The development of thrombotic disorders in humans is one of the most common causes of morbidity and mortality in the Western world. Thrombotic disease can be classified in broad terms as that which occurs in the venous system of low flow and pressure or in the high-flow and pressure arterial system. Certain basic distinctions can be drawn between arterial and venous thrombosis, such as the composition of the thrombi (platelet rich in arterial and fibrin rich in venous) and the presence of vascular wall damage (atheroma) in arterial thrombosis. However, the distinctions are not absolute, and there are common underlying mechanisms. Perturbation of hemostasis is central to the pathogenesis of all thrombosis, even though it differs in nature depending on location. Transient or long-lasting environmental influences may play important roles in perturbing hemostasis and influencing risk for thrombosis in both the venous and the arterial systems. The term environmental is used in its broadest sense to embrace changes induced by diverse influences such as childbirth, hormone ingestion, surgery, diet, and smoking, by intercurrent disorders such as diabetes mellitus, hypertension and dyslipidemia, and hyperhomocystinemia, and by local changes to the vascular wall. Perturbation of hemostasis may also be in part genetically determined, and, when this is the case, its influence is potentially profound because of its life-long presence. Because of the late onset of the majority of thromboses, it is unlikely that genetic changes could be their sole determinant, and this points to the importance of gene–environment interactions in disease.
There is a rapidly growing literature on the relationship between the genetics of hemostatic factors, the environment, and thrombotic occlusion. Most of this literature concerns variation in the genes for blood coagulation factors, inhibitors, fibrinolytic factors, and platelet-membrane receptors. The aim of our review is to summarize this literature, focusing on the common genetic variations (a prevalence approximately 1/100 or greater is used as a rough, not an absolute, guide, and the term polymorphism is used regardless of clinical effect) that could potentially have important influence on the health of populations. Certain changes, such as partial or total gene deletions, tend to be of low prevalence and will not be considered. Furthermore, the specific mutations in certain genes predisposing to thrombosis are essentially “private” to a relatively small number of families (such as those in the genes for antithrombin, protein C, and protein S) and will not be considered. At its conclusion, an evaluation will be made of the overall importance of genetics in determining the course of these common disorders, and a perspective on gene–environment interactions will be presented.
Venous thromboembolic disease
Venous thromboembolic disorders form a spectrum of conditions characterized by in situ thrombus formation and the variable presence of embolic manifestations. The clinical presentation depends to a large extent on the site and extent of thrombus formation and on the underlying cause. In the absence of genetic deficiencies, thrombosis occurs in the older population, largely in the context of marked environmental influences such as surgery, obesity, and underlying malignancy. In contrast, at the other extreme, familial thrombosis associated with mutations in genes for protein S, protein C, or antithrombin is associated with younger age of onset, lesser environmental stimulus, and thrombus formation often in unusual sites. There is an increasing awareness of the importance of hemostatic mechanisms in the pathogenesis of venous thrombosis. The hemostatic process comprises coagulation and fibrinolytic systems with activators, zymogens, cofactors, surfaces (such as platelets), and inhibitors. The function and regulation of these composite systems and factors have been extensively studied. Understanding their functional integration in vivo, however, still presents a major challenge. A starting point for a simplified model of integration envisages a balance between procoagulant (clot-promoting) and anticoagulant (clot-preventing) mechanisms. This balance serves to prevent an explosive generation of thrombin when coagulation is triggered. The balance is determined by the (functional) levels of all coagulation and fibrinolytic factors, with increases in the levels of coagulation factors and decreases in the levels of both fibrinolytic factors and natural inhibitors being procoagulant. The role of inhibitory mechanisms in preventing venous occlusion was established after the identification of the link between inherited deficiencies of antithrombin, protein C, protein S, and venous thrombosis during the 1960s and the 1970s. Thegeneral importance of the anticoagulant pathway involving protein C and protein S was established by the widespread prevalence among white populations and the clinical consequences of the phenotype of activated protein C resistance (APCR) and its main causative gene mutation, factor V G1691A (factor V Arg506Gln, factor V Leiden). There has been slower appreciation of the procoagulant effect of increased levels of coagulation factors in venous disease, but recent work has highlighted the likely importance of prothrombin and factor VIII levels in this regard.
Genetic polymorphisms and venous thrombosis
Factor V
After the identification of inherited deficiencies of coagulation inhibitors, antithrombin,1 protein C,2 and protein S3 and of the associated high prevalence of thrombosis (thrombophilia), venous thrombosis was commonly regarded as a single gene disorder. This view was conditioned largely by results of studies with small numbers of patients/families with an unusually high prevalence or severity of thrombosis. The recognition of APCR/factor V G1691A, as a prevalent risk factor for venous thrombosis in the general population,4-7 resulted in a clearer view of the episodic nature of thrombosis and of the importance of other genetic and acquired risk factors in the onset of thrombosis. A revised view of genetic predisposition toward venous thrombosis suggests that single gene defects confer increased risks that do not necessarily lead to thrombosis without interaction with other genetic or environmental risk factors.8-10 Venous thrombosis is now therefore widely considered a multicausal disorder. The main features distinguishing factor V G1691A from mutations in the genes for antithrombin, protein C, and protein S are the reduced risk associated with its inheritance11 and its high prevalence in white populations.
Factor V is a 300-kd multidomain glycoprotein, the gene of which has 25 exons. The relationship between factor V G1691A and the intermediate phenotype, APCR, is broadly established.12 The variant factor V Gln506 is activated by thrombin but is less readily inactivated by APC than factor V Arg506. There are 2 primary cleavage inactivation sites, Arg506 and Arg306. Several reports have investigated the kinetics of cleavage inactivation of factor Va. One group found that initial cleavage is at Arg506 and that this promotes more rapid cleavage of Arg306, which is the main inactivation step.13 Another group found that cleavage is random at the 2 sites but is much slower at Arg306.14 Protein S, the cofactor to APC, accelerates cleavage of Arg306.15 APC inactivation of factor Va Gln506 is delayed. According to the first view, this is because there is no enhanced cleavage at Arg306 by prior cleavage at Arg506, whereas the alternative view is that only the slow rate of cleavage at Arg306 can occur. An additional effect on phenotype is suggested by the finding that factor V functions as an anticoagulant because it is a cofactor in the degradation of factor VIIIa by APC (and protein S).16 This anticoagulant cofactor function requires cleavage of intact (as opposed to activated) factor V by APC; the C-terminal region of the factor V B domain is necessary for this, and cleavage at Arg506 appears critical.17 Because factor V Gln506 cannot be cleaved at this position, it cannot act effectively as a cofactor in factor VIIIa degradation. The relative importance of the delayed cleavage of factor Va Gln506 by APC and of the impaired anticoagulant cofactor function of factor V Gln506 in contributing to reduced endogenous anticoagulation in vivo has yet to be defined.
Regardless of the precise pattern of cleavage by which the factor V Arg506Gln substitution causes APCR or which property of the mutant factor V/Va is most important, its inheritance increases the risk for venous thrombosis. This was shown initially using the Leiden Thrombophilia Study, a large population-based case-control study of venous thrombosis,18 and then in the large prospective US Physicians Health Study19 and in numerous other reports. There is a high frequency of factor V G1691A in white persons (approximately 5%), and the polymorphism arose from a common source 20,000 to 30,000 years ago (founder effect).20 The high prevalence, coupled with its moderate to high (approximately 8-fold) risk, has enabled its interactions with other relatively uncommon genetic risk factors (in protein C, protein S, and antithrombin)21-23 and with environmental factors (such as oral contraceptives)24 25 to be studied.
The thrombotic risk of factor V G1691A/APCR is not confined to the deep veins of legs. Cerebral vein thrombosis is more common in carriers.26 However, factor V G1691A/APCR does not appear to be a major risk factor for all venous thromboembolic events, implying that regional factors can dominate risk. For example, hip and knee replacements are associated with a high risk for postoperative venous thrombosis. However, in a large (825-patient) retrospective study, there was no excess thrombosis in carriers of the genotype (31% [confidence interval, CI; 15% to 47%] in carriers; approximately 26% [CI, 22% to 29%] in noncarriers).27 There is evidence that patients with isolated pulmonary embolism do not have a greatly increased risk conferred by factor V G1691A/APCR.28These studies suggest that certain types or magnitudes of stimuli toward thrombosis, provided by some surgical procedures or by certain coexisting illnesses, might be so dominant that factor V G1691A/APCR provides only a minor contribution to overall risk.
Current outstanding issues concerning the factor V G1691A polymorphism relate to the management of clinically affected and unaffected carriers of factor V G1691A. It is unclear whether factor V G1691A increases the risk for recurrence after a first thrombosis. If this is the case, it might suggest that treatment of the initial event should be more intense in carriers. This is an important issue because of the known increased risk for severe bleeding caused by prolongation of oral anticoagulant treatment. Conflicting results on the frequency of recurrence have been reported29-33 because of either the small number of patients studied or the short period of follow-up. Well-designed studies on the prevalence of recurrence and on optimum duration of anticoagulation are now needed. Additionally, there are no studies that give clear guidance on the optimum management of clinically unaffected carriers.
Some patients have APCR but not factor V 1691A. The frequency of this combined genotype/phenotype has not often been reported and is therefore uncertain. There have, however, been 2 reports of patients with nucleotide changes resulting in alternative amino acid substitutions at factor V Arg306 (to Thr and to Gln).34,35These mutations appear to be private rather than of polymorphic frequency. The population frequency of APCR in the absence of factor V G1691A will depend on the specificity of the functional APCR assay for factor V G1691A. A recent report using the Leiden Thrombophilia Study and an APCR assay nonspecific for the gene change to analyze samples demonstrated that APCR in the absence of factor V G1691A confers a definite risk for thrombosis.36 The carriers of factor V G1691A were excluded from analysis, and normalized APC sensitivity ratios were determined in 337 patients with venous thrombosis and in 455 controls. A dose-response relationship was observed between APCR and risk for thrombosis, with a 4-fold difference between highest and the lowest quartiles of APCR. Elevated factor VIII levels are known to influence APCR, but the relationship remained after adjustment was made for this.
Another approach to elucidate alternative causes of APCR considered the influence of the genetic background of factor V on APCR. A haplotype termed HR2 was defined by 5 restriction enzyme-cutting sites in exon 13 and a single sequence variation in exon 16.37 The presence of this haplotype was found to be increased in patients heterozygous for factor V G1691A who had severe APCR, and it was increased in noncarriers of factor V Arg506Gln with APCR. One study has suggested that the HR2 haplotype is a mild risk factor for thrombosis.38 In this case-control study of 205 patients with thrombosis and 394 matched controls, the odds ratio (OR) for increased risk associated with the haplotype was 1.8 (CI, 1.1 to 2.8). In a large, retrospective, multicenter cohort study, the combination of the HR2 haplotype with other genetically determined risk factors was assessed.39 Combination of the HR2 haplotype with antithrombin, protein C, and protein S deficiencies did not increase the calculated risk for thrombosis associated with the latter group. However, when persons with factor V G1691A also carried the HR2 haplotype, there was an increase in the risk for thrombosis; compare relative risk (RR) 4.2 (CI, 1.6 to 11.3) for factor V G1691A with RR 10.9 (CI, 2.9 to 40.6) for both. The authors of the latter report add a caution concerning the limited number of thrombotic events in their study. The polymorphic sites within the haplotype do not explain why the haplotype should alter APCR. There are 2 amino acid substitutions coded by the haplotype, 1299His/Arg and 1736Met/Val, but these appear to be neutral. It has been suggested recently in abstract form40 that the functional basis for the effects of different haplotypes could arise from an altered distribution of the normal functional isoforms of factor V and may involve differences in glycosylation. The prevalence of this haplotype in Italian, Indian, and Somalian populations has been estimated to be 8% to 10%, suggesting a common ancestral origin.
Prothrombin
There was little in the literature relating prothrombin, a 72-kd zymogen, and venous thrombosis before 1996. A common polymorphism that increases the risk for venous thrombosis was then identified. This is a substitution in the 3′-untranslated region of prothrombin at nucleotide 20 210, G to A,41 identified by sequencing the coding, 5′and 3′UT regions of the gene in 28 selected patients with familial history of thrombosis. Eighteen percent of these patients had the 20 210A allele compared to 1% of healthy controls. The increased risk for venous thrombosis conferred by the polymorphism was determined in the Leiden Thrombophilia Study. Of the patients, 6.2% had the A allele compared to 2.3% of controls, giving a relative risk for thrombosis of 2.8. The prothrombin 20 210A allele is associated with elevated prothrombin levels, and this appears to be responsible for the increased risk for thrombosis. The precise mechanism by which prothrombin levels are altered has yet to be determined. Increased efficiency of polyadenylation of mRNA transcripts has been suggested. Results of several studies and an overall summary investigation indicate that the prevalence in the white population is 2% (CI, 1.4% to 2.6%)42,43 and confirm that the A allele is a risk factor for venous thrombosis, including cerebral vein thrombosis.44 The reported variation in population prevalence (0. 7 to 4. 0) is caused both by the small populations studied and by variations within populations: southern European populations have a higher prevalence of the A allele than northern European populations.43 A perplexing finding was a weak (RR, 1.7; CI, 0.9 to 3.2), nonsignificant association of the A allele with venous thrombosis in the US Physicians Health Study, despite a high population prevalence (3.9%).45 Haplotype analysis has shown that this polymorphism also arose from a founder 20 000 to 30 000 years ago.46 There is as yet little information on the risk for recurrent thromboembolism in carriers of the prothrombin 20 210A allele. In a single prospective study, 492 patients with (42 patients) and without (450 patients) the allele were investigated for 24 months.47 The probability of recurrence was 8% (CI, 0 to 16.7) and 12.2% (CI, 8.8 to 15.6), respectively, suggesting that long-term treatment with oral anticoagulants is not justified. Although the polymorphism may confer weak risk for thrombosis when an isolated defect, its effect may be more important when in combination with other genetic risk factors. Thus, in a retrospective study of 112 patients with factor V G1691A compared to 17 patients heterozygous for the factor V gene change and for prothrombin 20 210A, the risk for recurrence of thrombosis after a first event was greater (OR, 2.6; CI, 1.3 to 5.1) in those patients with both risk alleles.33This finding suggests the need for more prolonged anticoagulation in carriers of both risk alleles after a first thrombotic event.
Emerging candidate genes
Factor XIII
Factor XIII is a transamidase comprised of 2 A and 2 B subunits in a tetrameric structure (A2B2) of 320 kd.48 The role of activated factor XIII lies mainly in accelerating the formation of shear- and fibrinolysis-resistant cross-linked fibrin; a deficiency of factor XIII is associated with severe bleeding.48 Thrombin cleaves a 37-amino acid peptide from the A subunit. The gene for the A subunit codes for a polypeptide of 75 kd.49 In excess of 20 mutations have been described in the A subunit gene that relate to factor XIII deficiency by leading to absence of the A subunit.50-55 In addition 4 common coding polymorphic sites have been described at Val34Leu, Pro564Leu, Val650Ile, and Glu651Gln.51 54 The polymorphism coding, G/T, for factor XIII Val34Leu produces a coding change in the A subunit only 3 amino acids from the thrombin cleavage activation site at position Arg37-Gly38.
The Val34Leu polymorphism has been associated with a protective effect against venous thrombosis in a case-control study of 221 patients and 254 healthy controls.56 Patients with thrombosis had an increased frequency of the Val/Val genotype (63% versus 49%) and a lower frequency of the Val/Leu genotype (31% versus 42%) than controls. In a logistic regression model, the factor XIII genotype was shown to be independent of factor V G1691A as a predictor of thrombosis. A second report57 of 189 patients and 187 controls found no association of the heterozygous Val34Leu change with thrombosis, but the homozygous Leu34 was strongly associated with a protective effect (OR, 0.16; CI, 0.05 to 0.5). The proposed protective Leu allele appears to be associated with increased activation of factor XIII58 and enhanced fibrin cross-linking in in vitro assay systems.50 59 This finding of enhanced activation, coupled to an apparent protective effect against venous thrombosis, is intriguing and is the subject of ongoing investigation.
Endothelial cell protein C/activated protein C receptor
Endothelial cell protein C/activated protein C receptor (EPCR) is a 43-kd endothelial cell membrane type I intercalated receptor for protein C.60,61 There is a body of evidence accumulating that suggests that this receptor is an important component of the protein C anticoagulant pathway.62,63 It is found predominantly on large vessels and serves to ensure high local concentration of protein C for activation by the thrombin–thrombomodulin complex.64 The gene for the human EPCR contains 4 exons and has a number of potential polymorphic sites.65 An early report (abstract) has identified a 23-bp insertion in exon 3, which duplicates the preceding 23 bases and results in a STOP 6 codons downstream from the insertion point.66 The clinical significance of the insertion was examined in 149 patients with venous thrombosis and 404 controls. A crude OR for this mutation was 4.6 (CI, 1.1 to 19.7), suggesting that gene mutation of EPCR may predispose to venous thrombosis.
Thrombomodulin
Intuitively, it might be expected that thrombomodulin gene variation could predispose to thrombosis, given the overall importance of the protein C anticoagulant pathway in maintaining blood fluidity. Thrombomodulin is an endothelial cell membrane intercalated proteoglycan of approximately 100-kd. It functions as a thrombin receptor and transforms the specificity of bound thrombin so that it loses its procoagulant functions and acquires a greatly increased ability to activate protein C. Several coding sequence changes have been identified in families by screening for mutations in the thrombomodulin gene.67-69 However, there is still limited information on the effects on function of these genetic changes, and the families have been too small to provide decisive information on the relationship with thrombosis. The only common polymorphism identified, C/T coding for Ala455Val,70 appears not to be associated with thrombosis. It appears that any gene changes altering the function or expression of thrombomodulin are probably private mutations.
Factor VIII(-related)
Increased factor VIII levels have been shown to be associated with thrombosis in several studies. In the Leiden Thrombophilia Study, factor VIII elevated more than 150 IU/dL, measured as factor VIII coagulant activity, was shown to have an associated relative risk of 4.8 (CI, 2.3 to 10).71 Increased risk for elevated factor VIII has been confirmed by a study of patients referred for unexplained thrombosis who were largely free of markers of inflammation.72 To date, no common genetic variation in the factor VIII gene has been identified that might account for this phenotypic variation.73 However, there is good evidence that factor VIII levels may be genetically determined. Von Willebrand factor (vWF) and blood group are well-known determinants of the factor VIII level in plasma, and factor VIII levels have been shown to cluster in families.74
Genes other than those of hemostasis
Among the range of factors that can potentially interact with hemostatic genetic determinants, the blood level of homocysteine is of interest in the current context. Homocysteine is a sulfhydryl amino acid arising from the metabolism of methionine. A high level of homocysteine, hyperhomocystinemia, has attracted much recent interest as a potential risk factor for both venous and arterial thrombotic disease, not least because dietary supplementation with folic acid affords a safe and inexpensive therapeutic option. One of the 2 enzymatic pathways of remethylation of homocysteine to methionine involves methylene tetrahydrofolate reductase (MTHFR). The gene for MTHFR contains a common polymorphism, C677T, and this can potentially interact with hemostatic gene polymorphisms. Inconsistent results have been obtained in the numerous published studies, reviewed comprehensibly elsewhere.75 Hyperhomocystinemia appears to be only a weak risk factor for venous thrombosis,76,77 and this may account for the variable outcomes both when the C677T polymorphism has been studied as a sole risk factor and when in combination with hemostatic genetic risk factors.75
Arterial occlusive disease
Cardiovascular disease is a generalized disorder of the vascular tree characterized by long-term atheromatous plaque formation and culminating in atherothrombotic obstructive lesions that lead to tissue damage. In many subjects there are coexistent atheromatous lesions in the coronary, carotid, aorto-iliac, and femoropopliteal arteries that can present as various syndromes according to the vessel that is predominantly affected by the process. Angina and acute myocardial infarction (MI) are the clinical manifestations of the chronic development of coronary artery atheroma, with the final pathologic process of plaque rupture and coronary thrombosis. The underlying processes that lead to atheroma formation and coronary thrombosis are complex and involve multiple interrelated systems that regulate vasoactivity, adhesion molecules and their ligands, lipid metabolism, and the coagulation and fibrinolytic pathways. A body of evidence implicates underlying insulin resistance with associated clustering of cardiovascular risk in subjects with and without diabetes as 1 of the common factors linking some of these processes.78-80 The mechanisms involved are multifactorial, and effects of insulin/insulin resistance on nitric oxide production, lipid metabolism, hemostasis, and blood flow are implicated in subjects with diabetes and a proportion of subjects without diabetes.
Although environmental influences arising from a more sedentary and overweight population may account in part for the rise in MI seen in Western societies in the past century, genetic background can also be expected to play a role in influencing disease. An increasing number of studies, many of which are cited below, have been focused on young survivors of MI, and some of these have addressed the possibility of interaction between hemostatic polymorphisms and acquired influences such as smoking, diet, and metabolic changes. The rationale for use of such highly selected patients is that the burden of atherosclerosis in them can be expected to be less. Consequently, dominant pathogenic mechanisms for MI in these patients may more likely involve perturbed hemostatic balance.
Support for the concept of disordered hemostatic balance in MI comes from major prospective and case-control studies of vascular risk that report that elevated concentrations of hemostatic proteins, such as fibrinogen, factor VII, vWF, PAI-1, and tPA relate to vascular risk and cardiovascular outcome.81-85 These are clearly different risk factors than those associated with venous thrombosis. Obstruction of a coronary artery involves a thrombus the size of a small pea, whereas there may be a large mass of clot in an obstructed vein. Part of the reason for these differences must lie in the nature of disease in the respective vascular beds. Arterial disease occurs in the context of a high-flow, high-pressure system in which atheroma formation dominates and plaque rupture with exposure of tissue factor probably provides the stimulus for the initiation of clot formation. In a current model, plaque rupture, endothelial cell damage, or both, leads to exposure of the subendothelial layer that facilitates the binding of vWF. This leads to binding of vWF to the glycoprotein (Gp) Ib/IX platelet receptor and platelet adhesion. Alternatively, platelets may directly bind to collagen through their collagen receptors. These reactions lead to a conformational change in the platelet GpIIb/IIIa integrin that facilitates shear rate-dependent binding of both vWF and fibrinogen and platelet aggregation.
Coagulation gene polymorphisms
Fibrinogen
Relationship between plasma levels and disease, influence of polymorphisms on plasma levels.
Of all the components of the coagulation system, elevated fibrinogen has been most consistently associated with occlusive vascular disorders. Investigations such as the Northwick Park Heart Study and the Gothenburg and PROCAM studies have prospectively related fibrinogen to MI and stroke outcomes.81,82,86 Evidence from the Scottish Heart Health Study indicates that increased fibrinogen also clusters with other cardiovascular risk markers, including hypertension, diabetes, smoking, and peripheral vascular disease.87 Fibrinogen provides a link between smoking and the development of arterial disease because of the influence of smoking on fibrinogen levels.88
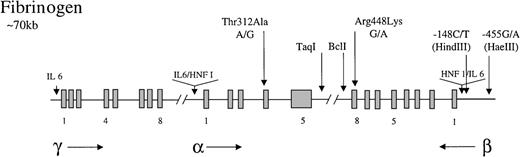
Fibrinogen is a 340-kd glycoprotein consisting of 3 nonidentical polypeptides (α, β, and γ) linked by disulfide bonds. The 3 polypeptides are encoded by 3 fibrinogen genes clustered on the long arm of chromosome 4.89 The cis andtrans acting factors that regulate fibrinogen transcription have been extensively studied. The 3 genes contain promoter regions with TATA and CAAT boxes and a number of sequence elements that confer tissue (liver)-specific and enhanced expression.89 These include hepatic nuclear factor 1 (HNF 1; 5′ to the α and β genes) and IL-6-responsive elements (5′ to all genes) (Figure1).90 91 Fibrinogen levels are subject to much biologic variation, with elevated circulating levels arising particularly as a consequence of the acute-phase response. The IL-6 responsive elements are thought to be important for mediating this effect. The acute-phase response arising from viral infection or from smoking is strongly implicated in the development of cardiovascular disease.
Representation of the organization of the fibrinogen gene locus.
The 3 genes for the polypeptides are represented along with the direction in which they are transcribed (horizontal arrows). Exons (blocks) are occasionally numbered. Common polymorphisms discussed in the test are indicated by vertical arrows, as are tissue-specific (HNF 1) and enhancer (IL-6) elements.
Representation of the organization of the fibrinogen gene locus.
The 3 genes for the polypeptides are represented along with the direction in which they are transcribed (horizontal arrows). Exons (blocks) are occasionally numbered. Common polymorphisms discussed in the test are indicated by vertical arrows, as are tissue-specific (HNF 1) and enhancer (IL-6) elements.
Humphries et al92,93 were the first to investigate the potential role of genetics in the regulation of fibrinogen levels. Multiple polymorphic sites have since been identified in the 3 genes, but, apart from some more recent interest in the α chain, most studies have focused on β chain polymorphisms. This is because of the suggestion arising from in vitro pulse chase studies in Hep G2 cells incubated with L-[35S] methionine that β chain synthesis is rate limiting in the production of mature fibrinogen.94 However, no evidence is available to suggest that this might be the case in vivo. Studies of the heritability of fibrinogen support a role of genetics, with genetic contributions to variance of 51% reported by Hamsten et al,95 48% in young subjects from the Kibbutzim study,96 and 65% in 191 female twin pairs (unpublished data). The 2 most studied polymorphisms are those detected by theBclI restriction enzyme (BclI polymorphism, located in the 3′ region of the β chain) and by the HaeIII restriction enzyme, the −455 G/A polymorphism (in the 5′ promoter region of the β chain). Of these, the latter (which is in linkage disequilibrium with the former) has been favored by many investigators because of its proximity to the IL-6-responsive element and the HNF 1 element. Humphries et al92,93 reported that 9% and 5% of fibrinogen variability could be explained by the β chain polymorphisms detected by BclI and HaeIII, respectively, whereas 4.2% was determined by an α chain fibrinogen gene polymorphism detected using TaqI. A mainly consistent finding of large studies of cardiovascular disease has been confirmation that levels of fibrinogen are associated with these genetic variations. In the ECTIM study97 of 565 patients and 668 controls, 10 polymorphisms of the β chain were found to be in linkage disequilibrium. In multivariate analysis, the −455G/A was found to be independently associated with plasma levels (P < .0003). This association was only found, though, in smokers. In the study termed EARS, 585 offspring of fathers with MI and 1106 control were studied.98 The −455A allele was strongly associated (P < .001) with elevated levels in males, but the association was also strongly influenced by gender and smoking. The Copenhagen City Heart Study has a large (n = 9127) general population sample.99 The −455A allele was associated with fibrinogen level in both genders, and an increase of 1 SD in circulating fibrinogen was associated with a 20% increase in OR for ischemic heart disease. In a study of 923 patients undergoing coronary angiography, this polymorphism was associated with fibrinogen levels (P < .0002), and an association between genotype and elevated levels after bypass surgery in 207 patients was also observed.100 Finally, in 885 male patients with coronary artery disease selected from the study REGRESS, there was a significant (P < .05) association between baseline fibrinogen and genotype.101
Polymorphisms and disease.
Although there is compelling evidence of associations between fibrinogen level and arterial disease and between fibrinogen level and certain polymorphisms, the relationship between fibrinogen polymorphisms and disease is altogether less clear. Certain studies, some large, suggest an association. In the ECTIM study, a number of associations between fibrinogen and severity of atheroma, assessed by coronary angiography, were documented.97 The rarer allele of the 4 β chain polymorphisms exhibiting associations with fibrinogen levels (BclI, C448, −455, and −1420) was significantly more common in subjects with more severe coronary artery disease. No association was found, however, between genotype and MI. Positive findings were reported in a small study of 187 subjects with type 2 diabetes, of whom those with coronary artery disease had a higher prevalence of the G allele of the −455 G/A polymorphism.102 The −455 G/A polymorphism has also been positively related to the progression of atheroma, though in this case it was the A allele that was associated with deleterious effects.101 The BclI polymorphism has been related to MI in a cohort of patients from the GISSI-2 study.103This study was small (102 patients, 173 controls) and unusual because its participants were selected from patients with 1 or more first-degree relatives who had MI or stroke before 65 years of age. There was a significant difference in allele frequency in patients than in controls (P = .002). In multivariate analysis, genotype was associated with risk of MI (OR, 2.4; CI, 1.2 to 4.6). The Austrian Stroke Prevention Study examined 399 invited “normal” subjects for associations between the β chain −148C/T polymorphism, fibrinogen levels, and carotid atherosclerosis.104 The T/T group (n = 25) demonstrated higher grades of atherosclerosis than carriers of the C allele (P = .003). The T/T genotype was a significant (OR, 6.17; CI, 1.70 to 22.3) predictor of disease in a multivariate model. Oddly, the fibrinogen level itself was a much weaker predictor of disease, and there was no association between genotype and fibrinogen level. Carter et al105 reported a strong association between β 448 genotype and cerebrovascular disease in 149 female patients compared with healthy controls. The β promoter polymorphism G/A −455 has been related to stroke in Japanese subjects, and the C/T 148 has been related to carotid atheroma.106
In contrast to these positive associations between genotype and disease, 2 very large studies showing the association between genotype and fibrinogen level failed to establish an association between genotype and disease. The Copenhagen City Heart Study99found an association between fibrinogen level and ischemic heart disease, with an increase in 1 SD in level increasing the OR for disease by approximately 20% (P < .01 for females,P < .005 for males). However, though the −455A allele was associated with fibrinogen levels in both genders (see above), its frequency was not influenced by disease. Either the increase (approximately 4% to 10% of the level associated with AA genotype) is secondary to other more critical influences or it is simply insufficient to influence the course of disease. Similar negative associations were observed in 2 other large studies.100,107Despite the association between genotype and level, noted above in the study by Gardemann et al,100 no association was found between −455G/A polymorphism and either coronary artery disease or MI. In the study by Wang et al,107 though fibrinogen was related to the onset and severity of coronary disease, there was no relationship between the polymorphism and disease.
A polymorphism in the coding region of the α chain (Thr312Ala) has been described108 that is of some interest because of its proximity to the factor XIII cross-linking site at position 328.109 Additionally, it lies within the region of the fibrinogen α chain that is involved in promoting the dissociation of the factor XIII A and B subunits and that enhances factor XIII activation.110 It has been reported that the substitution polymorphism at α Thr312Ala is associated with more rigid and less porous fibrin gel structures,109 suggesting this polymorphism as a potentially important candidate for involvement in thrombotic disorders. However, in a recent study by ECTIM of 585 patients with MI and 658 controls, there was no difference in the frequency of the genotypes.111 In a study of mortality rates after stroke, though, patients with the Ala312 coding allele had a dose-related significant reduction in survival than subjects homozygous for the Thr312 allele.112 This study also describes an increased frequency of the Ala312 allele in patients with pulmonary embolus compared to those with DVT alone or with normal controls. These findings do lend some support for the hypothesis that the Ala312 allele influences clot stability, though further clinical and in vitro studies are required to clarify this issue.
The most striking point about these association studies is their inconsistency in relating genotype, fibrinogen level, and arterial disease. In the study103 in which there are universally clear associations of BclI polymorphism and MI (GISSI-2), the unambiguous associations almost certainly arose because of the unusual selection criteria (familial cardiovascular disease) for the small number of patients (also see below).
Factor VII
Relationship between plasma levels and disease, influence of polymorphisms on plasma levels.
Interest in the relationship between factor VII and cardiovascular disease was stimulated by the finding from the Northwick Park Heart Study that elevated levels were related to fatal but not to nonfatal MI. In this prospective study of white males (n = 1511) aged 40 to 64 years at the time of recruitment, 109 developed a first major coronary artery disease event. Factor VIIC (ie, factor VII determined by clotting assay using factor VII-deficient plasma) was found to be strongly associated with coronary risk (117% compared with 107%;P < .001) with a 1 SD increase in factor VII associated with a 62% increase in risk over the first 5 years of the study.81 These results are often reported to have been supported by a second prospective study, PROCAM.86,113 In PROCAM, 130 coronary heart disease events occurred in 8 years of follow-up. However, in this study the results for factor VII were not decisive. Factor VIIC was elevated in patients who had coronary events (112% compared to 109%; P < .023), but in multiple logistic regression analysis, factor VIIC was not an independent risk factor for coronary events. A third prospective study, the Edinburgh Artery Study, also failed to confirm factor VII as an independent predictor of coronary disease.114 Interestingly, in the ECTIM study, the controls had uniformly higher levels of factor VII than the patients, which tended to militate against its major role as a cardiovascular risk marker.115 A number of other cross-sectional studies have provided conflicting conclusions about the importance of factor VII.116-118 A particular complication with factor VII is the methodology of its assay. The clotting assay, factor VIIC, reflects the total factor VII antigen, but it can also be influenced by the amount of factor VII that is activated (factor VIIa) in the plasma. The latter can be measured directly with an assay that utilizes truncated tissue factor as stimulant.119 It has been suggested that the factor VIIC assay used in the Northwick Park Heart Study is particularly sensitive to factor VIIa. There is, however, no good evidence that factor VIIa is an informative marker with respect to the prediction of cardiovascular disease.120
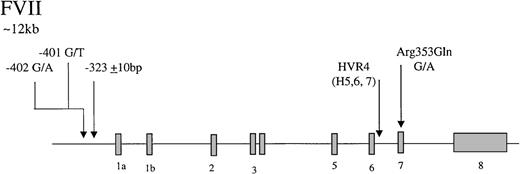
Factor VII is an inactive vitamin K-dependent zymogen synthesized in the liver and secreted as a single-chain glycoprotein of 48-kd. On contact with tissue factor, it is converted to the 2-chain active form by selective proteolysis by factor Xa, and the factor VIIa/tissue factor complex then activates factor X and factor IX. The gene coding for factor VII has 5 identified polymorphic sites that may associate with circulating levels of the gene product (Figure2), accounting for up to 30% of the variance in factor VII levels in plasma.121-125 In the promoter, a decanucleotide insertion at position −323 is in strong linkage disequilibrium with a single base substitution (A/G) at codon 353 (Arg/Gln) in exon 8. Two additional promoter polymorphisms arise at −401 (G to T) and −402 (G to A). A further common polymorphism has been described in hypervariable region 4 of intron 7, HVR4, with 3 different length alleles termed H5, H6, and H7. The relative influence of each polymorphic site on factor VII levels has yet to be fully resolved. The Arg353Gln site was noted initially to associate with a 20% to 30% variance in factor VII levels in males and females and in different ethnic groups.126 Many subsequent studies have confirmed that carriers of the allele coding for Gln353 have lower factor VII levels. It has been demonstrated recently127 that factor VII Gln353 is secreted with lower efficiency in vitro. It can be concluded that the Arg/Gln substitution is responsible for significant variation in level, with factor VII Gln353 approximately 20% lower. An association of triglyceride with factor VII level has been reported in several studies.128,129 A study of 215 pairs of twins has shown that genetic influences account for 57% of variation in factor VII levels and that there is a significant genetic correlation between factor VII and triglycerides.129 The association between factor VII and triglycerides has been attributed to the presence of the factor VII Arg353.128 In the Rotterdam Study, a large population-based study, an association of triglycerides with factor VII Arg353 was observed only in females.130 Returning to the promoter polymorphisms, the effects of the −401 and −402 polymorphisms have been studied using reporter gene analysis and in relation to the levels of factor VII in Swedish males.125Both polymorphisms strongly influence the binding properties of nuclear proteins and alter transcriptional activity in vitro. The rare −401T allele is associated with reduced transcription and reduced plasma factor VII levels. The rare −402A allele, in contrast, confers increased transcriptional activity and is associated with elevated levels in plasma. Together, they are associated with up to 30% variation in the factor VII/factor VIIa levels.
Representation of the organization of the factor VII gene,
together with the location of common polymorphisms discussed in the text.
Representation of the organization of the factor VII gene,
together with the location of common polymorphisms discussed in the text.
Polymorphisms and disease.
The relationship of certain of these polymorphisms to disease is highly controversial. In a study of a cohort of 453 patients with MI and 476 controls from the ECTIM study, the Arg353Gln polymorphism was found to relate strongly to levels of factor VII in cases and controls.115 There was, however, no difference in genotype frequencies between these 2 large groups. Similar results were obtained in a case-control study of 270 patients investigated for chest pain by coronary angiography.117 A case-control study in Sweden of 94 men with MI before the age of 45 years was reported by Moor et al.120 Using data from this study, Doggen et al131 calculated that there was a nonsignificant trend for an association of Arg353 with MI (OR, 1.81; CI, 0.79 to 4.13). The largest published case-control study, SMILE, in the Netherlands, included 560 patients and 644 controls.131 It found the expected association of factor VII levels with Arg353Gln in the control samples. However, Arg353 was associated with a reduced, rather than an increased, risk for MI (OR, 0.80; CI, 0.60 to 1.06), which was more pronounced in patients younger than 50 (OR, 0.49; CI, 0.28 to 0.84). Conclusions of this large study are that a genetic propensity to high factor VII levels is not associated with a risk for MI and that an elevated factor VII level itself is not a causal determinant. In contrast to these studies with largely negative results, a study of 165 Italian subjects with familial MI and 225 controls showed that there are higher risks associated with the common coding allele for both the Arg353Gln polymorphism and the hypervariable region in intron 7.132 The protection from MI associated with the homozygous Gln allele was large (OR, 0.08; CI, 0.01 to 0.9). It is interesting to note again (see “Fibrinogen”) that this small study derived from the GISSI 2 population provided strong associations between genotype and disease, whereas larger studies failed. Why should the same mutation be highly associated with MI in Italy but be apparently protective of disease in a study that was 3 times larger in the Netherlands? The issue of the unique manner of selection of patients in the Italian study must be considered as a possible influencer of outcome. Because these patients all had familial arterial disease, it is highly likely that there are associated important genetic factors that strengthened any associated risk. Similar overestimates of strengths of associated risks by family-based studies have been noted in venous thrombotic disease.133
There has been single study of factor VII genotype and levels in relation to atherothrombotic stroke in 317 patients, confirmed by computed tomography.134 The results again demonstrate the consistent finding of a strong relationship between genotype and levels of factor VII, but there is no indication that either is related to disease.
Emerging candidate genes
Factor XIII
Relatively little information is available relating changes in factor XIII level or activity to human arterial disease. Two animal studies have provided evidence for a role for circulating factor XIII in the pathogenesis of both MI and thromboembolic disease.135,136 In a canine electrically induced MI model treated with a factor XIII inhibitor, fibrinolytic therapy led to increased clot lysis compared to animals not pretreated with the inhibitor.135 In a ferret model of pulmonary embolus, factor XIII cross-linking is associated with increased resistance to exogenous tPA therapy.136 Both studies conclude that activated factor XIII has an important role in fibrinolysis-resistant clot formation and may affect outcomes in these 2 pathologically distinct syndromes that have fibrin formation as a common feature.
Two published case-control studies report associations between factor XIII Val34Leu and a history of MI. In a study of subjects with angiographically proven coronary artery disease and a history of MI determined by World Health Organization criteria, factor XIII Leu34 was reported to be significantly less common in those with a history of MI.137 In those subjects with Leu34 who had had a previous MI, there were higher levels of the fibrinolytic inhibitor PAI-1, the PAI-1 4G/4G genotype was more common, and there was evidence of interactions with other clotting factors and with features of the insulin-resistance syndrome.138,139 In the same population there was no evidence of relationships between other coding polymorphisms in the A subunit and cardiovascular disease.140 A mixed postmortem and case-control study of survivors of MI in Finnish subjects again suggested a protective effect of Leu34, but it was unable to reproduce the PAI-1 4G/4G genotype associations.141 Interestingly this study found the prevalence of the protective allele to be lowest in the Eastern Kainuu region, which has the highest prevalence of MI, similar to that reported in the high-risk Asian community in England.142
A single study of factor XIII Val34Leu in computed tomography-confirmed stroke was carried out in 612 patients.143 There were no differences in the prevalence of Leu34 in subjects with atherothrombotic stroke, though there was a marginally significant increase in Leu34 in subjects with a diagnosis of primary intracerebral hemorrhage. These results are consistent with the findings in relation to MI in that Leu34 may be protective against thrombosis though involved in the pathogenesis of hemorrhagic disorders. However, the small numbers in this study require that these results be viewed as preliminary.
Factor V/prothrombin
The factor V G1691A and prothrombin G20 210A polymorphisms in arterial disease have been subjects of numerous reports. Many of these—some large—concerned with their association with arterial disease in young, middle-aged, and elderly populations have had negative results.19,43,45,144-148 In contrast, some studies report positive associations, particularly when the interaction of these polymorphisms with environmental factors has been formally evaluated. For example, a study of factor V G1691A in 88 young women with MI and 388 controls found an OR for MI of 2.4 (CI, 1. 0 to 5.9).149 The variant had little effect on nonsmokers, whereas it led to a large-risk increase in smokers (OR, 3.6; CI 0.9 to 4.4), resulting in a 32-fold increase in OR for carriers who smoked compared to noncarriers who did not smoke. Furthermore, the prothrombin G20 210A polymorphism was associated with a high risk for MI in this population (OR, 4.0; CI, 1.1 to 15.1).150 This risk was again amplified in smokers (OR, 43.3; CI, 6.7 to 281). In a large case-control study of primarily middle aged men with MI (560 patients and 646 controls; SMILE), a small increase in risk for MI was associated with both polymorphisms.151 The risk associated with these polymorphisms was amplified in smokers (OR, 6.1; CI, 3.0 to 12.5) and in those with metabolic risk factors for MI (OR, 3.2; CI, 1.5 to 6.7). A large (n = 826) population study of males and females undergoing ultrasound imaging of femoral and carotid arteries found associations between APCR and stenosis at either location.152 Decreases in APCR were associated stepwise with increased stenosis in the presence and the absence of the factor V polymorphism.
EPCR/thrombomodulin
The 23-bp insertion in the EPCR (see above) has been investigated in a single study of MI and reported in abstract form.66 It was found in 4 of 203 patients and 0 of 195 controls, suggesting an association with MI. Until recently, there has been little published work relating changes in thrombomodulin expression and arterial thrombosis. A large (n = 14,170) case-cohort prospective study of coronary heart disease and atherosclerosis, the ARIC study, has now found an inverse relationship between plasma thrombomodulin levels and risk for incident coronary heart disease.153 This suggests that low thrombomodulin levels could be associated with increased risk for coronary heart disease. Support for genetically determined levels of thrombomodulin has been provided by a recent study of a family with a private mutation in this gene.154 There are 2 polymorphisms in the thrombomodulin gene, the C/T change coding for Ala455Val (mentioned above) and G/A change coding for Ala25Thr.155,156 One report has suggested an association between Ala455Val and MI,157 but this has not been confirmed.158 The Ala25Thr dimorphism has been studied in the SMILE study, and the data are suggestive that it is associated with increased risk for MI, particularly when it interacts with smoking (OR, 8.8; CI, 1.8 to 42.2) or with metabolic risk factors for the disease (OR, 4.4; CI, 0.9 to 21.3).156 There is no biochemical evidence showing that Thr25 alters expression or function of the protein.
Fibrinolysis gene polymorphisms
tPA
tPA is the main endothelial cell-derived blood activator of the fibrinolytic system. It is a 70-kd serine proteinase activator of plasminogen whose catalytic efficiency is greatly increased by the presence of fibrin.159 For many years it was believed that impaired fibrinolysis caused by the decreased function of tPA might be a risk factor for thrombosis. Recent interest has been on elevated, rather than reduced, levels of tPA as a risk marker.160Both the US Physicians Health and the ECAT prospective studies have found an increased risk for future MI in persons with elevated levels of tPA.85,161 Possible explanations for this apparently paradoxic association are that elevated tPA levels may reflect preexisting disease or that they may be caused by increased PAI 1, with which tPA forms an inactive complex. Certainly, the predictive influence of tPA in patients with angina is greatly influenced by adjustments for insulin resistance, inflammation, and endothelial cell markers.162
Numerous nucleotide sequence differences have been identified within the tPA gene locus. That which has been studied most in respect to cardiovascular disease is the Alu insertion/deletion within the intron between exons 8 and 9.163 A relationship between the polymorphism and the phenotype has yet to be established; a study of genetically typed umbilical endothelial cells found no difference in basal tPA expression over 24 hours between different genotypes.164 It has been suggested that there may be an association between the Alu repeats and thrombosis.165However, no association was found in the US Physicians Health Study or in 114 Italian patients selected for a familial history of cardiovascular events.166 167
PAI-1
Relationship between plasma levels and disease, influence of polymorphisms on plasma levels.
PAI-1 is a member of the SERPIN family, and its main function is as the fast-acting inhibitor of tPA.168 PAI-1 exists in the circulation in molar excess over tPA, and it is generally accepted that this prevents the development of systemic fibrino(geno)lysis and permits local clot lysis without systemic bleeding.168 It appears that fibrin shields fibrinolytic activators from the inhibitory effects of PAI-1, and it has been suggested that in this way fibrin facilitates its own destruction. In spite of the inherent difficulties that this concept introduces, it seems that elevated concentrations of PAI-1 are fairly consistently associated with vascular risk. High concentrations of PAI-1 occur in the atheromatous plaque,169,170 and these changes are more marked in patients with diabetes. Circulating PAI-1 has been related to vascular outcome in a number of studies.80,171,172 These associations occur during clustering of other vascular risk markers in the presence of insulin resistance with or without diabetes mellitus.78,79 173 It remains to be determined whether the associations result from any causal effect of elevated PAI-1.
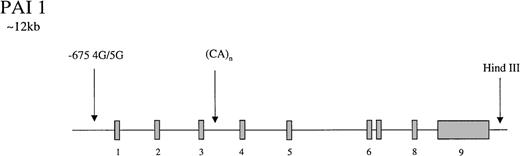
The gene for PAI-1 codes for a protein of 50-kd. It has several polymorphic loci (Figure 3), including a 3′ HindIII site, a CA(n) dinucleotide repeat in intron 3,174 and a 4G/5G insertion/deletion −675 bp from the start site of the promoter.175 The latter showed differential transcriptional responses to IL-1 in HepG2 cells with higher rates of PAI-1 synthesis in cells containing the 4G/4G genotype. It was suggested that the 4G site only binds an enhancer whereas the 5G allele binds both an enhancer and a suppressor, leading to lower rates of transcription with the 5G genotype.175 In a population of 145 young patients with MI and 95 healthy controls, Dawson et al174 found the dinucleotide repeat and the HindIII sites to be associated with variability in PAI-1 concentrations which, at the HindIII locus, appeared to be triglyceride dependent. Subsequently, the 4G/5G promoter site was reported to exhibit genotype-specific responses to triglyceride, with the highest levels of PAI-1 in 4G/4G persons with elevated triglyceride levels.176,177 These findings have been supported by laboratory studies in which a triglyceride-responsive region has been identified adjacent to the 4G/5G site.178 Considerable interest has been generated by these observations, and several studies have attempted to tease out the relative contributions of gene and environment to circulating PAI-1 levels. In a small study of 25 healthy twins (12 monozygotic), additive genetic influences accounted for 71.4% of variance in PAI-1 levels, but environment only contributed 13.2%.179 Oddly, in this study, none of the genetic influence was attributed to the 4G/5G locus, and it was suggested that either further PAI-1 gene polymorphisms or a quantitative trait locus was responsible for the genetic contribution to levels. However, a study of 5 common polymorphisms in the PAI-1 gene in a French population reported no association with PAI-1 levels.180 In support of the latter study, it has been reported that metabolic features of the insulin-resistant state are more important than genetic influences, accounting for 49% of PAI-1 variance in men.181 Numerous studies have reported that levels of PAI-1 in homozygous 4G persons are approximately 25% higher than they are in 5G/5G subjects.182-187
Representation of the organization of the PAI 1 gene,
together with the location of common polymorphisms discussed in the text.
Representation of the organization of the PAI 1 gene,
together with the location of common polymorphisms discussed in the text.
Polymorphisms and disease.
In a small study of young Swedish males with premature cardiovascular disease and controls, there were no differences in prevalence of either the dinucleotide repeat or the HindIII genotypes.174 Identification of the promoter 4G/5G site led to a flurry of publications with a mixture of results. In this study, a higher prevalence of the 4G allele was observed in the group of young Swedish males with MI than in controls.184 Similar results were obtained in a small number of subjects with type 2 diabetes and myocardial infarction.177 In a mixed-gender population of 453 patients undergoing coronary angiography, of whom 166 had a history of MI, the 4G allele was significantly associated with PAI-1 levels and with MI; the relationship with MI and the genotype were strengthened in those with established atheroma.183 In a study of 1179 healthy subjects and their first-degree relatives, the 4G allele was associated with significantly higher risk for MI (OR, 1.62; CI, 1.17 to 2.25).188 A small study has reported that the 4G allele is related to the development of acute coronary syndromes.189However, other studies, including the large ECTIM,182 the US Physicians Health Study,190 and SMILE,191failed to show any relationship between genotype and MI. A recent large study of 2565 patients undergoing coronary angiography has reported an association between the 4G allele and coronary artery disease.192 In the total sample, there was elevated risk associated with the 4G/4G genotype for coronary artery disease (OR, 1.31; CI, 1.04 to 1.65). The polymorphism was also a risk for the severity of disease when groups at high risk (smokers, persons with hypertension) were selected.
Despite a number of publications on this topic, many of the reports are conflicting and there is no consistently clear evidence that PAI-1 polymorphisms are associated with MI. A meta-analysis of many studies suggested a weak, significant effect of PAI-1 genotype on MI.193 In the context of the obvious reservations regarding meta-analysis, these finding indicate that, at most, PAI-1 genotype has a minor influence on the course of atherothrombotic disorders.
A single, large case-control study of more than 600 subjects with cerebrovascular disease, confirmed by computed tomography, failed to demonstrate any differences in genotype frequency compared than in a control group.194 Additionally, in this study there was no relationship between genotype and PAI-1 levels.
Platelet-membrane glycoproteins
The normal functional activity of platelets is dependent on the presence of platelet-membrane glycoproteins that have an important role in platelet adhesion and aggregation.195,196 Glycoprotein (Gp) IIb/IIIa (also known as integrin αIIb/β3) and Gp Ib-IX-V are membrane receptors of this family, which bind a variety of circulating ligands under differing shear conditions to promote clot formation.197 The importance of these receptors was originally noted by their absence in Glanzmann thrombasthenia (Gp IIb/IIIa deficiency) and Bernard Soulier syndrome (Gp Ib-IX-V deficiency).198
The importance of different ligands in platelet activation differs at different flow shear rates. Gp Ib-IX-V binds predominantly to the vWF molecule, which is itself tethered to damaged and exposed subendothelial collagen to promote platelet adhesion. This process activates the platelet and alters the conformation of the Gp IIb/IIIa receptor; at high shear rates it binds vWF and leads to platelet aggregation, but at lower shear rates fibrinogen is more efficient in promoting this process.197
Certain platelet surface proteins and receptors are involved in the direct interaction of platelets with collagen. The most important of these appear to be the Gp Ia/IIa complex (also known as VLA-2 and integrin α2/β1) and Gp VI.195 Both receptors were found to be deficient in patients with mild bleeding diatheses whose platelets exhibited impaired responses to collagen.199 200
Gp IIb/IIIa
Gp IIb is a disulfide-linked protein of 150-kd that associates with the 90-kd polypeptide Gp IIIa. Many coding polymorphisms in Gp IIb/IIIa have been identified in normal populations and approximately 18 mutations thought to be involved in the pathogenesis of Glanzmann thrombasthenia have been described. In Gp IIIa a common point mutation results in the substitution of Pro to Leu at position 33. The wild-type Leu (PLA1) is found in approximately 85% of the white population, whereas the Pro33 substitution (PLA2) is present in 15%.201
In April 1996 Weiss et al202 published the first report of an association between the Gp IIIa PLA2 allele and risk for myocardial infarction. In a small study of 71 patients with a diagnosis of MI or unstable angina admitted to a coronary care unit, it was reported that the PLA2 allele was overrepresented in subjects with coronary artery disease than in 68 normal controls (39.4% versus 19.1%, respectively). In the total population, PLA2 was associated with a 2.8-fold increase in risk for coronary artery disease, whereas in a subgroup of 21 subjects younger than 60 with disease, an OR of 6.2 was reported. This study was shortly followed by a case report of the death from MI of an Olympic athlete who was found to be homozygous for the PLA2 allele.203 In the 2 to 3 years after the publication of this association study, there have been a number of largely negative reports.204-209 Of particular note among these are the results of the prospective US Physicians Health Study of 374 patients with MI and 704 controls204 and the ECTIM study of 620 MI and 700 controls,205 both of which showed no suggestion of an association between the PLA2 allele and MI. Most of these studies with negative results carried out subgroup analysis to try to confirm the findings of Weiss et al202 in relation to age; again, most results were negative. Exceptions were a cohort analyzed by Carter et al210 that showed a statistically significant increase in the PLA2 allele in a small group of subjects younger than 47 and a comprehensive study of a number of prothrombotic genetic risk factors in young survivors of MI.211 In the latter case-control study of 200 survivors of MI younger than 45, none of the common polymorphisms of coagulation and fibrinolysis (factor V G1691A, prothrombin G20 210A, factor VII Arg355Gln, PAI 1 4G/5G) were strongly associated with MI. In contrast, the OR for carriers of PLA2 was 1.84 (CI, 1.12 to 3.03), and this was increased in 51 carriers who smoked, OR 13.7 (CI, 6.41 to 31.2). It was concluded that in smokers who carried the PLA2 allele, approximately 50% of early onset MI could be attributed to interaction of these 2 risk factors.
Two studies have reported an association between PLA2 and extent of coronary atheroma estimated by coronary angiography. In 1 study this association was weak (OR, 1.5; CI, 1.01 to 2.26) for the presence of more than 50% stenosis in more than 1 vessel,206 and in the other PLA2 was associated with severe coronary artery disease in patients at low risk.212 Several similar studies have reported no association with coronary atheroma.209,213 214
Two studies have addressed whether PLA2 influences outcome in patients undergoing coronary artery procedures. Walter et al215studied 318 patients followed up for 30 days and reported an OR of 5.26 for stent occlusion (CI, 1.55 to 17.8) for subjects with the PLA2 allele. Mamotte et al213 investigated the risk for coronary artery restenosis after angioplasty and reported no relationship to PLA2.
Four major studies (including the prospective US Physicians Health Study) of a total of 1140 patients with cerebrovascular disease and 1612 controls have failed to show a relationship between the PLA2 allele and disease.204,216,217 Carter et al216looked at stroke in patients younger than 47 years from their large case-control study and reported a prevalence of the PLA2 allele of 50% in this group, findings similar to those reported by the same group in young patients with MI.206 These results must be confirmed in a separate study of young patients with stroke.
Gp Ib-IX-V complex
Four gene products comprise the Gp Ib-IX-V receptor complex.196 Gp Ibα, approximately 140-kd, is disulfide bonded to Gp Ibβ, approximately 25-kd, and these polypeptides are noncovalently associated with Gp IX, approximately 22-kd and Gp V, approximately 82-kd. Two polymorphisms have attracted interest. A length polymorphism in Gp Ibα results from a variable number of tandem repeats (VNTR) of 39-bp coding for 13 amino acids in the glycosylated region (macroglycopeptide). Up to 4 polymorphic forms result, designated D, C, B, A, in order of increasing number of repeats (1, 2, 3, or 4 repeats). The function of the macroglycopeptide is thought to be that of a spacer, keeping the ligand-binding region well above the platelet surface. The second polymorphism, C to T at position 3550, results in a Thr145Met substitution and is linked to the HPA-2 alloantigen system.
A study of 101 patients with acute coronary disease, 104 patients with cerebrovascular disease, 95 patients with venous thrombosis—with matched controls—has reported an association of genotypes with arterial but not venous disease.218 For coronary disease, the associated risk of the C/B genotype was given by the OR of 2.84 (CI, 1.28 to 6.41); for cerebrovascular disease, the OR was 2.83 (CI, 1.16 to 7.07). Similarly, there was an association of Met145 with coronary disease (OR, 2.09; CI, 0.98 to 4.49). In contrast to these positive associations, no association was found between MI and Thr145Met in a case-control study of 200 young patients.211
Gp Ia/IIa complex
This collagen receptor consists of an approximately 167-kd (α2) and an approximately 130-kd (β1) polypeptide. What has been of great interest is the finding that though the receptor is expressed at low density on the platelet surface (1000 to 3000 copies), there is a wide, approximately 10-fold, variation within normal persons that results in variability of response to collagen.219 A genetic association of the density variation with linked sequence polymorphisms within the α2 gene has been reported. The variation is particularly associated with a silent exonic dimorphism at position 807, C/T. In a study of patients with von Willebrand disease (vWD), it was found that the 807C allele, associated with low receptor density, was higher in patients with-type I vWD, suggesting it might increase the tendency for bleeding.220
The significance of this polymorphism to arterial disease has been evaluated in a large study (n = 2237) of male patients undergoing coronary angiography.221 An association was found between the T allele (predicting high-receptor density) and MI in patients younger than 62 years mean age (OR, 1.57; CI, 1.14 to 2.13). Conversely, a large (n = 546) case-control study of MI was unable to establish an association of the T allele, (OR, 0.88; CI, 0.74 to 1.05), even for homozygous carriers.222 In a small (n = 45) case-control study of patients younger than 50 years with stroke, an association of the T allele with disease was found (OR, 3.02; CI, 1.20 to 7.61).
Genes other than those of hemostasis
A consistent finding in case-control and cross-sectional studies has been an association between hyperhomocystinemia and atherothrombotic disease.223,224 This association has not, however, always been found in prospective studies, for reasons that could include nutritional or genetic differences between the populations studied.75 The MTHFR C677T polymorphism has been studied extensively as a possible genetic determinant of the increased risk associated with the phenotype. A meta-analysis of 13 studies of this polymorphism in 3281 patients with cardiovascular disease (and their controls) has failed to support this link between genotype, phenotype, and disease.225
Conclusions and perspectives
A casual glance at a rapidly expanding literature could easily lead to the conclusion that we are well on the way to establishing the genetic basis for vascular disorders, such is the number of polymorphisms that have been investigated (see summary Table1). There has clearly been appreciable progress in relation to venous thromboembolic disease whereby the functional and clinical consequences of certain polymorphisms are accepted. Venous disease occurs in the context of a low-pressure, low-flow system in which atheroma does not occur. The most common genetic risk determinant for venous disease alters the function of the protein C anticoagulant pathway, whereas other genetic risk factors alter levels or activities of certain coagulation factors. Although environmental influences are undoubtedly important, genetic changes can play a crucial role and even sometimes lead to apparentlyspontaneous venous disease.
Summary of the outcome of investigations of hemostatic polymorphisms
| Polymorphism . | Phenotype . | Relationship of Phenotype to Disease . | Association of Genotype With Disease . |
|---|---|---|---|
| Venous thromboembolic disease | |||
| Factor V G1691A, Arg506Gln | APCR | Clearly established | Clear risk factor alone or in combination with other risk factors, genetic or acquired |
| Prothrombin G20210A | Altered levels, mechanism uncertain | Most likely | Moderate risk factor alone or in combination with other risk factors |
| Factor V HR2 haplotype | Slight APCR; glycosylation isoforms altered? | Suggestive | Weak risk factor alone or in combination with factor V G1691A? |
| Factor XIII Val34Leu | Increased activation rate | Suggestive | Preliminary results suggest 34Leu is protective against thrombosis |
| EPCR 23-bp repeat | Predicted altered expression on cell surface | Unknown | Preliminary results suggest it a weak/moderate risk factor |
| Arterial occlusive disease | |||
| Fibrinogen β chain −455G/A | Associated with altered level | Established, but causal? | Inconsistent |
| Fibrinogen β chainBcl I | Associated with altered level | Established, but causal? | Inconsistent |
| Fibrinogen α Thr313Ala | Altered clot structure? | Unknown | Inconsistent |
| Factor VII Arg353Gln | Altered level | Inconsistent | Inconsistent |
| Factor VII HVR4 | Probably altered level | Inconsistent | Unknown |
| Factor VII −402G/A | Altered level | Inconsistent | Unknown |
| Factor VII −401G/T | Altered level | Inconsistent | Unknown |
| Factor VII −323 0/10 | Altered level | Inconsistent | Unknown |
| Factor XIII Val34Leu | Increased activation rate | Unknown | Preliminary results suggest 34Leu is protective against MI |
| Factor V G1691A, Arg506Gln | APCR | Inconsistent | Possibly in selected patients and in combination with acquired risk factors |
| Prothrombin G20210A | Altered level | Unknown | Possibly in selected patients, and in combination with acquired risk factors |
| EPCR 23-bp repeat | Predicted altered expression on cell surface | Unknown | Preliminary results suggest it may be a weak risk factor |
| Thrombomodulin Ala455Thr | Unknown | Suggestive | Inconsistent |
| Thrombomodulin Ala25Thr | Unknown | Suggestive | Preliminary results suggest 25Thr associated with MI |
| tPA Alu insertion/deletion | Unlikely | Paradoxical | Inconsistent |
| PAI-1 −675 4G/5G | Inconsistent | Suggestive | Inconsistent |
| PAI-1 (CA)n | Suggestive of altered level | Suggestive | Unknown |
| PAI-1 Hind III | Suggestive of altered level | Suggestive | Unknown |
| Gp IIIa Pro33Leu | No effect on fibrinogen binding, but increased sensitivity to activation? | Unknown | Inconsistent |
| Gp IαVNTR | Unknown | Unknown | Preliminary results show association |
| Gp Ib; Iα C3550T, Thr145Met | Unknown | Unknown | Inconsistent |
| Gp Ia/IIa, α2 C807T | Associated with altered surface expression of receptor, altered collagen binding | Unknown | Inconsistent |
| Polymorphism . | Phenotype . | Relationship of Phenotype to Disease . | Association of Genotype With Disease . |
|---|---|---|---|
| Venous thromboembolic disease | |||
| Factor V G1691A, Arg506Gln | APCR | Clearly established | Clear risk factor alone or in combination with other risk factors, genetic or acquired |
| Prothrombin G20210A | Altered levels, mechanism uncertain | Most likely | Moderate risk factor alone or in combination with other risk factors |
| Factor V HR2 haplotype | Slight APCR; glycosylation isoforms altered? | Suggestive | Weak risk factor alone or in combination with factor V G1691A? |
| Factor XIII Val34Leu | Increased activation rate | Suggestive | Preliminary results suggest 34Leu is protective against thrombosis |
| EPCR 23-bp repeat | Predicted altered expression on cell surface | Unknown | Preliminary results suggest it a weak/moderate risk factor |
| Arterial occlusive disease | |||
| Fibrinogen β chain −455G/A | Associated with altered level | Established, but causal? | Inconsistent |
| Fibrinogen β chainBcl I | Associated with altered level | Established, but causal? | Inconsistent |
| Fibrinogen α Thr313Ala | Altered clot structure? | Unknown | Inconsistent |
| Factor VII Arg353Gln | Altered level | Inconsistent | Inconsistent |
| Factor VII HVR4 | Probably altered level | Inconsistent | Unknown |
| Factor VII −402G/A | Altered level | Inconsistent | Unknown |
| Factor VII −401G/T | Altered level | Inconsistent | Unknown |
| Factor VII −323 0/10 | Altered level | Inconsistent | Unknown |
| Factor XIII Val34Leu | Increased activation rate | Unknown | Preliminary results suggest 34Leu is protective against MI |
| Factor V G1691A, Arg506Gln | APCR | Inconsistent | Possibly in selected patients and in combination with acquired risk factors |
| Prothrombin G20210A | Altered level | Unknown | Possibly in selected patients, and in combination with acquired risk factors |
| EPCR 23-bp repeat | Predicted altered expression on cell surface | Unknown | Preliminary results suggest it may be a weak risk factor |
| Thrombomodulin Ala455Thr | Unknown | Suggestive | Inconsistent |
| Thrombomodulin Ala25Thr | Unknown | Suggestive | Preliminary results suggest 25Thr associated with MI |
| tPA Alu insertion/deletion | Unlikely | Paradoxical | Inconsistent |
| PAI-1 −675 4G/5G | Inconsistent | Suggestive | Inconsistent |
| PAI-1 (CA)n | Suggestive of altered level | Suggestive | Unknown |
| PAI-1 Hind III | Suggestive of altered level | Suggestive | Unknown |
| Gp IIIa Pro33Leu | No effect on fibrinogen binding, but increased sensitivity to activation? | Unknown | Inconsistent |
| Gp IαVNTR | Unknown | Unknown | Preliminary results show association |
| Gp Ib; Iα C3550T, Thr145Met | Unknown | Unknown | Inconsistent |
| Gp Ia/IIa, α2 C807T | Associated with altered surface expression of receptor, altered collagen binding | Unknown | Inconsistent |
Data on each polymorphism are summarized in terms of the nature of its phenotype, what is known of the relationship between the phenotypic change (not necessarily genetically determined) and the disease, and the direct association of the genotype to the disease.
In contrast, there is little clarity in relation to arterial disease (see Table 1). The initial promise that genetic risk factors might contribute appreciably to an explanation of the development of arterial disease has largely been unfulfilled, and the expectations raised by early reports of positive associations have been tempered by inconsistent results with almost all genes studied. Arterial disease occurs in a high-pressure, high-flow system with atheromatous disease as a dominant feature. Hemostatic factors have been extensively studied as possible risk determinants for disease. The most consistent associations have been found for fibrinogen. Although it is formally possible that fibrinogen is merely a marker of another underlying process, such as the acute-phase reaction,226 a strong case can be made that fibrinogen is causally involved in disease. For each of the other hemostatic factors, though a causal role is highly plausible, the evidence for this is less strong. It is interesting to note that hemostatic risk factors for atherothrombotic disorders are largely those that have been associated to some extent with classical risk markers such as features of insulin resistance. These observations indicate the importance of environmental influences and emphasize the complexity of the processes involved in vascular disease. When individual hemostatic factors do contribute to risk, it is in concert with myriad other metabolic factors. This understanding further increases the importance of lifelong risk interactions and may suggest an explanation for some of the inconsistencies in case-control studies of arterial disease. The available studies have often been too small to be informative in terms of the interactions between environment and genetic polymorphisms. Additionally, some of the problems in identifying causal genetic markers are related to difficulties associated with the precise definition of the clinical phenotype under study. These have been compounded by inadequate consideration of issues surrounding the distribution of polymorphisms in the population. Examples of factors that may be responsible for some of the observed inconsistencies are as follows. (1) The effects of common single-gene changes are often minor; study size and power are therefore critical issues. (2) There is plurality in clinical endpoints (MI, unstable angina, coronary artery disease, progression of arterial disease, stroke), and these have been often selected in a post-data collection search for significance. (3) Genetic polymorphisms may vary appreciably within and between racial groups. (4) Numerous studies have paid insufficient attention to minimizing recruitment bias in the selection of patients, controls, or both. (5) It is likely that the greatest effect of the gene polymorphism will most often be in association with (a) specific (set of) environmental changes that may vary between study cohorts and that will require very large studies to define.
Additionally, the importance of heritability of risk factors has generally not been fully appreciated. The apparent paradox of a firm relationship between gene and protein and protein and disease, but inconsistent gene–disease relationships, must be related to the quantitative contribution of heritability to the phenotype (circulating protein levels or density of expression in the case of receptors). Taking fibrinogen as an example of a plasma protein, there is a clear link between circulating levels and all forms of atherothrombotic disorders. Although most studies indicate that fibrinogen polymorphisms are related to levels, the relationship between fibrinogen genotype and disease remains uncertain. Most reports have estimated the contribution of individual polymorphisms on the variance of plasma levels to be approximately 5%. Formal heritability studies suggest that thetotal genetic influence on levels is approximately 50%. It follows from this that any currently known polymorphism in the fibrinogen gene locus can only contribute to a small (less than 10% of the effect of total level) extent to vascular risk and may therefore be undetectable in most sample sizes. If the genetic components that contribute 100% of the heritable variance in fibrinogen could be identified, they would be expected to have a predictive power for disease similar to fibrinogen levels. A key issue for the future, therefore, must be the identification of all the major heritable influences on variance. Some of these may lie within or immediately around the gene of interest and have to date evaded detection, but others may lie outside the gene loci. Fibrinogen is a strong case for further study, for the reasons mentioned above. For those factors in which the relationship between circulating levels of a protein (or its activity, or its receptor density) and disease is weak, establishing relationships between genetic determinants of variance and disease is likely to remain difficult even if the major genetic influences on heritability are identified. It can be concluded that the study of population genetics of polygenic disorders, such as arterial thrombotic disease, would ideally require prior knowledge of the relationship between the protein level/receptor density and disease, the degree of heritability of variance in the plasma levels of the protein/receptor density, and the genetic determinants of heritability.
In future studies, attention should be paid to identifying more clearly the optimum clinical phenotype and, in general, to applying a more rigorous approach to the clinical aspects. Because it is highly unlikely that single genetic polymorphisms will be sole determinants of disease, future studies should be designed specifically to investigate interactions between potential genetic polymorphisms and acquired risk factors. They should be designed at their outset to minimize recruitment bias and to have clear and precisely defined clinical endpoints. Because of these requirements, they should be larger than are currently used, unless of course they are early hypothesis-forming studies. A realistic position with regard to radical treatments for MI must be adopted. It must be clear from the above, and from the slow progress developing gene delivery systems, that gene therapy to alter hemostatic polymorphic phenotypes is a weak option. The immediate value of the genetic approach lies in increasing the understanding of mechanisms of disease and thereby giving the real opportunity for developing novel and selective drug-based therapies for the prevention of disease.
There is a growing number of potential genetic risk factors for occlusive thrombotic disease. This review has concentrated on hemostatic genes implicated in disease. The perspective in this field may change because of the international drive to complete the sequence of the human genome. There may be many novel genes involved in blood vessel development or in blood cell function awaiting identification and characterization that could play important roles in modifying the disease process. It might be that the hemostatic genes have to interplay with other genetic influences before they can influence significantly disease progression. Consequently, though there will no doubt continue to be expanding interest in this area, substantial further development may benefit from a clearer understanding of the pathophysiologic processes involved in the development of vessel disease in atherothrombotic disorders.
Supported by grants from the British Heart Foundation and the Stroke Association.
Reprints:David A. Lane, Department of Hematology, Imperial College School of Medicine, Charing Cross Campus, Hammersmith, London W6 8RP, United Kingdom; e-mail: d.lane@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.