Granzyme A (GrA) and B (GrB) together with perforin are the main constituents of cytotoxic granules of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. The cytotoxic proteins are released to deliver a lethal hit during contact between the CTL or NK cell and target cell. With the use of an enzyme-linked immunosorbent assay for antigenic levels, we showed in a recent study that plasma of patients with activated CTLs and NK cells contain elevated levels of extracellular GrA. In this study, we determined the form and proteolytic capacity of this extracellular GrA detected in plasma. With the use of various assays, we show that part of the extracellular GrA circulates in the mature conformation and is bound to proteoglycans that protect it against inactivation by protease inhibitors, such as antithrombin III and -2-macroglobulin, whereas another part of GrA circulates as a complex with antithrombin III. Finally, with the use of a novel assay for active GrA, we demonstrate that some plasma samples with high levels of extracellular GrA contain active GrA. These results suggest that various forms of extracellular GrA occur in vivo and that the regulation of GrA activity may be modified by proteoglycans. These data support the notion that granzymes may exert extracellular functions distant from the site of CTL or NK cell interaction with their target cells.
Granzymes A (GrA) and B (GrB) together with perforin are effector molecules of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. The concerted action of these molecules induce apoptosis of the target cell. According to the classic model of degranulation, granzymes enter the target cell via pores formed by multimerization of perforin molecules in the target cell membrane.1,2 An alternate model has been proposed in which GrB undergoes receptor-dependent endocytosis, followed by delivery to the cytosol via perforin-mediated permeabilization of the internalized vesicle.3,4 A specific receptor for GrA may also exist, suggesting that granzymes may have extracellular signaling functions.5
Granzymes are packaged in cytotoxic granules with negatively charged proteoglycans (PG) containing the glycosaminoglycan, chondroitin sulfate A.6-8 After granule exocytosis, a tryptase-like activity is secreted extracellularly that remains associated with PG complexes, but it is unclear whether this is due entirely to GrA.6,7,9 CTL and NK cells may also constitutively secrete the granzyme in the absence of the PG.10 Therefore, free or complexed GrA could reach the extracellular space where the proteinase mediates such biologic effects as cytokine induction5 or killing pathogen-infected bystander cells.11 However, the action of the extracellular granzymes would be bridled by circulating antiproteinases. Previous studies have shown that human GrA is inhibited by antithrombin III (ATIII)12 and alpha-2-macroglobulin (α2M)13 in vitro, suggesting granzymes in vivo would be rapidly inactivated by circulating antiproteinases.
We demonstrated recently that patients with various viral diseases or with rheumatic arthritis have elevated levels of circulating GrA and GrB.14 It was not clear, however, whether GrA detected by the GrA-ELISA (called hereafter antigenic GrA ELISA [enzyme-linked immunosorbent assay]) was the circulating zymogen, the free active GrA, active GrA complexed to PG, or granzyme inactivated by plasma-associated inhibitors. The aim of the present study was to investigate the nature of circulating GrA. We developed specific ELISAs for GrA-ATIII complexes and for GrA with a mature conformation. In addition, we established size exclusion chromatography conditions that allowed the detection of GrA bound to PG complexes. With the use of these assays, we show that GrA either was inactivated by ATIII or was circulated in the active form bound to PG.
Materials and methods
Reagents
Sepharose CL4B and CNBr-activated Sepharose 4B were purchased from Pharmacia Biotech AB (Uppsala, Sweden), benzamidine hydrochloride hydrate from Acros Organics (Geel, Belgium), and phenylmethylsulfonyl fluoride from Sigma Chemical Company (St Louis, MO).
Proteins
GrA was purified to homogeneity from interleukin 2 (IL-2)-activated mononuclear cells as described.15 It was radiolabeled with125I, according to the Iodo-gen method that yielded a specific activity of 1.6 MBq per μg of protein. Recombinant human GrA zymogen-containing culture medium of HepG2 cells infected with GrA recombinant vaccinia virus was a gift from Dr A. Kummer (Free University Hospital, Amsterdam, The Netherlands). Cathepsin C (sequence quality) and ATIII were obtained from Boehringer Mannheim GmbH (Mannheim, Germany).
Antibodies
Monoclonal antibodies (mAbs) recognizing native GrA and GrB were produced and purified as described previously.14 The mAb anti-ATIII-0 used for the detection of GrA-ATIII complexes was produced and purified as described.16 The mAb M1 directed against inactivated (complexed) α2M has been described before.17
Clinical samples
Plasma samples from a recipient of a renal transplant who developed a cytomegalovirus (CMV) infection were kindly provided by Dr R. J. M. ten Berge (Academic Medical Center, Amsterdam, The Netherlands). The diagnosis of CMV infection was based on the appearance of anti-CMV immunoglobulin M (IgM) antibodies with seroconversion to anti-CMV IgG antibodies and CMV-positive buffy coat cultures. Blood from patients with Dengue fever were provided by Dr M. Juffrey (Djokyakarta, Indonesia). Those patients were part of a larger study of the immunopathogenesis of Dengue fever. Results of those studies will be described in detail elsewhere. All blood samples were collected in tubes containing 0.1 mg/mL soybean trypsin inhibitor, 10 mmol/L EDTA, and 20 mmol/L benzamidine. Plasma samples were obtained by centrifugation at 1300g for 10 minutes and stored at −70°C until tested.
Biotinylation of antibodies
Purified antibodies were biotinylated with the use of long chain biotinyl-N-hydroxysuccinimide ester sulfonic acid (Pierce Chemical Co, Rockford, IL), according to the instructions of the manufacturer.
ELISA for antigenic GrA
The ELISA used for the detection of soluble GrA was modified from that described previously.14 Briefly, mAb GA29 was used as a catching mAb and biotinylated mAb GA28 was used as the detecting mAb. The ELISA procedure will be described and referred to as standard ELISA procedure hereafter. Microtiterplates (Nunc Maxisorb Immunoplate, Nunc, Copenhagen, Denmark; 100 μL/well) were coated with the catching mAb (2 μg/mL in 0.1 mol/L sodium carbonate/bicarbonate buffer, pH 9.6 [CB]) for 16 hours at room temperature. After washing 5 times with phosphate-buffered saline (PBS)/0.02% (w/v) Tween-20, which was repeated after each incubation step, residual binding sites were blocked by 30 minutes of incubation with 2% (v/v) cow milk in PBS. Samples were appropriately diluted in High Performance ELISA buffer (Department of Immune Reagents, CLB, Amsterdam, The Netherlands) and incubated for 1 hour. After a washing procedure, plates were then incubated with an excess of biotinylated detecting mAb in PBS/0.1% (w/v) Tween-20 (PT) containing 1% (v/v) normal mouse serum for 1 hour. Thereafter, the plates were incubated for 30 minutes with streptavidin-polymerized horseradish peroxidase (Department of Immune Reagents, CLB) in High Performance ELISA buffer, whereby bound peroxidase was visualized by incubation with a solution of 100 μg/mL of 3,3′,5,5′-tetramethylbenzidine (Merck, Darmstadt, Germany) and 0.003% (v/v) H2O2 in 0.11 mol/L sodium acetate buffer, pH 5.5. The reaction was stopped by addition of an equal volume of 2 mol/L H2SO4 to the wells. Finally, the absorbance at 450 nm was read on a Titer-Tek Multiscan plate reader (Labsystems, Helsinki, Finland).
ELISA for GrA in the mature conformation
GrA in the mature conformation was specifically detected with an ELISA (performed according to the standard procedure described above), in which mAb GA 29 (2 μg/mL) was used as the catching and biotinylated GA21 (1 μg/mL), recognizing GrA in the mature conformation only, as the detecting mAb. The specificity of mAb GA21 was derived from experiments in which the mAb was shown to bind to GrA only after activation of the zymogen by Cathepsin C.18
ELISA for active GrA with the use of biotinylated ATIII
Active GrA can form stable complexes with the plasma proteinase inhibitor ATIII.12 This property of GrA is the basis for a novel ELISA we developed to assess proteolytically active GrA at a pg/mL level (assay to be described elsewhere). Briefly, samples to be tested were incubated in plates coated with mAb GA29. Plates were washed, whereby active GrA bound to the plates was detected by an incubation of biotinylated ATIII in the presence of heparin.
ELISA for GrA-ATIII complexes
GrA-ATIII complexes were detected in a standard ELISA system in which mAb GA29 (2 μg/mL) was used as the catching mAb and biotinylated mAb aATIII-0 (0.5 μg/mL), directed against ATIII, was used as the detecting mAb.
Culturing and degranulation of lymphokine activated killer (LAK) cells
LAK cells were generated from peripheral blood mononuclear cells isolated from citrated (0.01 mol/L) plasma by Percoll 1.078 density centrifugation. Peripheral blood mononuclear cells were cultured (Iscoves modified Dulbecco's medium) in the presence of 1000 U/mL IL-2 (Chiron Corp, Emeryville, CA) for 6-10 days. Cells were washed 5 times in PBS and then lysed for 30 minutes on ice in lysis buffer [ie, 50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, and 1% (w/v) Nonidet P-40 (NP40; Sigma)]. The lysate was centrifuged for 10 minutes at 10 000 rpm to remove the nuclei. Both medium and lysate were stored at −70°C until used.
For degranulation experiments, peripheral blood mononuclear cells cultured for 6 days with IL-2 were washed five times in Iscoves modified Dulbecco's medium, after which the cells were resuspended at a concentration of 1 × 106 cells/mL in Iscoves modified Dulbecco's medium, 0.01% (w/v) BSA, 0.2 ng/mL PMA and incubated with 3 mAbs against CD2 (4B2, 6G4, and Hic 27; CLB; each at a concentration of 1 μg/mL) for 5 hours. The supernatant was collected by centrifugation for 10 minutes at 1800 rpm to remove intact cells and stored as “degranulation medium” at −70°C until used.
Affinity purification of GrA
MAb GA 21 was precipitated from conditioned hybridoma medium by 50% ammonium sulfate, dissolved in PBS, and coupled to CNBr-activated Sepharose 4B (30 mg protein to 1 g of Sepharose), according to manufacturer's instructions. The beads were incubated with GrA-containing medium or lysate diluted in PBS/0.1% (w/v) Tween-20, washed, and put into a column. Bound GrA was eluted from the column by gradually decreasing the pH of the elution buffers (25 mmol/L K2HPO4/KH2PO4, 125 mmol/L NaCl, pH 7.0; 25 mmol/L K2HPO4/KH2PO4; 125 mmol/L NaCl, pH 6.0; 25 mmol/L Na acetate, 125 mmol/L NaCl, pH 5.0; 25 mmol/L NaAcetate, 125 mmol/L NaCl, pH 4.0; 25 mmol/L glycine, 125mmol/L NaCl, pH 2.5). GrA in the fractions was measured with the ELISA for antigenic GrA. GrA-containing fractions were pooled and dialysed against 25 mmol/L Na acetate/125 mmol/L NaCl, pH 5.2. Samples were concentrated (Centriprep concentrator; Amicon; Beverly, MA) and stored at −70°C until used.
SDS-PAGE electrophoresis and Western blotting
Protein samples were separated on 12.5% (w/v) SDS-PAGE gels. Proteins were visualized either by autoradiography (radiolabeled proteins) or by silver staining or with Western blotting by specific antibodies. For this latter process, proteins were transferred onto nitrocellulose sheets (Schleicher and Schuell Inc, Germany), according to standard laboratory procedures. After blotting, the nitrocellulose sheets were incubated in PBS containing 4% (w/v) nonfat dry milk (ELK; Campina Melkunie BV, Eindhoven, The Netherlands) for 30 minutes to block the residual binding sites. Next, the sheets were incubated for 1 hour with mAb GA419 at 2 μg/mL in PT/0.4% (w/v) dry milk. The blots were then washed 3 times, each for 10 minutes, in PT and probed with horseradish peroxidase-conjugated rat anti-mouse immunoglobulins (Department of Immune Reagents, CLB) for 30 minutes. After a second extensive washing procedure, the sheets were stained by incubation with 4-Chloro-1-naphthol (Sigma) and 3,3′-Diaminobenzidine (Sigma) at 1.4 mmol/L and 2.3 mmol/L in PBS/0.01% H2O2(v/v), respectively. The reaction was stopped by incubating the blots in distilled water.
Size exclusion chromatography
Protein samples were run on a 60-mL Sepharose CL4B column equilibrated in PBS/0.01% (w/v) Tween-20, with or without 350 mmol/L NaCl, at a flow rate of 0.5 mL/min. The size exclusion chromatography column was calibrated by assessing the elution profile of standard proteins. The void volume was determined with Dextran Blue 2000 (Pharmacia).
Results
Interaction of conventionally purified GrA with ATIII and 2M
Plasma contains several inhibitors, such as ATIII, α2M, and α1-proteinase inhibitor, that potentially inhibit GrA.20 Hence, we studied the contribution of each of these inhibitors. 125I-labeled conventionally purified GrA was added to plasma in the presence or absence of heparin, and complex formation with inhibitors was studied by gel electrophoresis. In the absence of heparin (heparin potentiates the inhibitory activity of ATIII toward various proteases21including GrA12), GrA was bound to a large protein (Figure1A, lane 2) that after reduction migrated at approximately 200 kD (Figure 1A, lane 7). This pattern is characteristic of the interaction of proteases with α2M,22 which has an Mr of 720 kDa and 180 kDa under nonreduced and reduced conditions, respectively. Indeed, these bands were precipitated by an mAb that reacts with complexed α2M (Figure 1A, lanes 5 and 7). In the presence of heparin, however, GrA predominantly formed complexes migrating at approximately 90 kDa and 160 kDa in plasma (Figure 1B, lane 2). Because these complexes only occurred with heparin, we speculated that the bands represented the interaction of dimeric GrA (46 kDa)19with one or two molecules of ATIII (58 kDa). After reduction, a single band was identified at 80 kDa (Figure 1B, lane 6) that agrees with the expected behavior for complexes containing monomeric GrA (23 kDa) and ATIII (58 kDa). Furthermore, the complex could be precipitated with an mAb that recognizes ATIII (Figure 1B, lane 4 and 8).
Complex formation of radiolabeled granzyme A (GrA) to proteinase inhibitors in plasma.
Approximately 1 pg of 125I-GrA was incubated with 2 μL of plasma for 30 minutes at 20°C. Next, samples were either prepared for SDS-PAGE directly or incubated with 2 mL monoclonal antibody (mAb) coupled to Sepharose beads (2 mg/mL) for 30 minutes followed by recovery of the Sepharose-bound proteins that then were prepared for SDS-PAGE. After electrophoresis, the gel was dried and labeled; GrA was visualized by autoradiography. (A) Complex formation of125I-radiolabeled GrA in EDTA plasma: lane 1, GrA; lane 2, GrA incubated with EDTA plasma; lane 3, GrA incubated with plasma in the presence of phenylmethylsulfonyl fluoride; lane 4, immunoprecipitation of purified GrA with mAb M1; lane 5, immunoprecipitation of GrA in EDTA plasma with mAb M1; lane 6 and 7, as lanes 4 and 5 respectively, but under reducing conditions. (B) Complex formation of 125I-radiolabeled GrA in heparin plasma: lane 1, GrA; lane 2, GrA incubated with heparin plasma; lane 3, GrA incubated in heparin plasma in the presence of phenylmethylsulfonyl fluoride; lane 4, immunoprecipitation of purified GrA in heparin plasma with mAb a-antithrombin III; lane 5, 6, 7, and 8 same as lane 1, 2, 3, and 4, respectively, but under reducing conditions.
Complex formation of radiolabeled granzyme A (GrA) to proteinase inhibitors in plasma.
Approximately 1 pg of 125I-GrA was incubated with 2 μL of plasma for 30 minutes at 20°C. Next, samples were either prepared for SDS-PAGE directly or incubated with 2 mL monoclonal antibody (mAb) coupled to Sepharose beads (2 mg/mL) for 30 minutes followed by recovery of the Sepharose-bound proteins that then were prepared for SDS-PAGE. After electrophoresis, the gel was dried and labeled; GrA was visualized by autoradiography. (A) Complex formation of125I-radiolabeled GrA in EDTA plasma: lane 1, GrA; lane 2, GrA incubated with EDTA plasma; lane 3, GrA incubated with plasma in the presence of phenylmethylsulfonyl fluoride; lane 4, immunoprecipitation of purified GrA with mAb M1; lane 5, immunoprecipitation of GrA in EDTA plasma with mAb M1; lane 6 and 7, as lanes 4 and 5 respectively, but under reducing conditions. (B) Complex formation of 125I-radiolabeled GrA in heparin plasma: lane 1, GrA; lane 2, GrA incubated with heparin plasma; lane 3, GrA incubated in heparin plasma in the presence of phenylmethylsulfonyl fluoride; lane 4, immunoprecipitation of purified GrA in heparin plasma with mAb a-antithrombin III; lane 5, 6, 7, and 8 same as lane 1, 2, 3, and 4, respectively, but under reducing conditions.
ELISA for the detection of GrA-ATIII complexes
The experiments above demonstrated the importance of ATIII as an inhibitor of GrA in vitro. To learn whether ATIII interacted with GrA in vivo, we developed an ELISA that measured GrA-ATIII complexes in clinical samples. In this ELISA, an mAb was used to capture GrA, and a biotinylated anti-ATIII mAb served to detect bound complexes. The positive control consisted of purified GrA mixed with various concentrations of ATIII in the presence of 100 U/mL heparin in PBS/0.1% (w/v) Tween-20 for 30 minutes at room temperature. Indeed, a dose-dependent increase was observed in the ELISA (Figure2A). The specificity of the GrA-ATIII ELISA was confirmed by showing minimal reactivity for mixtures containing benzamidine-inactivated GrA and ATIII plus heparin (result not shown) and GrA plus ATIII without heparin (Figure 2A). Of importance, complex formation between purified GrA and ATIII markedly reduced the detection of granzyme in the antigenic assay (Figure 2B). Similar results were obtained when a mixture containing GrA and ATIII was tested in the presence of plasma (Figure 3A and 3B).
In vitro complex formation between granzyme A (GrA) and antithrombin III (ATIII), results in a decrease in GrA antigenic recovery.
Isolated GrA (18 ng) was incubated with a 200-fold excess of ATIII in the presence or absence of heparin (10 U) in phosphate-buffered saline/0.1% (w/v) Tween-20 for 30 minutes at 20°C. Various dilutions of the mixtures were then tested in the enzyme-linked immunosorbent assay (ELISA) for GrA-ATIII complexes (A) or in the ELISA for antigenic GrA (B) as described in “Materials and Methods.” The X-axis represents the final concentration of GrA in the wells, the Y-axis is the observed OD at 450 nm.
In vitro complex formation between granzyme A (GrA) and antithrombin III (ATIII), results in a decrease in GrA antigenic recovery.
Isolated GrA (18 ng) was incubated with a 200-fold excess of ATIII in the presence or absence of heparin (10 U) in phosphate-buffered saline/0.1% (w/v) Tween-20 for 30 minutes at 20°C. Various dilutions of the mixtures were then tested in the enzyme-linked immunosorbent assay (ELISA) for GrA-ATIII complexes (A) or in the ELISA for antigenic GrA (B) as described in “Materials and Methods.” The X-axis represents the final concentration of GrA in the wells, the Y-axis is the observed OD at 450 nm.
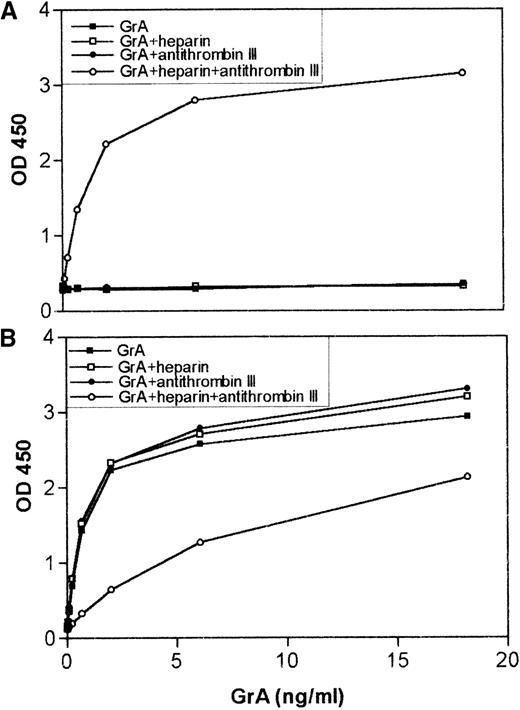
Addition of granzyme A (GrA) to EDTA plasma results, in the presence of heparin, in GrA-antithrombin III (ATIII) complex formation and a decrease in antigenic recovery.
GrA (18 ng) was incubated with 5 μL of EDTA-plasma either in or not in the presence of 10 U of heparin for 30 minutes at 20°C. As a control, GrA was also incubated with plasma containing 0.1 mol/L benzamidine. Before analysis, the samples were diluted to a final concentration of 7.5 ng/mL GrA. (A) GrA-ATIII complexes and (B) antigenic GrA present in the mixtures were measured and represented as described in the legend for Figure 2.
Addition of granzyme A (GrA) to EDTA plasma results, in the presence of heparin, in GrA-antithrombin III (ATIII) complex formation and a decrease in antigenic recovery.
GrA (18 ng) was incubated with 5 μL of EDTA-plasma either in or not in the presence of 10 U of heparin for 30 minutes at 20°C. As a control, GrA was also incubated with plasma containing 0.1 mol/L benzamidine. Before analysis, the samples were diluted to a final concentration of 7.5 ng/mL GrA. (A) GrA-ATIII complexes and (B) antigenic GrA present in the mixtures were measured and represented as described in the legend for Figure 2.
GrA-ATIII complexes in plasma samples
Levels of soluble (antigenic) granzymes are elevated in the blood of patients with systemic viral infections compared with levels present in healthy individuals.14 To establish whether antigenic GrA as well as GrA-ATIII complexes were present in vivo, plasma samples of 30 patients with Dengue fever, a systemic viral infection in which levels of antigenic GrA are increased, were tested. As a control, both antigenic GrA and GrA-ATIII complexes were measured in a group of 17 healthy individuals. Indeed, GrA-ATIII complexes were detectable in plasma at low levels in the healthy individuals (median level of 15 pg/mL; range 3-124), whereas, similarly as for antigenic GrA (Figure 4A), levels of the complexes were elevated in the plasma of patients with a systemic viral infection like Dengue fever (median level 282 pg/mL; range 56-5058) (Figure 4A). Overall, the levels of GrA and GrA-ATIII complexes were positively correlated (for Dengue fever Spearman r = 0.4325,P = .017) (Figure 4B). However, in some patients, the GrA levels were 3 to 4 times as high as the GrA-ATIII levels and vice versa. Because differences between antigenic GrA and GrA-ATIII levels may, among others, depend on the stage of the infection, longitudinal plasma samples from a renal transplant recipient who developed a CMV infection after surgery were tested (Figure 4B). It was found that levels of GrA-ATIII were higher than those of antigenic GrA; however, their course was comparable.
Levels of antigenic granzyme A (GrA) and GrA-antithrombin III (ATIII) complexes are correlated.
(A) Levels of antigenic GrA and GrA-ATIII complexes in plasma samples of healthy individuals or subjects with Dengue fever. The lines represent the median level for each group. (B) Correlation between GrA antigen and GrA-ATIII levels in plasma of patients (n = 30) with Dengue fever (Spearman r = 0.4025; P = .017). (C) Course of GrA antigen and GrA-ATIII complex levels in a renal transplant recipient that developed a cytomegalovirus (CMV) infection after surgery. The day of surgery was appointed day 0, the arrow represents the time of diagnosis of CMV infection. GrA and GrA-ATIII complexes were detected by enzyme-linked immunosorbent assays described in “Materials and Methods.” Note the difference in the scale for antigenic GrA (left Y-axis) and GrA-ATIII complexes (right Y-axis).
Levels of antigenic granzyme A (GrA) and GrA-antithrombin III (ATIII) complexes are correlated.
(A) Levels of antigenic GrA and GrA-ATIII complexes in plasma samples of healthy individuals or subjects with Dengue fever. The lines represent the median level for each group. (B) Correlation between GrA antigen and GrA-ATIII levels in plasma of patients (n = 30) with Dengue fever (Spearman r = 0.4025; P = .017). (C) Course of GrA antigen and GrA-ATIII complex levels in a renal transplant recipient that developed a cytomegalovirus (CMV) infection after surgery. The day of surgery was appointed day 0, the arrow represents the time of diagnosis of CMV infection. GrA and GrA-ATIII complexes were detected by enzyme-linked immunosorbent assays described in “Materials and Methods.” Note the difference in the scale for antigenic GrA (left Y-axis) and GrA-ATIII complexes (right Y-axis).
GrA circulates in plasma in its mature conformation
GrA is synthesized with a 26 amino acid hydrophobic prepeptide followed by a 2 amino acid residue propeptide. Maturation of GrA to the proteolytical active conformation occurs by Cathepsin C-mediated removal of the propeptide.18 To ensure that the antigenic GrA ELISA did not react with the zymogen as well as the mature enzyme, an assay was designed that specifically recognized the latter. To achieve this goal, the mAbs prepared previously14 were tested for binding to pro-GrA or mature GrA. The mAb, GA21, failed to react with recombinant zymogen (Figure 5A), but removal of the propeptide yielded a reactivity comparable to GrA purified from IL-2 stimulated mononuclear cells. An assay (called hereafter the mature conformation GrA ELISA) was constructed with the use of mAb GA29 and GA21 as the catching and detecting antibodies, respectively. The levels of granzyme detected by the antigenic and mature conformation GrA ELISAs were determined in 98 plasma samples from patients with Dengue fever (Figure 5B). GrA levels measured by these techniques were highly correlated (Spearmanr = 0.8840, P < .0001), indicating that most of the circulating GrA detected in plasma by the antigenic GrA ELISA was, indeed, the granzyme in the mature conformation. Furthermore, the discrepant antigenic GrA and GrA-ATIII levels noted above cannot be explained by the presence of circulating pro-GrA that is unable to form complexes with ATIII.
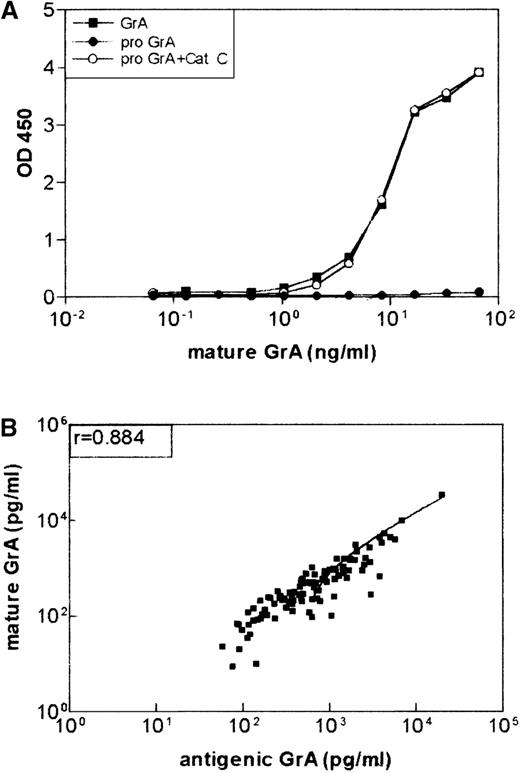
Antigenic granzyme A (GrA) as measured by enzyme-linked immunosorbent assay (ELISA) is in the mature conformation.
(A) Monoclonal antibody (mAb) GA 21 recognizes mature GrA and not the zymogen. Recombinant GrA zymogen (6 ng ) was incubated with cathepsin C (2 μg) as described previously19 and tested in the ELISA with mAb GA29 as a catching mAb and biotinylated GA 21 as the detecting mAb. Natural GrA (6 ng) was used as a control. (B) Correlation between levels of antigenic GrA and mature GrA in plasma of patients (n = 100) with Dengue fever (Spearman r = 0.884;P < .0001). Levels of soluble GrA and GrA in the mature conformation were detected by ELISAs, described in “Materials and Methods.”
Antigenic granzyme A (GrA) as measured by enzyme-linked immunosorbent assay (ELISA) is in the mature conformation.
(A) Monoclonal antibody (mAb) GA 21 recognizes mature GrA and not the zymogen. Recombinant GrA zymogen (6 ng ) was incubated with cathepsin C (2 μg) as described previously19 and tested in the ELISA with mAb GA29 as a catching mAb and biotinylated GA 21 as the detecting mAb. Natural GrA (6 ng) was used as a control. (B) Correlation between levels of antigenic GrA and mature GrA in plasma of patients (n = 100) with Dengue fever (Spearman r = 0.884;P < .0001). Levels of soluble GrA and GrA in the mature conformation were detected by ELISAs, described in “Materials and Methods.”
Recovery of active GrA in plasma
Because the results indicated that the 2 forms of GrA circulate in plasma: mature GrA and GrA-ATIII, we postulated that the mature form might be protected from the inhibitor by an interaction with its carrier PG, serglycin. Presuming GrA secreted from LAK cells was bound to serglycin,6 we determined if the addition of plasma selectively reduced the recovery of the free GrA. Purified GrA and LAK cell degranulate were incubated for 30 minutes at room temperature with EDTA, plasma and antigenic GrA levels were measured (Figure6). Although the recovery of conventionally purified GrA was poor (Figure 6A), only a minor reduction in the level of GrA was observed when plasma was added to the degranulate (Figure6B). More important, the capacity of plasma to reduce the level of isolated GrA was prevented by the addition of benzamidine. Therefore, the results suggest that plasma associated inhibitors reacted with isolated GrA to block detection in the antigenic GrA ELISA. Incubation in the presence of heparin also resulted in less antigenic recovery.
Comparison of the antigenic recovery of conventionally purified granzyme A (GrA) versus immunoaffinity purified GrA.
Detection by antigenic enzyme-linked immunosorbent assay of 18 ng conventionally purified GrA (A) and 18 ng GrA present in a degranulate (B) after incubation with 5 μL EDTA plasma for 30 minutes at 20°C.
Comparison of the antigenic recovery of conventionally purified granzyme A (GrA) versus immunoaffinity purified GrA.
Detection by antigenic enzyme-linked immunosorbent assay of 18 ng conventionally purified GrA (A) and 18 ng GrA present in a degranulate (B) after incubation with 5 μL EDTA plasma for 30 minutes at 20°C.
Immunopurification of GrA by mAb GA21
To verify that GrA-PG complexes are rather resistant to inactivation by plasma inhibitors, we then repeated the experiments described above with isolated complexes. Sepharose 4B beads coupled with mAb GA21 were incubated with LAK cell degranulate, the beads were washed and then packed into a column. GrA was then eluted by gradually decreasing the pH of the elution buffers (150 mmol/L NaCl) to pH 2.5, and, at that pH, GrA was found to elute from the column. The GrA purified in this manner appeared to be >95% homogeneous as estimated by SDS-PAGE and Western blotting (results not shown). With the use of conversion of the chromogenic substrate BLT as a readout, it was found that the activity of the preparation was comparable with that of conventionally purified GrA (result not shown). Contamination with GrB and perforin was negligible (data not shown). The macromolecular state of the immunoaffinity purified GrA was then assessed by applying the preparation (180-200 ng) to a Sepharose CL4B column and eluting under isotonic conditions; elution under the same conditions of a degranulate-containing GrA was used as a control. As shown in Figure7A, <1% of the GrA present in the degranulate and approximately 6% in the immunopurified preparation eluted in the void volume at the position of Dextran blue, whereas most of the applied protein could not be recovered, suggesting that this immunoaffinity purified GrA was contained in large macromolecular complexes. Rinsing the column with a buffer containing 500 mmol/L NaCl resulted in the elution of most of the GrA applied to the column (result not shown). When the immunopurified GrA preparation was applied to the column and eluted under high salt conditions (500 mmol/L), the immunoaffinity purified GrA eluted with a heterogeneous elution profile, with most of the protein eluting at a position of approximately 21-30 kDa (Figure 7A). The heterogeneity of the elution profile presumably was caused by interaction of GrA free of oppositely charged macromolecules with the column material. This overall difference in elution pattern under high and low salt conditions is typical for GrA bound to PG.23
Behavior of immunopurified granzyme A (GrA) in size exclusion chromatography, antithrombin III (ATIII) complex formation, and antigenic recovery experiments.
(A) Size exclusion chromatography of 220 ng immunopurified GrA under physiologic (150 mmol/L) and high salt (500 mmol/L) conditions. The elution profile of GrA-containing degranulate (applied degranulate contained 170 ng antigenic GrA) under physiological salt conditions was shown as a control. The presence of antigenic GrA in the different experiments was assessed by enzyme-linked immunosorbent assay as described in “Materials and Methods.” Detection of 37.5 ng immunopurified GrA in the antigenic assay (B) or as GrA-ATIII complexes after incubation with 5 μL EDTA plasma for 30 minutes at 20°C in the presence and absence of heparin (C).
Behavior of immunopurified granzyme A (GrA) in size exclusion chromatography, antithrombin III (ATIII) complex formation, and antigenic recovery experiments.
(A) Size exclusion chromatography of 220 ng immunopurified GrA under physiologic (150 mmol/L) and high salt (500 mmol/L) conditions. The elution profile of GrA-containing degranulate (applied degranulate contained 170 ng antigenic GrA) under physiological salt conditions was shown as a control. The presence of antigenic GrA in the different experiments was assessed by enzyme-linked immunosorbent assay as described in “Materials and Methods.” Detection of 37.5 ng immunopurified GrA in the antigenic assay (B) or as GrA-ATIII complexes after incubation with 5 μL EDTA plasma for 30 minutes at 20°C in the presence and absence of heparin (C).
Antigenic recovery of immunopurified GrA in the presence of normal plasma
To determine if the high molecular weight GrA reacted with plasma-associated inhibitors, immunoaffinity purified GrA was added to plasma, and the remaining mature GrA was determined by the antigenic GrA ELISA. As shown in Figure 7B, plasma minimally reduced the levels of antigenic GrA (Figure 7B), suggesting GrA complexes that presumably contain PG are protected from plasma-associated proteinase inhibitors. When the immunoaffinity purified GrA was mixed with plasma containing heparin, additional GrA-ATIII complex formation was observed (Figure7C), but the levels were significantly less than the reaction that occurred after incubation of the same amount of conventionally purified GrA with plasma (result not shown).
Size exclusion chromatography of plasma containing high GrA levels
To test whether the antigenic GrA in patient samples circulate as a high molecular weight complex, size exclusion chromatography was performed. Plasma samples (n = 2) that contained high levels of antigenic GrA and GrA-ATIII complexes were applied to a 60-mL Sepharose CL4B column, and the eluted fractions were tested for the presence of antigenic GrA and GrA-ATIII complexes (Figure8). GrA-ATIII complexes eluted with a heterogeneous profile of molecules with sizes ranging between slightly larger than IgG (150 kD) to somewhat smaller than HSA (67 kD). Of note, aspecific interactions between GrA-ATIII complexes and the gel filtration matrix may have contributed to the apparent heterogeneity of the elution profile. Alternatively, the apparent molecular heterogeneity might also be caused by the presence of various complexes, ranging from dimer GrA complexed to two ATIII molecules to monomeric GrA complexed to ATIII. Unfortunately, the limited amount of plasma prevented a thorough analysis of the composition of the complexes. No antigenic GrA could be detected in the eluate (which was comparable with immunopurified GrA), demonstrating that GrA-ATIII complexes and antigenic GrA represented different forms of the circulating granzyme. These results suggest that, in plasma, antigenic GrA is complexed to macromolecular structures from which it can dissociate under high salt conditions, whereas GrA complexed to ATIII is not. Moreover, the results also suggest that the antigenic GrA ELISA does not detect GrA-ATIII complexes; therefore, the protease-serpin complex and antigenic GrA represent different forms of the circulating granzyme.
Antigenic granzyme A ( GrA) and GrA-antithrombin III (ATIII) complexes detected in plasma represent different forms of the protein. Size exclusion chromatography of 750 μL of plasma from a patient with a cytomegalovirus infection containing high levels of antigenic GrA (619 pg/mL) and GrA-ATIII (1.4 ng/mL) complexes. Plasma was eluted under physiologic salt (150 mmol/L) conditions from a 60-mL size exclusion column. Antigenic GrA and GrA-ATIII complexes were detected by enzyme-linked immunosorbent assays as described in “Material and Methods.” As internal markers IgM (Mr 750 kD, fraction 34), IgG (Mr 150 kD, fraction 67), and HSA (Mr 67 KD, fraction 73) were measured.
Antigenic granzyme A ( GrA) and GrA-antithrombin III (ATIII) complexes detected in plasma represent different forms of the protein. Size exclusion chromatography of 750 μL of plasma from a patient with a cytomegalovirus infection containing high levels of antigenic GrA (619 pg/mL) and GrA-ATIII (1.4 ng/mL) complexes. Plasma was eluted under physiologic salt (150 mmol/L) conditions from a 60-mL size exclusion column. Antigenic GrA and GrA-ATIII complexes were detected by enzyme-linked immunosorbent assays as described in “Material and Methods.” As internal markers IgM (Mr 750 kD, fraction 34), IgG (Mr 150 kD, fraction 67), and HSA (Mr 67 KD, fraction 73) were measured.
Proteolytic activity of soluble GrA present in plasma
To formally establish that a portion of the antigenic GrA in plasma represents the proteolytically active granzyme, biotinylated ATIII, rather than an anti-GrA mAb, was used to detect captured GrA. The sensitivity of this novel ELISA was approximately 50 pg/mL. Plasma samples (n = 3) containing relatively high levels of antigenic GrA (ng/mL range) were separated by small-size exclusion chromatography columns (Sepharose CL4B), and fractions were assayed for antigenic GrA, GrA-ATIII, and active GrA. After elution from the column, the samples with high antigenic GrA levels, indeed, contained the proteolytic active form (result not shown), because, in the presence of heparin, binding of biotinylated ATIII was found. Under the same conditions, biotinylated ATIII did not bind to GrA when the GrA-inhibitor benzamidine was added during the incubations, indicating the requirement of active GrA for this binding.
Discussion
Increasing evidence in the literature suggests an extracellular function of GrA.5,24,25 However, the relevance of these in vitro observations is difficult to evaluate because, until recently, no techniques were available to study the presence of GrA in biological fluids. We have described ELISAs for GrA and GrB antigens in biological fluids.14 We observed that low levels of granzymes occur in plasma of healthy individuals, whereas higher levels were found in patients undergoing a CTL response. Although these results establish the presence of extracellular granzyme, the functional state was unclear. Theoretically, granzymes may occur in biological fluids in various forms: zymogen, mature proteinase either free or complexed to PG, and inactivated proteinase bound to inhibitors such as ATIII12 or α2M.13 By using a combination of new ELISA methods, we show that plasma-associated GrA is either complexed to the plasma inhibitor ATIII or bound to PG. Furthermore, our results suggest that the interaction with PG may protect GrA from inactivation by plasma-associated inhibitors.
Although granule exocytosis results in the delivery of granule contents to the target cell, some may diffuse to the extracellular space. To minimize adventitious proteolysis, GrA then would be inactivated by proteinase inhibitors, such as ATIII,12α2M,13 and α1-proteinase inhibitor.20 Our results show that GrA, indeed, is inactivated in plasma by α2M and ATIII, although we found no indication for a role of α1-proteinase inhibitor (data not shown). Furthermore, the results for the ELISA that detects GrA-ATIII established the existence of these complexes in plasma samples, supporting the role of ATIII as an inhibitor of GrA in vivo. Because the entire panel of anti-GrA mAbs (>90) did not recognize GrA complexed to α2M, an ELISA could not be devised to assess the contribution α2M might have in the binding of extracellular GrA. This outcome could be predicted on the basis of the trapping mechanism that serine proteases undergo in the face of α2M,26 thereby obscuring the binding epitopes recognized by the mAbs.
The levels of GrA antigen and GrA-ATIII complex overall showed a significant correlation (Spearman r = 0.4325;P = .017; n = 30), but in some patients rather high levels of GrA antigen were detected in the absence of detectable GrA-ATIII complexes. This study clearly suggested that more than one form of GrA besides ATIII-inactivated protease is present extracellularly. By using an ELISA that measures only mature GrA, zymogen GrA was not detected. Hence, the discrepancy between antigenic and GrA-ATIII complex levels could not be explained by the presence of progranzyme in the circulation.
When the conventionally purified GrA was incubated with plasma, virtually none of the protease was detected. Of note, the lost immunological reactivity was not observed when the active site of GrA was inhibited by low molecular weight inhibitors, such as benzamidine or phenylmethylsulfonyl fluoride. This finding suggested that an interaction of GrA with proteinase inhibitors in the added plasma was responsible for the reduced recovery. Consistent with this notion, the incubation of conventionally isolated GrA with purified ATIII and heparin masked the detection of the granzyme in the antigenic ELISA (see Figure 2).
To account for the discrepant circulating levels of antigenic GrA and GrA-ATIII complexes, we hypothesized that GrA is secreted as a macromolecular complex with the PG, serglycin. In this form, GrA would be unable to interact with proteinase inhibitors. The antigenic recovery of the immunopurified GrA was, indeed, greater than conventionally purified granzyme. Size exclusion chromatography under isotonic conditions showed that only about 6% of the GrA immunoaffinity purified from LAK cell lysate eluted in the void volume of the column, whereas the remaining protein did not elute at all. Because most of the remaining protein eluted after rinsing the column with a buffer containing 500 mmol/L NaCl, these proteins were presumably complexed to macromolecular structures. However, when performed at 1 mol/L NaCl, immunopurified GrA eluted with a molecular weight of approximately 21-30 kDa. Although the components of this high molecular weight GrA complex have not been determined, the results are consistent with reports showing the majority of the granzyme remains complexed to serglycin following granule exocytosis.23 27An analysis of plasma from patients with high levels of GrA revealed the presence of a similar high molecular weight complex that did not contain ATIII. To firmly establish that this high molecular weight complex contained active GrA, we constructed an ELISA that detects active GrA with biotinylated ATIII. This technique is superior to conventional chromogenic assays that lack sensitivity as well as specificity. By using this assay in combination with molecular sieve chromatography, the plasma of a patient with an ongoing CTL response was found to contain a high molecular weight form of active GrA that was devoid of ATIII.
Two hypotheses were made to explain the presence of different forms of GrA in plasma. In the first hypothesis, during activation of CTL, GrA complexed with PG is released into the intercellular cleft by degranulation. However, during activation of the CTL, GrA is also released directly without prior storage into the granules.10 Because this GrA is not complexed to PG, these proteins will be inactivated immediately by proteinase inhibitors like ATIII. In the second hypothesis, we postulate the existence of different structures of PG to which GrA binds with varying affinities. After release of GrA-PG into the intercellular space, low affinity GrA-PG complexes dissociate rapidly, resulting in inactivation of the free GrA by proteinase inhibitors, whereas GrA bound with high affinity to PG does not interact with proteinase inhibitors. According to this model, soluble GrA in plasma consists of high affinity GrA-PG complexes as well as GrA that has been dissociated from PG to be inhibited by ATIII and possibly other inhibitors.
In conclusion, although GrA circulates predominantly as an inactive complex with ATIII, the results of this study indicate that a portion of macromolecular GrA retains proteolytic activity that cannot be inactivated by plasma-associated proteinase inhibitors. The identification of active GrA in plasma supports the hypothesis that the granzyme may exert extracellular functions distant from the site of degranulation.
Reprints:E. H. A. Spaeny-Dekking, CLB, Sanquin Blood Supply Foundation, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal