Activation of the p38 mitogen-activated protein kinase (MAPK) pathway is important for some T-cell functions, but its role in intrathymic development is unclear. To investigate the function of p38 MAPK during the late stages of thymocyte differentiation, pharmacologic and genetic manipulations were used to inhibit p38 MAPK activity in developing thymocytes. Ligation of the T-cell antigen receptor (TCR) on either thymocytes or a thymocyte cell line resulted in p38 MAPK activation. Selective pharmacologic inhibition of p38 MAPK activity with the pyridinyl imidazole drug SB203580 severely impaired the development of mature CD4+ and CD8+ single positive (SP) thymocytes from their CD4+CD8+ double positive (DP) precursors in fetal thymic organ culture (FTOC). Further, pharmacologic or genetic suppression of p38 MAPK activity, the latter achieved by overexpressing a catalytically inactive p38 MAPK, resulted in a blockade of the DP-to-SP transition of a thymocyte cell line in a novel in vitro differentiation assay. Taken together, these data constitute the first demonstration that p38 MAPK plays a critical role in the DP-to-SP differentiation of thymocytes during late intrathymic development.
Mitogen-activated protein kinase (MAPK) signaling pathways regulate cellular responses to various environmental stimuli. MAPKs can be grouped into 3 structural families: extracellular signal related kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 kinases.1,2 The p38 family of MAPKs comprises 4 closely related serine/threonine kinases, p38α, β, γ, and δ,3 which are activated by phosphorylation on the threonine and tyrosine residues of a conserved threonine-glycine-tyrosine (TGY) motif4 by dual-specificity MAPK kinases.1
In mammalian cells, p38 MAPKs can be activated by multiple stimuli, including physical-chemical stress, proinflammatory cytokines, and growth factors.2 Activated p38 MAPKs phosphorylate and activate several transcription factors in vitro, including ATF-2, CHOP, Elk-1, MEF2C, and SAP-1.4-8 p38 MAPKs also activate by phosphorylation the eIF-4E protein kinases Mnk1 and Mnk29and the hsp27 protein kinase MAPKAP kinase-2.10,11 p38α and p38β are selectively inhibited by pyridinyl imidazole derivatives such as SB203580,12,13 whereas the kinase activities of p38γ and p38δ, or other MAPKs are unaffected.3,14 SB203580 has therefore been instrumental in identifying the biologic functions of p38α/β MAPKs.15
Little is known about the role of the p38 MAPK pathway in T-cell development and function. p38 MAPK is activated in immature16 and mature T cells17 on T-cell receptor (TCR) engagement, and it is implicated in the regulation of IL-2 production18 and T-cell proliferation in response to IL-2 and IL-7.19 Moreover, p38 MAPK activity was shown to be selectively induced in Th1 cells and to regulate their production of IFN-γ.20
In regard to intrathymic T-cell development, p38 MAPK activity was reported to be high in freshly isolated thymocytes, and it was proposed that this was due to intrathymic signals in vivo.21However, kinase activation by stress signals generated during mouse euthanasia or mechanical disruption of the thymus could not be formally excluded. Recently, transgenic mice expressing a dominant-negative p38 MAPK have been described.20 Thymocyte subpopulations, as minimally defined by expression of CD4 and CD8, were not affected in these animals. However, endogenous p38 MAPK activity was only partially suppressed in the transgenic mice, being thus likely that the remaining p38 MAPK activity was still sufficient to support thymocyte maturation. In another study,22 generation of mature thymocytes in fetal thymic organ cultures (FTOCs) was not blocked by the specific p38 MAPK inhibitor SB203580, although the use of a relatively low concentration of the inhibitor provided once at the initiation of the cultures, together with a rather long culture period, raises questions as to the effectiveness of SB203580 in these experiments. Thus, the signaling pathways leading to p38 MAPK activation in thymocytes and the relevance of this kinase cascade to T-cell development are still unclear.
In the current study, the functional coupling of p38 MAPK to TCR signaling in thymocytes was examined. Further, the relevance of the p38 MAPK pathway to late intrathymic development was investigated by using pharmacologic and genetic tools to inhibit p38 MAPK function in 2 model systems of thymocyte differentiation. The results show that engagement of the TCR on thymocytes can trigger p38 MAPK activation, and that p38 MAPK activity is essential for the progression of thymocytes from an immature CD4+CD8+ double positive (DP) to a mature single positive (SP) stage during late intrathymic development.
Materials and methods
Cells and reagents
Thymocytes were isolated from the thymi of 4- to 6-week-old C57BL/6 mice. The DP cell line Thy278/107 was derived from a spontaneous thymic tumor arising in a mouse, expressing a transgenic Vα11-TCRα chain.23 Surface expression of a Vβ3-TCRβ chain confers to Thy278/107 reactivity to the bacterial superantigen staphylococcal enterotoxin A (SEA).24 DCEK-ICAM is a murine fibroblast line transfected with I-Ek and ICAM-1.25 Cells were routinely grown in complete culture medium consisting of RPMI-1640 (HyClone, Logan, UT), supplemented with 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L HEPES (Biofluids, Rockville, MD), 2-mercapto-ethanol (2 × 10−5mol/L; Sigma Chemical Co, St. Louis, MO) and 10% fetal calf serum (FCS) (HyClone). SEA was purchased from Sigma. SB203580 was generously provided by John C. Lee (SmithKline Beecham Pharmaceuticals, King of Prussia, PA).
Cell stimulation and Western blot analysis
To reduce the high levels of basal p38 MAPK activity observed in freshly isolated thymocytes,21 these cells were maintained at 4°C for 3 hours before stimulation with anti-CD3 MAb. For TCR stimulation, thymocytes or Thy278/107 cells (1-2 × 106 in phosphate-buffered saline [PBS]) were incubated with 1 μg biotinylated anti-CD3 MAb (145-2C11 or 500A2; Pharmingen, San Diego, CA) on ice for 10 minutes. After removal of unbound antibody, cells were resuspended in 40 μL PBS and incubated at 37°C for varying periods with 100 μg/mL streptavidin (Sigma). Cells were pelleted and then lysed by resuspension in cold lysis buffer (1% Triton X-100, 50 mmol/L Tris at pH 8, 150 mmol/L NaCl, 5 mmol/L EGTA (pH 8), 1.5 mmol/L MgCl2, 10% glycerol, 200 μg/mL Na3VO4, 50 mmol/L NaF (pH 8), 100 μg/mL PMSF, and 1 μg/mL aprotinin, pepstatin and leupeptin). After a 15-minute incubation on ice, cell lysates were cleared by centrifugation, resolved by SDS-PAGE and transferred to nitrocellulose membrane (Amersham Intl, Little Chalfont, England). Membranes were blocked with PBS, 0.1% Tween 20 and 5% nonfat dry milk, and probed with a phospho-specific antibody to dually phosphorylated (Thr180/Tyr182) p38 MAPK (New England Biolabs, Beverly, MA), followed by antirabbit peroxidase-conjugated antibody (Amersham). Blots were developed using enhanced chemiluminiscence (Amersham).
Immune-complex kinase assay
Cell lysates were subjected to immunoprecipitation with a polyclonal antibody to the N-terminus of p38 MAPK detecting p38α and p38β (Santa Cruz Biotechnology Inc, Santa Cruz, CA). Immunoprecipitates were washed twice with lysis buffer and twice with kinase buffer (25 mmol/L Tris, ph 7.5, 5 mmol/L β-glycerolphosphate, 2 mmol/L dithiothreitol, 0.1 mmol/L Na3VO4, 10 mmol/L MgCl2). Kinase reactions were performed at 30°C in 50 μL kinase buffer supplemented with 200 μmol/L ATP and 2 μg GST-ATF-2 fusion protein (New England Biolabs). After 30 minutes, the reaction was finished by addition of 4× sample buffer. Samples were boiled and resolve by SDS-PAGE. Phosphorylation of GST-ATF-2 was visualized by Western blotting, using a phospho-specific antibody detecting ATF-2 when phosphorylated on Thr71 (New England Biolabs), followed by enhanced chemiluminiscence.
Thy278/107 differentiation assay
Thy278/107 cells (2 × 105) were cultured for 72 hours on semiconfluent monolayers of DCEK-ICAM cells pulsed overnight with SEA (50 ng/mL), as antigen-presenting cells (APCs). In experiments involving SB203580, Thy278/107 cells were incubated for 1 hour at 37°C/5% CO2 in complete culture medium, containing 10 μmol/L SB203580, before culture with APCs. The p38 MAPK inhibitor was left in the medium during the culture period. An equal volume of solvent (DMSO) was added to control cultures.
Flow cytometric analysis
For 2-color immunostainings, cells were resuspended in staining buffer (PBS, 1% bovine serum albumin [BSA], 0.1% sodium azide) and incubated with FITC- and PE-conjugated MAbs for 30 minutes on ice. For 3-color immunostainings, cells were first incubated with FITC- and biotin-conjugated MAbs for 30 minutes on ice, washed twice with staining buffer, followed by a 30-minute incubation on ice with PE-conjugated MAb and red670-streptavidin (Life Technologies, Gaithersburg, MD). After 2 washes, stained cells were analyzed using a FACScan flow cytometer and CellQuest software (Becton Dickinson, Mountain View, CA). All antibodies were purchased from Pharmingen.
Plasmids and transfections
An expression vector for a catalytically inactive form of human p38β13 was transfected into Thy278/107 cells (10 μg/107 cells) by electroporation (250 V, 960 μF) using a BioRad Gene-Pulser (Bio-Rad Laboratories, Hercules, CA). Stable transfectants were selected in complete culture medium containing 800 μg/mL Geneticin (G418; Life Technologies).
Fetal thymic organ cultures (FTOCs)
Thymic lobes from day 16 C57BL/6 mouse fetuses were cultured on microporous membranes on top of 1.5 mL of complete culture medium in 6-well plates (Transwell; Costar, Cambridge, MA) at a density of 10 to 15 lobes per well. Organ cultures were maintained at 37°C, in a 5% CO2 atmosphere for 4 days in the presence or absence of 30 μmol/L SB203580. An equal volume of the solvent (DMSO) was added to control cultures. Medium was replaced every 24 hours with complete culture medium containing fresh inhibitor. At the end of the culture period, thymic lobes were strained through nylon mesh to release thymocytes, and viable cells were counted by trypan blue dye exclusion.
Results
TCR-mediated signals activate p38 MAPK in thymocytes
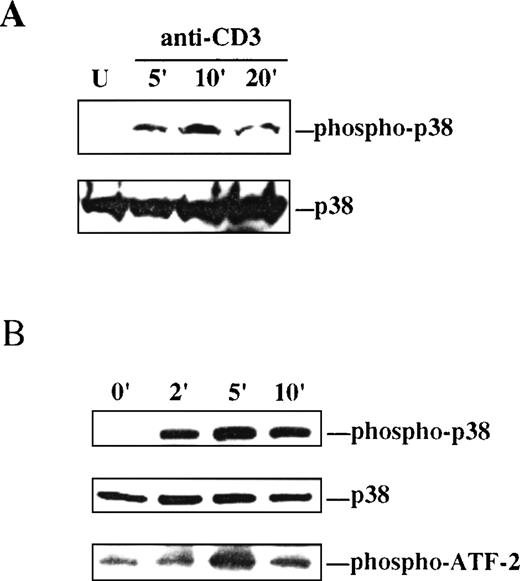
As an initial step to study the involvement of the p38 MAPK pathway in intrathymic T-cell development, the capacity of TCR-initiated signaling to activate p38 MAPK in thymocytes was investigated. Dual threonine/tyrosine phosphorylation of the conserved TGY motif in the catalytic domain of p38 MAPK is an absolute requirement for activation of its kinase activity.4 Thus, phosphorylation at the TGY motif on antibody-mediated engagement of the TCR was assessed by Western blot analysis as a measure of p38 MAPK activation. Ligation of the TCR complex on thymocytes with anti-CD3 MAb resulted in a rapid but transient phosphorylation of p38 MAPK in the TGY activation motif, as determined by immunoblotting with a phospho-specific antibody that recognizes the dually phosphorylated form of p38 MAPK (Figure1A).
p38 MAPK is activated by ligation of the TCR on thymocytes and Thy278/107 cells.
A, Lysates were prepared from unstimulated (U) or anti-CD3-stimulated thymocytes at the indicated times after stimulation. Active p38 MAPK in the lysates was detected by immunoblotting with a phospho-specific antibody to the dually phosphorylated form of p38 MAPK (upper). The membrane was stripped and reprobed with a polyclonal antibody to p38α MAPK to test for equal protein abundance (lower). B, Thy278/107 cells were stimulated with anti-CD3 MAb for increasing times, after which cells were lysed. Phospho-p38 and total p38α MAPK in the lysates were detected as in A (upper and middle). In vitro kinase assay reactions were performed as described in Materials and Methods using GST-ATF-2 as substrate. Phospho-ATF-2 was detected by immunoblotting with an antibody against Thr71-phosphorylated ATF-2 (lower).
p38 MAPK is activated by ligation of the TCR on thymocytes and Thy278/107 cells.
A, Lysates were prepared from unstimulated (U) or anti-CD3-stimulated thymocytes at the indicated times after stimulation. Active p38 MAPK in the lysates was detected by immunoblotting with a phospho-specific antibody to the dually phosphorylated form of p38 MAPK (upper). The membrane was stripped and reprobed with a polyclonal antibody to p38α MAPK to test for equal protein abundance (lower). B, Thy278/107 cells were stimulated with anti-CD3 MAb for increasing times, after which cells were lysed. Phospho-p38 and total p38α MAPK in the lysates were detected as in A (upper and middle). In vitro kinase assay reactions were performed as described in Materials and Methods using GST-ATF-2 as substrate. Phospho-ATF-2 was detected by immunoblotting with an antibody against Thr71-phosphorylated ATF-2 (lower).
Activation of p38 MAPK by TCR signaling was next examined in the DP thymocyte line Thy278/107. As observed in thymocytes, ligation of the TCR on Thy278/107 with anti-CD3 MAb resulted in a rapid and time-dependent phosphorylation of p38 MAPK at the TGY activation motif that correlated with the induction of kinase activity measured as threonine phosphorylation of exogenous ATF-2 (Figure 1B). Together, these results show that p38 MAPK can be activated by TCR-initiated signals in immature T cells.
Pharmacologic inhibition of p38 MAPK activity impairs thymocyte DP-to-SP differentiation in FTOC
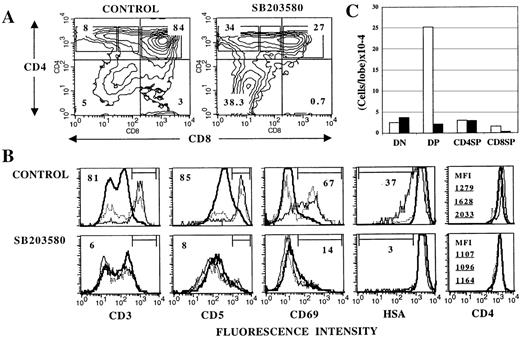
In view of the result showing that p38 MAPK can be activated on TCR engagement in thymocytes, and because transition from an immature DP to a mature SP stage during late intrathymic development is driven by signals emanating from the TCR, I next asked whether p38 MAPK could play a role in the DP-to-SP differentiation of thymocytes. To address this question, the effects of SB203580, a specific membrane-permeable inhibitor of p38α12 and p38β13 MAPKs (hereafter referred to collectively as “p38 MAPK”), on thymocyte differentiation in day 16 FTOCs26 were investigated. At this stage of fetal development, CD4−CD8− double negative (DN) and DP but not mature SP cells are present in the thymus,27thus providing a way to examine the DP-to-SP transition in vitro. Day 16 thymic lobes were cultured in the absence or presence of 30 μmol/L SB203580 (added fresh daily) for 4 days. At this point, cell yields were determined and thymocyte subsets phenotyped by flow cytometry. The average cell recovery in the presence of SB203580 was 28% that of untreated cultures. As seen in Figure 2 (A and C), this reduction in thymocyte number was due mainly to a depletion of DP thymocytes, which in turn may reflect a blockade in the DN-to-DP transition, increased DP differentiation into more mature thymocytes, or augmented cell death within the DP population. Because the absolute number of DN or SP cells was not coordinatedly increased (Figure 2C), a major DN-to-DP blockade or increased DP differentiation to more mature stages does not appear likely. Thymocyte cell death was selectively increased in DP cells of SB203580-treated cultures compared with untreated FTOCs (data not shown), accounting at least in part for the reduction in the number of DP thymocytes observed in the presence of SB203580. This suggests that p38 MAPK could play a role in the transduction of survival signals28 in DP thymocytes.
Inhibition of p38 MAPK activity with SB203580 impairs DP-to-SP differentiation in FTOC.
Thymic lobes from day 16 fetuses were cultured for 4 days in the absence (control) or in the presence of 30 μmol/L SB203580, and analyzed by flow cytometry for surface marker expression. A, Contour plots of CD4/CD8 expression. Numbers indicate the percentage of cells in each quadrant. B, Histograms represent expression of the indicated surface molecule on DP (thick solid line), CD4+CD8lo (dotted line) and CD4+CD8− (thin solid line) cells gated as shown in CD4/CD8 plots. Percentages of cells within the marked region are given for CD4+CD8− cells. For CD4 expression, mean fluorescence intensity (MFI) values for DP (top), CD4+CD8lo (middle), and CD4+CD8− (bottom) cells are shown to appreciate the difference in CD4 levels in the subpopulations. C, Mean cell recovery per lobe (5 experiments) for DN, DP, CD4 SP, and CD8 SP cells (as defined by quadrants in A) from control (white bars) and SB203580-treated (black bars) FTOCs. In each case, SEM did not exceed 15% of the mean.
Inhibition of p38 MAPK activity with SB203580 impairs DP-to-SP differentiation in FTOC.
Thymic lobes from day 16 fetuses were cultured for 4 days in the absence (control) or in the presence of 30 μmol/L SB203580, and analyzed by flow cytometry for surface marker expression. A, Contour plots of CD4/CD8 expression. Numbers indicate the percentage of cells in each quadrant. B, Histograms represent expression of the indicated surface molecule on DP (thick solid line), CD4+CD8lo (dotted line) and CD4+CD8− (thin solid line) cells gated as shown in CD4/CD8 plots. Percentages of cells within the marked region are given for CD4+CD8− cells. For CD4 expression, mean fluorescence intensity (MFI) values for DP (top), CD4+CD8lo (middle), and CD4+CD8− (bottom) cells are shown to appreciate the difference in CD4 levels in the subpopulations. C, Mean cell recovery per lobe (5 experiments) for DN, DP, CD4 SP, and CD8 SP cells (as defined by quadrants in A) from control (white bars) and SB203580-treated (black bars) FTOCs. In each case, SEM did not exceed 15% of the mean.
CD8 SP thymocytes, like DP cells, were severely reduced in SB203580-treated FTOC, both in proportion and absolute number (Figure2A and C). These cells were predominantly mature TCRαβ+thymocytes. CD8+TCRγδ+ cells were less markedly affected by SB203580 (data not shown). In contrast to CD8 SP cells, CD4+CD8lo/−cells were markedly increased in percentage (Figure 2A) in the presence of SB203580. Further phenotypic analysis of these cells revealed that unlike control cultures, where transition from a DP to a CD4+CD8−stage was accompanied by gradual up-regulation of surface CD3, CD5, CD69, CD4, and partial down-regulation of heat stable antigen (HSA) (Figure 2B Upper), all these developmental events were substantially impaired in SB203580-treated FTOCs (Figure 2B Lower).
The phenotypically abnormal CD4+CD8lo/−cells observed in SB203580-treated organ cultures could result from a lack of effect of SB203580 on the developmentally regulated down-regulation of CD8 in DP cells. Alternatively, they could be generated as a consequence of an as-yet-unidentified regulatory action of SB203580 on CD8 expression not related to differentiation events. To discriminate between these possibilities and to exclude potential harmful effects of SB203580 on DP or stromal cells as the cause of the altered thymocyte development, experiments were performed using a novel in vitro system for the DP-to-SP transition, based on the cell line Thy278/107.
DP-to-SP differentiation of a thymocyte cell line in vitro is inhibited by SB203580
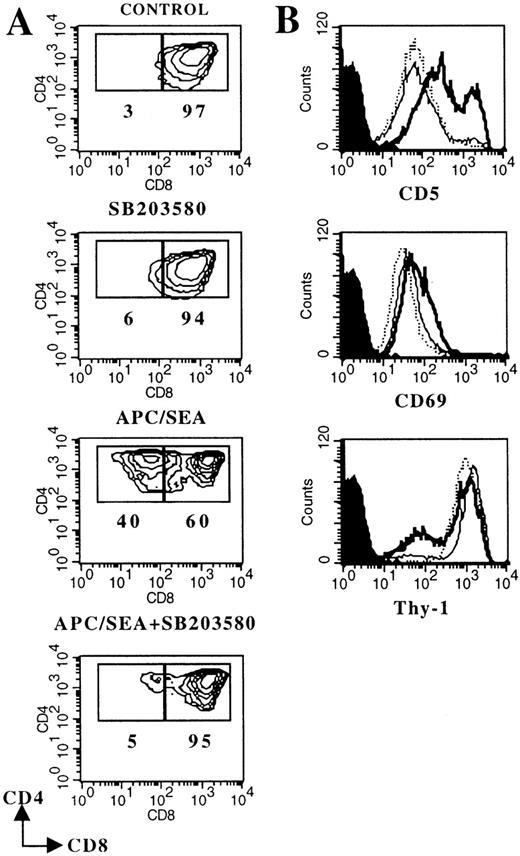
The thymocyte line Thy278/107 was derived from a spontaneous thymic tumor arising in a mouse expressing a transgenic Vα11-TCRα chain.23 In addition to the expression of both CD4 and CD8 coreceptors (Figure 3A), Thy278/107 resembles a subpopulation of DP thymocytes in the expression of CD69 (Figure 3B), which is regarded as typical of DP cells that have received early TCR signals.29 Coculture of Thy278/107 cells with SEA-pulsed APCs (but not with unloaded APCs; not shown) triggered several events that closely mirror progression to a CD4 SP stage in vivo27 and in FTOC (Figure 2), including CD8 down-modulation, CD5 and CD69 up-regulation, and decreased expression of Thy-1 (Figure 3). Of note, TCR/CD3 expression was constitutively high on Thy278/107 cells and was not significantly affected by stimulation with SEA-pulsed APCs (data not shown). Thus, inducible TCR up-regulation could not be used as a marker of maturation in this system.
DP-to-SP differentiation of the thymocyte cell line Thy278/107 is blocked by SB203580.
Thy278/107 cells (2 × 105) were cultured without (control) or with 10 μmol/L SB203580, or cocultured with SEA-pulsed APCs in the absence or in the presence of the p38 MAPK inhibitor. After 3 days, cultures were analyzed by flow cytometry. A, Contour plots of CD4/CD8 expression. Numbers represent the percentage of Thy278/107 cells in the indicated region. B, Histograms represent CD5, CD69 and Thy-1 expression on control (dotted line) Thy278/107 cells, or cells cocultured with SEA-pulsed APCs in the absence (thick solid line) or in the presence (thin solid line) of 10 μmol/L SB203580. Shaded curves represent background staining with isotype-matched FITC- or PE-conjugated rat immunoglobulins. Cell recoveries in this particular experiment were: 30.6 × 105 (control); 33.6 × 105 (SB203580); 14.3 × 105 (APC/SEA) and 8.0 × 105 (APC/SEA/SB203580).
DP-to-SP differentiation of the thymocyte cell line Thy278/107 is blocked by SB203580.
Thy278/107 cells (2 × 105) were cultured without (control) or with 10 μmol/L SB203580, or cocultured with SEA-pulsed APCs in the absence or in the presence of the p38 MAPK inhibitor. After 3 days, cultures were analyzed by flow cytometry. A, Contour plots of CD4/CD8 expression. Numbers represent the percentage of Thy278/107 cells in the indicated region. B, Histograms represent CD5, CD69 and Thy-1 expression on control (dotted line) Thy278/107 cells, or cells cocultured with SEA-pulsed APCs in the absence (thick solid line) or in the presence (thin solid line) of 10 μmol/L SB203580. Shaded curves represent background staining with isotype-matched FITC- or PE-conjugated rat immunoglobulins. Cell recoveries in this particular experiment were: 30.6 × 105 (control); 33.6 × 105 (SB203580); 14.3 × 105 (APC/SEA) and 8.0 × 105 (APC/SEA/SB203580).
All the phenotypic changes induced in SEA-stimulated Thy278/107 cells were severely suppressed in the presence of 10 μmol/L SB203580 (Figure 3). In contrast to DP thymocytes in FTOC, SB203580 has no significant effect on the viability of the DP cell line, as indicated by the similar cell recoveries observed in SB203580-treated and untreated cultures of Thy278/107 without APCs. Also, SB203580 did not induce significant CD8 down-regulation in these cells (Figure 3). It is also unlikely that SB203580 had harmful effects on APCs in this system, as it did not affect the viability, MHC class II/ICAM-1 expression, or function of APCs (data not shown). Together, these results clearly indicate that TCR-mediated signals leading to DP-to-SP differentiation of Thy278/107 cells require p38 MAPK activity. Further, they show that TCR-mediated down-regulation of CD8 in DP cells is inhibitable by SB203580, suggesting that in SB203580-treated FTOCs loss of CD8 surface expression by DP thymocytes is not likely driven by differentiation signals.
Expression of a catalitically inactive p38 MAPK impairs the DP-to-SP differentiation of Thy278/107 cells
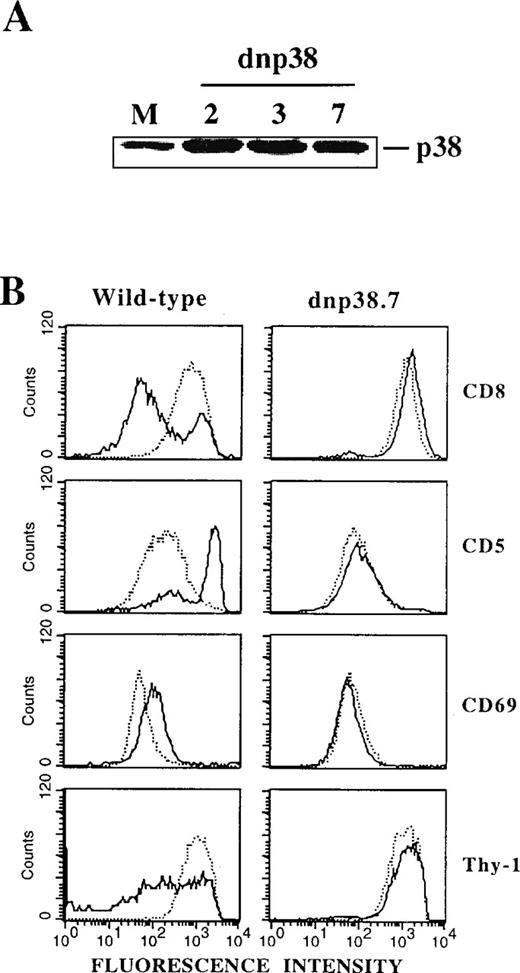
To further substantiate a direct involvement of p38 MAPK in the DP-to-SP transition and to exclude as yet uncharacterized effects of SB203580, a catalytically inactive mutant of p38 MAPK13 was overexpressed in Thy278/107 cells. Levels of immunoreactive p38 MAPK were markedly increased in different stably transfected lines compared with mock transfectants (Figure 4A), indicating efficient expression of the mutant protein. Cells expressing the kinase-dead p38 MAPK retained their DP phenotype and wild-type levels of surface TCR/CD3 (data not shown). Strikingly, differentiation induced by SEA-pulsed APCs was severely impaired in these transfectants (Figure 4B), as previously observed for Thy278/107 cells in the presence of SB203580 (Figure 3). Thus, either pharmacologic or genetic inhibition of p38 MAPK activity resulted in a marked impairment of the TCR-mediated DP-to-SP transition of Thy278/107 cells, strongly indicating that DP-to-SP thymocyte differentiation is critically dependent on an activated p38 MAPK.
Expression of a catalytically inactive mutant of p38 MAPK blocks the DP-to-SP differentiation of Thy278/107 cells.
(A) Cell lysates from Thy278/107 cells transfected with empty vector (mock; M) and 3 independent lines established after transfection of a catalytically inactive mutant of human p38β MAPK (dnp38) were immunoblotted with a polyclonal antibody to p38 MAPK to test for protein abundance. (B) Thy278/107 cells (wild-type) or a line expressing a kinase-dead mutant of p38 MAPK (dnp38.7) were cultured alone (dotted line) or with SEA-pulsed APCs (solid line). After 3 days, cultures were analyzed for the expression of the indicated surface markers by flow cytometry.
Expression of a catalytically inactive mutant of p38 MAPK blocks the DP-to-SP differentiation of Thy278/107 cells.
(A) Cell lysates from Thy278/107 cells transfected with empty vector (mock; M) and 3 independent lines established after transfection of a catalytically inactive mutant of human p38β MAPK (dnp38) were immunoblotted with a polyclonal antibody to p38 MAPK to test for protein abundance. (B) Thy278/107 cells (wild-type) or a line expressing a kinase-dead mutant of p38 MAPK (dnp38.7) were cultured alone (dotted line) or with SEA-pulsed APCs (solid line). After 3 days, cultures were analyzed for the expression of the indicated surface markers by flow cytometry.
Discussion
Little is known about the signal transduction pathways and transcriptional mechanisms that control the generation of T cells in the thymus. Collectively, this study shows that the p38 MAPK pathway is activated by TCR-initiated signals in thymocytes and that activated p38 MAPK plays a critical role in the intrathymic differentiation of DP thymocytes into mature SP cells.
According to recent models of thymocyte development,29,30suitable recognition of MHC on stromal cells by the TCR on CD4+CD8+CD69−CD3lothymocytes results in down-regulation of both CD4 and CD8 coreceptors, TCR/CD3 up-regulation and CD69 expression. Faster reexpression of CD4 relative to CD8 appears to generate a population of CD4hiCD8loCD69+CD3int/hicells that has been shown to contain the precursors of both mature CD4 and CD8 SP thymocytes.31,32 At this intermediate state, the cells appear to activate a TCR-dependent lineage-specific differentiation program that results in selective suppresion of CD4 or CD8 synthesis and further modulation of maturation-associated surface markers.27 29
The presence of DP cells expressing CD69 and intermediate levels of CD3 in SB203580-treated FTOCs, similarly to control cultures (Figure 2B), indicates that early TCR signaling in the DP compartment is not affected by inhibition of p38 MAPK activity. In contrast, progression to a more mature stage characterized by a CD4+CD8lophenotype and upregulated expression of CD4, CD5, CD69, and CD3 appears to be critically dependent on p38 MAPK activity. Although CD4+CD8lo/− thymocytes were present in SB203580-treated FTOCs (Figure 2A), these cells are not apparently the result of differentiation but rather of an inhibitory effect of SB203580 on CD8 expression observed specifically in DP cells in FTOC conditions, as suggested by the phenotypic similarity between CD4+CD8lo/−and DP cells in the presence of the inhibitor (Figure 2B). The mechanism by which SB203580 negatively regulates CD8 expression in DP thymocytes in FTOC remains unknown.
A specific requirement for p38 MAPK in the transition of thymocytes from a CD4+CD8+CD69+ to a more mature stage of development was further evidenced using a novel in vitro DP-to-SP differentiation assay. In this model system, TCR-mediated differentiation of the DP cell line Thy278/107 into phenotypically more mature CD4+CD8lo/−cells was blocked by SB203580, or by overexpression of a kinase-dead version of p38 MAPK. The latter finding is particularly significant as it excludes potential effects of SB203580 derived from its interaction with molecules different from p38 MAPK.33 34 Together, these data strongly indicate that p38 MAPK activity is essential for the DP-to-SP differentiation of thymocytes.
In apparent contrast to these results, thymocyte subsets appeared normal in transgenic mice expressing a catalytically inactive form of p38α MAPK.20 However, endogenous p38 MAPK activity was only partially suppressed in these animals. Accordingly, it is conceivable that the level of p38 MAPK activity in the transgenic mice was still sufficient to drive thymocyte development. Alternatively, p38β but not p38α could be the isoform of p38 MAPK relevant to late intrathymic development, an issue deserving further investigation.
In another study,22 generation of mature thymocytes in FTOCs from day 17 mice was not blocked by SB203580. One explanation for these differing results is that day 17 precursors of SP cells, as opposed to those in day 16 thymi (this study), have already received p38 MAPK-dependent signals and no longer require p38 MAPK activity to complete maturation. Alternatively, effective suppression of p38 MAPK activity in FTOC could require daily addition of fresh inhibitor during the culture period, as performed in this study but not in that of Sugawara et al,22 to overcome the apparent instability of SB203580 in culture16 and/or the potentially limited availability of drugs in organ culture conditions.35
Both cytosolic kinases and nuclear transcription factors have been identified as substrates of p38 MAPK in vitro.15 However, whether they are physiological targets of p38 MAPK in T cells or relevant to T-cell development is largely unknown. Although it is unlikely that the TCR is the only surface molecule on thymocytes linked to the p38 MAPK pathway,16,21 it is nonetheless conceivable that TCR-mediated activation of p38 MAPK in immature thymocytes, as shown in the current study, encompasses a mechanism to coordinate in space and time36 p38 kinase activity with the availability of TCR-induced p38 MAPK-activated transcription factors. Further studies will be required to identify these transcription factors ultimately controlling gene expression during late thymocyte development.37
Irrespective of the precise mechanisms involved, it is apparent from the current study that p38 MAPK plays a critical role in the DP-to-SP transition of thymocytes. Interestingly, a similar role has been previously ascribed to the ERK pathway,38,39 whereas the JNK cascade is apparently dispensable for thymocyte development.40 However, ERK activation appears to be particularly important for development of CD4 rather than CD8 SP cells,38 39 although the data presented here demonstrate a requirement for p38 MAPK activity in the generation of both lineages of mature thymocytes. Interestingly enough, the developmental blockade observed in FTOCs treated simultaneously with ERK and p38 MAPK inhibitors was similar to that in organ cultures treated with SB203580 only (E. Fernández, unpublished data), suggesting that p38 MAPK is critical at an earlier stage than ERK activity, and/or that p38 MAPK and ERKs regulate partly different events during the DP-to-SP transition. How the p38 MAPK and ERK signaling pathways are coordinated to regulate gene expression influencing the fate of developing thymocytes remains to be elucidated.
Acknowledgments
I wish to thank Ada M. Kruisbeek for providing the Thy278/107 cell line, John C. Lee for SB203580, Jiahuai Han for the expression vector for kinase-dead p38 MAPK, and the NIAID Animal Care Branch for animal husbandry. I am also grateful to Jon Shuman, William Magner, Francisco Borrego, and Balbino Alarcón for critical reading of the manuscript.
Reprints:Dr Edgar Fernández, Centro de Biologı́a Molecular Severo Ochoa, CSIC-Universidad Autónoma de Madrid, Cantoblanco, Madrid 28049, Spain; e-mail: efernandez@cbm.uam.es.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal