After bone marrow transplantation (BMT) using T-cell–depleted marrow from an unrelated donor or HLA-mismatched related donor, the risk of developing lymphoproliferative disease associated with the Epstein-Barr virus (EBV) ranges from 1% to 25%. We have shown that administration of donor-derived EBV-specific cytotoxic T lymphocytes (CTL) is effective prophylaxis and treatment for this complication, and we routinely generate CTL for high-risk patients. However, EBV lymphoma can occur in recipients of matched-sibling transplants for whom CTL are unavailable or in patients for whom CTL administration is contraindicated. We report on 3 such patients, who were successfully and safely treated with rituximab, a CD20 monoclonal antibody. The patients remain disease free 7, 8, and 9 months, respectively, after therapy. We conclude that CD20 antibody may be a useful alternative treatment strategy in patients with EBV lymphoma after BMT.
The risk of B-cell lymphoproliferative disease (LPD) after bone marrow transplantation (BMT) has been associated with the receipt of bone marrow from HLA-mismatched family members or unrelated donors. If the infused donor marrow is treated with antibodies that selectively deplete T cells to decrease the risk of graft-versus-host disease (GVHD), Epstein-Barr virus (EBV) LPD occurs in 12% to 25% of patients.1-4 Treatment of LPD has focused largely on enhancing the immune response to EBV. Adoptive immunotherapy with unselected donor leukocytes, which contain high precursor frequencies of cytotoxic T lymphocytes (CTL) reactive with EBV in most seropositive individuals, has proved to be effective.5 However, such unmanipulated products may also contain a high frequency of alloreactive T lymphocytes and induce GVHD.3-6 To avoid the problem of alloreactivity, we routinely generate donor-derived EBV-specific CTL for patients at high risk of EBV LPD.7-9This strategy has reconstituted immunity to EBV and reduced the rate of EBV lymphoma in recipients of marrow from unrelated or mismatched donors from 11.5% to 0% when used prophylactically.8,9EBV-specific CTL have also been efficacious as therapy.9However, there are some limitations to using CTL. Occasionally, EBV-associated LPD develops in low-risk patients after matched-sibling BMT when prophylactic CTL are not available. Furthermore, in patients in whom EBV LPD develops after autologous BMT or intensive chemotherapy, use of donor-derived T cells is not a therapeutic option. There are also certain clinical situations in which CTL therapy may produce morbidity or be ineffective. For example, adoptively transferred CTL may not persist in patients receiving steroids at the time of treatment or may cause inflammation in patients with bulky or infiltrative disease in which morbidity from CTL-induced inflammatory responses occur.9,10 Finally, resistance to the infused CTL can develop through mutations of EBV epitopes recognized by the CTL.11
Other immunologic treatments for EBV-associated LPD include anti–B-cell antibodies, such as anti-CD21 and anti-CD24.12However, the antibodies used in studies of these agents are not currently available for routine clinical applications and did not have consistent activity against EBV lymphomas arising after BMT.13 Recently, a chimeric human-mouse antibody against the B-cell antigen CD20 (rituximab) was approved by the US Food and Drug Administration (FDA) for the treatment of relapsed CD20-positive low-grade or follicular lymphoma.14 15 Its potential efficacy in the treatment of other B-cell malignant diseases is currently being investigated. We here demonstrate in 3 patients with posttransplantation lymphoma that anti-CD20 is well tolerated and can be used successfully.
Study design
Transplantation procedure
BMT was performed according to institutional review board (IRB)-approved protocols. One patient received marrow from a matched sibling and 2 received marrow from matched unrelated donors. All patients received cytarabine (3 g/m2 of body surface area [BSA]; 6 doses) and cyclophosphamide (45 mg/kg of body weight; 2 doses), with mesna (45 mg/kg of body weight, divided into 5 doses) administered before and 3, 6, 9, and 12 hours after each dose of cyclophosphamide.16 Antithymocyte globulin was administered to recipients of unrelated-donor marrow as part of the conditioning regimen to enhance immunosuppression. The patients also received total-body irradiation in 8 fractions, for a total of 12 Gy (matched-sibling marrow recipient) or 14 Gy (unrelated-donor marrow recipients). Marrow from the unrelated donors was depleted of T lymphocytes with use of the anti–T-cell antibodies CD6 and CD8 and rabbit complement.16 Beginning 2 days before BMT, both the recipient of the matched-sibling marrow and the recipients of the matched-unrelated-donor marrow received cyclosporine in a dosage that was adjusted to attain plasma levels of 200 to 350 ng/mL. The recipient of the T-cell–replete matched-sibling marrow received additional prophylaxis with a short course of methotrexate.
Detection of EBV DNA and meaning of levels
Peripheral blood mononuclear cells (PBMC) were isolated from blood samples on lymphopreparation gradients (10-40 mL each). Genomic DNA was then isolated on an anion exchange column (Qiagen, Valencia, CA) from 1 × 106 to 1 × 107 mononuclear cells, and samples of 0.01 to 103 ng were amplified with primer sequences that detect a single copy of an EBV-DNA BamHI-C segment, as previously described.17 Positivity was defined as a detectable EBV-specific signal on Southern blotting with 0.01 ng of DNA from BL2/B95-8 or IB4 cells (which both contain 2 integrated EBV genomes per cell) diluted in 1 mg of DNA from an EBV genome-negative cell line, BL41. We previously demonstrated that the EBV load in peripheral blood can be used as a direct measure of the onset of posttransplantation LPD.17 Measurement of EBV DNA in healthy donors showed the median EBV genome copy number per microgram of peripheral blood DNA was 20 (range, 0-400). The onset of posttransplantation LPD is usually preceded by an increase in EBV load, and a 1 to 3 log increase in EBV DNA has been found to be highly predictive of subsequent development of EBV lymphoma.
Immunophenotyping
For cell-surface phenotyping, PBMC were incubated with combinations of fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated monoclonal antibodies to CD3, CD4, CD8, CD16, CD56, CD19, and CD20 (Becton Dickinson, San Jose, CA). Cells were analyzed on a MoFlo flow cytometer (Cytomation, Fort Collins, CO).
Results and discussion
Patients
Case 1: High-risk patient with no available CTL.
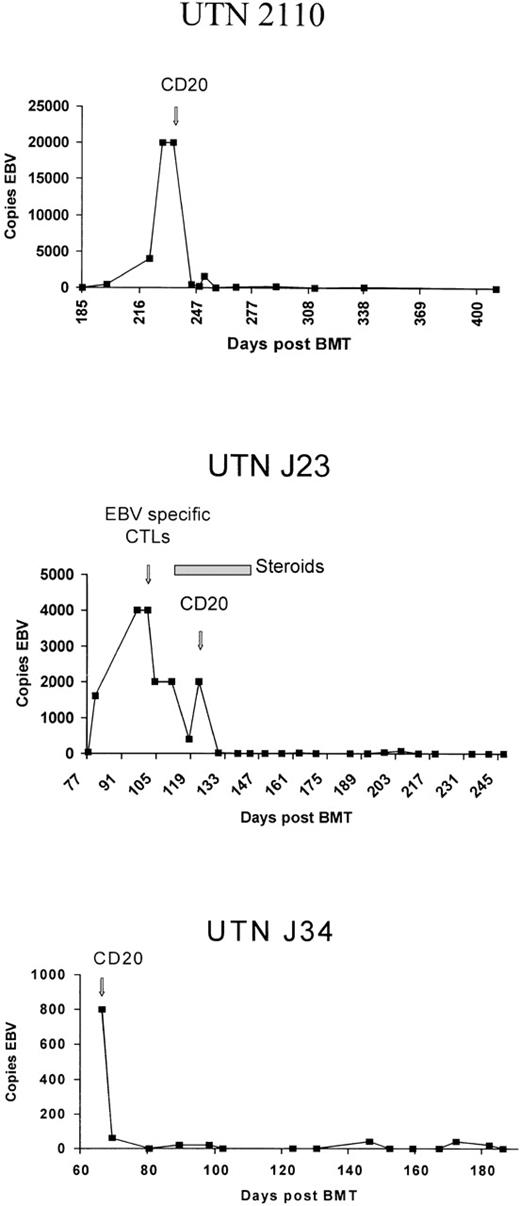
UTN2110, a 2-year-old boy with acute myelogenous leukemia (ALL) in second clinical remission, received an HLA-identical, T-cell–depleted marrow transplant from an unrelated donor. His posttransplantation course was complicated by GVHD of the liver and skin. On day 228 after BMT, fever, hepatomegaly, diffuse cervical and inguinal adenopathy, and elevated levels of transaminases developed. The patient was found to have extremely high EBV DNA levels (20 000 genomes/μg) in the peripheral blood, which we previously showed correlate strongly with the development of EBV lymphoma.17 Donor-derived EBV-specific CTL were not available, and the preexisting GVHD precluded use of donor lymphocytes. On day 237 after BMT, the patient received a single dose of rituximab (375 mg/m2 of BSA). As shown in Figure1, DNA copy numbers decreased within 7 days after the treatment, accompanied by normalization of levels of transaminases and resolution of clinical findings and symptoms. The patient remains disease free 9 months after treatment with rituximab.
Epstein-Barr virus DNA levels in 3 patients before and after administration of rituximab.
Epstein-Barr virus DNA levels in 3 patients before and after administration of rituximab.
Case 2: High-risk patient with pulmonary infiltrates receiving steroid therapy.
UTNJ23, a 4-year-old boy with relapsed ALL, was given a T-cell–depleted graft from a matched unrelated donor. The patient's EBV DNA levels increased from 40 to 4000 genomes per μg over 3 weeks. On day 90 after BMT, he received 1 dose of 2 × 107 donor-derived EBV-specific CTL in an IRB-approved protocol.18 The following week, an intercurrent upper respiratory tract infection with fever and a left lower lobe pulmonary infiltrate developed. The pulmonary infiltrate was thought to be likely due to the respiratory tract infection, although the possibility that it was caused by an inflammatory response mediated by the infused CTL could not be excluded.
After initiation of steroid therapy, the patient improved clinically, but the pulmonary infiltrates recurred when the steroids were discontinued. EBV DNA concentrations decreased initially in response to the CTL but, as shown in Figure 1, increased again when the corticosteroid dosage was increased. Additional CTL were not administered because of concern that this might exacerbate the patient's respiratory condition, so the patient received 1 dose of rituximab (375 mg/m2 of BSA). EBV DNA decreased rapidly to undetectable levels, the pulmonary infiltrates resolved, and the patient remains clinically well 8 months after therapy. Although it is impossible to distinguish the relative contributions of the EBV-specific CTL and the CD20 antibody to the therapeutic response, CD20 antibody was a useful alternative to an additional dose of CTL in this clinical situation, where there was concern that the CTL might exacerbate the patient's respiratory insufficiency.
Case 3: EBV LPD in a low-risk BMT recipient with no CTL available.
UTNJ34, a 13-year-old boy with chronic myelogenous leukemia, received an unmanipulated bone marrow transplant from a matched sibling. After BMT, he received a short course of steroid therapy for grade I GVHD. Thirty-three days after BMT, the patient presented with persistent fever; an extensive infection workup yielded negative results. On day 60 after BMT, bilateral cervical and inguinal tenderness and adenopathy and splenomegaly developed. EBV DNA levels became markedly elevated at the time the adenopathy appeared. A lymph node biopsy showed histologic characteristics of large-cell lymphoma, and in situ hybridization was positive for EBV DNA. Because the patient was at low risk for posttransplantation EBV lymphoma, donor-derived EBV-specific CTL were not available for him. Therefore, he received 1 dose of rituximab (375 mg/m2 of BSA), which resulted in clinical improvement in adenopathy, resolution of fever, and a decrease in EBV DNA to normal levels. Seven months after treatment, the patient remains well and EBV DNA continues to be undetectable.
Rituximab is a CD20 antibody recently approved by the FDA for therapy of relapsed CD20-positive follicular lymphoma.14,15 Because this antibody has a human component, its half life is longer than that of murine antibodies: rituximab has induced profound B-cell depletion for 6 months. We phenotyped peripheral blood from the 3 BMT patients in this study, starting 5 months after the administration of rituximab therapy to the time of the most recent follow-up (Table 1). The 2 patients given rituximab 9 months ago and 7 months ago, respectively, have normal B-cell numbers and levels of immunoglobulins. The third patient, who was given the CD20 infusion 8 months ago, continues to have profound B-cell deficiency and hypogammaglobulinemia. None of our patients had an increase in opportunistic infections. There is one other report of CD20 administration after BMT.19 The patient also responded rapidly to the first dose of this therapy, which led us to choose a single dose with subsequent close monitoring of EBV DNA levels. Although these results need to be confirmed in larger series, they do illustrate a potential role for therapy with CD20 as an adjuvant to approaches using T-cell immunotherapy to treat EBV lymphoma.
Phenotyping results and levels of immunoglobulins in 3 patients before and after administration of rituximab
| Patient . | Day after BMT . | Day after Rituximab Administration . | Absolute Lymphocyte Count (×106/mL) . | Total CD19/20 Cells (×106/mL) . | Total CD3 Cells (×106/mL) . | Immunoglobulins at most recent follow-up (g/L)* . |
|---|---|---|---|---|---|---|
| UTN2110 | 411 | 174 | 2180 | 380 | 1350 | |
| 509 | 258 | 2368 | 497 | 1560 | ||
| 535 | 284 | 1716 | 480 | 1100 | IgG, 7.9; IgA, 0.7; IgM, 0.6 | |
| UTNJ23 | 248 | 124 | 2140 | 9 | 1370 | |
| 278 | 154 | 3050 | 2 | 700 | ||
| 311 | 187 | 1950 | <1 | 937 | ||
| 342 | 218 | 2880 | <2 | 1411 | IgG, 1.5; IgA, 0.004; IgM, 0.009 | |
| UTNJ34 | 189 | 115 | 490 | 3 | 264 | |
| 238 | 164 | 820 | 311 | 262 | ||
| 257 | 183 | 540 | 145 | 227 | ||
| 288 | 214 | 1050 | 283 | 546 | IgG, 11.9; IgA, 0.5; IgM, 0.9 |
| Patient . | Day after BMT . | Day after Rituximab Administration . | Absolute Lymphocyte Count (×106/mL) . | Total CD19/20 Cells (×106/mL) . | Total CD3 Cells (×106/mL) . | Immunoglobulins at most recent follow-up (g/L)* . |
|---|---|---|---|---|---|---|
| UTN2110 | 411 | 174 | 2180 | 380 | 1350 | |
| 509 | 258 | 2368 | 497 | 1560 | ||
| 535 | 284 | 1716 | 480 | 1100 | IgG, 7.9; IgA, 0.7; IgM, 0.6 | |
| UTNJ23 | 248 | 124 | 2140 | 9 | 1370 | |
| 278 | 154 | 3050 | 2 | 700 | ||
| 311 | 187 | 1950 | <1 | 937 | ||
| 342 | 218 | 2880 | <2 | 1411 | IgG, 1.5; IgA, 0.004; IgM, 0.009 | |
| UTNJ34 | 189 | 115 | 490 | 3 | 264 | |
| 238 | 164 | 820 | 311 | 262 | ||
| 257 | 183 | 540 | 145 | 227 | ||
| 288 | 214 | 1050 | 283 | 546 | IgG, 11.9; IgA, 0.5; IgM, 0.9 |
BMT indicates bone marrow transplantation.
Normal ranges are 6.4 to 13.4 g/L for IgG, 0.7 to 2.6 g/L for IgA, and 0.4 to 1.8 g/L for IgM.
Might CD20 therapy eventually replace adoptive immunotherapy with donor-derived T cells or CTL? There are potential detrimental effects of CD20 therapy. The profound B-cell depletion may further exacerbate immunodeficiency in transplant recipients, although eventual recovery should occur because CD20 is not expressed on B-cell precursors. It is also unclear whether a lack of EBV-infected B cells will retard recovery of EBV-specific immunity and thereby allow the development of lymphomas later in the posttransplantation course. Moreover, it is unclear whether depletion of the B-cell reservoir of EBV will result in development of a primary infection in the future. Finally, CD20 therapy may cause selection of a CD20-negative population of proliferating B cells, as has been reported in a few patients with lymphoma.20 Ultimately, both CD20 and EBV-specific CTL may be needed to achieve optimal results.
Mortality from EBV lymphoma may be reduced in other ways. Studies have shown that the risk is lowered if methods that deplete donor B cells as well as donor T cells are used. In a large review of patients whose marrow transplants were treated with the CAMPATH series of antibodies, the EBV-LPD rate was < 2%.21 A low rate also occurred after elutriation, which removes > 90% of B cells from the donor graft.22 In another study, addition of a monoclonal antibody that depleted B cells to the T-cell–depletion regimen reduced the occurrence of EBV-LPD from 7 of 19 historical controls to none of the next 19 patients.23 Hence, it seems likely that the introduction of modified methods of graft manipulation, coupled with the availability of antibody and cellular-based therapies, should substantially reduce future mortality from EBV lymphoma in BMT recipients.
Supported by grant CA 61384 from the National Cancer Institute.
Reprints:Helen Heslop, Center for Cell and Gene Therapy, Baylor College of Medicine, 1102 Bates Street, Suite 1140, Houston, TX 77030; e-mail: hheslop@bcm.tmc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal