Glutathione S-transferases (GSTs) have been associated with outcome in human cancers treated with cytotoxic chemotherapy. In a case-control study, we investigated the association between polymorphisms within theGSTM1, GSTT1, and GSTP1 genes and risk of relapse in childhood acute lymphoblastic leukemia (ALL). Cases were relapsed patients. Controls were successfully treated patients with a minimum follow-up of 5 years. The null genotype (absence of both alleles) for GSTM1 or GSTT1 conferred a 2-fold (OR = 0.5, 95% CI = 0.23-1.07, P = .078) and 2.8-fold (OR = 0.36, 95% CI = 0.13-0.99, P = .048) reduction in risk of relapse, respectively, relative to the presence of the GSTM1 or GSTT1 gene. The GSTP1Val105/Val105 genotype showed a 3-fold decrease in risk of relapse (OR = 0.33, 95% CI = 0.09-1.23,P = .099) in comparison to the combined category of Ile105/Val105 and Ile105/Ile105 genotypes. No particular associations with relapse were observed for the GSTP1polymorphism at codon 114. The risk of relapse when having 1 of the low-risk genotypes (GSTM1 null, GSTT1 null,GSTP1 Val105/Val105) decreased 1.9-fold (OR = 0.53, 95% CI = 0.24-1.19, P = .123), and the risk when having 2 or 3 low-risk genotypes 3.5-fold (OR = 0.29, 95% CI = 0.06-1.37, P = .118), compared with individuals having no low-risk genotype (P for trend = .005). Our results suggest that polymorphisms within genes of the GST superfamily may be associated with risk of relapse in childhood ALL.
Glutathione S-transferases (GSTs) are a family of cytosolic enzymes involved in the detoxification of various exogenous as well as endogenous reactive species.1,2 GSTs function as dimers by catalyzing the conjugation of mutagenic electrophilic substrates to glutathione. In humans, 4 major subfamilies of GSTs can be distinguished and are designated as GSTα, GSTμ, GSTθ, and GSTπ.3 Each of these subfamilies is composed of several members, some of which display genetic polymorphism. Within the GSTμ subfamily, the gene coding for GSTM1 exhibits a deletion polymorphism, which in case of homozygozity (GSTM1 null) leads to absence of phenotypic enzyme activity.4 A similar mechanism is described for GSTT1 within the GSTθ subfamily,5 whereas the gene coding for GSTP1, a member of the GSTπ subfamily, displays polymorphisms within its coding region at codon 105 (Ile105Val) and codon 114 (Ala114Val).6-10 The coding region polymorphisms within GSTP1 have been suggested to confer different catalytic activities.11 12
Important environmental carcinogens (eg, benzo[a]pyrene and other polyaromatic hydrocarbons) are detoxified through the GST system.1,2,13 In this context, interindividual differences in GST enzyme activity mediated by polymorphic genes have been suggested to confer varying susceptibility to environmental cancer.2,13 Several groups have investigated the association of GSTM1, GSTT1, and GSTP1 genotype status with various malignancies such as smoking-induced lung cancer and bladder, breast, or gastrointestinal cancers.10,14-21Some of these studies observed an increased risk for individuals with GST genotypes with lower enzyme activities.10,14-16,19-21GSTs may also confer resistance to cytotoxic chemotherapeutic agents used to treat cancer.22,23 However, in contrast to the role of GSTs in environmental carcinogenesis, GST genotypes conferring lower enzyme activity may be of advantage for individuals undergoing chemotherapeutic treatment for neoplastic disease because reduced detoxification potentially enhances effectiveness of cytotoxic drugs. Anticancer drugs that have been shown to be substrates for GSTs are, for example, chlorambucil, melphalan, cyclophosphamide metabolites, and steroids.22,24,25 Indirect evidence for a role of GSTs in modulating drug effects through deactivation of drug-generated hydroperoxides or other reactive oxygene species exists for adriamycin, mitomycin C, carboplatin, and cisplatin.22,26 27
Childhood acute lymphoblastic leukemia (ALL) is the most common malignant disease of childhood and can be cured in up to 70% of cases by the application of intensive multiagent chemotherapeutic regimens.28,29 To assess whether genetic polymorphisms in GST genes are associated with therapeutic success in childhood ALL, we conducted a matched case-control study of relapsed and nonrelapsed patients selected from the multicenter ALL-BFM 86 and ALL-BFM 90 trials of childhood ALL conducted by the Berlin-Frankfurt-Münster (BFM) study group.30 31 The questions addressed in this study were as follows: (1) What are the frequencies and what is the interrelation of the GSTM1, GSTT1, and GSTP1genotypes; (2) how are these genotypes related to important clinical risk factors such as gender, age at diagnosis, initial white blood cell count (WBC), and immunophenotype in childhood ALL; (3) what is the association of specific GST genotypes with the risk of relapse; and (4) what is the effect of GST genotype combinations on risk of relapse in childhood ALL?
Patients and methods
Patients
This study uses data from the ALL-BFM 86 and ALL-BFM 90 trials in childhood ALL, conducted by the BFM study group. In these multicenter trials, pediatric patients up to 18 years from up to 96 different treatment centers in Germany, Austria, and Switzerland were enrolled after informed consent was obtained. Design, conduct, analysis, and results of the ALL-BFM 86 and ALL-BFM 90 trials are described in detail elsewhere.30-32 In both trials, treatment was stratified into 3 branches (standard, intermediate, and high risk), primarily according to the leukemic cell mass estimate and treatment response. The leukemic cell mass estimate, the so-called risk factor, is a composite variable calculated from the initial blast count in the peripheral blood and the sizes of liver and spleen below the costal margin in cm (risk factor = 0.2 × log (no. of blood blasts/μL + 1) + 0.06 × liver size + 0.04 × spleen size). Treatment response is based mainly on the in vivo prednisone response. Prednisone poor response is defined as the presence of ≥ 1000/μL peripheral blood blasts on treatment day 8 after a 7-day monotherapy with prednisone and a single intrathecal application of methotrexate on treatment day 1. In ALL-BFM 86 standard risk patients had < 1000/μL peripheral blood blasts on treatment day 8, a risk factor of < 0.8, no central nervous system (CNS) disease, and no mediastinal mass. Intermediate risk was defined as < 1000/μL peripheral blood blasts on treatment day 8, a risk factor ≥ 0.8, or a risk factor < 0.8 and CNS disease and/or presence of a mediastinal mass. High-risk patients had ≥ 1000/μL peripheral blood blasts on treatment day 8, or > 5% blasts in the bone marrow at treatment day 40, or acute undifferentiated leukemia. In ALL-BFM 90 standard-risk patients had < 1000/μL peripheral blood blasts on treatment day 8, a risk factor of < 0.8, no CNS disease, no mediastinal mass, and no T-cell ALL. Intermediate risk was defined as < 1000/μL peripheral blood blasts on treatment day 8, a risk factor ≥ 0.8, or a risk factor < 0.8 and CNS disease and/or presence of a mediastinal mass or T-cell ALL. High-risk patients had ≥ 1000/μL peripheral blood blasts on treatment day 8, or ≥ 5% blasts in the bone marrow at treatment day 33, or were positive for a t (9;22) or bcr/abl fusion RNA. Treatment in both trials used standard drugs (eg, prednisone, vincristine, daunorubicin, L-asparaginase, cyclophosphamide, ifosfamide, cytarabine, 6-mercaptopurine, 6-thioguanine, and methotrexate) and, in parts of the study group, cranial radiotherapy. With the exception of high-risk patients in ALL-BFM 90, all patients received induction, consolidation, and reinduction treatment, followed by maintenance therapy. Some patients in the intermediate-risk group in ALL-BFM 86 received a late intensification protocol. High-risk patients in ALL-BFM 90 were treated on a shorter induction regimen and continued on an intensive rotational consolidation schedule but did not receive the regular reinduction protocol.
Study design
Of 998 protocol patients enrolled in the ALL-BFM 86 trial, 472 patients (348 successfully treated patients; 124 relapsed patients) and, of the 2178 protocol patients enrolled in the ALL-BFM 90 trial, 575 patients (465 successfully treated patients; 110 relapsed patients) had spare, unstained peripheral blood or bone marrow smears available, obtained at 1 or more time points during the treatment period. The smears were stored at −20°C and were wrapped in parafilm.
Exclusion criteria for patients in this study were preexisting neoplastic disease, preexisting immunologic or hematologic disorders, genetic syndromes, initial CNS disease, and deviations from the therapy protocol. This left 406 patients from trial ALL-BFM 86 (308 successfully treated patients; 98 relapsed patients) and 539 patients from trial ALL-BFM 90 (440 successfully treated patients; 99 relapsed patients). Of the remaining total of 197 relapsed patients, all with an available remission peripheral blood or bone marrow smear were included as cases into the study group if they could be matched to a successfully treated patient with an available remission peripheral blood or bone marrow smear (control individual) according to the following criteria: sex, age at diagnosis (± 6 months), WBC at diagnosis (± 10 000/μL), immunophenotype, trial, risk group, and treatment arm within the branch (risk group) of the respective trial. The latter criterion assured similarity of treatment between cases and controls. Controls had to have a minimum follow-up of 5 years. In case of relapses occurring later than 5 years of diagnosis, the follow-up for the control subject had to be at least as long as the time from date of initial diagnosis to date of relapse diagnosis in the case subject. If more than 1 control subject was available, the subject with the closest initial WBC at diagnosis with reference to the case subject was chosen. Remission peripheral blood or bone marrow smears were used to avoid misclassification of genotypes in the study subjects caused by genetic instability-mediated, possible leukemic clone-specific alterations within the investigated GST genes. This procedure led to the identification of 64 case subjects and 64 individually matched control subjects.
Genotype analysis
Peripheral blood or bone marrow was carefully scraped off the frozen microscopic slides by using sterile surgical scalpel blades. The material was then transferred to microcentrifuge tubes. After each patient, surgical blades, laboratory bench covers, and gloves of the person performing the isolation procedure were changed. DNA was then isolated by using a polymerase chain reaction (PCR) template isolation system (Boehringer Mannheim, Mannheim, Germany) and the DNA yield estimated by measuring the optical density at 260 nm in a spectrophotometer. Genotypes for GSTM1 and GSTT1 were determined by PCR as previously described by Chen et al.33This assay places individuals in 2 categories for each GSTM1and GSTT1, one being either homozygous or heterozygous forGSTM1 or GSTT1 and the other having a homozygous deletion of GSTM1 (GSTM1 null) or GSTT1(GSTT1 null). Thus, the assay cannot distinguish between heterozygotes and homozygotes for GSTM1 and GSTT1, but places them into 1 category. The GSTP1 codon 105 genotype was analyzed according to Harries et al.10 This assay distinguishes homozygotes for the Ile105 allele, heterozygotes (Ile105/Val105), and homozygotes for the Val105 allele. The GSTP1 codon 114 genotype was analyzed according to Harris et al34 using the restriction enzyme AciI instead of Cac8I. In this assay homozygotes for the Ala114 allele, heterozygotes (Ala114/Val114), and homozygotes for the Val114 allele can be distinguished. Negative controls in both PCR assays consisted of similar reaction mixtures as regular samples, but did not contain DNA. Twenty-nine subjects with an available additional remission peripheral blood or bone marrow smear were genotyped twice for the 4 investigated GST genotypes. No deviations from initial genotyping results were observed.
Statistical analysis
For descriptive purposes, frequencies of characteristics and common factors affecting risk of relapse were obtained at the beginning of the analysis. To investigate the interrelationships between GSTM1,GSTT1, and GSTP1 genotypes, and their associations with sex, age at diagnosis, WBC at diagnosis, immunophenotype, and risk group, Spearman correlation coefficients were computed. The association between GST genotypes and occurrence of relapse was examined with conditional logistic regression analysis to calculate odds ratios (OR) and their 95% confidence intervals (CI). GST genotypes and genotype combinations were used as categorical variables in the analyses. Computations were performed with SAS software (SAS-PC Version 6.04, SAS Institute, Cary, NC).
Results
Table 1 shows the distribution of matching variables and genetic analyses (DNA index and karyotype) in relapsed case subjects and successfully treated control subjects. Case and control group showed equal distributions of the matching variables. Thirty-five matched pairs were selected from the ALL-BFM 86 trial and 29 pairs from the ALL-BFM 90 study. There was a higher percentage of males compared with females. At diagnosis, the majority of patients were from 1 to 9 years of age and, with the exception of a single matched pair, all patients presented with an initial WBC of < 50 000/μL. Phenotypically, 54 matched pairs were common (c)-ALLs (diagnostic criteria: terminal deoxynucleotidyl transferase [TdT]+, CD19+, CD10+, cytoplasmic IgM−, cell surface Ig−) and 10 pairs were pre-B-ALLs (diagnostic criteria: TdT+, CD19+, CD10 ±, cytoplasmic IgM+, cell surface Ig−). All subjects selected were either part of the standard-risk group (23 matched pairs) or the intermediate-risk group (41 matched pairs). No study subject was a prednisone poor-responder or part of the high-risk group. For DNA index (ratio of DNA content of leukemic G0/G1 cells to normal diploid cells) and karyotype analyses, most of the patients in both groups had no information available. Table 2 displays the characteristics of relapses in the 64 case subjects of this study. Most of the treatment failures occurred between 2 and 5 years after the date of initial diagnosis and were isolated bone marrow relapses or combined relapses in the bone marrow and CNS. Table3 shows the treatment protocols of standard- and intermediate-risk patients in ALL-BFM 86 and 90. Drugs that are known GST substrates are indicated as well as potential or indirect substrates of GSTs. In addition to a previously described association (P < .05) between the GSTP1 codon 105 and codon 114 genotypes,34 which is due to the almost absent Ile105/Val114 haplotype (see below), no particular correlations among the GSTM1, GSTT1, andGSTP1 genotypes or between the genotypes and sex, age at diagnosis, WBC at diagnosis, immunophenotype, and risk group were observed (data not shown).
Characteristics of relapsed case subjects and successfully treated matched control subjects with acute lymphoblastic leukemia selected from trials ALL-BFM 86 and ALL-BFM 90
| . | No. of Subjects and Prevalence (%) . | |
|---|---|---|
| Cases (n = 64) . | Controls (n = 64) . | |
| Trial | ||
| ALL-BFM 86 | 35 (54.7) | 35 (54.7) |
| ALL-BFM 90 | 29 (45.3) | 29 (45.3) |
| Sex | ||
| Male | 42 (65.6) | 42 (65.6) |
| Female | 22 (34.4) | 22 (34.4) |
| Age (y) | ||
| <1 | 1 (1.6) | 1 (1.6) |
| 1-9 | 56 (87.5) | 56 (87.5) |
| 10-14 | 6 (9.4) | 7 (10.9) |
| 15-18 | 1 (1.6) | — |
| WBC* (103/μL) | ||
| <10 | 42 (65.6) | 41 (64.1) |
| 10-<50 | 21 (32.8) | 22 (34.4) |
| ≥50 | 1 (1.6) | 1 (1.6) |
| Immunophenotype | ||
| c-ALL† | 54 (84.4) | 54 (84.4) |
| Pre-B-ALL‡ | 10 (15.6) | 10 (15.6) |
| Risk group1-153 | ||
| Standard | 23 (35.9) | 23 (35.9) |
| Intermediate | 41 (64.1) | 41 (64.1) |
| High | — | — |
| DNA index1-155 | ||
| <1.16 | 30 (46.9) | 30 (46.9) |
| ≥1.16 | 12 (18.8) | 7 (10.9) |
| Not examined | 22 (34.4) | 27 (42.2) |
| Genotype | ||
| Normal | 4 (6.3) | 10 (15.6) |
| 11q23 Aberrations | — | 1 (1.6) |
| t(1;19) | 1 (1.6) | — |
| t(9;22) | — | — |
| Other | 15 (23.3) | 13 (20.3) |
| Not examined | 44 (68.8) | 40 (62.5) |
| . | No. of Subjects and Prevalence (%) . | |
|---|---|---|
| Cases (n = 64) . | Controls (n = 64) . | |
| Trial | ||
| ALL-BFM 86 | 35 (54.7) | 35 (54.7) |
| ALL-BFM 90 | 29 (45.3) | 29 (45.3) |
| Sex | ||
| Male | 42 (65.6) | 42 (65.6) |
| Female | 22 (34.4) | 22 (34.4) |
| Age (y) | ||
| <1 | 1 (1.6) | 1 (1.6) |
| 1-9 | 56 (87.5) | 56 (87.5) |
| 10-14 | 6 (9.4) | 7 (10.9) |
| 15-18 | 1 (1.6) | — |
| WBC* (103/μL) | ||
| <10 | 42 (65.6) | 41 (64.1) |
| 10-<50 | 21 (32.8) | 22 (34.4) |
| ≥50 | 1 (1.6) | 1 (1.6) |
| Immunophenotype | ||
| c-ALL† | 54 (84.4) | 54 (84.4) |
| Pre-B-ALL‡ | 10 (15.6) | 10 (15.6) |
| Risk group1-153 | ||
| Standard | 23 (35.9) | 23 (35.9) |
| Intermediate | 41 (64.1) | 41 (64.1) |
| High | — | — |
| DNA index1-155 | ||
| <1.16 | 30 (46.9) | 30 (46.9) |
| ≥1.16 | 12 (18.8) | 7 (10.9) |
| Not examined | 22 (34.4) | 27 (42.2) |
| Genotype | ||
| Normal | 4 (6.3) | 10 (15.6) |
| 11q23 Aberrations | — | 1 (1.6) |
| t(1;19) | 1 (1.6) | — |
| t(9;22) | — | — |
| Other | 15 (23.3) | 13 (20.3) |
| Not examined | 44 (68.8) | 40 (62.5) |
WBC: White blood cell count.
Common acute lymphoblastic leukemia; see “Results” or definition.
Pre-B-cell acute lymphoblastic leukemia; see “Results” for definition.
Therapy stratification in risk groups is mainly based on initial leukemic cell mass estimate and initial treatment response; see “Materials and Methods” for exact definition.
Ratio of DNA content of leukemic G0/G1cells-to-normal diploid lymphocytes.
Classification of relapses in 64 case subjects by ALL-BFM trial
| . | No. of Subjects and Prevalence (%) . | |
|---|---|---|
| ALL-BFM 86 (n = 35) . | ALL-BFM 90 (n = 29) . | |
| Time to relapse (y) | ||
| <2 | 7 (20.0) | 6 (20.7) |
| 2-5 | 25 (71.4) | 19 (65.5) |
| >5 | 3 (8.6) | 4 (13.8) |
| Type of relapse | ||
| Isolated relapse | ||
| Bone marrow | 19 (54.3) | 16 (55.2) |
| CNS | 3 (8.6) | — |
| Testis | 3 (8.6) | 2 (6.9) |
| Other | — | 1 (3.4) |
| Combined relapse | ||
| Bone marrow/CNS | 5 (14.3) | 7 (24.1) |
| Bone marrow/testis | 4 (11.4) | 2 (6.9) |
| Bone marrow/CNS/testis | 1 (2.9) | — |
| Other | — | 1 (3.4) |
| . | No. of Subjects and Prevalence (%) . | |
|---|---|---|
| ALL-BFM 86 (n = 35) . | ALL-BFM 90 (n = 29) . | |
| Time to relapse (y) | ||
| <2 | 7 (20.0) | 6 (20.7) |
| 2-5 | 25 (71.4) | 19 (65.5) |
| >5 | 3 (8.6) | 4 (13.8) |
| Type of relapse | ||
| Isolated relapse | ||
| Bone marrow | 19 (54.3) | 16 (55.2) |
| CNS | 3 (8.6) | — |
| Testis | 3 (8.6) | 2 (6.9) |
| Other | — | 1 (3.4) |
| Combined relapse | ||
| Bone marrow/CNS | 5 (14.3) | 7 (24.1) |
| Bone marrow/testis | 4 (11.4) | 2 (6.9) |
| Bone marrow/CNS/testis | 1 (2.9) | — |
| Other | — | 1 (3.4) |
CNS: central nervous system.
Treatment protocols3-150 for standard- and intermediate-risk patients in ALL-BFM 86 and ALL-BFM 90
| Drug . | ALL-BFM 86 . | ALL-BFM 90 . | Glutathione S-Transferase Substrate . | ||
|---|---|---|---|---|---|
| Dose . | Administered on Days . | Dose . | Administered on Days . | ||
| Induction | |||||
| Prednisone (orally) | 60 mg/m2/d | 1-28 | 60 mg/m2/d | 1-28 | Potential22 25 |
| Vincristine (IV) | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | |
| Daunorubicin (IV) | 40 mg/m2/d | 8, 15, 22, 29 | 30 mg/m2/d | 8, 15, 22, 29 | Indirect22,26 27 |
| L-Asparaginase (IV) | 10 000 IU/m2/d | 19, 22, 25, 28, 31, 34, 37, 40 | 10 000 IU/m2/d | 12, 15, 18, 21, 24, 27, 30, 33 | |
| Cyclophosphamide (IV) | 1000 mg/m2/d | 43, 71 | 1000 mg/m2/d | 36, 64 | Yes22 24 |
| Cytarabine (IV) | 75 mg/m2/d | 45-48, 52-55, 59-62, 66-69 | 75 mg/m2/d | 38-41, 45-48, 52-55, 59-62 | |
| 6-Mercaptopurine (orally) | 60 mg/m2/d | 43-70 | 60 mg/m2/d | 36-64 | |
| Methotrexate (IT) | 12 mg/d | 1, 45, 59 | 12 mg/d | 1, 15, 29, 45, 59 | |
| Consolidation | |||||
| 6-Mercaptopurine (orally) | 25 mg/m2/d | 1-56 | 25 mg/m2/d | 1-56 | |
| Methotrexate (24-h INF) | 5 g/m2/d | 8, 22, 36, 50 | 5 g/m2/d | 8, 22, 36, 50 | |
| Methotrexate (IT) | 12 mg/d | 8, 22, 36, 50 | 12 mg/d | 8, 2, 36, 50 | |
| L-Asparaginase (IM) | — | — | 25 000 IU/m2/d | 10, 24, 38, 52 | |
| Reinduction | |||||
| Dexamethasone (orally) | 10 mg/m2/d | 1-21 | 10 mg/m2/d | 1-21 | Yes22 25 |
| Vincristine (IV) | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | |
| Doxorubicin (IV) | 30 mg/m2/d | 8, 15, 22, 29 | 30 mg/m2/d | 8, 15, 22, 29 | Indirect22,26 27 |
| L-Asparaginase (IV) | 10 000 IU/m2/d | 8, 11, 15, 18 | 10 000 IU/m2/d | 8, 11, 15, 18 | |
| Cyclophosphamide (IV) | 1000 mg/m2/d | 36 | 1000 mg/m2/d | 36 | Yes22 24 |
| Cytarabine (IV) | 75 mg/m2/d | 38-41, 45-48 | 75 mg/m2/d | 38-41, 45-48 | |
| 6-Thioguanine (orally) | 60 mg/m2/d | 36-49 | 60 mg/m2/d | 36-49 | |
| Methotrexate (IT) | 12 mg/d | 38, 45 | 12 mg/d | 38, 45 | |
| Late intensification3-151 | |||||
| Prednisone (orally) | 100 mg/m2/d | 1-7, 15-21 | — | — | Potential22 25 |
| Vindesine (IV) | 3 mg/m2/d (max 5 mg) | 1, 8, 15, 22 | — | — | |
| Teniposide (IV) | 150 mg/m2/d | 1, 8, 15, 22 | — | — | |
| Ifosfamide (IV) | 1000 mg/m2/12 h | 1, 2 | — | — | Yes48 49 |
| Cytarabine (3-h INF) | 2000 mg/m2/12 h | 15, 16 | — | — | |
| Drug . | ALL-BFM 86 . | ALL-BFM 90 . | Glutathione S-Transferase Substrate . | ||
|---|---|---|---|---|---|
| Dose . | Administered on Days . | Dose . | Administered on Days . | ||
| Induction | |||||
| Prednisone (orally) | 60 mg/m2/d | 1-28 | 60 mg/m2/d | 1-28 | Potential22 25 |
| Vincristine (IV) | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | |
| Daunorubicin (IV) | 40 mg/m2/d | 8, 15, 22, 29 | 30 mg/m2/d | 8, 15, 22, 29 | Indirect22,26 27 |
| L-Asparaginase (IV) | 10 000 IU/m2/d | 19, 22, 25, 28, 31, 34, 37, 40 | 10 000 IU/m2/d | 12, 15, 18, 21, 24, 27, 30, 33 | |
| Cyclophosphamide (IV) | 1000 mg/m2/d | 43, 71 | 1000 mg/m2/d | 36, 64 | Yes22 24 |
| Cytarabine (IV) | 75 mg/m2/d | 45-48, 52-55, 59-62, 66-69 | 75 mg/m2/d | 38-41, 45-48, 52-55, 59-62 | |
| 6-Mercaptopurine (orally) | 60 mg/m2/d | 43-70 | 60 mg/m2/d | 36-64 | |
| Methotrexate (IT) | 12 mg/d | 1, 45, 59 | 12 mg/d | 1, 15, 29, 45, 59 | |
| Consolidation | |||||
| 6-Mercaptopurine (orally) | 25 mg/m2/d | 1-56 | 25 mg/m2/d | 1-56 | |
| Methotrexate (24-h INF) | 5 g/m2/d | 8, 22, 36, 50 | 5 g/m2/d | 8, 22, 36, 50 | |
| Methotrexate (IT) | 12 mg/d | 8, 22, 36, 50 | 12 mg/d | 8, 2, 36, 50 | |
| L-Asparaginase (IM) | — | — | 25 000 IU/m2/d | 10, 24, 38, 52 | |
| Reinduction | |||||
| Dexamethasone (orally) | 10 mg/m2/d | 1-21 | 10 mg/m2/d | 1-21 | Yes22 25 |
| Vincristine (IV) | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | 1.5 mg/m2/d (max 2 mg) | 8, 15, 22, 29 | |
| Doxorubicin (IV) | 30 mg/m2/d | 8, 15, 22, 29 | 30 mg/m2/d | 8, 15, 22, 29 | Indirect22,26 27 |
| L-Asparaginase (IV) | 10 000 IU/m2/d | 8, 11, 15, 18 | 10 000 IU/m2/d | 8, 11, 15, 18 | |
| Cyclophosphamide (IV) | 1000 mg/m2/d | 36 | 1000 mg/m2/d | 36 | Yes22 24 |
| Cytarabine (IV) | 75 mg/m2/d | 38-41, 45-48 | 75 mg/m2/d | 38-41, 45-48 | |
| 6-Thioguanine (orally) | 60 mg/m2/d | 36-49 | 60 mg/m2/d | 36-49 | |
| Methotrexate (IT) | 12 mg/d | 38, 45 | 12 mg/d | 38, 45 | |
| Late intensification3-151 | |||||
| Prednisone (orally) | 100 mg/m2/d | 1-7, 15-21 | — | — | Potential22 25 |
| Vindesine (IV) | 3 mg/m2/d (max 5 mg) | 1, 8, 15, 22 | — | — | |
| Teniposide (IV) | 150 mg/m2/d | 1, 8, 15, 22 | — | — | |
| Ifosfamide (IV) | 1000 mg/m2/12 h | 1, 2 | — | — | Yes48 49 |
| Cytarabine (3-h INF) | 2000 mg/m2/12 h | 15, 16 | — | — | |
All patients in our study received maintenance therapy with oral 6-mercaptopurine (50 mg/m2/d) and methotrexate (20 mg/m2/wk) for 24 mo.
Patients in the intermediate-risk group of ALL-BFM 86 were randomized to either receive late intensification after reinduction or not to receive it.
IV: intravenously; IT: intrathecally, doses were adjusted for children <3 y; INF: intravenous infusion; IM: intramuscularly, only given in ALL-BFM 90 where patients were randomized to consolidation protocols with or without IM L-Asparaginase.
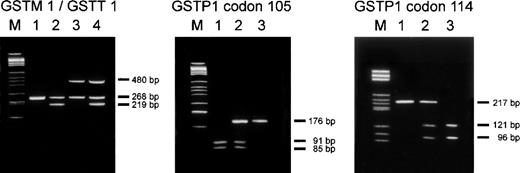
Figure 1 explains the PCR assays used for genotype analyses. Genotype prevalences in the entire study population were as follows: GSTM1 present: 50%, GSTM1 null: 50%;GSTT1 present: 85.2%, GSTT1 null: 14.8%;GSTP1 Ile105/Ile105: 50.8%,GSTP1 Ile105/Val105: 38.3%,GSTP1 Val105/Val105: 10.9%; andGSTP1 Ala114/Ala114: 78.1%,GSTP1 Ala114/Val114: 21.1%,GSTP1 Val114/Val114: 0.8%. Table4 shows the distribution of GSTM1,GSTT1, and GSTP1 genotypes in case and control subjects and the association of these genotypes with the occurrence of relapse. The risk of relapse for study subjects being GSTM1 null conferred a statistically nonsignificant 2-fold protection from relapse compared with individuals being either heterozygous or homozygous forGSTM1 (OR = 0.50; 95% CI = 0.23-1.07; P = .078). The GSTT1 null genotype conferred a statistically significant 2.8-fold reduction in risk of relapse relative to the presence of theGSTT1 gene (OR = 0.36, 95% CI = 0.13-0.99,P = .048). For GSTP1, the Ile105/Val105 and the Ile105/Ile105 genotypes did not differ with respect to their association with risk of relapse (OR = 1.0 for Ile105/Val105 relative to Ile105/Ile105). Thus, both groups were combined in 1 category to increase the variability for the assessment of risk of the Val105/Val105 genotype. A 3-fold decrease in risk of relapse (OR = 0.33, 95% CI = 0.09-1.23,P = .099) was associated with the Val105/Val105 genotype in comparison to the combined category (Ile105/Val105 and Ile105/Ile105). No particular association with relapse was observed for the GSTP1 polymorphism at codon 114. The OR for a combined category of Ala114/Val114and Val114/Val114 genotypes with reference to the Ala114/Ala114 genotype was 0.85 (95% CI = 0.38-1.88, P = .682).
Genotype analyses of GSTM1, GSTT1, andGSTP1.
Left panel: Agarose gel electrophoresis of PCR products from a multiplex PCR experiment for simultaneous assessment of GSTM1and GSTT1 status.33 The PCR products amplified from the GSTM1 and GSTT1 loci are 219 bp and 480 bp in size, respectively. A 268 bp fragment from the β-globin locus was coamplified for internal control purposes. Lane 1 shows an individual with a homozygous deletion of GSTM1 and GSTT1. Lane 2 shows an individual in which GSTM1 can be detected butGSTT1 is homozygously deleted. In lane 3, GSTM1 is absent while a PCR product from the GSTT1 locus can be detected. In lane 4 both GSTM1 and GSTT1 are present. M = DNA size standard. Middle and right panel: Detection ofGSTP1 codon 105 and codon 114 genotypes by agarose gel electrophoresis of PCR products after digestion with the restriction endonucleases BsmAI and AciI, respectively.10 34 The sequence polymorphism at GSTP1 codon 105 creates a restriction site for BsmAI within the resulting 176 bp PCR product, leading to the generation of 2 fragments of 91 bp and 85 bp, respectively. Thus, in individuals homozygous for this polymorphism (Val105/Val105) the 176 bp PCR product is completely digested into 2 fragments (lane 1). Lane 2 displays a heterozygous individual (Ile105/Val105), and in lane 3 an individual homozygous for the Ile105-coding allele is shown. The polymorphism at GSTP1 codon 114 leads to the loss of an AciI restriction site. Thus, individuals homozygous for the Val114 allele only show the undigested 217 bp PCR product (lane 1). Heterozygous individuals (Ala114/Val114) show the undigested product and 2 additional fragments (121 bp and 96 bp) resulting from its digestion (lane 2), and individuals homozygous for Ala114 are characterized by the sole presence of the digestion products of 121 bp and 96 bp (lane 3). M = DNA size standard.
Genotype analyses of GSTM1, GSTT1, andGSTP1.
Left panel: Agarose gel electrophoresis of PCR products from a multiplex PCR experiment for simultaneous assessment of GSTM1and GSTT1 status.33 The PCR products amplified from the GSTM1 and GSTT1 loci are 219 bp and 480 bp in size, respectively. A 268 bp fragment from the β-globin locus was coamplified for internal control purposes. Lane 1 shows an individual with a homozygous deletion of GSTM1 and GSTT1. Lane 2 shows an individual in which GSTM1 can be detected butGSTT1 is homozygously deleted. In lane 3, GSTM1 is absent while a PCR product from the GSTT1 locus can be detected. In lane 4 both GSTM1 and GSTT1 are present. M = DNA size standard. Middle and right panel: Detection ofGSTP1 codon 105 and codon 114 genotypes by agarose gel electrophoresis of PCR products after digestion with the restriction endonucleases BsmAI and AciI, respectively.10 34 The sequence polymorphism at GSTP1 codon 105 creates a restriction site for BsmAI within the resulting 176 bp PCR product, leading to the generation of 2 fragments of 91 bp and 85 bp, respectively. Thus, in individuals homozygous for this polymorphism (Val105/Val105) the 176 bp PCR product is completely digested into 2 fragments (lane 1). Lane 2 displays a heterozygous individual (Ile105/Val105), and in lane 3 an individual homozygous for the Ile105-coding allele is shown. The polymorphism at GSTP1 codon 114 leads to the loss of an AciI restriction site. Thus, individuals homozygous for the Val114 allele only show the undigested 217 bp PCR product (lane 1). Heterozygous individuals (Ala114/Val114) show the undigested product and 2 additional fragments (121 bp and 96 bp) resulting from its digestion (lane 2), and individuals homozygous for Ala114 are characterized by the sole presence of the digestion products of 121 bp and 96 bp (lane 3). M = DNA size standard.
Distribution of GSTM1, GSTT1, GSTP1 genotypes and their association with the occurrence of relapse in 64 case subjects and 64 successfully treated matched control subjects with acute lymphoblastic leukemia from ALL-BFM trials 86 and 90
| . | No. of Subjects and Prevalence (%) . | OR (95% CI) . | P Value . | |
|---|---|---|---|---|
| Cases . | Controls . | |||
| GSTMI | ||||
| Present | 37 (57.8) | 27 (42.2) | 1.004-150 | |
| Null | 27 (42.2) | 37 (57.8) | 0.50 (0.23-1.07) | .078 |
| GSTT1 | ||||
| Present | 59 (92.2) | 50 (78.1) | 1.004-150 | |
| Null | 5 (7.8) | 14 (21.9) | 0.36 (0.13-0.99) | .048 |
| GSTP1 codon 105 | ||||
| Ile/Ile | 33 (51.6) | 32 (50.0) | ||
| Ile/Val | 27 (42.2) | 22 (34.4) | 1.004-151 | |
| Val/Val | 4 (6.3) | 10 (15.6) | 0.33 (0.09-1.23) | .099 |
| GSTP1 codon 114 | ||||
| Ala/Ala | 52 (81.3) | 48 (75.0) | 1.004-150 | |
| Ala/Val | 11 (17.2) | 16 (25.0) | 0.85‡ (0.38-1.88) | .682 |
| Val/Val | 1 (1.6) | — | ||
| . | No. of Subjects and Prevalence (%) . | OR (95% CI) . | P Value . | |
|---|---|---|---|---|
| Cases . | Controls . | |||
| GSTMI | ||||
| Present | 37 (57.8) | 27 (42.2) | 1.004-150 | |
| Null | 27 (42.2) | 37 (57.8) | 0.50 (0.23-1.07) | .078 |
| GSTT1 | ||||
| Present | 59 (92.2) | 50 (78.1) | 1.004-150 | |
| Null | 5 (7.8) | 14 (21.9) | 0.36 (0.13-0.99) | .048 |
| GSTP1 codon 105 | ||||
| Ile/Ile | 33 (51.6) | 32 (50.0) | ||
| Ile/Val | 27 (42.2) | 22 (34.4) | 1.004-151 | |
| Val/Val | 4 (6.3) | 10 (15.6) | 0.33 (0.09-1.23) | .099 |
| GSTP1 codon 114 | ||||
| Ala/Ala | 52 (81.3) | 48 (75.0) | 1.004-150 | |
| Ala/Val | 11 (17.2) | 16 (25.0) | 0.85‡ (0.38-1.88) | .682 |
| Val/Val | 1 (1.6) | — | ||
Reference category.
Reference category was a combined category of Ile105/Ile105 and Ile105/Val105 genotypes, because OR for Ile105/Val105 compared with Ile105/Ile105 showed equal risk in both categories.
OR for the combined category of Ala114/Val114 and Val114/Val114 genotypes with reference to the Ala114/Ala114 category.
OR: Odds ratio; CI: confidence interval.
Four different haplotypes have been described forGSTP1.7-9GSTP1*A has Ile at codon 105 and Ala at codon 114. GSTP1*B has Val at codon 105 and Ala at codon 114. GSTP1*C has both Val at codon 105 and 114, andGSTP1*D has Ile at position 105 and Val at position 114. The PCR genotyping method used in this study does not allow precise determination of GSTP1 haplotypes, but haplotypes can be estimated according to their order of occurrence.9GSTP1*A is by far the most common haplotype, followed byGSTP1*B and GSTP1*C. The GSTP1*D haplotype is very rare. Table 5 shows the distribution of estimated GSTP1 haplotypes in our study population. Our sample size of 128 patients combined with the distribution ofGSTP1 haplotypes over the 6 categories did not allow a reasonable, matched assessment of the association of GSTP1haplotypes with risk of relapse. However, the most protection from relapse with reference to the GSTP1*A/GSTP1*A category was conferred by the GSTP1*B/GSTP1*B andGSTP1*B/GSTP1*C genotypes with an OR of 0.50 (P = .418) and 0.33 (P = .317), respectively. These 2 genotypes carry Val at codon 105 in both of their alleles. The ORs for the GSTP1*A/GSTP1*B andGSTP1*A/GSTP1*C categories in comparison to theGSTP1*A/GSTP1*A category were 0.70 (P = .465) and 1.00 (P > .99), respectively. Thus, although hampered by small numbers, in haplotype analyses, patients homozygous for Val at codon 105 seemed to have the greatest protection from relapse.
Distribution of estimated GSTP1 haplotypes in 64 case subjects and 64 successfully treated matched control subjects with acute lymphoblastic leukemia from ALL-BFM trials 86 and 90
| . | No. of Subjects and Prevalence (%) . | |
|---|---|---|
| Cases . | Controls . | |
| GSTP1*A/GSTP1*A | 32 (50.0) | 31 (48.4) |
| GSTP1*A/GSTP1*B | 19 (29.7) | 13 (20.3) |
| GSTP1*A/GSTP1*C | 8 (12.5) | 10 (15.6) |
| GSTP1*B/GSTP1*B | 2 (3.1) | 5 (7.8) |
| GSTP1*B/GSTP1*C | 2 (3.1) | 5 (7.8) |
| GSTP1*C/GSTP1*D | 1 (1.6) | — |
| . | No. of Subjects and Prevalence (%) . | |
|---|---|---|
| Cases . | Controls . | |
| GSTP1*A/GSTP1*A | 32 (50.0) | 31 (48.4) |
| GSTP1*A/GSTP1*B | 19 (29.7) | 13 (20.3) |
| GSTP1*A/GSTP1*C | 8 (12.5) | 10 (15.6) |
| GSTP1*B/GSTP1*B | 2 (3.1) | 5 (7.8) |
| GSTP1*B/GSTP1*C | 2 (3.1) | 5 (7.8) |
| GSTP1*C/GSTP1*D | 1 (1.6) | — |
See “Results” for explanation of haplotypes.
Regarding binding properties of specific substrates by GSTs in the body, it was suggested that, besides specific substrates, GST isozymes display overlapping limited substrate specifity toward a variety of substances.22 Such an overlap in substrate specificity suggests that different GST genotype combinations may confer varying levels of GST activity. For this reason, we investigated the association of GST genotype combinations with risk of relapse in our study population of children with ALL. The reference category consisted of study subjects who did not have any of the genotypes that were shown to be protective in the above described analyses of GSTM1,GSTT1, and GSTP1 genotypes. These were individuals withGSTM1 present, GSTT1 present, and GSTP1genotypes other than GSTP1Val105/Val105. GSTM1 null, GSTT1null, and GSTP1 Val105/Val105(corresponding to GSTP1 haplotypesGSTP1*B/GSTP1*B and GSTP1*B/GSTP1*C) were considered as protective GST genotypes (low-risk genotypes). In Table6, the distribution of low-risk GST genotypes and their association with risk of ALL relapse is shown. The risk of relapse when having 1 low-risk GST genotype decreased 1.9-fold (OR = 0.53, 95% CI = 0.24-1.19, P = .123) and the risk when having 2 or 3 low-risk genotypes decreased 3.5-fold (OR = 0.29, 95% CI = 0.06-1.37, P = .118), compared with the reference group (no low-risk genotype). The decrease of risk with increasing numbers of low-risk genotypes was statistically significant (Pfor trend = .005).
Distribution of potential glutathione S-transferase low-risk genotypes and their association with the occurrence of relapse in 64 case subjects and 64 successfully treated matched control subjects with acute lymphoblastic leukemia from ALL-BFM trials 86 and 90
| . | No. of Subjects and Prevalence (%) . | OR (95% CI) . | P Value . | |
|---|---|---|---|---|
| Cases . | Controls . | |||
| Genotype combinations | ||||
| —No low risk genotype6-150 | 33 (51.6) | 20 (31.3) | 1.006-151 | |
| —1 low risk genotype | 27 (42.2) | 28 (43.8) | 0.53 (0.24-1.19) | 0.123 |
| —2 or 3 low risk genotypes | 4 (6.3) | 16 (25.0) | 0.29 (0.06-1.37) | 0.118 |
| . | No. of Subjects and Prevalence (%) . | OR (95% CI) . | P Value . | |
|---|---|---|---|---|
| Cases . | Controls . | |||
| Genotype combinations | ||||
| —No low risk genotype6-150 | 33 (51.6) | 20 (31.3) | 1.006-151 | |
| —1 low risk genotype | 27 (42.2) | 28 (43.8) | 0.53 (0.24-1.19) | 0.123 |
| —2 or 3 low risk genotypes | 4 (6.3) | 16 (25.0) | 0.29 (0.06-1.37) | 0.118 |
Low-risk genotypes: GSTM1 null, GSTT1 null, andGSTP1 Val105/Val105 (corresponding toGSTP1 haplotypes GSTP1*B/GSTP1*B, orGSTP1*B/GSTO1*C).
Reference category.
OR: Odds ratio; CI: confidence interval.
Discussion
In our study population, the prevalence of theGSTM1 and GSTT1 null genotype was 50.0% and 14.8%, respectively. Thus, our findings within the whole study sample are not different from the results observed by Chen et al35 in a study on GSTM1 and GSTT1genotypes in childhood ALL. In their study, the GSTM1 null genotype was detected in 55.2% of white childhood ALL patients and 53.5% of normal controls, and the GSTT1 null genotype was found in 14.1% of white childhood ALL patients and 15.0% of their controls. To our knowledge, there are no reported genotype frequencies for GSTP1 in childhood ALL. However, the GSTP1 genotype distributions (Ile105/Ile105: 50.8%, Ile105/Val105: 38.3%, Val105/Val105: 10.9%; and Ala114/Ala114: 78.1%, Ala114/Val114: 21.1%, Val114/Val114: 0.8%) observed in our study population fall within the same range described for white populations in studies conducted on GSTP1 genotype frequencies and, for example, risk of breast cancer or other malignancies.9,10 19
In the analysis of the relationships of GSTM1,GSTT1, and GSTP1 genotypes with important clinical risk factors such as gender, age, and WBC at diagnosis, and immunophenotype in childhood ALL, we did not detect any particular associations. This is in accordance with the above mentioned study by Chen et al35 who reported on a suggestive association of the GST double null genotype (GSTM1 and GSTT1 null) with WBC at diagnosis and DNA index (ploidy), but did not find any significant relationship. We were not able to appropriately address the question of an association between DNA ploidy measurements and GST genotypes because a major part of our patients had no data available (see Table1). In a study by Hall et al,36 however, on protein expression of μ class GST and event-free survival in childhood ALL, no obvious associations between GST μ expression and prognostic features were observed either.
In the past, several studies addressed the association of GST protein expression and leukemia outcome.36-40 For example, in the above mentioned study by Hall et al,36 a 3-fold increased risk of childhood ALL relapse (95% CI = 1.25-7.26) in patients expressing μ class GST compared with nonexpressing patients was observed. In contrast, very little information is available on the role of GST genotypes in leukemia. To our knowledge, this is the first study to show a statistically significant association between GST genotypes and outcome in childhood ALL. In our study, the null genotype for GSTM1 or GSTT1 conferred a 2-fold (OR = 0.5, 95% CI = 0.23-1.07, P = .078) and 2.8-fold (OR = 0.36, 95% CI = 0.13-0.99, P = .048) reduction in risk of relapse, respectively, relative to the presence of the GSTM1 orGSTT1 gene. For GSTP1, the Val105/Val105 genotype showed a 3-fold decrease in risk of relapse (OR = 0.33, 95% CI = 0.09-1.23,P = .099) in comparison to the combined category of Ile105/Val105 and Ile105/Ile105 genotypes. Thus, we detected a statistically significant association of the GSTT1 genotype with risk of relapse, and strongly suggestive results for theGSTM1 and GSTP1 codon 105 genotypes. The biologic plausability of our results is supported by a statistically significant (P for trend = 0.005) decrease in risk of relapse with increasing number of low-risk genotypes (GSTM1 null,GSTT1 null, GSTP1Val105/Val105). With regard to the small sample size in our study and the relatively low prevalence of theGSTT1 null and the GSTP1Val105/Val105 genotypes (Table 4), as well as the small group of patients with 2 or 3 low-risk genotypes (Table 6), our results encourage the prospective evaluation of the contribution of GST genotypes to therapeutic outcome in larger patient populations. Such prospective studies should also include assays to evaluate if patients who are heterozygous for GSTM1 or GSTT1 do differ from patients homozygous for GSTM1 orGSTT1. In addition, a potential modulatory effect of the GSTM1*A and GSTM1*B alleles on therapeutic outcome would be of investigative interest. These 2 distinct GSTM1alleles have recently been suggested to confer varying susceptibility to, for example, breast and bladder cancer, and may therefore also display different enzyme activities.41 42 This information may help clarify the issue of the potential existence of variability with regard to therapeutic outcome in GSTT1 and GSTM1positive patient populations and increase our knowledge on the role of GST genotypes in childhood ALL.
In the only other study addressing the association of GST genotypes and outcome in childhood ALL, Chen et al35reported on GSTM1 and GSTT1 genotypes and their impact on event-free survival (EFS), hematologic remission, and time to isolated CNS relapse in 161 childhood ALL patients from 3 consecutive trials conducted at St Jude Children's Research Hospital. Except for a tendency of higher CNS relapse-free survival at 5 years among patients with the GSTM1 null genotype (P = .054), no particular associations between GSTM1 and GSTT1genotypes, and outcome of childhood ALL were detected in that study.35 Because 2 different study designs were used, the diverging results between the study by Chen et al35 and ours could be explained several ways. In our study, case and control groups were highly homologous concerning the matching factors, assuring similarity between both groups with regard to treatment and all clinical prognostic features used for patient stratification in the ALL-BFM 86 and 90 trials. However, the matching criteria set forth in our study led to the selection of a particular subgroup of the entire ALL patient population. Thus, our results are not generalizable to all childhood ALL patients, but are restricted to B-cell precursor ALL of standard- and intermediate-risk. In contrast, Chen et al35looked at 161 patients derived from 3 consecutive trials representing a selection from the St Jude Children's Research Hospital trials of childhood ALL Total XI, Total XII, and Total XIIIA. This strategy led to the analysis of a more diverse patient population and to more generalizable results. However, a possible effect of GST genotypes in certain subgroups may have been diluted. Another reason could be the different drug regimens applied in the trials at St Jude Children's Research Hospital and in the ALL-BFM trials. Unfortunately, our study population was too small to appropriately assess the association of GST genotypes and site of relapse. With regard to the results of Chen et al35 (tendency of higher CNS relapse-free survival among patients with the GSTM1 null genotype) and the notion of tissue-specific expression of GSTs,1,36 this is an important question for the future. Another important aspect for future studies will be the contribution of GST genotypes to leukemia outcome under consideration of cytogenetically and/or molecular genetically defined patient populations. This, because GST genotypes, may be associated with leukemogenesis in specific, cytogenetically, and/or molecular genetically defined leukemia subsets.43-45 If such an association exists, the evaluation of GST genotypes in association with leukemia outcome will have to take cytogenetic and/or molecular genetic information into account. Unfortunately, in our patient population cytogenetic data were only available for 34.4%, making an analysis in a matched setting impossible.
One last issue to be discussed relates to the mechanism by which GSTs modulate response to cytotoxic chemotherapy. On one hand, GST-family isozymes have relatively low binding affinities to specific substrates, and on the other hand, they recognize and/or detoxify a broad range of substrates.22 As a consequence, a variety of anticancer drugs are proven or potential substrates or binding partners of GSTs. However, the mechanism by which GSTs may modulate resistance to anticancer drugs is still a matter of debate. The major mechanism suggested is still the conjugation with glutathione.1 Other studies propose additional possibilities, involving the binding of drugs and/or their removal from the cell.46 47 Growing understanding of the mechanism of action will help design clinical studies for a more specific assessment of the role of GSTs in drug resistance. Another open question is the contribution of specific GST isozymes to the metabolism of distinct anticancer drugs. For example, to our knowledgeGSTT1 has not yet been associated with the detoxification or binding of any of the anticancer drugs used in ALL-BFM 86 and ALL-BFM 90. Although overlapping substrate specificities may infer involvement of GSTT1 in the detoxification or binding of at least some of the drugs previously described as substrates for other GST isozymes, this issue remains unproven. The results of the current study should inspire further studies that evaluate such specific substrate associations for GSTT1 and other GST isozymes, as well.
In conclusion, our results suggest an association of GST genotypes with therapeutic outcome in childhood precursor B-cell ALL. However, the contribution of genetic interindividual variability in the GST system has to be evaluated in larger, well-characterized patient populations. Nevertheless, GST genotypes may be useful for the future development of individual patient risk profiles to optimize cancer chemotherapeutic regimens.
Acknowledgments
We thank all the participants of the ALL-BFM 86 and 90 studies for their cooperation and Nicolai Götz for excellent data management. We are particularly thankful to Edelgard Odenwald for her careful organization of spare bone marrow and peripheral blood smears.
M.St. is a recipient of a “Kind-Philipp-Rückkehrstipendium” through the “Kind-Philipp-Stiftung” within the “Stifterverband für die Deutsche Wissenschaft,” Essen, Germany.
Supported in part by the “Madeleine Schickedanz-Kinderkrebs-Stiftung,” Fürth, Germany, and the Lions Club, Rinteln, Germany.
Reprints:Karl Welte, Department of Pediatric Hematology and Oncology, Children's Hospital, Hannover Medical School, Carl-Neuberg-Str. 1, 30625 Hannover, Germany; e-mail:welte.karl@mh-hannover.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal