Castleman disease (CD) is a lymphoproliferative disorder of unknown etiology that is associated with the development of secondary tumors, including B-cell lymphoma. Human herpesvirus 8 (HHV-8) (Kaposi's sarcoma–associated herpesvirus) sequences have been described in some cases of multicentric Castleman disease (MCD). Using a monoclonal antibody against an HHV-8–latent nuclear antigen, we show that HHV-8 is specifically associated with a variant of MCD in which HHV-8–positive plasmablasts that show λ light-chain restriction localize in the mantle zone of B-cell follicles and coalesce to form microscopic lymphomas in some cases. Furthermore, we show that the frank plasmablastic lymphoma that develops in patients with this plasmablastic variant of MCD is also positive for HHV-8 and λ light chain. Plasmablastic lymphoma associated with MCD is a new disease entity associated with HHV-8 infection.
It is now widely accepted that human herpesvirus 8 (HHV-8) plays a major role in the pathogenesis of Kaposi's sarcoma (KS).1-4 Both molecular and large sero-epidemiological studies have confirmed the association between HHV-8 and KS.5-7 HHV-8 sequences are also found in cells of primary effusion lymphoma (PEL).8-11 However, PEL is a rare disease in the West, and there are therefore no sero-epidemiological studies to link HHV-8 directly to the etiopathogenesis of PEL. Furthermore, the majority of PEL cells are co-infected by Epstein-Barr virus (EBV), making it difficult to interpret the exact role of HHV-8 in this tumor. Soulier and colleagues12 first identified HHV-8 DNA in Castleman disease (CD) biopsies by means of the polymerase chain reaction (PCR). Other groups have since confirmed this finding,13-17 but the prevalence of HHV-8 in the different types of CD and its relationship to the development of related lymphoma remain unclear.
As originally described by Castleman et al in 1956,18 CD comprises a benign localized mass of lymphoid tissue. Histologically, the lesion is characterized by the presence of large follicles separated by vascular lymphoid tissue containing lymphocytes. This histological form is known as the hyaline vascular (HV) type of CD.
Subsequently, a variant that is distinguished by the presence of sheets of plasma cells in the interfollicular zone was described and is referred to as the plasma cell variant of CD.19 Plasma cell variant CD is frequently multicentric, presents as a systemic lymphoproliferative disorder often associated with immunological abnormalities,20 and has a poorer prognosis than the localized HV type.21-24 Multicentric CD (MCD) has been described in human immunodeficiency virus (HIV)–infected individuals; however, it may be difficult to distinguish HIV-related lymphadenopathic changes from those of CD.25
Patients with MCD often develop secondary tumors, such as KS, non-Hodgkin lymphoma (NHL), Hodgkin disease, and plasmacytoma.25-27 In one study, 25% of patients with MCD developed NHL,21 and immunoblastic or plasmablastic B-cell lymphoma is the most frequent subtype described.21 28
Recently, it was shown that HHV-8 is present in plasmablastic or immunoblastic cells in MCD,17 and these cells were subsequently shown to belong to the B-cell lineage.29 It is not known whether these cells are also a feature of HHV-8–negative CD, or whether the plasmablastic variant of CD is specifically associated with HHV-8. Moreover, the relationship between HHV-8 and lymphomas associated with MCD has not been explored.
Materials and methods
This study enrolled 8 HIV-1–positive patients with MCD and 12 HIV-negative patients with either localized CD of HV type (n = 7) or MCD (n = 5). The clinical records of the patients were retrieved where possible.
Paraffin-embedded blocks of formalin-fixed lymph nodes from patients with a diagnosis of CD were retrieved from the surgical pathology files of University College Hospital and from the department of pathology, Hôpital Cochin, Paris. Of these biopsies, 4 (cases 2, 3, 4, and 7) were part of a previous study.29 Four of the HIV-positive patients had undergone splenectomy, and paraffin-embedded spleen tissue was available in each of these cases. Paraffin-embedded tissue was also obtained from 3 of 4 lymphomas that developed in these patients (cases 3, 4, and 5). Standard hematoxylin/eosin staining was performed on all sections, and the histology was reviewed.
We used monoclonal antibodies (mAbs) against one of the latent nuclear antigens (LNAs) of HHV-8 encoded by viral open reading frame (orf) 73 to study the presence of HHV-8. We previously confirmed the specificity of these HHV-8 LNA mAbs by Western blot, immunoprecipitation, immunofluorescent assay, and fluorescence-activated cell sorter analysis.30 One of our antibodies, LN53, recognizes an EQEQE motif epitope in orf73; this motif is repeated more than 20 times in orf73, and this antibody reacts very well with antigen in paraffin-embedded tissue.29 30 We used this antibody here to identify cell types infected latently by HHV-8 in CD and lymphomas associated with CD.
Five μm sections were cut onto sialin-coated slides. Sections were deparaffinized with xylene and 100% ethanol and were heated in a 750 W microwave oven in Dako (High Wycombe, UK) antigen retrieval solution, pH 9.9, for 30 minutes. After treatment with 20% acetic acid, sections were incubated with mAb LN53 (dilution 1 in 500 in phosphate buffer saline, [PBS]) for 1 hour at 22°C. Slides were then washed twice with 0.1% Tween in PBS. Incubation of the primary antibody was followed by a streptavidin-biotin complex peroxidase (Vector, Burlingame, CA), and the sections were counterstained with hematoxylin. Adjacent sections were stained with CD20, CD30, and Ki-67 (Dako, High Wycombe, UK) and with antibodies to μ, α, and γ immunoglobulin (Ig) heavy chains and to κ and λ light chains (Dako). Sections from selected cases were double-stained with anti-Igλ chain (immunoperoxidase) followed by HHV-8 LNA (immunoalkaline phosphatase with fast blue chromagen).
Detection of EBER 1,2 messenger RNAs was performed with the use of fluorescein isothiocyanate (FITC)–labeled specific oligonucleotides. The hybridization product was detected with a mouse monoclonal anti-FITC antibody (Dako). As a third layer, the APAAP complex (Dako) was used with BCIP NBT as a chromogen and nuclear fast red as counterstaining.31
To perform amplification of HHV-8 and EBV by PCR, we deparaffinized sections from paraffin-embedded tissues using xylene and 100% ethanol, treated with proteinase K in lysis buffer for 2 hours at 60°C. HHV-8 DNA sequences were searched for by a nested PCR as previously described.32 We also investigated the presence of EBV DNA by PCR from 2 out of the 3 lymphoma samples with the use of primers as previously described.33
We also performed amplification of rearranged immunoglobulin heavy chain genes. DNA was extracted from sections of the immunoblastic lymphomas. In addition, in cases 1, 2, and 4, DNA extracts were enhanced for the presence of cells of interest by microdissection of confluent clusters of HHV-8–positive cells (microlymphomas) highlighted by immunostaining as described.34 All DNA extracts were subjected to PCR amplification of the Ig heavy-chain gene from framework 3 of the V region to the J region with the use of consensus primers and the semi-nested protocol previously reported.35 Each experiment was accompanied by appropriate positive and negative controls, and all test samples were amplified in triplicate. Products were analyzed on 10% polyacrylamide mini-gels, followed by ethidium bromide staining.
Results
The results are summarized in Table 1.
Results of study
| Patient . | Age . | Sex . | Related Disease . | HIV Status . | Diagnosis and Sites of Disease . | Site of Biopsy Tested . | Plasmablasts . | HHV-8 LNA . | Plasmablasts Light-Chain Restriction . | Microscopic Lymphoma . | Clinical Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | KS, skin | + | MCD PC LNs, spleen | Spleen | + | + | Lambda | Yes | Died 6 months after the diagnosis of CD owing to a blast crisis where leukocyte count was 38 000 with 85% “immunoblasts” |
| 2 | 62 | M | + | MCD PC LNs, spleen | Lymph node Spleen | + + | + + | Lambda Lambda | No Yes | Died 7 months after the diagnosis of CD owing to disease progression and ketoacidosis | |
| 3 | 46 | M | + | MCD PC LNs, spleen Lymphoma | Lymph node Spleen Muscle | + + + | + + + | Lambda Lambda U | No No — | 9 months after a splenectomy for CD, developed plasmablastic lymphoma and died 10 months later owing to PD | |
| 4 | 47 | F | KS, skin & palate | + | MCD PC LNs, spleen Lymphoma | Lymph node Spleen Pharynx | + + + | + + + | Lambda Lambda Lambda | No Yes — | Died 9 months after the diagnosis of CD with pancytopenia and PD. Also had plasmablastic lymphoma in pharynx |
| 5 | 60 | M | KS, skin | + | MCD PC LNs Lymphoma | Lymph node Lymph node | + + | + + | Lambda Lambda | No — | Died within weeks after the diagnosis of lymphoma, cause unknown |
| 6 | 35 | M | KS, skin and LNs | + | MCD PC LNs | Lymph node | + | + | Lambda | No | Lost to follow-up |
| 7 | 40 | M | + | MCD PC LNs | Lymph node | + | + | Lambda | No | Died 9 months after the diagnosis of CD, owing to progressive AIDS | |
| 8 | 37 | M | KS, LN | + | MCD PC LNs | Lymph node | + | + | Lambda | No | Alive |
| 9 | 32 | M | PEL | − | MCD PC LNs | Lymph node | + | + | Lambda | No | Alive |
| 10 | 73 | F | − | MCD PC LNs | Lymph node | + | + | Lambda | No | Alive, but progressive CD | |
| 11 | 43 | F | − | LCD HV | Lymph node | − | − | No | No | Alive | |
| 12 | 48 | M | − | LCD HV | Lymph node | − | − | No | No | Lost to follow-up | |
| 13 | 31 | F | − | MCD PC LNs | Lymph node | − | − | No | No | Alive | |
| 14 | 64 | F | − | LCD HV | Lymph node | − | − | No | No | Alive for more than 10 years | |
| 15 | ? | M | − | LCD HV | Lymph node | − | − | No | No | Lost to follow-up | |
| 16 | 19 | F | − | LCD HV | Lymph node | − | − | No | No | Alive | |
| 17 | 16 | M | − | LCD HV | Lymph node | − | − | No | No | Alive | |
| 18 | 65 | M | − | MCD PC LNs | Lymph node | − | − | No | No | Alive | |
| 19 | 45 | M | − | MCD PC LNs | Lymph node | − | − | No | No | Alive | |
| 20 | 44 | F | − | LCD HV | Lymph node | − | − | No | No | Alive |
| Patient . | Age . | Sex . | Related Disease . | HIV Status . | Diagnosis and Sites of Disease . | Site of Biopsy Tested . | Plasmablasts . | HHV-8 LNA . | Plasmablasts Light-Chain Restriction . | Microscopic Lymphoma . | Clinical Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | M | KS, skin | + | MCD PC LNs, spleen | Spleen | + | + | Lambda | Yes | Died 6 months after the diagnosis of CD owing to a blast crisis where leukocyte count was 38 000 with 85% “immunoblasts” |
| 2 | 62 | M | + | MCD PC LNs, spleen | Lymph node Spleen | + + | + + | Lambda Lambda | No Yes | Died 7 months after the diagnosis of CD owing to disease progression and ketoacidosis | |
| 3 | 46 | M | + | MCD PC LNs, spleen Lymphoma | Lymph node Spleen Muscle | + + + | + + + | Lambda Lambda U | No No — | 9 months after a splenectomy for CD, developed plasmablastic lymphoma and died 10 months later owing to PD | |
| 4 | 47 | F | KS, skin & palate | + | MCD PC LNs, spleen Lymphoma | Lymph node Spleen Pharynx | + + + | + + + | Lambda Lambda Lambda | No Yes — | Died 9 months after the diagnosis of CD with pancytopenia and PD. Also had plasmablastic lymphoma in pharynx |
| 5 | 60 | M | KS, skin | + | MCD PC LNs Lymphoma | Lymph node Lymph node | + + | + + | Lambda Lambda | No — | Died within weeks after the diagnosis of lymphoma, cause unknown |
| 6 | 35 | M | KS, skin and LNs | + | MCD PC LNs | Lymph node | + | + | Lambda | No | Lost to follow-up |
| 7 | 40 | M | + | MCD PC LNs | Lymph node | + | + | Lambda | No | Died 9 months after the diagnosis of CD, owing to progressive AIDS | |
| 8 | 37 | M | KS, LN | + | MCD PC LNs | Lymph node | + | + | Lambda | No | Alive |
| 9 | 32 | M | PEL | − | MCD PC LNs | Lymph node | + | + | Lambda | No | Alive |
| 10 | 73 | F | − | MCD PC LNs | Lymph node | + | + | Lambda | No | Alive, but progressive CD | |
| 11 | 43 | F | − | LCD HV | Lymph node | − | − | No | No | Alive | |
| 12 | 48 | M | − | LCD HV | Lymph node | − | − | No | No | Lost to follow-up | |
| 13 | 31 | F | − | MCD PC LNs | Lymph node | − | − | No | No | Alive | |
| 14 | 64 | F | − | LCD HV | Lymph node | − | − | No | No | Alive for more than 10 years | |
| 15 | ? | M | − | LCD HV | Lymph node | − | − | No | No | Lost to follow-up | |
| 16 | 19 | F | − | LCD HV | Lymph node | − | − | No | No | Alive | |
| 17 | 16 | M | − | LCD HV | Lymph node | − | − | No | No | Alive | |
| 18 | 65 | M | − | MCD PC LNs | Lymph node | − | − | No | No | Alive | |
| 19 | 45 | M | − | MCD PC LNs | Lymph node | − | − | No | No | Alive | |
| 20 | 44 | F | − | LCD HV | Lymph node | − | − | No | No | Alive |
HIV indicates human immunodeficiency virus; HHV-8 LNA, human herpesvirus 8 latent nuclear antigen; KS, Kaposi's sarcoma; MCD PC, multicentric Castleman disease, plasma cell type; LNs, lymph nodes; CD, Castleman disease; U, unsatisfactory staining; PD, progressive disease; LCD HV, localized Castleman disease, hyalin vascular type; PEL, primary effusion lymphoma.
Clinical features
Of the 8 HIV-1–infected patients, 7 were male and 1 female. Of the 12 HIV-negative patients, 6 were male and 6 female. All the patients with MCD had either lymph node and splenic involvement or enlargement of multiple lymph nodes. Of the 8 HIV-positive patients with MCD, 5 also had Kaposi's sarcoma. One of the HIV-negative patients with HHV-8–positive MCD also had PEL. In all patients, at least 1 lymph node was biopsied, and sections were made available for immunostaining. Of the patients with HIV and MCD, 4 developed a plasmablastic lymphoma. Patient 1 developed a hematological blast crisis 6 months after the diagnosis of MCD. He presented to the emergency department and died with a leukocyte count of 38 000 comprising 85% plasmablasts. No lymphoma tissue was available in this case. Patient 3 developed plasmablastic lymphoma, diagnosed from a biopsy of a mass in an arm muscle, 9 months after the diagnosis of MCD. Patient 4 was diagnosed with plasmablastic lymphoma of the pharynx and MCD in the lymph nodes. He previously had enlarged lymph nodes that were not biopsied. Patient 5 developed plasmablastic lymphoma in lymph nodes 6 months after the diagnosis of MCD.
Histology and Immunohistochemistry
Lymph node biopsies from 7 out of 12 HIV-negative patients showed the classic features of hyaline vascular CD (Table 1). Enlarged lymphoid follicles comprised an eosinophilic lymphocyte–depleted and vascular follicle center surrounded by a broad concentric mantle of small lymphocytes. Between the follicles there was lymphocyte-rich tissue containing only occasional plasma cells. Both mantle cells and interfollicular B-cells were polytypic when immunostained for immunoglobulin light chains.
Biopsies from 13 patients, including all 8 HIV-positive cases, showed the features of MCD (Table 1). The germinal centers were somewhat less hyalinized, and the mantle was frequently not as broad as in hyaline vascular CD. The main difference lay in the interfollicular infiltrate, which was rich in mature plasma cells. Sections of spleen showed similar alterations in the white pulp follicles, with a concentric zone of fibrosis around the white pulp that contained large numbers of plasma cells. The red pulp was normal. Both mantle zone B-cells and interfollicular plasma cells expressed polytypic immunoglobulin light chains.
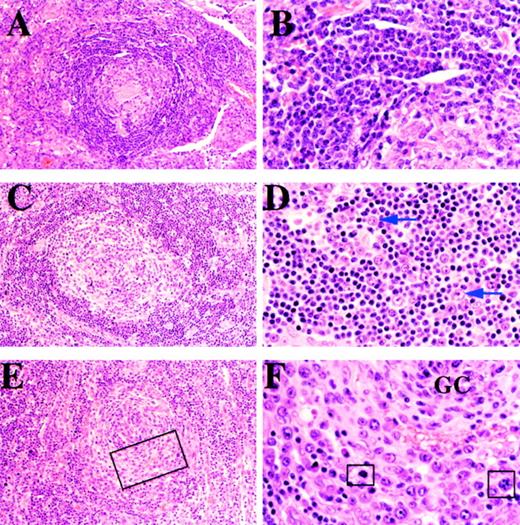
The biopsies from all 8 HIV-positive MCD cases and from 2 of the HIV-negative MCD cases showed an additional feature in the mantle zone. In contrast to usual MCD (Figure 1A and 1B), the mantle zone contained numbers of larger cells approximately twice the size of mantle zone lymphocytes and characterized by a moderate amount of amphophilic cytoplasm and a large vesicular nucleus containing 1 or sometimes 2 prominent nucleoli (Figure 1C and 1D). We have elected to use the term “plasmablast” to describe these cells although many of them have classical immunoblastic features. In 3 cases, these cells were particularly prominent and displaced the residual mantle zone to the periphery (Figure 1E and 1F).
Cases 19, 7, and 8.
(A) and (B): A lymph node from case 19 shows features of plasma cell variant CD (panels A and B). (A) Follicle comprises a hyalinized germinal center surrounded by a broad mantle. (B) High magnifcation showing uniform population of small lymphocytes in the mantle zone. (C) and (D): A lymph node from case 7, an HIV-positive patient, shows features of plasmablastic variant CD. (C) The follicle comprises a partially hyalinized germinal center surrounded by a well-formed mantle. (D) High magnification of mantle zone showing small lymphocytes and scattered transformed plasmablasts, 2 of which are arrowed. (E) and (F): A lymph node from case 8, an HIV-positive patient with plasmablastic CD. (E) The follicle shows a poorly defined mantle zone that is partially replaced by a concentric infiltrate of plasmablasts. (F) High magnification of area within the rectangle in Figure 1E, showing the germinal center (GC) surrounded by plasmablasts that have replaced the mantle. Some cells in mitosis are highlighted in squares.
Cases 19, 7, and 8.
(A) and (B): A lymph node from case 19 shows features of plasma cell variant CD (panels A and B). (A) Follicle comprises a hyalinized germinal center surrounded by a broad mantle. (B) High magnifcation showing uniform population of small lymphocytes in the mantle zone. (C) and (D): A lymph node from case 7, an HIV-positive patient, shows features of plasmablastic variant CD. (C) The follicle comprises a partially hyalinized germinal center surrounded by a well-formed mantle. (D) High magnification of mantle zone showing small lymphocytes and scattered transformed plasmablasts, 2 of which are arrowed. (E) and (F): A lymph node from case 8, an HIV-positive patient with plasmablastic CD. (E) The follicle shows a poorly defined mantle zone that is partially replaced by a concentric infiltrate of plasmablasts. (F) High magnification of area within the rectangle in Figure 1E, showing the germinal center (GC) surrounded by plasmablasts that have replaced the mantle. Some cells in mitosis are highlighted in squares.
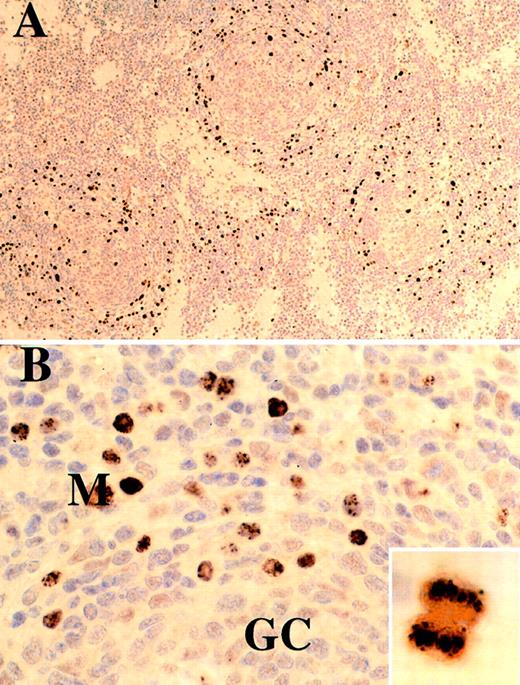
The mantle zone plasmablasts were CD20+, CD30− and showed striking homogeneous or stippled nuclear staining for HHV-8 LNA, which was also seen in scattered interfollicular cells (Figures2A, 2B, and 3A). LNA is known to tether HHV-8 DNA to chromosomes during mitosis,36 and this is well illustrated in the inset of Figure 2B. These cells expressed IgM with λ immunoglobulin light-chain restriction, in striking contrast to the IgM-negative polytypic interfollicular plasma cells (Figure 3B, 3C, and3D). Double-stained preparations showed that HHV-8 expression was confined to λ-positive cells both in the mantle zone and in isolated interfollicular cells (Figure 3F). The proliferation fraction of the mantle zone plasmablasts judged by Ki-67 expression was nearly 100% (Figure 3E). HHV-8 LNA staining was negative in all 7 cases of HV CD and in the cases of MCD in which plasmablasts were not seen in the mantle zone.
Case 10, lymph node from an HIV-negative patient stained for HHV-8.
(A) Low magnification showing mantle zone distribution of HHV-8–positive cells. (B) High magnification showing stippled and/or diffuse nuclear staining. Inset shows a cell in mitosis; there is localization of HHV-8 DNA to chromosomes by antiLNA antibody.
Case 10, lymph node from an HIV-negative patient stained for HHV-8.
(A) Low magnification showing mantle zone distribution of HHV-8–positive cells. (B) High magnification showing stippled and/or diffuse nuclear staining. Inset shows a cell in mitosis; there is localization of HHV-8 DNA to chromosomes by antiLNA antibody.
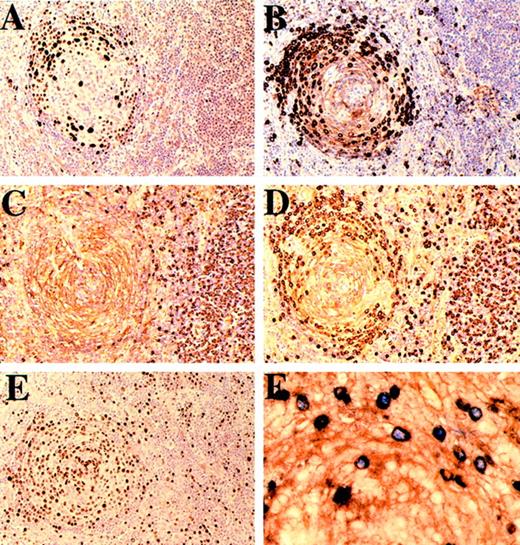
Cases 8 and 10.
(A-E): Case 8. Follicle and interfollicular plasma cells stained (A) for HHV-8, (B) for μ heavy chain, (C) for κ light chain, (D) for λ light chain, and (E) with Ki-67. The mantle zone plasmablasts show nuclear positivity for HHV-8, express μ heavy chain, and show λ light-chain restriction, while the μ negative interfollicular plasma cells are polytypic with respect to light chains. Most, if not all, of the plasmablasts are in cycle. (F) Case 10. Mantle zone immunoblasts double-stained for HHV-8 (blue) and λ light chain (brown). HHV-8–positive nuclei are exclusively within λ-positive cells.
Cases 8 and 10.
(A-E): Case 8. Follicle and interfollicular plasma cells stained (A) for HHV-8, (B) for μ heavy chain, (C) for κ light chain, (D) for λ light chain, and (E) with Ki-67. The mantle zone plasmablasts show nuclear positivity for HHV-8, express μ heavy chain, and show λ light-chain restriction, while the μ negative interfollicular plasma cells are polytypic with respect to light chains. Most, if not all, of the plasmablasts are in cycle. (F) Case 10. Mantle zone immunoblasts double-stained for HHV-8 (blue) and λ light chain (brown). HHV-8–positive nuclei are exclusively within λ-positive cells.
In sections of spleen from 3 cases, HHV-8–positive plasmablasts coalesced to form small confluent clusters either adjacent to or replacing some follicles or forming isolated lesions within the red pulp (Figure 4A, 4B, and 4C). These lesions were quite distinct from the more disaggregated plasmablasts in the mantle zones although the cytology of the individual cells was similar. Like the mantle zone plasmablasts, these microlymphomas showed unequivocal λ light-chain restriction (Figure 4D). Sections of the 3 frank lymphomas (cases 3, 4, and 5) showed that they were composed of confluent sheets of plasmablasts with cytological characteristics similar to those of the individual transformed cells in the mantle zones and the small cohesive clusters of cells described above (Figure5A and 5B). In 2 of these cases (cases 4 and 5), the plasmablastic lymphomas also showed λ light-chain restriction (Figure 5C), but the results of staining for light chains were unsatisfactory in case 3.
Case 1.
Section of spleen showing (A) a microlymphoma surrounding a splenic arteriole comprising (B) HHV-8–positive cells with (C) plasmablastic morphology. (D) Immunostain for κ (left) and λ (right); there is λ light-chain restriction.
Case 1.
Section of spleen showing (A) a microlymphoma surrounding a splenic arteriole comprising (B) HHV-8–positive cells with (C) plasmablastic morphology. (D) Immunostain for κ (left) and λ (right); there is λ light-chain restriction.
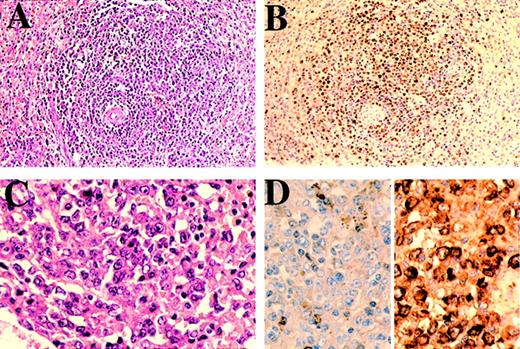
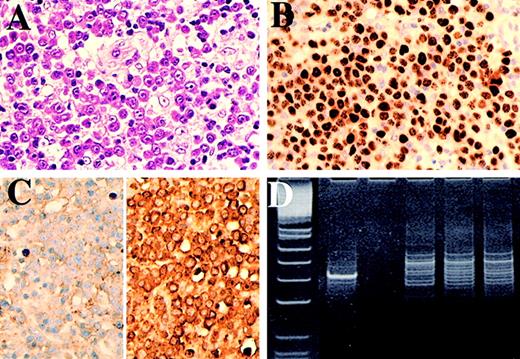
Case 5.
(A) Lymph node showing frank plasmablastic lymphoma, (B) immunostained for HHV-8 and (C) for κ (left) and λ (right) light chains. The HHV-8–positive lymphoma cells show λ light-chain restriction. (D) Polyacrylamide gel electrophoresis of immunoglobulin heavy-chain gene PCR products. Lane 1: PhiXHinfI molecular weight markers (the marker band immediately below the positive control band is 82bp variant CD). Lane 2: positive control B-cell lymphoma. Lane 3: negative control (no DNA). Lanes 4, 5, and 6: repeat amplifications of DNA showing a reproducible dominant band indicating an expanded clone.
Case 5.
(A) Lymph node showing frank plasmablastic lymphoma, (B) immunostained for HHV-8 and (C) for κ (left) and λ (right) light chains. The HHV-8–positive lymphoma cells show λ light-chain restriction. (D) Polyacrylamide gel electrophoresis of immunoglobulin heavy-chain gene PCR products. Lane 1: PhiXHinfI molecular weight markers (the marker band immediately below the positive control band is 82bp variant CD). Lane 2: positive control B-cell lymphoma. Lane 3: negative control (no DNA). Lanes 4, 5, and 6: repeat amplifications of DNA showing a reproducible dominant band indicating an expanded clone.
PCR and EBER in situ hybridization
PCR for HHV-8 confirmed viral DNA in all cases where plasmablasts were present, but in none of the other cases.
EBV DNA was not detectable by PCR in the 2 plasmablastic lymphomas tested. In situ hybridization with EBER probes showed rare (fewer than 1%) positive cells in 1 of the 3 plasmablastic lymphomas tested. In 3 of 5 HHV-8–positive MCD lymph nodes tested, EBER was positive in a few of the lymphocytes, but in all cases the plasmablasts in the mantle zone were negative.
PCR for immunoglobulin gene rearrangement confirmed monoclonality in 2 (cases 4 and 5) of the plasmablastic lymphomas tested (Figure 5D); however, this could not be confirmed in the other lymphoma (case 3) or in DNA extracted from microdissected microlymphomas.
Discussion
IgM-positive immunoblasts have previously been described in the interfollicular region of MCD, but not in the mantle zone.20 37 We now show that HHV-8 is present in a unique population of plasmablasts present in the mantle zone of a subset of cases of MCD. These plasmablasts are not present in HHV-8–negative MCD or in HV CD. HHV-8–positive MCD should thus be considered a distinct subtype and specifically designated as a plasmablastic variant of MCD. Confluent clusters of HHV-8–positive blasts are present in some of these cases, indicating that isolated HHV-8 plasmablasts have coalesced to form foci of microlymphoma. We suggest that this could herald the development of frank HHV-8–positive plasmablastic lymphoma as seen in 3 of our cases (3, 4, and 5). One further case (case 1) developed a hematological “plasmablastic crisis,” although material from the peripheral blood smears was not available to investigate HHV-8 expression. Plasmablastic lymphoma associated with MCD represents a new B-cell neoplasm associated with HHV-8 infection.
Whether occurring as separated cells in the mantle zone, in small confluent clusters (microlymphomas), or in large lymphomatous sheets, the HHV-8–positive cells in MCD invariably expressed cytoplasmic IgM λ (Figures 3B, 3D, 3F, 4D, and 5C), suggesting that these cells in all circumstances comprise a monoclonal population.
Interestingly, 2 groups have previously described a monotypic, λ light-chain–restricted population of cells in CD.38 39However, these cells comprised mature (IgG- or IgA-positive) plasma cells in the interfollicular zones. In contrast, the HHV-8–positive cells we describe here are immature cells with blastic morphology that are seen predominantly in the mantle zones and exclusively express IgM.
We were unable to confirm monoclonality of the HHV-8–positive microlymphomas and 1 of the 3 frank lymphomas by PCR, even after enriching tissue samples for HHV-8–positive cells by microdissection. We believe that the most likely explanation for this is that the Ig genes of the HHV-8–positive plasmablasts are hypermutated, as has previously been shown for HHV-8–positive PEL cells.40 In 4 out of 7 cases of PEL reported by Matolcsy et al,40 the framework 3 primer target regions contained multiple mutations that would affect hybridization with consensus primers. Futhermore, in 1 case of immunoblastic lymphoma associated with CD described by Soulier et al,12 monoclonality could be demonstrated by Southern blot, but not by PCR analysis using consensus primers. Frozen lymphoma tissue, which is a requirement for the use of family-specific PCR primers or Southern blotting, was not available from our cases. The consistent expression of λ light-chain restriction in all the HHV-8–positive plasmablasts in all cases in our study is intriguing, and the possibility that the λ light chain is involved in the mechanism of HHV-8 entry into cells should be explored. If this is the case, the plasmablastic proliferation, while monotypic, may not be monoclonal. There is, however, no precedent for a monotypic, polyclonal B-cell lymphoproliferative disorder.
An in situ hybridization study has indicated that a proportion of the cells positive for HHV-8 in CD are potentially productively infected, as they express a number of lytic HHV-8 genes.41 In some of our cases, up to 50% of the cells in the mantle zone were HHV-8–positive plasmablasts, and most of these cells were in cycle, as shown by the frequency of mitoses (Figures 1F, 2B inset) and nuclear expression of Ki-67 (Figure 3E), indicating that the vast majority of these cells were not undergoing lytic infection. However, HHV-8 may express a larger repertoire of genes during latency in these plasmablasts, compared with the limited number of latent genes expressed in PEL cells.42
The introduction of highly active antiretroviral therapy (HAART) has led to a decline in the incidence of KS and to the resolution of established lesions in some patients.43 However, HAART is not associated with a decrease in the incidence of NHL.43Current studies suggest that HHV-8–positive MCD has a poorer prognosis than HHV-8–negative cases.16,17 A group of aggressive CD cases occurring or progressing in HIV-infected patients on HAART have been described.44,45 These patients were all positive for HHV-8, and the authors of one study suggested that HHV-8–positive MCD in HIV-infected patients should be considered a medical emergency.45 Although the cases we describe in the current paper were not part of a controlled study, the HIV-positive/HHV-8–positive cases had a particularly poor prognosis (Table 1), and most died within 12 months of the diagnosis, owing to MCD progression. One patient with microscopic lymphoma progressed to plasmablastic lymphoma despite the introduction of HAART, and one additional patient with MCD died owing to a blastic crisis. The presence of HHV-8–positive plasmablasts in MCD identifies a distinct subgroup with a very poor prognosis, especially in HIV-infected individuals. Future studies should determine whether these cases should all be managed as NHL and be treated with systemic chemotherapy.
Apart from their association with HHV-8, the lymphomas associated with MCD and PEL appear to be distinct disorders. Clinically, PEL presents predominantly as an effusion, with only rare examples of lymph node involvement.46,29 Moreover, in contrast to the HHV-8–positive plasmablasts in plasmablastic MCD and related plasmablastic lymphoma, PEL cells express CD30, and in nearly all cases described in HIV-infected patients, the lymphoma cells are co-infected with EBV.5,29,47 Furthermore, the vast majority of PEL cells do not express immunoglobulin,40 which is highly expressed in our cases of plasmablastic lymphoma. The plasmablastic lymphoma cells associated with MCD have exactly the same phenotypic features as the plasmablasts in MCD, including IgM λ expression and lack of EBV infection. This suggests that these frank lymphomas are a further step in the pathogenesis of this subtype of MCD. However, because of a lack of fresh material, we have been unable to confirm this using molecular markers of clonality. The poor prognosis of HIV-positive patients with MCD and the current reluctance to perform routine lymph node biopsies in such cases mean that it is difficult to be certain how many patients with the HHV-8–positive plasmablastic form of MCD also have microscopic or frank lymphoma.
This study supports a role for HHV-8 as a lymphomagenic agent. We have shown that HHV-8 is associated with a specific plasmablastic form of MCD. Plasmablastic lymphoma associated with CD should be added to the list of tumors associated with HHV-8 infection.
Supported by the Leukaemia Research Fund, the Cancer Research Campaign, l'Académie Nationale de Médecine, la Fondation Cancer et Solidarité, and GlaxoWellcome.
N.D. and T.D. contributed equally to this work; P.G.I. and C.B.are the senior authors.
Reprints:Peter G. Isaacson, Department of Histopathology, Rockefeller Building, UCL, University Street, London, WC1E 6JJ, United Kingdom; e-mail:p.isaacson@ucl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal