Leukemia relapse is a major cause of treatment failure for patients with acute leukemia given allogeneic bone marrow transplantation (BMT). This study evaluated whether a reduction of the dosage of cyclosporine-A (Cs-A) used for graft versus host disease (GVHD) prophylaxis could reduce relapse rate (RR) in children with acute leukemia given BMT. Fifty-nine children who had transplantation from HLA-identical siblings were randomized to receive Cs-A intravenously at a dosage of 1 mg/kg/d (Cs-A1) or of 3 mg/kg/d (Cs-A3) until patients were able to tolerate oral intake. Subsequently, both groups received Cs-A orally at a dosage of 6 mg/kg/d, with discontinuation 5 months after BMT. The probability of developing grade II-IV acute GVHD was 57% for the Cs-A1 group versus 38% for the Cs-A3 group (P = .06); the probability of developing chronic GVHD was 30% for the Cs-A1 group and 26% for the Cs-A3 group (P = NS). Three patients died of grade IV acute GVHD: 2 were in the Cs-A1 and the third in the Cs-A3 group. The RR was 15% for the Cs-A1 group and 41% for the Cs-A3 group (P = .034); 1-year transplant-related mortality estimates were 17% and 7%, respectively (P = NS). With a median observation time of 44 months from BMT, the 5-year event-free survival for children belonging to Cs-A1 and Cs-A3 groups was 70% and 51%, respectively (P = .15). Our data demonstrate that the use of low Cs-A doses is associated with a statistically significant reduction of leukemia relapse, probably due to an increased graft versus leukemia effect.
Allogeneic bone marrow transplantation (BMT) is an established treatment modality for patients with acute leukemia. Unfortunately, the success of BMT is offset by the risk of leukemia relapse and of death for transplant-related causes, mainly graft versus host disease (GVHD), infectious complications, and toxicity of the conditioning regimen.
Acute GVHD is a common immunologic complication that occurs in 40% to 50% of allogeneic BMT recipients during the early posttransplant period; chronic GVHD can be observed in 30% to 60% of long-term survivors.1,2 Even though GVHD remains a major contributor to both early and late mortality and morbidity after BMT,2-5 large retrospective trials have demonstrated lower relapse rates in patients who developed acute1,6-9 or chronic GVHD (or both).9-12 In fact, as extensively demonstrated in animal models, donor immunocompetent cells contribute to the antileukemic efficacy of BMT through the so-called graft versus leukemia (GVL) reaction.13,14 Further clinical evidence supporting the relevance of GVL effect in the eradication of malignant cells escaping the preparative regimen includes a higher incidence of leukemia relapse observed after syngeneic or T-cell–depleted transplants.8
This said, it is not surprising that, to reduce the risk of relapse, several efforts have been made to identify, in patients with leukemia, the best strategy of GVHD prevention, able to avoid the occurrence of severe GVHD, but sparing the GVL reaction.
So far, cyclosporine-A (Cs-A) has been the most commonly used agent for GVHD prophylaxis after allogeneic BMT and, alone or in combination with methotrexate, it is included in the GVHD prophylaxis schedule of more than 70% of transplant recipients.15 The introduction of Cs-A in the clinical practice has resulted in better prevention of GVHD, even though a relevant proportion of patients still experience this complication. On the other hand, because of its immunomodulating action on donor T cells, Cs-A could impair the GVL effect.
In this regard, Bacigalupo et al demonstrated in a randomized trial performed in adults that patients who received Cs-A at a dosage of 1 mg/kg/d had a significant reduction of relapse rate (RR) and, despite an increased GVHD incidence, a better leukemia-free survival, as compared to those who received Cs-A at the dose of 5 mg/kg/d.16
On the basis of this experience and considering the reported low GVHD incidence in childhood,1 we evaluated in a multicenter, randomized, prospective clinical trial whether, in children with acute leukemia given an allogeneic BMT from an HLA-compatible sibling, the use of Cs-A at a dose of 1 mg/kg/d could be associated with a lower risk of leukemia relapse and a better event-free survival (EFS), in comparison to children treated with Cs-A at a higher dose (3 mg/kg/d).
Patients and methods
Study design
The study was designed as a prospective, multicenter, centrally randomized trial aimed at evaluating the impact of 2 different Cs-A dosages for acute GVHD prophylaxis on the clinical outcome of children with leukemia given allogeneic BMT from an HLA-identical sibling. The major end point of the study was to determine whether the use of low-dose Cs-A (1 mg/kg/d) could reduce RR as compared to standard-dose Cs-A (3 mg/kg/d). Secondary end points were the occurrence of acute and chronic GVHD, transplant-related mortality (TRM), and EFS in the 2 groups.
Eligibility criteria for patients to be enrolled in the study were age between 1 and 18 years, a diagnosis of acute lymphocytic leukemia (ALL) in first, second, or third complete remission (CR) or of acute myelogenous (AML) in first or second CR, the availability of an HLA-genotypically identical sibling, and a written informed consent from the parents.
To calculate the number of patients to be enrolled in this study a 2-sided sample size evaluation method based on the log-rank test was used. Considering a study significance level of 0.05 and a study power of 0.80 and supposing an estimated probability of RR at 18 months of 0.10 for the first arm and 0.40 for the second arm of the study, the required number of cases resulted in 28 patients per arm with an accrual time of 24 months and an overall study duration of 48 months. To monitor the results of the trial, an interim analysis was performed 1 year after study beginning and, subsequently, every year. It was decided that patient enrollment should be closed when the difference in RR between the 2 arms reached a P value of .01.
Randomization was centralized at CINECA (North East Inter-University Italian Computing Center, Bologna, Italy) by one of the investigators (R.R.), who was not involved in the clinical management of the patients, and performed using a 1:1 allocation ratio. Patients were stratified according to diagnosis (ALL or AML) and disease phase (first CR versus more advanced disease). The analysis was based on the intention to treat principle. The institutions participating in the study were not blinded as to Cs-A dose.
Treatment protocol
Pretransplant preparative regimens were assigned according to the institutional protocols at each clinical site, on the basis of the underlying disease, and on the age of the recipient. Supportive care was standardized within each center and uniformly applied to the patients in both groups. Usually, empirical broad-spectrum antibiotic therapy was started when children became febrile, and antifungal therapy was used in the presence of either clinical evidence of fungal infection or fever persisting after 3 days of antibiotic therapy. As prophylaxis for Pneumocystis carinii pneumonia, patients received oral cotrimoxazole, starting from the day of engraftment. To reduce the risk of infections, children received a commercial immunoglobulin preparation intravenously at a dosage of 400 mg/kg every week, starting the week before transplantation and ending 3 months after the allograft. Cytomegalovirus (CMV) serologic status was studied before transplantation in all children and in their donors. Forty of the 59 BMT recipients studied were seropositive, as well as 35 of the 59 donors. The expression of pp65 human CMV matrix protein was monitored in all patients to detect CMV reactivation.17 Patients experiencing CMV reactivation were usually treated with ganciclovir or foscarnet at conventional doses.
The day of transplantation was designated as day 0. Between day −7 and −3 before BMT, patients were randomized to receive Cs-A at a dosage of 1 mg/kg/d (Cs-A1 group) or 3 mg/kg/d (Cs-A3 group) from day −1. The assigned dose of Cs-A was administered intravenously in 2 divided 2-hour infusions. Subsequently, when patients were able to tolerate oral intake, they received Cs-A orally at a dosage of 6 mg/kg/d in 2 divided doses, with a gradual reduction until discontinuation, in absence of chronic GVHD, 5 months after BMT. No other agent was used for GVHD prophylaxis.
Dose modifications of Cs-A were allowed in presence of serum creatinine levels more than 2 times baseline, whereas Cs-A whole blood levels did not influence the assigned drug dose. Moreover, physicians were free to increase the dose of Cs-A for patients experiencing acute GVHD if this was considered indicated.
At each participating center, the development of acute and chronic GVHD was monitored throughout the study and graded by a single investigator, blinded to the randomization arm of the patients. Tissue biopsy samples were obtained to confirm the diagnosis of GVHD whenever clinically indicated and feasible. Treatment of acute and chronic GVHD was administered according to the protocols in use at each institution.
Patient demographics and characteristics
From May 1993 to May 1996, 59 patients (33 males and 26 females) were centrally randomized and assigned to one of the treatment arms. Patients were treated in 7 Italian pediatric BMT centers participating in AIEOP (Italian Association of Pediatric Hematology/Oncology) BMT group and listed in the appendix. All subjects randomized underwent BMT from an HLA-identical sibling and were evaluated in the data analysis.
HLA class I and II antigen serologic typing of donors and recipients was performed by standard National Institutes of Health microlymphocytotoxicity; low-resolution generic oligotyping was used to confirm the DRB1 identity in 38 donor/recipient pairs.
At time of BMT, patients' median age was 8 years (range 1-18 years). Forty-seven of the 59 patients enrolled had ALL; 15 children were transplanted in first CR, 28 in second CR, and the remaining 4 in third CR. Twelve patients had AML; 9 were given BMT in first CR, and the remaining 3 in second CR.
Karyotype analysis at diagnosis was available in 48 of the 59 children (84%). For the purpose of this study, ALL patients with hyperdiploid karyotype and AML patients with inversion of chromosome 16, translocation 8;21 and 15;17 were classified as having good prognosis (7 cases). Chromosomal abnormalities classified as poor prognosis features (7 cases) included ALL with translocation 9;22 and 4;11, as well as AML with monosomy of chromosomes 5, 7, anomalies of 11q or translocation 6;9. Patients with normal karyotype, t12;21 or other cytogenetic abnormalities, as well as those with unavailable cytogenetic data, were assigned to an intermediate risk category (45 cases, Table 1).
Clinical characteristics of the patients belonging to the 2 arms of the study
| . | Cs-A Dose . | P Value . | |
|---|---|---|---|
| 1 mg/kg/d . | 3 mg/kg/d . | ||
| Cs-A1 group . | Cs-A3 group . | ||
| No. of patients | 30 | 29 | |
| ALL | 24 | 23 | |
| 1st CR | 10 | 5 | |
| 2nd CR | 13 | 15 | NS |
| 3rd CR | 1 | 3 | |
| AML | 6 | 6 | |
| 1st CR | 6 | 3 | NS |
| 2nd CR | 0 | 3 | |
| ALL immunophenotype | |||
| Common | 15 | 13 | |
| Pre-B | 3 | 5 | |
| B | 0 | 1 | NS |
| Early-T | 4 | 2 | |
| T | 2 | 2 | |
| AML FAB | |||
| M0 | 1 | 0 | |
| M1 | 0 | 2 | |
| M3 | 1 | 2 | NS |
| M4 | 1 | 0 | |
| M5 | 2 | 2 | |
| M6 | 1 | 0 | |
| WBC at diagnosis (× 109/L) | 38 (0.5-532) | 24 (0.9-750) | NS |
| Cytogenetics | |||
| Unfavorable | 5 | 2 | |
| Intermediate | 21 | 24 | NS |
| Favorable | 4 | 3 | |
| Extramedullary disease at diagnosis (Yes/No) | 4 | 3 | NS |
| Interval diagnosis—1st CR (d) | 41 (15-126) | 42 (13-97) | NS |
| Interval last CR—BMT (mo) | 3 (1-11) | 2.5 (0.5-13) | NS |
| First relapse | |||
| On therapy | 4 | 6 | NS |
| Off therapy | 10 | 15 | |
| Recipient | |||
| Sex (M/F) | 15/15 | 18/11 | NS |
| Age at diagnosis (years) | 4 (0.5-16) | 7 (1.4-14) | NS |
| Age at BMT (years) | 7 (0.9-17) | 9 (1.8-18) | NS |
| Donor | |||
| Sex (M/F) | 16/14 | 17/12 | NS |
| Age (years) | 11 (1-21) | 7 (1-25) | NS |
| Sex match | 17 | 14 | |
| Sex mismatch | |||
| M → F | 7 | 7 | NS |
| F → M | 6 | 8 | |
| Conditioning regimen | |||
| TBI + Thio + Cy | 14 | 15 | |
| TBI + VCR + Cy | 7 | 7 | NS |
| Bu + Cy + Melphalan | 9 | 7 | |
| Number of cells infused (× 108/Kg) | 3.4 (0.3-7.6) | 3.2 (0.2-9.0) | NS |
| G-CSF after BMT (yes/no) | 25/5 | 20/9 | NS |
| . | Cs-A Dose . | P Value . | |
|---|---|---|---|
| 1 mg/kg/d . | 3 mg/kg/d . | ||
| Cs-A1 group . | Cs-A3 group . | ||
| No. of patients | 30 | 29 | |
| ALL | 24 | 23 | |
| 1st CR | 10 | 5 | |
| 2nd CR | 13 | 15 | NS |
| 3rd CR | 1 | 3 | |
| AML | 6 | 6 | |
| 1st CR | 6 | 3 | NS |
| 2nd CR | 0 | 3 | |
| ALL immunophenotype | |||
| Common | 15 | 13 | |
| Pre-B | 3 | 5 | |
| B | 0 | 1 | NS |
| Early-T | 4 | 2 | |
| T | 2 | 2 | |
| AML FAB | |||
| M0 | 1 | 0 | |
| M1 | 0 | 2 | |
| M3 | 1 | 2 | NS |
| M4 | 1 | 0 | |
| M5 | 2 | 2 | |
| M6 | 1 | 0 | |
| WBC at diagnosis (× 109/L) | 38 (0.5-532) | 24 (0.9-750) | NS |
| Cytogenetics | |||
| Unfavorable | 5 | 2 | |
| Intermediate | 21 | 24 | NS |
| Favorable | 4 | 3 | |
| Extramedullary disease at diagnosis (Yes/No) | 4 | 3 | NS |
| Interval diagnosis—1st CR (d) | 41 (15-126) | 42 (13-97) | NS |
| Interval last CR—BMT (mo) | 3 (1-11) | 2.5 (0.5-13) | NS |
| First relapse | |||
| On therapy | 4 | 6 | NS |
| Off therapy | 10 | 15 | |
| Recipient | |||
| Sex (M/F) | 15/15 | 18/11 | NS |
| Age at diagnosis (years) | 4 (0.5-16) | 7 (1.4-14) | NS |
| Age at BMT (years) | 7 (0.9-17) | 9 (1.8-18) | NS |
| Donor | |||
| Sex (M/F) | 16/14 | 17/12 | NS |
| Age (years) | 11 (1-21) | 7 (1-25) | NS |
| Sex match | 17 | 14 | |
| Sex mismatch | |||
| M → F | 7 | 7 | NS |
| F → M | 6 | 8 | |
| Conditioning regimen | |||
| TBI + Thio + Cy | 14 | 15 | |
| TBI + VCR + Cy | 7 | 7 | NS |
| Bu + Cy + Melphalan | 9 | 7 | |
| Number of cells infused (× 108/Kg) | 3.4 (0.3-7.6) | 3.2 (0.2-9.0) | NS |
| G-CSF after BMT (yes/no) | 25/5 | 20/9 | NS |
Data are expressed as median and range. P values were calculated using Mann-Whitney rank-sum test, Student's t test, χ2 test, or Fisher exact test, as appropriate. TBI indicates total-body irradiation; Thio, thiotepa; Cy, cyclophosphamide; VCR, vincristine; Bu, busulfan; G-CSF, granulocyte-colony stimulating factor; WBC, white blood cell count.
Pretransplant conditioning regimen consisted of: (1) fractionated total body irradiation (TBI, 1200 cGy in 6 fractions over 3 days), thiotepa (10 mg/kg in 2 divided doses), and cyclophosphamide (60 mg/kg/d for 2 days) in 29 cases; (2) fractionated TBI, vincristine (1.5 mg/m2 intravenously followed by 2.5 mg/m2continuous infusion over 5 days), and cyclophosphamide (1800 mg/m2/d for 2 days) in 14 cases; and (3) busulfan (16 mg/kg in 16 divided doses over 4 days), cyclophosphamide (60 mg/kg/d for 2 days), and melphalan (140 mg/m2 in a single dose) in the remaining 16 cases. All patients received an unmanipulated marrow inoculum.
Further details on the characteristics of the patients and donors, pretransplant disease history, and comparison between the 2 arms of the study are reported in Table 1. The 2 groups were comparable for all the variables studied.
Definitions
Myeloid and platelet engraftments were defined as the first of 3 consecutive days with neutrophils more than 0.5 × 109/L and unsupported platelets more than 50 × 109/L, respectively. Patients were considered assessable for engraftment if they survived at least 7 days after the transplant.
Acute and chronic GVHD were classified according to previously published criteria.18-20 Children with sustained donor engraftment and surviving more than 14 days and more than 100 days after the transplant were assessable for occurrence and severity of acute and chronic GVHD, respectively.
Statistical analysis
Data were analyzed as of August 31, 1998. EFS, TRM, RR, GVHD occurrence, as well as neutrophil and platelet engraftment curves after transplantation (starting point), were calculated by the Kaplan-Meier method23 and compared using the log-rank test. In the EFS analysis, both relapse and death in remission due to any cause were considered treatment failures, whereas in the RR analysis, only disease relapse was considered failure. In the TRM analysis, all deaths not due to leukemia recurrence were considered failures. Results were expressed as probability (%) and 95% confidence intervals (95% CI). A multivariate Cox proportional hazard regression model24 was also used separately for TRM, RR, and EFS, with the following variables: diagnosis, disease status, donor sex and age, recipient sex and age, Cs-A dose, and acute GVHD and chronic GVHD occurrence. Student's t test and Mann-Whitney rank-sum test were used to compare differences in continuous variables between groups, and χ2 test or Fisher exact test were used to compare percentages, as appropriate. All P values were 2-sided and a value less than .05 was considered statistically significant. Pvalues more than .1 were reported as not significant (NS), whereas those between .05 and .1 were reported in detail. The SAS package (SAS Institute, Cary, NC) was used for the analysis of the data.
Results
The median follow-up for Cs-A1 and Cs-A3 group patients was 44 months (range 21-66) and 43 months (range 23-63) for survivors (P = NS), and 9 months (range 1-20) and 8 months (range 1-40) for deceased patients (P = NS), respectively. Cs-A was administered intravenously for a median of 17 days (range 6-45 days) to patients belonging to the Cs-A1 group and for a median of 18 days (range 11-62 days) to patients of the Cs-A3 group (P = NS).
Engraftment
Marrow engraftment was observed in all patients enrolled in the study. Neutrophil recovery was achieved within 3 weeks in all children. The median time for myeloid engraftment was 11 days (range 8-22 days) and 10 days (range 7-19) for children belonging to the Cs-A1 and Cs-A3 group, respectively (P = NS). Platelet engraftment was reached in 55 of the 59 patients analyzed, the remaining 4 patients having died before platelet recovery. Median time for platelet engraftment was 23 days (range 12-159 days) in the Cs-A1 group and 22 days (range 10-203 days) in the Cs-A3 group, with no significant difference between the 2 arms.
GVHD
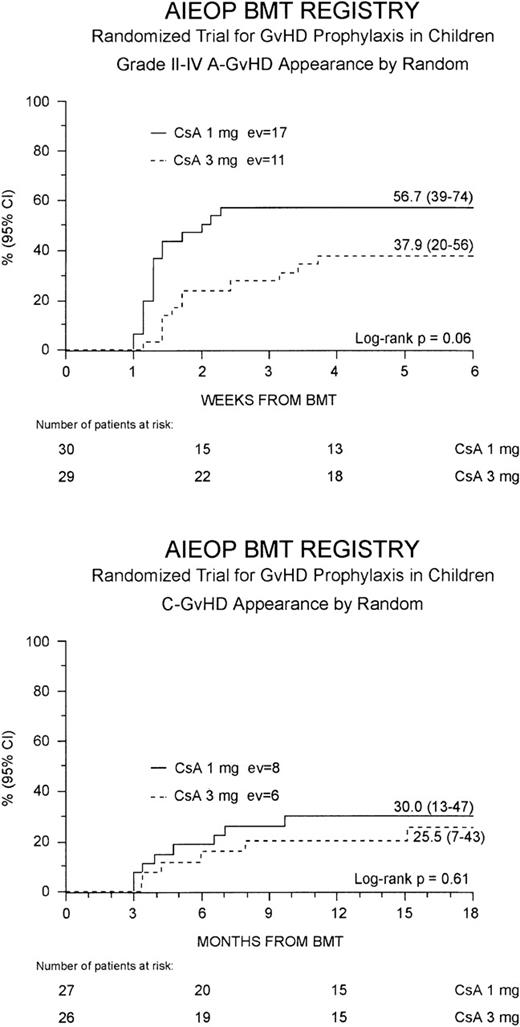
Grade II to IV acute GVHD developed in 28 (47%) of the 59 patients at a median of 10 days after the transplant (range 7-26 days). This resulted in a cumulative probability of 48% (35-60%) at 100 days after BMT. Children randomized to the Cs-A1 group had a greater cumulative probability of developing grade II to IV acute GVHD in comparison to those belonging to the Cs-A3 group: 57% (39-74%) versus 38% (20-56%), respectively, P = .06 (Figure1, top). Grade III to IV acute GVHD was observed in 12 children (20%), 8 belonging to the Cs-A1 group and 4 to the Cs-A3 group. The cumulative probability of developing grade III to IV acute GVHD was 27% (11-43%) for the Cs-A1 group, and 14% (1-26%) for the Cs-A3 group (P = NS). Median duration of grade II to IV acute GVHD in surviving patients was 21 days (range 4-56) in the Cs-A1 group and 26 days (range 5-74) in the Cs-A3 group (P = NS).
Development of GVHD.
The cumulative probability of developing grade II to IV acute GVHD (top) and chronic GVHD (bottom) for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line) is shown. EV = number of events occurring in each arm of randomization.
Development of GVHD.
The cumulative probability of developing grade II to IV acute GVHD (top) and chronic GVHD (bottom) for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line) is shown. EV = number of events occurring in each arm of randomization.
Table 2 shows the details on organ involvement by acute GVHD. The Cs-A1 group had a higher incidence of gastrointestinal (58% vs. 35%) and liver GVHD (42% vs. 17%), as well as a greater frequency of involvement of at least 2 (65% vs. 39%) or 3 (39% vs. 9%) organs. However, only for the last comparison (3-organ involvement) was the difference between the 2 arms statistically significant (P < .05).
GVHD incidence in the 2 groups
| . | Cs-A Dose . | P Value . | |
|---|---|---|---|
| 1 mg/kg/d . | 3 mg/kg/d . | ||
| Cs-A1 group . | Cs-A3 group . | ||
| Acute GVHD (59 evaluable patients) | |||
| Number of patients | 30 | 29 | |
| Absent | 4 (13.3%) | 6 (20.7%) | |
| Grade I | 9 (30%) | 12 (41.4%) | |
| Grade II | 9 (30%) | 7 (24.1%) | NS |
| Grade III | 4 (13.3%) | 2 (6.9%) | |
| Grade IV | 4 (13.3%) | 2 (6.9%) | |
| Organ involvement in acute GVHD (49 patients) | |||
| Number of patients | 26 | 23 | |
| Skin | 10 (38.5%) | 13 (56.5%) | NS |
| Intestine | 1 (4.3%) | NS | |
| Skin + intestine | 5 (19.2%) | 4 (17.4%) | NS |
| Skin + liver | 1 (3.8%) | 2 (8.7%) | NS |
| Intestine + liver | 1 (4.3%) | NS | |
| Skin + intestine + liver | 10 (38.5%) | 2 (8.7%) | .016 |
| Chronic GVHD (53 evaluable patients) | |||
| Number of patients | 27 | 26 | |
| Absent | 19 (70.4%) | 20 (76.9%) | |
| Limited | 6 (22.2%) | 5 (19.2%) | NS |
| Extensive | 2 (7.4%) | 1 (3.9%) | |
| . | Cs-A Dose . | P Value . | |
|---|---|---|---|
| 1 mg/kg/d . | 3 mg/kg/d . | ||
| Cs-A1 group . | Cs-A3 group . | ||
| Acute GVHD (59 evaluable patients) | |||
| Number of patients | 30 | 29 | |
| Absent | 4 (13.3%) | 6 (20.7%) | |
| Grade I | 9 (30%) | 12 (41.4%) | |
| Grade II | 9 (30%) | 7 (24.1%) | NS |
| Grade III | 4 (13.3%) | 2 (6.9%) | |
| Grade IV | 4 (13.3%) | 2 (6.9%) | |
| Organ involvement in acute GVHD (49 patients) | |||
| Number of patients | 26 | 23 | |
| Skin | 10 (38.5%) | 13 (56.5%) | NS |
| Intestine | 1 (4.3%) | NS | |
| Skin + intestine | 5 (19.2%) | 4 (17.4%) | NS |
| Skin + liver | 1 (3.8%) | 2 (8.7%) | NS |
| Intestine + liver | 1 (4.3%) | NS | |
| Skin + intestine + liver | 10 (38.5%) | 2 (8.7%) | .016 |
| Chronic GVHD (53 evaluable patients) | |||
| Number of patients | 27 | 26 | |
| Absent | 19 (70.4%) | 20 (76.9%) | |
| Limited | 6 (22.2%) | 5 (19.2%) | NS |
| Extensive | 2 (7.4%) | 1 (3.9%) | |
Three patients (5%) died due to grade IV acute GVHD on day +39, +40, and +42, respectively. The first 2 children belonged to the Cs-A1 group and the third to the Cs-A3 group.
First-line acute GVHD treatment consisted of corticosteroids in all cases. Median duration of steroid treatment in surviving patients was 30 days (range 9-253) for Cs-A1 patients and 22 days (range 6-270) for Cs-A3 patients (P = NS). Within the group of patients experiencing acute GVHD, 59% of children belonging to the Cs-A1 group, and 55% of those belonging to the Cs-A3 group increased Cs-A dosage. Second-line treatment with antilymphocyte globulin or monoclonal antibodies was started in the 3 children who subsequently died of acute GVHD and in 1 patient belonging to the Cs-A3 group, who recovered from grade III acute GVHD.
Chronic GVHD developed in 14 of the 53 assessable patients (26%). In all cases, chronic GVHD followed acute GVHD and no case of de novo disease was observed. The cumulative probability of developing chronic GVHD was 28% (15-41%), with a median time from BMT to chronic GVHD occurrence of 9 months (range 3-18 months). Eleven children, 6 in the Cs-A1 group and 5 in the Cs-A3 group, had limited skin chronic GVHD, and 3 children, 2 in the Cs-A1 group and 1 in the Cs-A3 group, had the extensive form of the disease (Table 2). The Kaplan-Meier estimate of chronic GVHD occurrence was 30% (13-47%) for the Cs-A1 group and 26% (7-43%) for the Cs-A3 group (P = NS; Figure1, bottom). Median Karnofsky score of patients with chronic GVHD was 90% (range 50-100%), with no difference between the 2 randomization arms.
The CMV serologic status of the patients and their donors did not have any influence on GVHD occurrence (data not shown).
Transplant-related morbidity and mortality
Regimen-related toxicity was mild or moderate and mainly limited to mucosal and gastrointestinal involvement. Details on organ involvement and severity, as well as comparisons between the 2 arms of the study, are listed in Table 3. No difference was observed in incidence and severity of toxicity between the 2 arms of the study.
Regimen-related toxicity
| . | Cs-A Dose . | P Value . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg/kg/d (n = 30) Toxicity Grade . | 3 mg/kg/d (n = 29) Toxicity Grade . | ||||||||||
| 0 . | 1 . | 2 . | 3 . | 4 . | 0 . | 1 . | 2 . | 3 . | 4 . | ||
| Heart | 28 | 1 | 1 | 28 | 1 | NS | |||||
| Liver | 25 | 1 | 3 | 1 | 21 | 5 | 3 | NS | |||
| GI | 14 | 11 | 5 | 12 | 14 | 3 | NS | ||||
| Mucosal | 2 | 12 | 15 | 1 | 2 | 9 | 17 | 1 | NS | ||
| Lung | 29 | 1 | 29 | NS | |||||||
| Kidney | 25 | 2 | 2 | 1 | 25 | 2 | 2 | NS | |||
| CNS | 27 | 1 | 2 | 26 | 2 | 1 | NS | ||||
| Bladder | 26 | 4 | 26 | 1 | 2 | NS | |||||
| . | Cs-A Dose . | P Value . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mg/kg/d (n = 30) Toxicity Grade . | 3 mg/kg/d (n = 29) Toxicity Grade . | ||||||||||
| 0 . | 1 . | 2 . | 3 . | 4 . | 0 . | 1 . | 2 . | 3 . | 4 . | ||
| Heart | 28 | 1 | 1 | 28 | 1 | NS | |||||
| Liver | 25 | 1 | 3 | 1 | 21 | 5 | 3 | NS | |||
| GI | 14 | 11 | 5 | 12 | 14 | 3 | NS | ||||
| Mucosal | 2 | 12 | 15 | 1 | 2 | 9 | 17 | 1 | NS | ||
| Lung | 29 | 1 | 29 | NS | |||||||
| Kidney | 25 | 2 | 2 | 1 | 25 | 2 | 2 | NS | |||
| CNS | 27 | 1 | 2 | 26 | 2 | 1 | NS | ||||
| Bladder | 26 | 4 | 26 | 1 | 2 | NS | |||||
On the whole, 8 patients died in remission, as a result of nonleukemic BMT-related causes, at a median of 2 months after transplantation (range 1-40 months). As mentioned above, acute GVHD was the major cause of death in 3 children, and fatal infectious complications were reported in the other 5 children. One child developed fatal P carinii penumonia, 1 had CMV interstitial pneumonia, and 1 had aspergillus pneumonia. Bacterial infections were the reported cause of death in the last 2 patients (Table 4).
Causes of death in the 2 groups
| . | Cs-A Dose . | |
|---|---|---|
| 1 mg/kg/d . | 3 mg/kg/d . | |
| Cs-A1 group . | Cs-A3 group . | |
| Disease Progression | 4 | 10 |
| Transplant-related causes | ||
| Acute GVHD | 2 | 1 |
| CMV interstitial pneumonia | 1 | |
| P carinii pneumonia | 1 | |
| Aspergillus pneumonia | 1 | |
| Bacterial infection | 1 | 1 |
| Total | 9 | 13 |
| . | Cs-A Dose . | |
|---|---|---|
| 1 mg/kg/d . | 3 mg/kg/d . | |
| Cs-A1 group . | Cs-A3 group . | |
| Disease Progression | 4 | 10 |
| Transplant-related causes | ||
| Acute GVHD | 2 | 1 |
| CMV interstitial pneumonia | 1 | |
| P carinii pneumonia | 1 | |
| Aspergillus pneumonia | 1 | |
| Bacterial infection | 1 | 1 |
| Total | 9 | 13 |
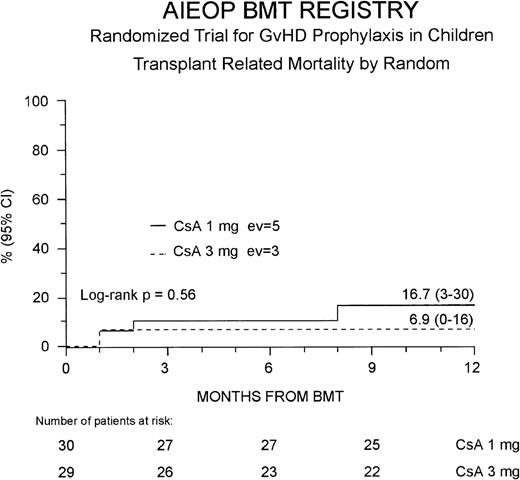
The 1-year TRM probability was 12% (4-21%) for the entire study population; it was 17% (3-30%) and 7% (0-16%) for the Cs-A1 and Cs-A3 groups, respectively (P = NS, Figure2). Because 1 more patient belonging to the Cs-A3 group died in remission 40 months after the transplant due to a bacterial infection, ultimately the 5-year probability of dying in remission was identical in the 2 groups (17% for the Cs-A1 group and 14% for the Cs-A3 group, respectively, P = NS).
Probability of transplant-related mortality.
The cumulative 1-year probability of TRM is shown for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line). EV = number of events occurring in each arm of randomization.
Probability of transplant-related mortality.
The cumulative 1-year probability of TRM is shown for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line). EV = number of events occurring in each arm of randomization.
Relapse probability
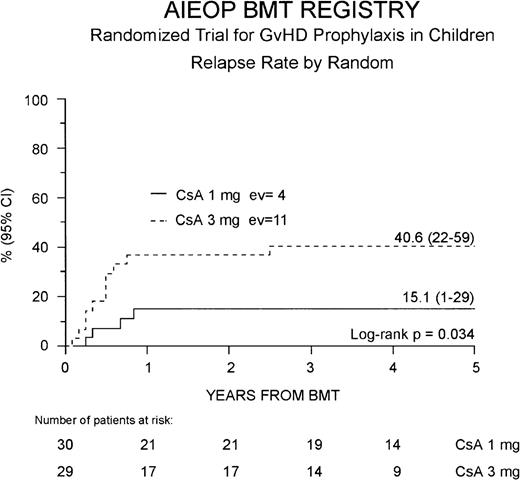
Fifteen of the 59 patients enrolled (25%) presented a leukemia relapse at a median of 6 months after BMT (range 1.6-31 months), and the overall cumulative RR for the entire cohort of patients enrolled in the study was 29% (16-41%). Four of the 15 relapsed patients were in the Cs-A1 group and 11 in the Cs-A3 group. Cumulative RR was 15% (1-29%) for the Cs-A1 group and 41% (22-59%) for the Cs-A3 group, respectively (P = .034, Figure3). No difference was observed in the time from BMT to relapse in the 2 randomization arms.
Probability of relapse.
Relapse probability is shown for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line). EV = number of events occurring in each arm of randomization.
Probability of relapse.
Relapse probability is shown for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line). EV = number of events occurring in each arm of randomization.
Fourteen of the 15 relapsed patients died due to disease progression; 1 child is alive with disease present (Table 4).
Survival and EFS
Thirty-seven (63%) of the 59 enrolled patients are surviving after BMT at a median of 44 months after the transplant (range 21-66 months). The 5-year Kaplan-Meier estimate of survival is 61% (47-74%) for the whole cohort of patients studied; it is 70% (54-86%) and 52% (33-71%), respectively, for the Cs-A1 and the Cs-A3 group (P = NS).
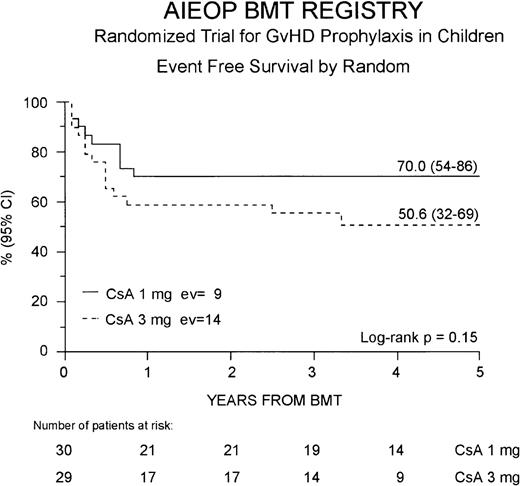
Thirty-six patients (61%) are alive in complete hematologic remission at a median observation time of 44 months from BMT (range 21-66 months). The cumulative EFS probability at 5 years for the entire study population is 59% (46-73%). Twenty-one (70%) of the 30 patients belonging to the Cs-A1 group are alive and disease-free, whereas in the Cs-A3 group, 15 (52%) of the 29 patients are alive in remission. The 5-year cumulative EFS probability is 70% (54-86%) for patients belonging to the Cs-A1 group, and 51% (32-69%) for patients belonging to Cs-A3 group, respectively (P = NS, Figure4).
Event-free survival.
The EFS for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line) is shown. EV = number of events occurring in each arm of randomization.
Event-free survival.
The EFS for the Cs-A1 group (continuous line) and the Cs-A3 group (dotted line) is shown. EV = number of events occurring in each arm of randomization.
No significant correlation was observed between sex, age at diagnosis, age at transplant, leukocyte count at diagnosis, immunophenotype or interval CR-transplant, and the clinical outcome of the patients, neither in univariate nor in multivariate models (data not shown).
Impact of diagnosis and GVHD
The analysis performed considering ALL patients separately from AML patients showed results similar to those observed in the whole study population. In fact, for the 47 patients affected by ALL the relapse probability was 20% (2-37%) for the Cs-A1 group and 47% (25-68%) for the Cs-A3 group (P = .06), whereas EFS was 67% (48-86%) for the Cs-A1 group and 45% (23-66%) for the Cs-A3 group (P = NS). Considering only the 12 patients affected by AML, the relapse probability was 0% for the Cs-A1 group and 17% (0-46%) for the Cs-A3 group, whereas EFS was 83% (54-100%) for the Cs-A1 group and 67% (29-100%) for the Cs-A3 group (P = NS in both cases).
Analyzing RR and EFS according to grade II to IV acute GVHD occurrence, irrespective of the arm of randomization, no difference was observed between patients developing grade II to IV acute GVHD and those without or with grade I acute GVHD. The 5-year RR was 30% (13-46%) for patients without or with grade I acute GVHD and 26% (8-44%) for those with grade II to IV acute GVHD, respectively (P = NS); EFS was 67% (51-84%) and 51% (31-71%) for subjects without or with grade I acute GVHD and for those with grade II to IV acute GVHD, respectively (P = NS).
By contrast, irrespective of the arm of randomization, the analysis of relapse and disease-free survival according to chronic GVHD occurrence showed a RR of was 33% (17-48%) for patients without chronic GVHD and 7% (0-21%) for those with chronic GVHD (P = 0.07). In the same way, EFS was 63% (47-78%) for patients without chronic GVHD and 83% (60-100%) for those with chronic GVHD (P = 0.09).
Discussion
Despite relapse, TRM, and GVHD occurrence, allogeneic BMT from HLA-identical siblings is curative in 50% to 70% of children with acute leukemia in first or second CR.22 25-29
New conditioning regimens are under evaluation for the treatment of acute leukemia. However, it is difficult to achieve a significant improvement of EFS only by means of more intensive conditioning regimens. For this reason, there is considerable interest in the optimal strategy to prevent severe GVHD, but sparing at best the GVL reaction.13 14
Cyclosporine-A, alone or in combination with methotrexate, is the most frequently used drug for prophylaxis of acute GVHD. Nevertheless, little consensus remains on the scheduling, dosage, and blood levels of the drug. Bacigalupo et al demonstrated in a cohort of adult patients given Cs-A alone as GVHD prophylaxis that the drug dosage during the first 10 days after BMT has a critical impact on the clinical outcome of the transplant procedure.16 In fact, in their experience, adult patients receiving Cs-A at a dose of 1 mg/kg/d had a significantly lower risk of relapse, a comparable TRM, and, when transplanted in first CR, a significantly better probability of EFS as compared to patients who received Cs-A at the dose of 5 mg/kg/d. An updated analysis of this study demonstrated that the survival advantage for the group given the lower Cs-A dose persists in the subgroup of younger patients.30
This trial compared the standard dosage of Cs-A used in children (3 mg/kg/d) during the first 3 weeks after BMT, with a significantly lower dose (1 mg/kg/d). Results show that the reduction of Cs-A dosage during the first 3 weeks after BMT determined a significant decrease (26%) in the RR for patients treated with 1 mg/kg/d. As expected, the Cs-A1 group patients had lower blood levels of the drug (data not shown), and they did experience a higher incidence of grade II to IV acute GVHD. Moreover, these patients had a greater incidence of gut and liver acute GVHD. However, the TRM directly attributable to acute GVHD was very limited: only 3 patients, 5% of the whole study population, died from this complication. The mortality due to infections, which in some cases could be related to the development of acute or chronic GVHD or to its treatment, was similar in the 2 groups as well, and the reduction of Cs-A dosage did not translate either into a longer period of steroid therapy for GVHD or into a higher incidence of chronic GVHD. In comparison to patients in the higher Cs-A dose group, the lower RR of patients belonging to Cs-A1 arm resulted in a 19% advantage in 5-year Kaplan-Meier estimate of EFS. Because children in the lower Cs-A1 dose group had a greater 1-year TRM, we cannot exclude that, with a greater number of patients, the advantage offered by the reduced RR would be offset by an increased number of transplant-related deaths.
To our knowledge, this is the only prospective randomized study performed on a pediatric population and specifically aimed at comparing 2 different GVHD prophylaxis regimes in children with acute leukemia undergoing BMT. This consideration is particularly noteworthy also because, as shown by the retrospective analysis by Ringdén et al,31 data on GVHD prophylaxis obtained in an adult population are not directly transferable to children.
It is reasonable to hypothesize that the reduction of RR in our patients was due to an increased GVL effect, capable of suppressing residual leukemia cells that escaped the cytotoxic effect of myeloablative therapy. As far as the biologic mechanisms of GVL effect are concerned, 3 distinct components have been proposed by Horowitz et al8: (1) an antileukemia effect related to the development of GVHD and mediated by the action of donor lymphocytes toward recipient widely distributed tissue antigens; its magnitude correlates with disease severity, and both acute and chronic GVHD are involved in the destruction of residual leukemia cells; (2) an allogeneic antileukemia effect, independent of clinically evident GVHD and directed against leukemia-associated or tissue-restricted antigens; and (3) an antileukemia effect of T cells, independent of GVHD and impaired by T-cell depletion. In that retrospective analysis, the greatest antileukemia effect was observed in chronic myeloid leukemia (CML), an intermediate effect in AML, and the smallest effect in ALL.8 Furthermore, in ALL no evidence was found for GVL occurring without clinically manifest GVHD.8 32
Cyclospoine-A can interfere with the GVL response. In fact, this agent, modulating the lymphocyte expansion through inhibition of production of interleukin-2 and its receptor and increasing production of transforming-growth factor β, may negatively influence T-cell function. Further evidence to the negative effect of Cs-A on GVL effect is provided by the observation that some relapsed patients have been put into a new remission by abrupt Cs-A discontinuation, often in association with the appearance of signs of GVHD.20 33
The analysis performed without considering the arm of randomization showed similar relapse rates in patients with grade 0 to I acute GVHD and in those with grade II to IV disease. This finding suggests that the stronger GVL effect observed in patients belonging to the Cs-A1 group could be independent from the development of acute GVHD or, alternatively, could be ascribable to a subclinical GVHD. Subjects who did develop chronic GVHD had a lower probability of relapse after the transplant than those who did not. Nevertheless, chronic GVHD incidence in the 2 arms of the study was similar, with only a 4.5% higher probability in the arm receiving the lower Cs-A dosage.
In conclusion, this study indicates that in pediatric patients, who probably have immunologic advantages for the induction of tolerance in comparison to adults, the use of Cs-A at 1 mg/kg/d is able to reduce the risk of leukemia recurrence, likely through an enhanced GVL effect. Whether this advantage in terms of leukemia control results into a better EFS remains to be definitively proved.
Supported in part by grants from AIRC (Associazione Italiana Ricerca sul Cancro) and IRCCS Policlinico S. Matteo to F.L.
Reprints:Franco Locatelli, Dipartimento di Scienze Pediatriche, Università di Pavia, IRCCS Policlinico San Matteo, P.le Golgi, 2, I-27100 Pavia, Italy; e-mail:f.locatelli@smatteo.pv.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal