell contribution to the blood was still significantly less than the contribution to other tissues. Thus, EKLF−/− Aγ-globin–expressing erythroid cells may also be partially defective in these chimeric mice. The 2 attempts to rescue the EKLF null phenotype (that described here and that of Lim et al21) have some important experimental design differences. First, there may be certain EKLF target genes that have critical role(s) only at the fetal liver stage of erythropoiesis. The chimeras could survive this developmental phase by virtue of blood production from non–ES cell-derived fetal liver stem cells, whereas the EKLF−/− embryos described in this article could not. Alternatively, wild-type stem cells, microenvironment components, or both within the adult bone marrow of chimeric animals may somehow nurture EKLF−/− erythroid cells. That is, there may be a non-cell-autonomous component to the observed survival of EKLF−/− Aγ+ erythroid cells in chimeric animals21 unavailable to erythroid cells within the fetal livers of the EKLF−/−Aγ+ mice.
The nature of alternative EKLF target genes remains undetermined. Genes encoding other globin chains, heme biosynthetic enzymes, glycolysis pathway enzymes, other transcription factors, transmembrane proteins, and cytoskeletal proteins all have CACC box elements in their promoters.9,22 Many fall within the consensus site for EKLF (NCN-CNC-CCN) predicted by the crystal structure of the related protein, Zif268, bound to DNA.3 However, it remains to be tested whether EKLF binds equally well to all such CACC box elements or just to a subset of them. A detailed examination of the spectrum of CACC sequences able to bind purified recombinant EKLF would be helpful in the resolution of the precise EKLF binding-site preferences. In short, it is difficult to be sure whether any of the erythroid promoters with important CACC box elements actually binds EKLF in vivo.
One approach to the problem of alternative EKLF target genes is to examine the expression of candidate genes in fetal liver cells derived from EKLF−/− embryos. We have previously examined the expression of GATA-1, EpoR, PBGD, βh1, and murine ε-y globin genes in EKLF−/− fetal liver cells and found them all to be EKLF independent.5 A possible explanation is that other Kruppel-like factors are able to function at many of these promoters in vivo. This may reflect the inability of EKLF to bind to these sites in vivo (a promoter context argument), or it may indicate that similar Kruppel-like factors, such as basic Kruppel-like factor (BKLF),23 can bind these sites and compensate for the loss of EKLF (a redundancy argument). These alternatives might be resolved by studying mice null for both EKLF and BKLF. A similar dilemma exists for GATA-1 null embryos, which also die from anemia.24 It has been suggested that GATA-2 may substitute for GATA-1 at many erythroid gene promoters.
One potentially important EKLF target gene is amino-levulinic acid synthetase (ALAS), the rate-limiting enzyme of the heme biosynthesis pathway. There are 2 overlapping CACC elements in the erythroid specific ALAS promoter at −49/−39,25 with an identical sequence (albeit on the reverse strand) to that found in the human β-globin promoter. This site binds EKLF in vitro, and the ALAS promoter is activated by EKLF in transient assays in a CACC element-dependent fashion.25 It remains to be determined whether EKLF is required for ALAS expression in fetal liver cells of developing embryos. Interestingly, ALAS null ES cells display a defect in erythroid cell maturation,26 and a defect in ALAS is responsible for the anemic zebrafish mutant,sautern.27 Nevertheless, it is likely that a significant amount of heme is produced in definitive and embryonic erythroid cells in the absence of EKLF. Although we did not make any direct measurements of heme, the positive staining for o-dianisidine suggests the presence of an intact heme moiety. Thus, we suggest that ALAS does not require EKLF for expression in vivo or that sufficient enzyme is produced in EKLF−/− erythroid cells from reduced mRNA levels to permit adequate heme production rates.
Erythroid cytoskeletal genes may also be dependent on EKLF for expression. Moreover, their reduced expression may contribute to the EKLF null phenotype. The promoters for many of the erythroid cytoskeletal proteins, including band 4.1, band 3, ankyrin, α- and β-spectrin, and band 7.1 have been cloned and sequenced. Many have GC-rich elements in their promoters, but few fit perfectly with the proposed EKLF consensus. To date, none of these genes has been tested for their dependence on EKLF for expression in vivo or in transient assays. Many have been disrupted in ES cells by homologous recombination, and others occur as spontaneous mouse mutants.28 Mice null for band 4.1, ankyrin, β-spectrin, α-spectrin all have hemolytic anemia of varying severity (reviewed in27,28). The most severe anemia occurs in mice with defective β-spectrin (ja/ja mice), with approximately 90% dying in the first 2 weeks of life.29 In all cases the phenotypes are milder that the EKLF null phenotype. Nevertheless, it remains possible that defective expression of multiple red cell cytoskeletal proteins could result in a severely anemic phenotype such as that present in EKLF−/− embryos.
When considering potential EKLF target genes, it is helpful to remember that EKLF−/− embryonic red cells are morphologically normal or nearly so. Thus, it may be reasonable to consider those genes that are selectively expressed in definitive cells as more likely candidates for EKLF target genes. One of the major differences between embryonic and definitive red cells is that definitive red cells extrude their nuclei. It is striking that most EKLF−/− erythroid fetal liver cells remain nucleated even in the presence of high levels of γ-globin (Figure 3). We originally suggested this was secondary to erythroid stress, but it remains possible that EKLF coordinates the process of enucleation itself.
Embryonic and definitive erythroid cells also differ in their metabolism. Definitive erythroid cells rely almost exclusively on anaerobic metabolism for the production of adenosine triphosphate from glucose. They are also dependent on the pentose-phosphate pathway for the generation of reducing power in the form of NADPH. Enzymes of the Embden–Myerhoff glycolysis pathway are certainly important for the metabolic function of definitive human erythroid cells. Defects in pyruvate kinase (PK), the rate-limiting enzyme of glycolysis, are common in humans and result in chronic hemolytic anemia. Like many erythroid genes, the PK gene contains closely associated CACC and GATA motifs in the proximal promoter. The CACC site is required for full expression in transient transfection assays and it could bind EKLF, though this remains to be tested. Additionally, an element 3.7 kb upstream of the transcriptional start site of the PK gene has erythroid-specific enhancer activity in transgenic mice and harbors a duplicated CACC element that fits well with the EKLF consensus.30 More work must be done to determine whether PK is truly EKLF dependent in vivo.
To make further progress in the identification of putative EKLF target genes, a cell-based functional assay would be helpful. For example, an immortalized EKLF−/− erythroid cell line would allow conditional reintroduction of EKLF and subtractive approaches to target gene discovery. Candidate target genes could also be examined in such a system. Our results suggest that attempts to reactivate γ-globin in adults that harbor mutations in the β-globin gene through antagonism of EKLF carry a significant risk for inducing additional defects in red blood cell function.
Acknowledgments
We thank Carlo Brugnara and Vivian Smith for hemoglobin electrophoresis. We also thank Bing Chuen-Lau and John Kim for expert technical assistance, Klar Kleman for hemoglobin isolation by isoelectric focusing, and Cedric Shackleton for his support in mass spectrometric studies. We thank Y. Fujiwara and K. Cunniff for help with animal husbandry and Elise Coghill for critical appraisal of this manuscript.
Supported by a Special Research Fellowship (3172-97) from The Leukemia Society of America and by a National Institutes of Health Northern California Comprehensive Sickle Cell grant (HL20895). The VG BioQ mass spectrometer was purchased through a National Institutes of Health Shared Instrumentation Program grant (RR06505).
Reprints:Stuart H. Orkin, Children's Hospital, Division of Hematology/Oncology, 300 Longwood Avenue, Boston, MA 02115; e-mail: orkin@rascal.med.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal