Previous studies have shown that thrombin generation in vivo caused a 92% decrease in factor IX (F.IX) activity and the appearance of a cleavage product after immunoblotting that comigrated with activated F.IX (F.IXa). Under these conditions, the fibrinolytic system was clearly activated, suggesting plasmin may have altered F.IX. Thus, the effect(s) of plasmin on human F.IX was determined in vitro. Plasmin (50 nM) decreased the 1-stage clotting activity of F.IX (4 μM) by 80% and the activity of F.IXa (4 μM) by 50% after 30 minutes at 37°C. Plasmin hydrolysis of F.IX yields products of 45, 30, 20, and 14 kd on reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and 2 products of 52 and 14 kd under nonreducing conditions. Plasmin-treated F.IX did not bind the active site probe, p-aminobenzamidine, or form an SDS-stable complex with antithrombin. It only marginally activated human factor X in the presence of phospholipid and activated factor VIII. Although dansyl-Glu-Gly-Arg-chloromethyl ketone inactivated–F.IXa inhibited the clotting activity of F.IXa, plasmin-treated F.IX did not. Plasmin cleaves F.IX after Lys43, Arg145, Arg180, Lys316, and Arg318, but F.IXa is not appreciably generated despite cleavage at the 2 normal activation sites (Arg145 and Arg180). Tissue plasminogen activator–catalyzed lysis of fibrin formed in human plasma results in generation of the 45- and 30-kd fragments of F.IX and decreased F.IX clotting activity. Collectively, the results suggest that plasmin is able to down-regulate coagulation by inactivating F.IX.
The processes of clot formation and lysis consist of a series of enzymatic reactions that involve the controlled activation of inactive zymogens and cofactors.1-4 The blood-clotting reactions result in the generation of thrombin, which mediates clot formation by catalyzing the conversion of soluble fibrinogen to insoluble fibrin. Fibrinolysis occurs upon generation of plasmin, which cleaves insoluble fibrin to fibrin degradation products. Experimental evidence indicates that a hemostatic balance exists between coagulation and fibrinolysis under normal conditions5,6 and that this balance is perturbed in response to injury7 and during disease.8-10 Components of the coagulation and fibrinolytic processes may not only regulate their respective pathways but may also interact with each other. For example, in the presence of thrombomodulin, thrombin activates a plasma carboxypeptidase (thrombin activable fibrinolysis inhibitor) whose resultant activity downregulates fibrinolysis.11 Conversely, plasmin has been shown in vitro to inactivate factor (F.)IX,12 activate F.VII13 and F.XII,14 and initially activate and then subsequently inactivate F.V15 and F.VIII.16 Plasmin's action on F.V, F.VII, F.VIII, F.IX, and F.XII may profoundly alter the levels of thrombin generated and significantly influence the resultant rate of coagulation.
The infusion of activated F.X (F.Xa) and phosphatidylcholine/phosphatidylserine (PCPS) vesicles results in thrombin generation in vivo.17-19 These animal models have been used to study the coagulation and fibrinolytic reactions that occur subsequent to thrombin generation in vivo. At elevated doses of F.Xa and PCPS, the regulation of hemostasis may be compromised, and the resultant phenotype is remarkably similar to the human disorder, disseminated intravascular coagulation (DIC).20 A study of the individual coagulation factors indicated that the clotting activity of F.IX decreased by 92% in this experimental model after F.Xa/PCPS infusion.21 In addition, a lower apparent molecular mass cleavage product of F.IX approximating the size of F.IXa was observed by immunoblotting following F.Xa/PCPS infusion. This result was unexpected because the F.IXa-like species produced as a result of thrombin generation would have been expected to complex with plasma antithrombin (AT) into a larger product. These results suggest that, in response to thrombin generation in vivo, either F.IXa was generated from the action of its physiologic activators—F.XIa22 or F.VIIa and tissue factor (TF)23—and further cleaved to an “inactive” protease, or that a different protease cleaved F.IX directly to a F.IXa-like product possessing decreased coagulant activity. These changes in F.IX were associated with a 10- to 25-fold increase in the functional activity and antigen levels of tissue plasminogen activator (tPA) and a 2.5-fold decrease in the antigen levels of α2—antiplasmin (α2-AP),19suggesting that increased generation of plasmin may have mediated the effect(s) on F.IX.
F.IX is synthesized in the liver as a single-chain glycoprotein (Mr 57 000), and the zymogen circulates in human plasma at approximately 5 μg/mL (0.09 μM).24 The complete nucleotide sequence of the F.IX gene and amino acid sequence of the mature protein has been determined.25 F.IX is activated in a 2-step process by either F.XIa22 or TF and F.VIIa.23 Initial cleavage occurs at Arg145 to yield a 2-chain inactive “intermediate” of 46 kd (F.IXα). Subsequent hydrolysis at Arg180 generates the fully active protease (F.IXaβ) consisting of an N-terminal 18-kd light-chain disulphide linked to a C-terminal 28-kd heavy chain. The physiologic importance of cleavage at Arg145 relates to an increased binding affinity between F.IXα and its essential cofactor in the tenase complex, F.VIIIa.26Hydrolysis at Arg180 generates the structural rearrangements necessary for the development of a catalytic region surrounding the active site serine, Ser365.27 28 The following studies describe a detailed characterization of F.IX cleavage by plasmin using purified proteins in vitro. The results indicate that plasmin-like fragments of F.IX are detectible in reactions of tPA-induced lysis of fibrin formed in human plasma. Plasmin catalyzes cleavage not only at the 2 activation sites of F.IX (Arg145 and Arg180) but also at at least 3 additional sites by which the molecule is inactivated. Collectively, the results suggest that plasmin has the potential to down-regulate coagulation by inactivating F.IX.
Materials and methods
Materials
Human prothrombin, F.IX, and F.X were purified from frozen plasma according to Bajaj et al.29 Human thrombin was prepared from prothrombin according to Lundblad et al.30 Bovine F.XIa was from Enzyme Research Laboratories (South Bend, IN). Human F.IX (4.1 μM) was activated to F.IXa with 500 nM of F.XIa for 2 hours at 37°C according to Lenting et al.26 Human F.X was activated to F.Xa according to Fujikawa et al.31 PCPS vesicles were prepared according to Barenholz et al,32 and their concentration was determined by assay of inorganic phosphorous.33 Human Glu-plasminogen was prepared from frozen plasma using lysine-Sepharose according to Castellino and Powell34 and activated to plasmin with urokinase according to Bajzar et al.35Dansylarginine-N-(3-ethyl-1,5-pentanediyl) amide (DAPA) was synthesized according to Nesheim et al.36 Human F.VIII was from Bayer (Darmstedt, Germany). Bovine serum albumin (BSA), p-aminobenzamidine (pAB), N-[2-Hydroxyethyl]piperazine-N'-[2-ethanesulfonic acid] (HEPES), porcine intestinal mucosa heparin, and Coomassie Blue R 250 were from Sigma (St. Louis, MO). The F.Xa and plasmin peptide substrates, S-2222 and S-2251, respectively, were from DiaPharma (Franklin, OH). tPA and human AT were generous gifts from Genentech (San Francisco, CA). The AT was further purified using heparin-Sepharose chromatography.37 The sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) molecular weight standards were from Pharmacia (Uppsala, Sweden). F.IX-deficient human plasma was from BioPool (Burlington, Ontario). The activated partial thromboplastin time (aPTT) reagent and the Coag-a-mate XC Plus were from Organon Teknika (Scarborough, Ontario). Valyl-phenyl-lysyl chloromethylketone (VFK-CMK) and 1,5-dansyl-glutamyl-glycyl-arginine-chloromethylketone (dEGR) were from Calbiochem (San Diego, CA). Normal human pooled plasma (NHP) was from Precision Biologicals (Dartmouth, Nova Scotia). Immobilon-P membranes were from Millipore (Bedford, MA). Prestained molecular weight standards were from Gibco/Bethesda Research Laboratories (Burlington, Ontario). The rabbit antiserum to human F.IX was generously supplied by Dr Arthur R. Thompson (University of Washington, Seattle, WA). Goat anti-rabbit immunoglobulin G (IgG) conjugated to horseradish peroxidase and SDS were from BioRad (Mississauga, Ontario). Western blot Chemiluminescene Reagent Plus was from Mandel Scientific (Guelph, Ontario).
Methods
Hydrolysis of F.IX and F.IXa by plasmin.
Purified F.IX or F.IXa (4 μM) was treated at 37°C with 50 nM of plasmin in 50 mM of HEPES per 0.15 mol/L NaCl, pH 7.4 (HBS) containing 5 of mM CaCl2 (HBS/Ca). After varying times of incubation, 2 aliquots were removed from the same reaction. One aliquot was added to an equal volume of SDS-PAGE sample buffer as previously described.38 The other aliquot was added to VFK-CMK (2.2 μM final concentration) in HBS and incubated on ice for 10 minutes prior to assay for F.IX clotting activity (see below). VFK-CMK (2.2 μM) completely inactivated plasmin activity toward both F.IX and the plasmin peptide substrate S-2251, but it had no effect on the F.IX/IXa coagulant activity at the concentrations of F.IX/IXa assayed.
Factor IX clotting assay.
This was performed using an aPTT clotting assay and F.IX-deficient human plasma as previously described.38 NHP was used as the standard, assigning 1 unit of F.IX clotting activity per milliliter of plasma.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
SDS-PAGE was carried out according to the method of Neville.39 Approximately 4 μg of F.IX protein was loaded per lane using 5%-to-15% or 5%-to-20% linear polyacrylamide gradient gels. The plasmin cleavage products of F.IX were visualized after 12 to 16 hours of staining at 22°C with 0.0016% Coomassie Blue R-250 in 5% acetic acid and 7.5% ethanol and destaining for 2 to 3 hours in 18% methanol and 9% acetic acid.
Binding of pAB to F.IX treated with F.XIa or plasmin.
The binding of the fluorescent active site probe pAB to F.IX treated with either F.XIa or plasmin was performed according to Lin et al.40 F.IX (4 μM) was incubated with either 500 nM of F.XIa or 50 nM of plasmin and 4 μM of pAB in HBS, pH 7.4. The reactions were performed at 37°C using a Perkin Elmer Luminescence Spectrometer (Model LS50B; Montreal, Quebec) with excitation and emission wavelengths of 336 nm and 376 nm, respectively, and a 350-nm cutoff filter in the emission beam.
Effect of plasmin on the interaction of F.IX and F.IXa with AT.
F.IX (4 μM) was incubated in HBS/Ca at 37°C with either no additions for 60 minutes (control) or with 50 nM of plasmin for 60 minutes (F.IXp) or with 500 nM of F.XIa for 2 hours (F.IXa). In some cases, F.IXa and F.IXp (4 μM of each) were further treated at 37°C with plasmin (50 nM for 60 minutes) and F.XIa (500 nM for 2 hours); samples were designated F.IXa/p and F.IXp/a, respectively. The plasmin was inactivated as described above. The F.IX samples were then either diluted for the clotting assay, prepared directly for nonreducing SDS-PAGE, or further incubated with AT (5 μM) and heparin (2 mg/mL) for 30 minutes at 37°C and prepared for nonreducing SDS-PAGE.
Activation of human factor X.
Factor VIII (60 U/mL) was activated with thrombin (1 nM) for 5 minutes at 37°C according to Astermark et al.41 Thrombin was inactivated by the addition of DAPA to a final concentration of 1μM. F.IX (4 μM) was treated at 37°C in HBS/Ca with either 50 nM of plasmin for 60 minutes or with 500 nM of F.XIa for 2 hours. The plasmin was inactivated as described above. F.IXa or plasmin-treated F.IX (0.3 nM of each) was incubated with human F.X (600 nM), F.VIIIa (0-10 U/mL), and PCPS vesicles (30 μM) according to Nishimura et al.42 Assay of F.Xa generated was carried out following the addition of S-2222 to 0.4 mM and measurement of the initial rates of p-nitoraniline produced at 405 nm and 37°C in a 96-well plate (Dynatech Immulon, VWR Scientific, Mississauga, Ontario) using a SpectroMax 250 plate reader (Molecular Devices, Sunnyvale, CA). The amount of F.Xa in the different reactions was calculated from a standard curve using purified human F.Xa.
Effect of plasmin-cleaved F.IX or dEGR-F.IXa on the clotting activity of F.IXa.
F.IX (4 μM) was treated at 37°C in HBS/Ca with either 50 nM of plasmin for 60 minutes or with 500 nM of F.XIa for 2 hours. The plasmin was inactivated as described above. Half of the F.IXa reaction was further treated with a 5-fold molar excess of dEGR-CMK (20 μM) for 20 minutes at 37°C and incubated for 30 minutes at 37°C to inactivate any free dEGR-CMK. The F.IX samples were assayed at 0.01 to 10 000 pmol/L in the clotting assay using a fixed concentration of F.IXa (10 pmol/L).
NH2-terminal sequencing of the plasmin cleavage products of F.IX.
F.IX (4 μM) was incubated with plasmin (50 nM) for 2 or 30 minutes in HBS/Ca at 37°C, and the plasmin was inactivated as described above. The fragments from the 2 plasmin-digested F.IX samples were subjected to dialysis, reducing SDS-PAGE, and blotting as previously described.38 Amino acid sequencing was performed by Dr Teng-Song Chen at The Hospital For Sick Children (Toronto, Ontario) directly from the blotted material using a Porton Gas-Phase Microsequencer (Model 2090; Tarzana, CA) with online phenylthiohydantoin (PTH) analysis. The PTH-amino acids were identified by HPLC and compared with a chromatogram containing all the amino acids, excluding cysteine.
tPA-catalyzed lysis of fibrin clots formed in human plasma and F.IX immunoblotting.
NHP was added to the wells of a Dynatech Immulon plate that contained separated aliquots of thrombin, CaCl2, and tPA. The final concentrations of thrombin, CaCl2, and tPA were 6 nM, 2 mM, and 0 to 10 nM, respectively. The plate was incubated at 37°C, and the absorbance at 405 nm was measured every 2 minutes for 74 minutes using a SpectroMax 250 plate reader. The material in the wells was then solubilized and prepared for reducing SDS-PAGE according to Neville.39 In cases where the fibrin clot had lysed completely, an aliquot was added to 2.2 μM of VFK-CMK prior to measurement of its F.IX clotting activity. In some cases, purified F.IX (4 μM) was digested with plasmin (50 nM) for various times (2-60 minutes) at 37°C and “spiked” into NHP before reducing SDS-PAGE to serve as a positive control. The proteins were transferred to an Immobilon-P membrane according to Towbin et al43 and probed sequentially with rabbit anti-human F.IX IgG and then goat anti-rabbit IgG conjugated to horseradish peroxidase. Detection of F.IX and its cleavage products was accomplished using the chemiluminescence reagents and exposing the blots to Kodak X-OMAT AR film before development in a Kodak M35A processor (Eastman Kodak, Rochester, NY).
Results
The effect of plasmin on the procoagulant activity of F.IX and F.IXa
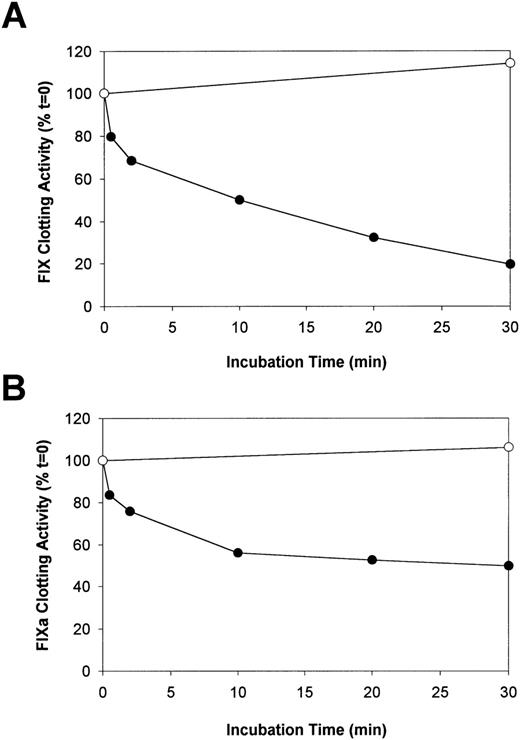
Human F.IX or F.IXa was incubated with plasmin, and aliquots were removed after varying times to measure the F.IX coagulant activity. In addition, other aliquots were removed simultaneously from the same reaction mixtures for analysis by reducing SDS-PAGE. Initial experiments indicated that the addition of plasmin (5-500 nM) to F.IX (4.0 μM) for up to 30 minutes at 37°C resulted only in reduced F.IX clotting activity, with no evidence of transient activation. A concentration of 50 nM was selected for subsequent study because this was the minimum concentration required to give a maximum effect over this time interval. The effect of plasmin on the clotting activity of F.IX is shown in Figure 1A. Plasmin decreased the clotting activity of F.IX from 100% to 50% after 10 minutes and to 20% after 30 minutes. The clotting activity of F.IX after 30 minutes without plasmin was 114% of the initial activity.
The effect of plasmin on the aPTT coagulant activity of F.IX and F.IXa.
Purified human F.IX or F.IXa (4 μM of each) was incubated either alone (control) or with plasmin (50 nM) in HBS/Ca, pH 7.4 at 37°C. At selected times, 2 aliquots of the same reaction mixtures were removed and assayed for either F.IX aPTT coagulant activity after addition to 2.2 μM of VFK-CMK (A and B) or analyzed by reducing SDS-PAGE (see Figure 2). The relative F.IX and F.IXa aPTT coagulant activity (normalized to the time = 0 sample in each case) versus incubation time with (closed circles) and without (open circles) added plasmin is illustrated in A and B, respectively.
The effect of plasmin on the aPTT coagulant activity of F.IX and F.IXa.
Purified human F.IX or F.IXa (4 μM of each) was incubated either alone (control) or with plasmin (50 nM) in HBS/Ca, pH 7.4 at 37°C. At selected times, 2 aliquots of the same reaction mixtures were removed and assayed for either F.IX aPTT coagulant activity after addition to 2.2 μM of VFK-CMK (A and B) or analyzed by reducing SDS-PAGE (see Figure 2). The relative F.IX and F.IXa aPTT coagulant activity (normalized to the time = 0 sample in each case) versus incubation time with (closed circles) and without (open circles) added plasmin is illustrated in A and B, respectively.
Plasmin also decreased the clotting activity of F.IXa, but the magnitude of the reduction (16%-25%) was less than was observed with F.IX (Figure 1B). This occurred during the initial 0.5 to 2 minutes of incubation (Figure 1B). Over the next 28 minutes, the clotting activity of F.IXa approached stable levels such that approximately 50% of its initial activity was present after 30 minutes of incubation with plasmin. The clotting activity of F.IXa after 30 minutes in the absence of plasmin was 106% of the initial activity.
SDS-PAGE analysis of plasmin cleavage of F.IX and F.IXa
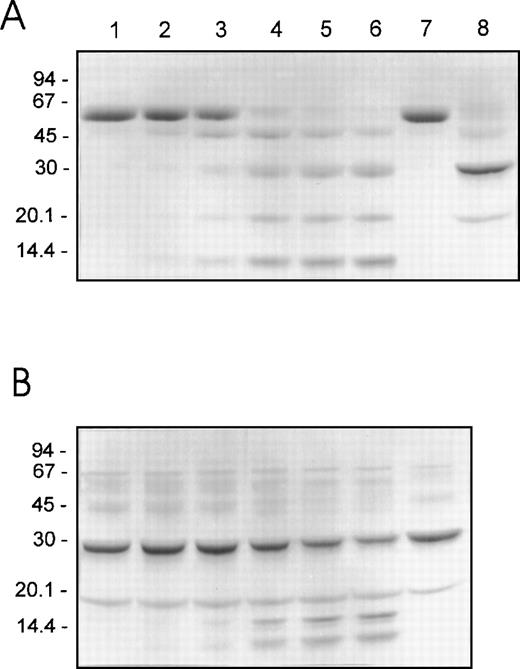
Reducing SDS-PAGE was performed using the same reactions and sampling times that were employed for the clotting assays in order to directly compare the plasmin cleavage profiles of F.IX and F.IXa with the changes in their clotting activities. The results observed for F.IX and F.IXa (4 μM) treated with 50 nM of plasmin are shown in Figure2A and 2B, respectively. The initial plasmin-mediated loss of F.IX coagulant activity after 0.5 to 2 minutes correlated with the hydrolysis of F.IX and the production of at least 4 protein fragments of 45, 30, 20, and 14 kd (Figure 2A, lanes 1-3). Thereafter, over the next 28 minutes, during which plasmin had the greatest inactivation effect on the F.IX clotting activity, the 45-kd fragment became converted into 3 more stable products of 30, 20, and 14 kd (Figure 2A, lanes 4-6). The 30- and 20-kd protein fragments comigrated with the heavy and light chains, respectively, of F.IX activated with F.XIa (Figure 2A, compare lanes 4-6 with lane 8).
Reducing SDS-PAGE of human F.IX and F.IXa after proteolysis by plasmin.
Purified human F.IX or F.IXa (4 μM of each) was incubated with plasmin (50 nM) as described in the legend to Figure 1. Aliquots (containing approximately 4 μg of F.IX protein) of F.IX (A) or F.IXa (B) were withdrawn into reducing SDS-PAGE sample buffer after 0, 0.5, 2, 10, 20, and 30 minutes of incubation with plasmin at 37°C (lanes 1-6, respectively). F.IX and F.IXa (A, lanes 7 and 8, respectively) or F.IXa (B, lane 7) incubated as described above for 30 minutes at 37°C but in the absence of added plasmin are also shown. The migration positions of the molecular weight standards (in kd) are shown at the left of each panel.
Reducing SDS-PAGE of human F.IX and F.IXa after proteolysis by plasmin.
Purified human F.IX or F.IXa (4 μM of each) was incubated with plasmin (50 nM) as described in the legend to Figure 1. Aliquots (containing approximately 4 μg of F.IX protein) of F.IX (A) or F.IXa (B) were withdrawn into reducing SDS-PAGE sample buffer after 0, 0.5, 2, 10, 20, and 30 minutes of incubation with plasmin at 37°C (lanes 1-6, respectively). F.IX and F.IXa (A, lanes 7 and 8, respectively) or F.IXa (B, lane 7) incubated as described above for 30 minutes at 37°C but in the absence of added plasmin are also shown. The migration positions of the molecular weight standards (in kd) are shown at the left of each panel.
The initial 16% to 25% loss in the clotting activity of F.IXa corresponded with the generation of at least 2 protein fragments of 16 (doublet) and 14 kd (Figure 2B, lanes 1-3). The generation of the 16- and 14-kd fragments increased over the next 28 minutes, during which time the F.IXa clotting activity decreased to and remained at approximately 50% of the initial level (Figure 2B, lanes 4-6). Densitometry indicated that the decreased staining intensity of the 30-kd heavy chain correlated very well with both the increase in the staining intensity of the 16- and 14-kd fragments as well as the decrease in the F.IXa activity (results not shown). These results suggest that plasmin inactivates the activity of F.IXa after cleavage of its 30-kd heavy chain.
The interaction of F.IXa and plasmin treated F.IX with pAB
The binding of pAB to the active site of F.IXa and F.IX treated with plasmin was studied by measuring the fluorescence change after cleavage of F.IX with F.XIa and plasmin, respectively. Treatment of F.IX with F.XIa resulted in a time-dependent increase in the pAB fluorescence, which reached maximal levels about 50 to 60 minutes following the addition of F.XIa (results not shown). In striking contrast, no increase in pAB fluorescence was observed when F.IX was incubated with plasmin (results not shown). The inability of F.IX cleaved by plasmin to bind pAB and display an increase in fluorescence suggests the active site was not developed.
The interaction of F.IX derivatives with AT and heparin
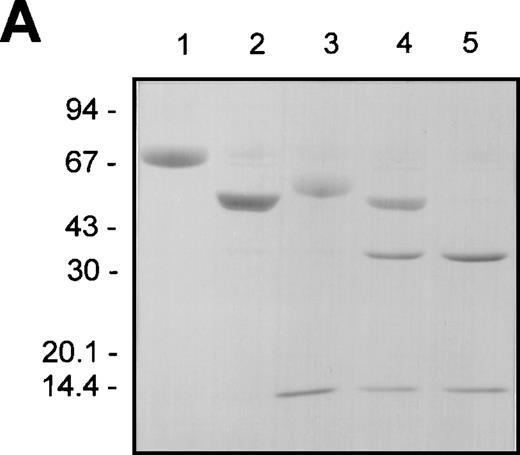
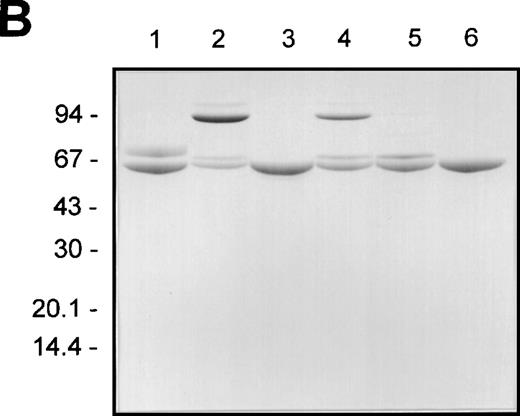
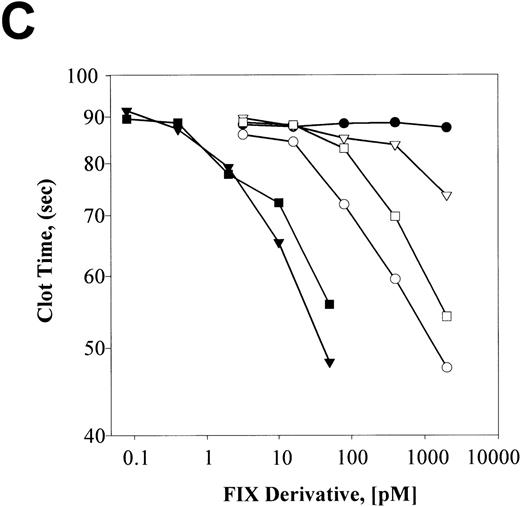
F.IX, F.IXa, plasmin-cleaved F.IX (F.IXp), F.IXa cleaved with plasmin (F.IXa/p), and plasmin-treated F.IX that was subsequently exposed to F.XIa (F.IXp/a) were compared with respect to their nonreducing SDS-PAGE staining patterns before and after incubation with AT and heparin (Figures 3A and B), respectively. Aliquots from these same reactions were also analyzed with respect to their F.IX clotting activities (Figure 3C).
The effect of plasmin or F.XIa on F.IX complexation with AT in the presence of heparin and aPTT coagulant activity.
The different F.IX derivatives were produced as described in “Materials and Methods.” Aliquots of the samples (containing approximately 3 μg of F.IX protein) were prepared for nonreducing SDS-PAGE without and with incubation with AT (5 μM) and heparin (2 mg/mL) for 30 minutes at 37°C (A and B, respectively). The different F.IX samples were also diluted in HBS/BSA and assayed for their F.IX aPTT coagulant activity (C). A and B illustrate the nonreducing SDS-PAGE analysis of the different F.IX samples without and with incubation with AT and heparin, respectively: F.IX (lane 1), F.IXa (lane 2), plasmin-treated F.IX (lane 3), plasmin-treated F.IXa (lane 4), F.IX treated with plasmin then F.XIa (lane 5). B also shows AT and heparin alone (lane 6). The migration positions of the molecular weight standards (in kd) are shown to the left of A and B. C illustrates the log F.IX aPTT clot time (in seconds) versus the log F.IX derivative concentration (in pmol/L): HBS/BSA buffer control (closed circles); F.IX (open circles); plasmin-treated F.IX (open downward triangles); F.IX treated with plasmin then F.XIa (open squares); F.IXa (closed downward triangles); plasmin-treated F.IXa (closed squares).
The effect of plasmin or F.XIa on F.IX complexation with AT in the presence of heparin and aPTT coagulant activity.
The different F.IX derivatives were produced as described in “Materials and Methods.” Aliquots of the samples (containing approximately 3 μg of F.IX protein) were prepared for nonreducing SDS-PAGE without and with incubation with AT (5 μM) and heparin (2 mg/mL) for 30 minutes at 37°C (A and B, respectively). The different F.IX samples were also diluted in HBS/BSA and assayed for their F.IX aPTT coagulant activity (C). A and B illustrate the nonreducing SDS-PAGE analysis of the different F.IX samples without and with incubation with AT and heparin, respectively: F.IX (lane 1), F.IXa (lane 2), plasmin-treated F.IX (lane 3), plasmin-treated F.IXa (lane 4), F.IX treated with plasmin then F.XIa (lane 5). B also shows AT and heparin alone (lane 6). The migration positions of the molecular weight standards (in kd) are shown to the left of A and B. C illustrates the log F.IX aPTT clot time (in seconds) versus the log F.IX derivative concentration (in pmol/L): HBS/BSA buffer control (closed circles); F.IX (open circles); plasmin-treated F.IX (open downward triangles); F.IX treated with plasmin then F.XIa (open squares); F.IXa (closed downward triangles); plasmin-treated F.IXa (closed squares).
Activation of F.IX with F.XIa resulted in the generation of a protein product of 45 kd after nonreducing SDS-PAGE (Figure 3A, lane 2). Cleavage of F.IX with plasmin resulted in the appearance of products of 52 and 14 kd (Figure 3A, lane 3). The 52-kd product stained weakly with Coomassie Blue, suggesting that it still possessed the activation peptide portion of F.IX. This was confirmed upon demonstration that the 52-kd product, but not the 14-kd product, was susceptible to cleavage by F.XIa. This resulted in the generation of a product of 37 kd (Figure3A, lane 5) and partial activation of its clotting activity (Figure3C). Treatment of F.IXa with plasmin resulted in the appearance of fragments of 37 and 14 kd (Figure 3A, lane 4).
F.IXa formed at least 2 larger complexes with AT plus heparin of 93 and 106 kd after nonreducing SDS-PAGE (Figure 3B, lane 2). This agrees with published observations.40 In contrast, the 52-kd product of plasmin-treated F.IX comigrated with AT under these conditions and closely approximated that observed in the absence of plasmin (Figure3B, compare lanes 3 and 6). Similarly, F.IX did not complex with AT in the presence of heparin (Figure 3B, lane 1). F.IXa/p formed reduced levels of the larger species of 93 and 106 kd with AT in comparison with that observed with F.IXa (Figure 3B, compare lanes 2 and 4). F.IXp/a did not form larger complexes with AT and heparin, and the resultant 60- and 69-kd protein products stained similarly with that observed when untreated F.IX was incubated with AT and heparin (Figure3B, compare lane 5 with lane 1).
The same reactions used for the AT/heparin binding studies were also assayed over a wide range of concentrations for their F.IX clotting activities. The times of F.IX treatment with either F.XIa or plasmin were chosen to preclude large amounts of residual F.IX and its cleavage intermediates from being present. F.IXp possessed a clotting activity that was significantly less than untreated F.IX but exhibited greater activity than the buffer control (Figure 3C). F.XIa treatment of F.IXp increased its clotting activity approximately 3- to 5-fold to levels that were intermediate between untreated F.IX and F.IXp. F.IXa was the most active species, and plasmin treatment of F.IXa inactivated approximately 50% of its clotting activity. Plasmin-treated F.IX possessed 3% to 4% of the clotting activity of the F.IX control reaction without added protease. It is possible that the very low clotting activity of plasmin-treated F.IX is due to the presence of trace amounts of uncleaved F.IX or residual activity associated with the completely hydrolyzed product. The results suggest that plasmin cleavage of F.IX inactivated its clotting activity but did not entirely eliminate its potential to be activated upon further incubation with purified F.XIa compared with the buffer control.
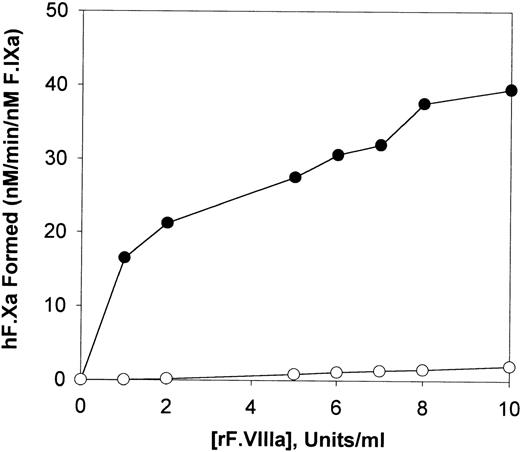
Activation of human factor X by F.IXa and plasmin-treated F.IX
Plasmin-cleaved F.IX and F.IXa were also compared with respect to their ability to activate human F.X with increasing amounts of F.VIIIa. As illustrated in Figure 4, F.X activation by plasmin-treated F.IX was only marginally affected by increasing the concentration of F.VIIIa compared with F.IXa. At the greatest level of F.VIIIa assayed (10 U/mL), plasmin-treated F.IX possessed approximately 5% of the F.X activating potential, as was observed with equivalent amounts of F.IXa (Figure 4).
The effect of F.VIIIa on the activation of human factor X.
Human F.X (600 nM) was incubated with 0.3 nM of F.IXa (closed circles) or 0.3 nM of plasmin-digested F.IX (open circles) in the presence of various concentrations of human F.VIIIa in TBS (tris buffered saline), pH 7.4 containing 0.1% BSA/5mM CaCl2and 30 mM of PCPS vesicles for 10 minutes at 37°C. The initial rate of cleavage of the chromogenic substrate S-2222 (0.4 mM final concentration) by human F.Xa generated is shown after determination of the F.Xa activity levels in the reactions with a standard curve constructed with known amounts of purified human F.Xa.
The effect of F.VIIIa on the activation of human factor X.
Human F.X (600 nM) was incubated with 0.3 nM of F.IXa (closed circles) or 0.3 nM of plasmin-digested F.IX (open circles) in the presence of various concentrations of human F.VIIIa in TBS (tris buffered saline), pH 7.4 containing 0.1% BSA/5mM CaCl2and 30 mM of PCPS vesicles for 10 minutes at 37°C. The initial rate of cleavage of the chromogenic substrate S-2222 (0.4 mM final concentration) by human F.Xa generated is shown after determination of the F.Xa activity levels in the reactions with a standard curve constructed with known amounts of purified human F.Xa.
The effect of dEGR-F.IXa and plasmin-treated F.IX on the clotting activity of F.IXa
The effect of increasing amounts of dEGR-F.IXa and plasmin-treated F.IX on the clotting activity of a fixed amount of F.IXa was also studied. The results indicate that when dEGR-F.IXa was present at 10, 100, 1000, and 10 000 pmol/L, the clot times of 10 pmol/L of F.IXa progressively increased from 63.6 seconds to 65.6 seconds, 71.1 seconds, 84.9 seconds, and 98.2 seconds, respectively. In contrast, when plasmin-treated F.IX was assayed at 10, 100, 1000, and 10 000 pmol/L, the clot times of 10 pmol/L of F.IXa remained for the most part unchanged from 63.6 seconds to 63.8 seconds, 63.5 seconds, 61.4 seconds, and 53.1 seconds, respectively. The shortened clot time of 10 pmol/L of F.IXa (53.1 seconds) upon assay with 10 000 pmol/L of plasmin-treated F.IX may reflect the presence of a low level of uncleaved F.IX that manifested its increased coagulant activity only upon assay at this elevated concentration. Collectively, the overall lack of a large competitive prolongation of the F.IXa aPTT clot times with increasing amounts of plasmin-treated F.IX suggests that the products of plasmin-cleaved F.IX failed to block the proper assembly of F.IXa into the tenase complex.
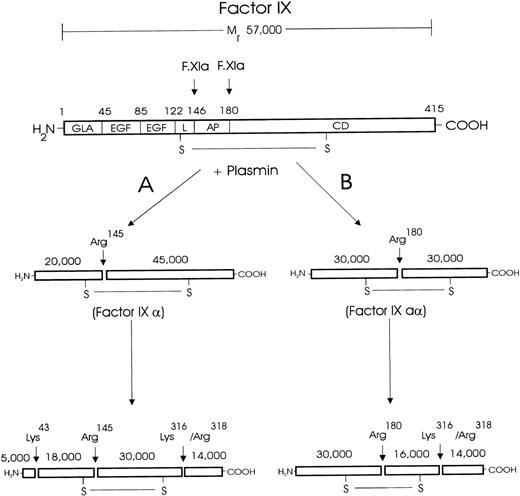
NH2-terminal sequencing of the plasmin cleavage products of F.IX
The data from the NH2-terminal sequencing of the plasmin cleavage products of F.IX are shown in Table1, and a schematic diagram of the plasmin cleavage sites within the F.IX molecule is presented in Figure5.
NH2-terminal sequence of human factor IX-derived fragments following plasmin digestion
| Cycle No. . | Plasmin Cleavage Products of Human F.IX . | |||||
|---|---|---|---|---|---|---|
| 45 kd . | 30 kd . | 20 kd . | 18 kd . | 16 kd . | 14 kd . | |
| 1 | A (20.0) | A (45.0)/Y (26.0)/V (20.0) | Y (142.0) | Q (29.0) | V (36.0)/G (4.0) | G (67.0)/S (10.0) |
| 2 | E (20.0) | E (36.0)/N (20.0)/V (10.0) | N (116.0) | Y (35.0) | V (35.0)/R (5.0) | R (90.0)/A (4.0) |
| 3 | T (10.0)/A (19.0) | T (30.0)/A (30.0)/S (15.0)/G (9.0) | S (66.0) | V (24.0) | G (36.0)/S (5.0) | S (48.0)/L (6.0) |
| 4 | V (14.0) | V (27.0)/G (7.0) | G (32.0) | D (27.0) | G (33.0)/A (6.0) | A (70.0)/V (4.0) |
| 5 | F (14.0) | F (33.0)/K (8.0)/E (5.0) | K (60.0) | G (17.0) | E (21.0)/L (3.0) | L (70.0) |
| 6 | P (10.0) | P (25.0)/L (12.0)/D (9.0) | L (56.0) | D (16.0) | D (27.0)/V (8.0) | V (50.0)/Q (8.0) |
| 7 | D (35.0)/E (7.0)/A (8.0) | Q (8.0) | A (30.0)/L (5.0) | |||
| 8 | K (40.0)/Q (6.0) | |||||
| Cycle No. . | Plasmin Cleavage Products of Human F.IX . | |||||
|---|---|---|---|---|---|---|
| 45 kd . | 30 kd . | 20 kd . | 18 kd . | 16 kd . | 14 kd . | |
| 1 | A (20.0) | A (45.0)/Y (26.0)/V (20.0) | Y (142.0) | Q (29.0) | V (36.0)/G (4.0) | G (67.0)/S (10.0) |
| 2 | E (20.0) | E (36.0)/N (20.0)/V (10.0) | N (116.0) | Y (35.0) | V (35.0)/R (5.0) | R (90.0)/A (4.0) |
| 3 | T (10.0)/A (19.0) | T (30.0)/A (30.0)/S (15.0)/G (9.0) | S (66.0) | V (24.0) | G (36.0)/S (5.0) | S (48.0)/L (6.0) |
| 4 | V (14.0) | V (27.0)/G (7.0) | G (32.0) | D (27.0) | G (33.0)/A (6.0) | A (70.0)/V (4.0) |
| 5 | F (14.0) | F (33.0)/K (8.0)/E (5.0) | K (60.0) | G (17.0) | E (21.0)/L (3.0) | L (70.0) |
| 6 | P (10.0) | P (25.0)/L (12.0)/D (9.0) | L (56.0) | D (16.0) | D (27.0)/V (8.0) | V (50.0)/Q (8.0) |
| 7 | D (35.0)/E (7.0)/A (8.0) | Q (8.0) | A (30.0)/L (5.0) | |||
| 8 | K (40.0)/Q (6.0) | |||||
Human F.IX (4 μM) was treated with plasmin (50 nM) for 2 and 30 minutes at 37°C in HBS/Ca, as described in “Materials and Methods.” Approximately 100 μg (1750 pmol) of F.IX was used for each reaction. The apparent molecular mass of the protein fragments is indicated with the amount of each amino acid (in pmol) after the number of cycles performed. Fragments of 45, 30, 20, 18, 16, and 14 kd represent the plasmin cleavage products of human F.IX.
Schematic diagram of plasmin cleavage and inactivation of human F.IX.
Human F.IX is composed of a light chain (residues 1-145), an activation peptide (residues 146-180), and a heavy chain (residues 181-415). The light chain comprises a domain containing 10-12 γ-carboxyglutamic acid (GLA) residues, 2 human epidermal growth factor (EGF)-like domains, and a linker (L) region.25 The activation peptide (AP) is cleaved and removed upon F.IX activation by F.VIIa/tissue factor or F.XIa. The heavy chain contains the catalytic domain (CD), which is made up of the active site formed by the triad of residues: Asp270, His221, and Ser365. The results of the NH2-terminal sequencing indicate that plasmin inactivates the coagulant activity of F.IX after hydrolysis at Lys43, Arg145, Arg180, Lys316, and Arg318. The results of reducing and nonreducing SDS-PAGE, NH2-terminal sequencing, and F.IX aPTT coagulant activity assays are collectively consistent only with plasmin hydrolysis following 2 pathways, which are indicated (A and B), as are the cleavage sites and the apparent molecular masses of the cleavage products. By this scheme, no single molecule is cleaved at both Arg145 and Arg180 and nowhere else. Thus, although plasmin can catalyze the activation cleavages individually, plasmin does not generate F.IXa.
Schematic diagram of plasmin cleavage and inactivation of human F.IX.
Human F.IX is composed of a light chain (residues 1-145), an activation peptide (residues 146-180), and a heavy chain (residues 181-415). The light chain comprises a domain containing 10-12 γ-carboxyglutamic acid (GLA) residues, 2 human epidermal growth factor (EGF)-like domains, and a linker (L) region.25 The activation peptide (AP) is cleaved and removed upon F.IX activation by F.VIIa/tissue factor or F.XIa. The heavy chain contains the catalytic domain (CD), which is made up of the active site formed by the triad of residues: Asp270, His221, and Ser365. The results of the NH2-terminal sequencing indicate that plasmin inactivates the coagulant activity of F.IX after hydrolysis at Lys43, Arg145, Arg180, Lys316, and Arg318. The results of reducing and nonreducing SDS-PAGE, NH2-terminal sequencing, and F.IX aPTT coagulant activity assays are collectively consistent only with plasmin hydrolysis following 2 pathways, which are indicated (A and B), as are the cleavage sites and the apparent molecular masses of the cleavage products. By this scheme, no single molecule is cleaved at both Arg145 and Arg180 and nowhere else. Thus, although plasmin can catalyze the activation cleavages individually, plasmin does not generate F.IXa.
The 45-kd fragment possessed an amino acid sequence matching the 6 amino acids from Ala146 to Pro151, indicating plasmin hydrolysis at Arg145. The 30-kd fragment possessed 3 amino acid sequences matching the 7 amino acids from Ala146 to Asp152, Val181 to Ala187, and Tyr1 to Glu7, indicating plasmin cleavage at Arg145, Arg180, and the NH2-terminus of intact F.IX, respectively. The 20-kd fragment possessed an amino acid sequence matching the 6 amino acids from Tyr1 to Leu6, indicating the NH2-terminus of intact F.IX. The 18-kd fragment possessed an amino acid sequence matching the 7 amino acids from Gln44 to Gln50, indicating plasmin cleavage at Lys43. This plasmin cleavage product of F.IX was either not present in sufficient amounts or resolved from the 20-kd protein fragment to be observed in the smaller gel systems used in the initial analysis (Figure 2). The 16-kd fragment possessed 2 amino acid sequences matching the 8 amino acids from Val181 to Lys188 and Gly317 to Gln324, indicating plasmin cleavage at Arg180 and Lys316, respectively. The 14-kd fragment possessed 2 amino acid sequences matching the 6 amino acids in F.IX from Gly317 to Val322 and Ser319 to Gln324, indicating plasmin cleavage at Lys316 and Arg318, respectively.
The SDS-PAGE and NH2-terminal sequencing results are consistent with 2 reaction pathways for the inactivation of F.IX by plasmin (Figure 5). Plasmin hydrolyzes F.IX initially at Arg145 (pathway A) or Arg180 (pathway B), but not at both sites within the same molecule, to generate F.IXα and F.IXaα, its 2 normal activation intermediates.22 23 Subsequently, F.IXα and F.IXaα are hydrolyzed at Lys43, Lys316, and Arg318 and at Lys316 and Arg318, respectively.
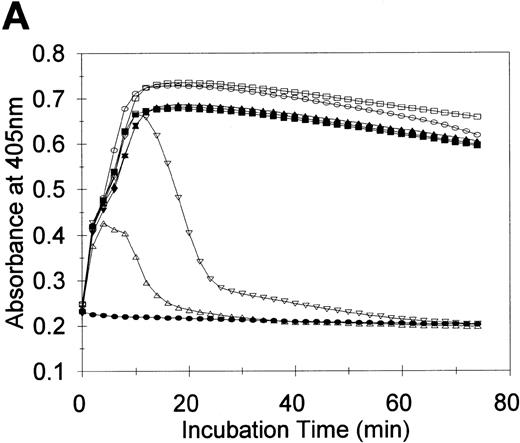
tPA-catalyzed lysis of fibrin in human plasma and F.IX immunoblotting
To test the potential relevance of the cleavage and inactivation of F.IX by plasmin in vitro, a series of tPA-induced fibrin clot lysis experiments were performed in NHP, and the reactions were subjected to F.IX immunoblotting. The time courses of clot lysis induced by tPA (0-10 nM), which occurred subsequent to fibrin formation in NHP catalyzed by thrombin in the presence CaCl2, are illustrated in Figure 6A. These same reactions were also subjected to F.IX immunoblotting to probe for the presence of plasmin-like fragments of F.IX (Figure 6B). Combining NHP with thrombin and CaCl2 resulted in maximal fibrin formation after 18 minutes (Figure 6A, closed squares). Inclusion of 0.2 nM or 0.5 nM of tPA with NHP, thrombin, and CaCl2 did not significantly change the time course of the turbidity profile from that observed with NHP, thrombin, and CaCl2 (Figure 6A, compare downward and upward closed triangles, respectively, with closed squares). Addition of 1 and 2 nM of tPA to NHP, thrombin, and CaCl2 increased the maximal turbidity at all times by 5% to 8% from that observed in the absence of tPA or with 0.2 and 0.5 nM of tPA (Figure 6A, compare open squares and open circles, respectively, with closed squares and downward and upward closed triangles). Significant fibrin clot lysis was observed only when 5 and 10 nM of tPA were each combined with NHP, thrombin, and CaCl2 (Figure 6A, downward and upward open triangles, respectively).
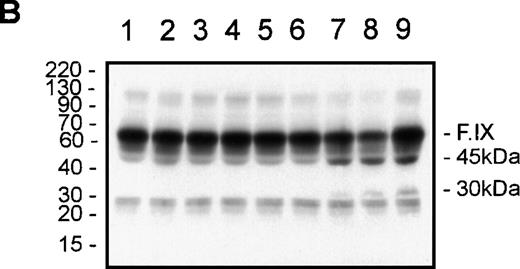
tPA-induced clot lysis and F.IX immunoblotting.
Fibrin clot lysis was monitored by measurement of the absorbance at 405 nm every 2 minutes at 37°C (A). At the end of the 74-minute time course, the reactions were either solubilized directly in reducing SDS-PAGE buffer for F.IX immunoblotting, or an aliquot was diluted in HBS/BSA for F.IX clotting assays prior to solubilization and reducing SDS-PAGE/F.IX immunoblotting (B). NHP (90 μL) was combined with either (final concentrations) thrombin (6 nM) and CaCl2 (2 mM) (closed squares in A; or lane 1 in B), or tPA (10 nM, closed circles in A; or lane 2 in B), or thrombin/CaCl2 and 0.2 nM of tPA (closed downward triangles in A; or lane 3 in B), or 0.5 nM of tPA (closed upward triangles in A; or lane 4 in B), or 1 nM of tPA (open squares in A; or lane 5 in B), or 2 nM of tPA (open circles in A; or lane 6 in B), or 5 nM of tPA (open downward triangles in A; or lane 7 in B), or 10 nM of tPA (open upward triangles in A; or lane 8 in B). Purified F.IX (4 μM) was digested with plasmin (50 nM) for 2 minutes at 37°C and added to NHP at 0.18 μM prior to solubilization and F.IX immunoblotting (B, lane 9). The positions of prestained molecular weight standards (in kd) are shown to the left of B. The migration positions of F.IX and the 45- and 30-kd immunoreactive species are shown to the right of B.
tPA-induced clot lysis and F.IX immunoblotting.
Fibrin clot lysis was monitored by measurement of the absorbance at 405 nm every 2 minutes at 37°C (A). At the end of the 74-minute time course, the reactions were either solubilized directly in reducing SDS-PAGE buffer for F.IX immunoblotting, or an aliquot was diluted in HBS/BSA for F.IX clotting assays prior to solubilization and reducing SDS-PAGE/F.IX immunoblotting (B). NHP (90 μL) was combined with either (final concentrations) thrombin (6 nM) and CaCl2 (2 mM) (closed squares in A; or lane 1 in B), or tPA (10 nM, closed circles in A; or lane 2 in B), or thrombin/CaCl2 and 0.2 nM of tPA (closed downward triangles in A; or lane 3 in B), or 0.5 nM of tPA (closed upward triangles in A; or lane 4 in B), or 1 nM of tPA (open squares in A; or lane 5 in B), or 2 nM of tPA (open circles in A; or lane 6 in B), or 5 nM of tPA (open downward triangles in A; or lane 7 in B), or 10 nM of tPA (open upward triangles in A; or lane 8 in B). Purified F.IX (4 μM) was digested with plasmin (50 nM) for 2 minutes at 37°C and added to NHP at 0.18 μM prior to solubilization and F.IX immunoblotting (B, lane 9). The positions of prestained molecular weight standards (in kd) are shown to the left of B. The migration positions of F.IX and the 45- and 30-kd immunoreactive species are shown to the right of B.
F.IX immunoreactive species of approximately 60 kd (F.IX zymogen), 45 kd, and 25 kd were detected in all the samples analyzed (Figure 6B, lanes 1-8). Over the concentration range of tPA studied with thrombin and calcium, increased levels of F.IX immunoreactive products of 45 and 30 kd were detected only in the samples with 5 and 10 nM of tPA (Figure 6B, lanes 7 and 8, respectively). The increased levels of the 45- and 30-kd species present in these 2 clot lysis reactions comigrated with purified F.IX digested with plasmin upon addition to NHP (Figure 6B, lane 9), and these were the only 2 reactions that displayed significant clot lysis (Figure 6A).
Clotting assays indicated that the F.IX activity decreased from 1.07 U/mL and 1.08 U/mL for NHP and NHP/10-nM tPA to 0.91 U/mL and 0.80 U/mL for NHP/ thrombin/CaCl2 and 5 and 10 nM of tPA, respectively. These results strongly suggest that the increased levels of the 45- and 30-kd fragments of F.IX observed were due to the increased plasmin activity toward both the fibrin and F.IX in plasma and that this increased plasmin activity decreased the F.IX coagulant activity to levels comparable to that observed in vitro (see Figure1A).
Discussion
It has been proposed that, in normal hemostasis, a balance exists between coagulation and fibrinolysis and, thus, thrombus formation and dissolution is finely regulated at the point of vascular injury.5,6 It has also been suggested that imbalance between these 2 systems may lead to pathologic thrombosis or bleeding.7-10 Both experimental44,45 and clinical46,47 observations support this proposal. In clinical DIC, the course is frequently complicated by bleeding consequent to the significant consumption and proteolysis of vital clotting factors during its development.48 Plasmin is a likely candidate as an instigator of the proteolysis observed. Experimental19,49 and clinical50,51 evidence have confirmed its excessive generation in fulminating DIC and, predictably, its specificity for fibrin would be compromised if the capacity of its natural inhibitor, α2-AP, were exceeded. However, numerous proteases may be active simultaneously during experimental or clinical DIC. In an animal model of human DIC, a drastic reduction in the functional activity of F.IX was observed.21 Substantial plasmin generation was documented in this model19 and could therefore have been responsible for the observed effects on F.IX. However, additional studies demonstrated that neutrophil elastase (NE), was also a candidate protease,21 and the precise details of NE-dependent proteolysis of F.IX has been reported.38 Consequently, to facilitate assigning priority of activity in the more complex situation in vivo, it was imperative initially to characterize in detail the profile of F.IX proteolysis by plasmin. The use of purified proteins in an in vitro system and analysis by a combination of SDS-PAGE, NH2-terminal sequence analysis, and functional assays permitted the unambiguous correlation between the specific plasmin cleavage sites within the F.IX molecule and the resultant product's physical and procoagulant characteristics.
The results reported confirm and extend the observations of Osterud and coworkers12 with respect to the cleavage and inactivation of F.IX by plasmin in vitro. The amino acid cleavage specificity of plasmin observed is in agreement with this protease's known preference for hydrolyzing substrates after either arginine or lysine residues.52 NH2-terminal sequence analysis indicated that plasmin initially cleaves F.IX at its 2 physiologic activation sites, Arg145 and Arg180, to generate 2 reaction intermediates, F.IXα (45 kd) and F.IXaα (30 kd), respectively. However, no transient evidence of plasmin-dependent activation of F.IX coagulant activity was observed by 1-stage F.IX clotting assays under any of the experimental conditions employed, including a wide range of plasmin concentrations (5-500 nM) or with the addition of phospholipid vesicles (50 μM). Moreover, the unavailability of a functional active site in the plasmin-digested F.IX products was demonstrated by the failure to bind pAB and form complexes with its physiologic inhibitor, AT, in the presence of heparin. An explanation for these findings is apparent from the additional observations from SDS-PAGE and NH2-terminal sequence analysis. Reducing SDS-PAGE demonstrated the generation of a 14-kd product concomitantly with the appearance of products of 45, 30, and 20 kd, and NH2-terminal sequence analysis suggested cleavage at Lys43, Lys316, and Arg318. Based on the known structural/functional relationships of the F.IX molecule,53 this pattern of degradation would have predictable consequences for the protein's coagulant function. Of major importance, cleavage of F.IX at Lys316 and Arg318 and generation of the 14-kd product would result in the loss of covalent attachment of a portion of the catalytic domain containing the active site serine residue, Ser365, given there are no disulphide bonds to link the light and heavy chains within the F.IX molecule C-terminal to Lys316 and Arg318. It is possible that this portion may remain noncovalently associated with the remainder of the F.IX molecule and retain some clotting activity. If this does occur, it was undetectable by the functional assays employed here. Kisiel and coworkers54 have demonstrated that α-thrombin cleaved human F.IX in the absence of calcium at Arg318, Arg327, and Arg338, rendering the product incapable of developing coagulant activity with or without prior treatment with purified F.XIa. Thrombin cleavage of human F.IX at Arg318 is identical to 1 of the plasmin cleavage sites reported here. Finally, plasmin cleavage at Lys43 would be expected to decrease the product's coagulant activity by removal of the γ-carboxylic acid residue domain, which is required for the optimal calcium-dependent interaction of Fs.IX/IXa with phospholipid surfaces.55 These observations suggest that any “active” F.IX coagulant species generated by plasmin proteolysis were either present at undetectable levels or rapidly converted to more “inactive” species. The inactivation of F.IXa, as assessed by functional assay, associated with the production of degradation products of 16 and 14 kd identified by SDS-PAGE is in line with this conclusion. However, the observation that some activation occurred when purified F.XIa was added to the terminal digest raises the possibility that, in a more complete plasma or whole-blood milieu, plasmin may initially activate and subsequently inactivate F.IX coagulant activity.
The results of the present studies indicate that plasmin hydrolyzes F.IX and F.IXa to products with decreased coagulant function. Should this occur in vivo, thrombin generation via the intrinsic pathway would be significantly reduced. It will be important to establish the relevance of these findings in the in vivo context to assess the role of plasmin and its importance relative to other proteases in the pathogenesis of DIC. This understanding is essential if more effective management strategies are to be developed for this serious condition. Although the purified system used aided in the specific characterization of the proteolytic process, it is clearly remote from the in vivo context. The results from the tPA-catalyzed fibrin clot lysis studies were reassuring in that they supported the conclusions drawn from the SDS-PAGE analyses using the purified system. Thus, in a more complete system, as in whole plasma, the inferences drawn and the information obtained was similar. Of particular importance was the observation that F.IX may be cleaved by plasmin during normal clot lysis. The high concentration of α2-AP (approximately 1.5 μM)56 in human plasma may have been expected to prevent F.IX cleavage by plasmin in a whole-plasma–based system. These data suggest that fibrin-bound plasmin may be protected from inhibition by physiologic levels of α2-AP, as other investigators have proposed.57 Nonetheless, it will now be important to apply this information to in vivo study in experimental and clinical DIC. The results of this and our previous study of the NE-dependent cleavage of F.IX38 should permit a more detailed analysis of F.IX degradation in such cases than was previously possible. Such studies should lead to a more detailed understanding of the role and hierarchy of these proteases during normal coagulation and fibrinolysis and in the pathogenesis of DIC.
Supported by grants from The Medical Research Council of Canada (MA-7667) and The Bayer/Canadian Red Cross Society Research and Development Fund (grant 9610).
A.R.G. is a Distinguished Research Professor of The Heart & Stroke Foundation of Ontario.
A preliminary report of these studies was presented at the XVIth Congress of The International Society of Thrombosis and Hemostasis, Florence, Italy, June 6-12, 1997.
Reprints:John A. Samis, Department of Pathology, Botterell Hall, Rm A222, Queen's University, Kingston, ON, Canada, K7L 3N6; e-mail: samisj@post.queensu.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal