Myelopoietins (MPOs) constitute a family of engineered, chimeric molecules that bind and activate the IL-3 and G-CSF receptors on hematopoietic cells. This study investigated the in vivo hematopoietic response of rhesus monkeys administered MPO after radiation-induced myelosuppression. Animals were total body irradiated (TBI) in 2 series, with biologically equivalent doses consisting of either a 700 cGy dose of Cobalt-60 (60Co) γ-radiation or 600 cGy, 250 kVp x-irradiation. First series: On day 1 after 700 cGy irradiation, cohorts of animals were subcutaneously (SC) administered MPO at 200 μg/kg/d (n = 4), or 50 μg/kg/d (n = 2), twice daily, or human serum albumin (HSA) (n = 10). Second series: The 600 cGy x-irradiated cohorts of animals were administered either MPO at 200 μg/kg/d, in a daily schedule (n = 4) or 0.1% autologous serum (AS) , daily, SC (n = 11) for 23 days. MPO regardless of administration schedule (twice a day or every day) significantly reduced the mean durations of neutropenia (absolute neutrophil count [ANC] < 500/μL) and thrombocytopenia (platelet < 20 000/μL) versus respective control-treated cohorts. Mean neutrophil and platelet nadirs were significantly improved and time to recovery for neutrophils (ANC to < 500/μL) and platelets (PLT < 20 000/μL) were significantly enhanced in the MPO-treated cohorts versus controls. Red cell recovery was further improved relative to control-treated cohorts that received whole blood transfusions. Significant increases in bone marrow-derived clonogenic activity was observed by day 14 after TBI in MPO-treated cohorts versus respective time-matched controls. Thus, MPO, administered daily was as effective as a twice daily schedule for multilineage recovery in nonhuman primates after high-dose, radiation-induced myelosuppression.
The major dose limiting sequelae, consequent to radiation and/or chemotherapy are neutropenia and thrombocytopenia. Protocols involving dose intensification and/or schedule compression will most likely exacerbate the duration and degree of myelosuppression associated with standard dose therapy. Although G-CSF or GM-CSF are used clinically to reduce the obligate neutropenic consequences of cytotoxic therapy, the effective treatment of thrombocytopenia has remained elusive.1-7 Recently, cytokines such as IL-11 and the cognate ligand (L) of the c-mpl receptor (Mpl-L) offer renewed potential for increasing platelet production after cytotoxic therapy.8-16 The Mpl-L, the full length, glycosylated, recombinant human (rHu) thrombopoietin (Tpo), and the nonpegylated or pegylated (PEG) truncated form (encompassing the Epo-like domain) of the Mpl-L, designated as megakaryocyte growth and development factor (MGDF), significantly induced recovery of thrombopoiesis in myelosuppressed nonhuman primates.8,11,12,17 The extension of these models to combination studies showed that the Mpl-L in combination with G-CSF augmented the G-CSF–induced recovery of neutrophils without evidence of lineage competition after radiation-induced myelosuppression.11,17 Furthermore, the combination of daniplestim, an engineered IL-3 receptor agonist with a truncated Mpl-L (Epo-like domain), enhanced hemopoietic regeneration and reduced clinical support requirements noted with respective daniplestim or truncated Mpl-L monotherapy in the irradiated nonhuman primate.18
Another approach to inducing multilineage hematopoietic recovery, subsequent to myelosuppressive therapy, has been to engineer new molecules such as chimeric growth factor (GF) receptor agonists that possess greater biologic activity than either GF agonist alone. Furthermore, the ability to engineer each GF component of the chimeric molecule offers the potential for enhancing the efficacy of GFs with narrow therapeutic windows when used as monotherapy, such as IL-3. These recently described families of chimeric proteins are composed of combined GF agonists for either IL-3 and G-CSF receptors (myelopoietin), IL-3 and Mpl-L receptors (promegapoietins), or Flt-3L and G-CSF receptors (progenipoietins).19-24
The combination of engineered IL-3 and G-CSF receptor agonists to form myelopoietin (MPO) was based on a consistent amount of in vitro and in vivo evidence, suggesting the combination would enhance both neutrophil and platelet regeneration within a favorable toxicity profile.25-31 In fact, the chimeric IL-3/G-CSF receptor agonist, MPO, had a significantly higher potency for multilineage CFU stimulatory activity on human CD34+ bone marrow cells compared with mixtures of the IL-3 and G-CSF receptor agonists.20 The in vivo priming effect of IL-3 for the action of other cytokines on early hematopoietic progenitors suggested that maximum stimulation would be achieved by the combination of IL-3 and G-CSF.32 In addition, the combined therapeutic administration of daniplestim (IL-3 receptor agonist) and G-CSF to high-dose, sublethally irradiated nonhuman primates further decreased the cytopenic durations and nadirs for both platelets and neutrophils relative to the respective cytokine monotherapy.33 Also, preliminary pharmacokinetic studies of the MPO molecule in normal rhesus monkeys demonstrated an increased biologic half-life and suggested that a reduced administration schedule would be biologically equivalent to the normally required twice daily schedule used with native, or the engineered IL-3, daniplestim.31,33 34 This study evaluated the preclinical efficacy of myelopoietin, using twice daily or daily administration schedules on multilineage hematopoietic recovery in a nonhuman primate model of high-dose, radiation-induced myelosuppression.
Materials and methods
Conduct of experiments
Experimental protocols evaluating the therapeutic efficacy of MPO were conducted using 2 models of radiation-induced myelosuppression. The same team of investigators conducted “Series 1” at the Armed Forces Radiobiology Research Institute (AFRRI), Bethesda, MD, and “Series 2” at the Greenebaum Cancer Center (GCC) at the University of Maryland, Baltimore, MD.
Animals
Male rhesus monkeys, Macaca mulatta, mean weight 3.5 ± 0.2 kg, were respectively housed in individual stainless steel cages in conventional holding rooms at the AFRRI or the Veterinary Resources Department at GCC in animal facilities accredited by the American Association for Accreditation of Laboratory Animal Care. Research was conducted according to the principles enunciated in the Guide for the Care and Use of Laboratory Animals,35 prepared by the Institute of Laboratory Animal Resources, National Research Council and research protocols approved by respective Institutional Animal Care and Use Committees.
Irradiation
Monkeys, after a prehabituation period, were irradiated in plexiglass restraining chairs for both protocols.First series. In the AFRRI protocol, monkeys received bilateral, total body irradiation (TBI) with Cobalt-60 (60Co) γ-radiation to a total midline tissue dose of 700 cGy at a dose rate of 40 cGy/min. Second series. In the GCC protocol, monkeys received unilateral TBI with 250 kVp x-radiation at 13 cGy/min in the posterior-anterior position, rotated 180° at the mid-dose (300 cGy) to the anterior-posterior position for completion of the total 600 cGy midline tissue exposure. The 700 cGy 60Co γ-radiation exposure is bioequivalent to the 600 cGy x-irradiation relative to hematopoietic myelosuppression (see Results, Table1). Dosimetry was performed using paired 0.5 cm3 ionization chambers, with calibration factors traceable to the National Institute of Standards and Technology.
Comparison of radiation-induced hematologic effects: 700 cGy 60Co γ-irradiation versus 600 cGy 250 kVp x-irradiation
| . | 700 cGy 60Co γ-Irradiation . | 600 cGy 250 kVp x-Irradiation . |
|---|---|---|
| Neutropenia duration (days) | 14.8 ± 0.7 | 16.2 ± 0.4 |
| ANC nadir (μL−1) | 0 ± 0 | 8 ± 4 |
| ANC recovery (days) | 19.7 ± 0.5 | 21.7 ± 0.4 |
| Thrombocytopenia duration (days) | 11.9 ± 1.6 | 10.4 ± 0.8 |
| PLT nadir (μL−1) | 5 000 ± 600 | 3 000 ± 1 000 |
| PLT recovery (days) | 20.7 ± 1.4 | 20.7 ± 0.9 |
| . | 700 cGy 60Co γ-Irradiation . | 600 cGy 250 kVp x-Irradiation . |
|---|---|---|
| Neutropenia duration (days) | 14.8 ± 0.7 | 16.2 ± 0.4 |
| ANC nadir (μL−1) | 0 ± 0 | 8 ± 4 |
| ANC recovery (days) | 19.7 ± 0.5 | 21.7 ± 0.4 |
| Thrombocytopenia duration (days) | 11.9 ± 1.6 | 10.4 ± 0.8 |
| PLT nadir (μL−1) | 5 000 ± 600 | 3 000 ± 1 000 |
| PLT recovery (days) | 20.7 ± 1.4 | 20.7 ± 0.9 |
Monkeys were total body irradiated to 700 cGy with60Co γ-irradiation (n = 10) or 600 cGy with 250 kVp x-irradiation (n = 11) as midline tissue doses and treated with control protein (HSA or autologous serum) according to protocol (Methods). Neutropenia is defined as an absolute neutrophil count (ANC) <500/μL and thrombocytopenia is defined as a platelet count (PLT) <20 000/μL. ANC and PLT recovery are the days required to reach sustained levels of ANC ≥500/μL and PLT ≥20 000/μL. Data represents mean values ± SEM.
Recombinant cytokines
Myelopoietin (SC-68420) (MPO), an engineered, chimeric hematopoietic growth factor that binds and activates the IL-3 and G-CSF receptors was produced in Escherichia coli through the use of a plasmid-based expression vector. It was expressed in insoluble inclusion bodies within the E coli cells. Washed inclusion bodies were solubilized in urea buffer and disulfide bonds formed through air oxidation after lowering the urea concentration. MPO was purified by ion exhange chromatography and filtration. Endotoxin levels were undetectable (Limulus amebocyte assay [Associates of Cape Cod, Inc, Woods Hole, MA]). Purified protein was stored as a frozen solution in 10 mmol/L Tris buffer, pH 8.0. In the previous study daniplestin (SC-55494) was provided by Searle R&D (St Louis, MO). G-CSF was purchased as Neupogen (Filgrastin) (Amgen Inc, Thousand Oaks, CA).
Study design
In each experimental series, animals were irradiated at day 0 and randomly assigned to a treatment protocol. First series, 700 cGy γ-irradiation protocols. On day 1 after 700 cGy γ-irradiation, animals received MPO at either 200 μg/kg/d (n = 4) or 50 μg/kg/d (n = 2), or HSA (Plasbumin-5, Miles Inc, Cutter Biological, Elkhart, IN). MPO or control protein, 15 μg/kg/d, n = 10, was injected twice daily (BID) as a divided dose (10-hour interval) as 1 mL subcutaneous (SC) injection bolus for 18 to 23 days. Administration was stopped when the white blood cell count reached 1 × 105/μL. Of the 4 animals injected with 200 μg/kg of MPO, 1 animal each was stopped after 18, 20, 22, and 23 days of administration. Both animals administered MPO at 50 μg/kg/d received the full 23-day schedule.Second series, 600 cGy x-irradiation protocols. On day 1 after 600 cGy x-irradiation, animals received MPO 200 μg/kg/d, n = 5 or 0.1% autologous serum (AS, n = 11) as control protein in 1 mL bolus SC injections once a day (QD) for 18 days.
Rationale for cytokine dose and schedule
The dose (50 μg/kg and 200 μg/kg) and administration schedule (BID vs QD) for MPO were based on several observations: (1) Native rhuIL-3 or engineered the IL-3 receptor agonist, daniplestim, have 20- to 50-fold less binding affinity for rhesus IL-3 receptor than the homologous, rhesus IL-3.36,37 (2) We previously demonstrated the enhanced therapeutic efficacy of daniplestim administered BID at 100 μg/kg/d relative to 25 μg/kg/d.31 (3) The MPO chimeric is constructed at approximately equimolar ratios of engineered IL-3 and G-CSF receptor agonists. Therefore, the 200 μg/kg dose of MPO provides approximately 100 μg/kg of engineered IL-3. (4) Monotherapy protocols that use native rhuIL-3 or daniplestim, required BID administration for optimal biologic response34 (Farese et al, unpublished observations, 1998). (5) The plasma pharmacokinetic parameters of daniplestim in irradiated monkeys31 and MPO in normal monkeys (Table 2, herein) suggested that an abbreviated schedule (QD vs BID) of MPO would induce an equivalent biologic response to the full BID schedule.
Plasma pharmacokinetic parameters for MPO after intravenous or subcutaneous administration to normal rhesus monkeys
| Pharmacokinetic Parameters . | Rhesus . |
|---|---|
| Intravenous* | |
| Dose (μg/kg) | 10 |
| Half-lives (h) | |
| First | 0.533 ± 0.051 |
| Second | 25.3 ± 14.0 |
| Clearance (mL/min/kg) | 0.415 ± 0.070 |
| VC (L/kg)† | 0.0220 ± 0.0026 |
| VSS (L/kg)† | 0.0706 ± 0.0481 |
| Subcutaneous* | |
| Dose (μg/kg) | 30 |
| CMAX (ng/mL) | 97.4 ± 29.1 |
| TMAX (h) | 3.3 ± 1.3 |
| AUC ([ng/mL]h)‡ | 806 ± 368 |
| Bioavailability (%) | 62.5 |
| Pharmacokinetic Parameters . | Rhesus . |
|---|---|
| Intravenous* | |
| Dose (μg/kg) | 10 |
| Half-lives (h) | |
| First | 0.533 ± 0.051 |
| Second | 25.3 ± 14.0 |
| Clearance (mL/min/kg) | 0.415 ± 0.070 |
| VC (L/kg)† | 0.0220 ± 0.0026 |
| VSS (L/kg)† | 0.0706 ± 0.0481 |
| Subcutaneous* | |
| Dose (μg/kg) | 30 |
| CMAX (ng/mL) | 97.4 ± 29.1 |
| TMAX (h) | 3.3 ± 1.3 |
| AUC ([ng/mL]h)‡ | 806 ± 368 |
| Bioavailability (%) | 62.5 |
Values are the mean (±SEM) for 3 monkeys.
VC and VSS are the apparent central compartment and steady state volumes of distribution, respectively.
Values are calculated from 0 to 24 hours for monkeys.
Clinical support
Hematologic evaluations
Peripheral blood.
Peripheral blood was obtained from the saphenous vein to assay complete blood (Baker, System 9000, Serono-Baker, Allentown, PA or Sysmex K-4500, Long Grove, IL) and differential counts (Wright-Giemsa Stain, Ames Automated Slide Stainer, Elkhart, IN). Assessment of hematologic evaluations has been previously described.31
Bone marrow.
Approximately 2 mL of heparinized-bone marrow (BM) was aspirated from the humerus and/or iliac crest of anesthetized primates (Ketaset, Fort Dodge, IA, 10 mg/kg intramuscular). Low density (< 1.077 g/cm3) mononuclear cells (MNC) were separated and cultured as previously described.31 GM-CFC and E-BFU derived colonies (> 50 cells) were expressed as the number of CFC/105 MNC. MK-CFC (10-50 cells/colony) are not distinguished from MK-BFC in this study, and are termed MK-CFC.
Pharmacokinetic analysis
The pharmacokinetics of MPO were determined in female rhesus monkeys (n = 3) after intravenous (IV) bolus (10 μg/kg) and SC (30 μg/kg) administration. MPO concentrations in plasma were determined by sandwich ELISA (enzyme-linked immunosorbent assay) procedures. The ELISA uses a murine monoclonal anti–G-CSF antibody, bound in microtiter plate wells, to bind MPO present in plasma samples. After removal of unbound plasma proteins by washing with buffer, MPO was quantified by the addition of goat polyclonal antidaniplestim antibody that is conjugated to horseradish peroxidase. The color produced by incubation with peroxidase substrate is read at 650 nm, and MPO concentrations are determined by reference to a standard curve prepared with MPO in plasma with a sensitivity limit of 0.150 ng/mL.
Statistical analysis
The Normal Scores Test was used to make pairwise comparisons of the durations of neutropenia and thrombocytopenia and evaluate the statistical significance between nadirs, the time to recovery and transfusion and antibiotic requirements. The tests were carried out using the software package StatXact (Cytel Software Corp, Cambridge, MA). The exact P values were obtained for all analysis.
Comparison of the MPO-treated cohorts with the combined daniplestim/G-CSF cohort in the published data set33 used a 1-way analysis of variance (ANOVA) across all groups, followed by linear contrasts representing each type comparison. Appropriate transforms were used to obtain homogeneous variance.
Bone marrow-derived clonogenic activities were analyzed by 2 methods. A repeated measures analysis was performed, followed by a 2-sided Dunnett's test to compare treatment days after TBI with baseline values. The comparisons of treated groups versus the time-matched, HSA- or AS-treated controls were made with a 1-way ANOVA, followed by a 1-sided Dunnett's test.
Results
Relative hematologic effects of total body exposure to 700 cGy γ-irradiation versus 600 cGy 250 kVp x-irradiation
The acute hematologic suppression consequent to a midline tissue dose of 700 cGy γ-irradiation is not significantly different from that observed consequent to 600 cGy of 250 kVp x-irradiation (Table 1). The key parameters, indicative of the relative degree of myelosuppression, namely, the duration of neutropenia, absolute neutrophil count (ANC) < 500/μL, 14.8 and 16.2 days,P = .065, and thrombocytopenia, platelet count (PLT) < 20 000/μL, 11.9 and 10.4 days, P = .207, absolute neutrophil (0 and 8/μL, P = .139), and platelet nadirs, 5 000 and 3 000/μL, P = .197, are not statistically different after exposure to 700 cGy 60Co γ-irradiation or 600 cGy 250 kVp x-irradiation, respectively (Table 1). Inspection of the BM-derived clonogenic activity noted in Figures 2 and 4 for the respective control-treated cohorts also substantiates the equivocal hematopoietic myelosuppressive effects of these different quality radiation exposures.
Pharmacokinetics of MPO in normal animals
Elimination of MPO from rhesus monkey plasma occurred in 2 phases after bolus IV administration of 10 μg/kg. The mean values of the first and second phase half-lives were 0.533 and 25.3 hours, respectively (Table 2). The total body clearance of MPO was 0.415 mL/min/kg. The central compartment (Vc) and steady state (VSS) volumes of distribution were 0.0220 and 0.0706 L/kg, respectively. These volume of distribution values are similar to the plasma volume and whole blood volume, respectively, and indicate little, if any, distribution from blood into other tissues.
The maximal plasma concentration (Cmax) after a 30 μg/kg SC dose was 97.4 ng/mL at 3.3 hours (TMAX). MPO was measurable in plasma up to 24 to 48 hours after SC administration. Mean area under the plasma concentration-time curves (AUC) was 806 (ng/mL) hour. The apparent SC bioavailability (based on separate groups of animals for IV and SC doses) was 62.5%. Human whole body clearance of rhIL-3 and G-CSF have been reported to be approximately 4 to 5 mL/min and 0.5-0.7 mL/min/kg, respectively. The whole body clearance of MPO that would be expected in man was estimated by using an allometric scaling procedure. Linear extrapolation predicted that human clearance for MPO would approximate 0.2 mL/min/kg.
First series, 700 cGy γ-irradiation, MPO, BID administration protocol
Neutrophil recovery.
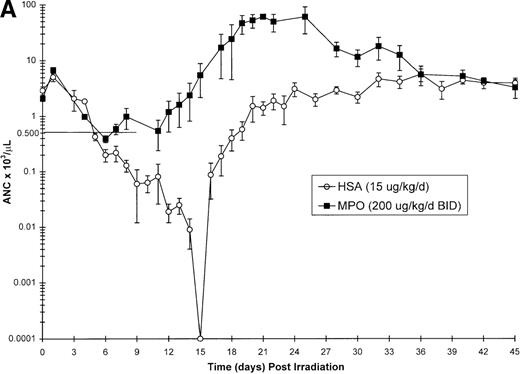
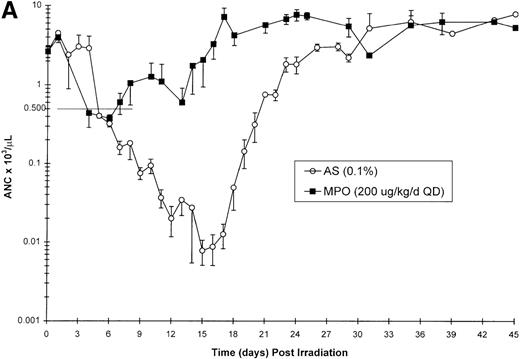
Neutrophil parameters were significantly improved by administration of MPO at a total daily dose of 200 μg/kg/d in a BID protocol after 700 cGy total body γ-irradiation. The duration of neutropenia (ANC < 500/μL) was decreased from 14.8 days in control-treated animals to 7.3 days (P = .008) and the neutrophil nadir was improved from absolute neutropenia in the control group to 390/μL (P = .005) (Table 3, Figure1A) in the MPO-treated cohort. The time to recovery of ANC to ≥ 500/μL was significantly improved from 19.7 days in the control-treated group to 12.3 days (P = .007) in the 200 μg/kg/d, BID, MPO-treated group. Consequently, the days on antibiotics were significantly reduced from 17.4 days for controls to 7.5 days (P = .005) for MPO-treated animals. Treatment with a lower dose of MPO (50 μg/kg/d), although less effective than the 200 μg/kg dose, improved the neutropenic duration (12.0 days), neutrophil nadir (36/μL), recovery time to ANC ≥ 500/μL (15.5 days), and days on antibiotics (12.5 days) relative to control-treated animals (Table 3).
Neutrophil and platelet-related parameters in sublethally γ-irradiated (700 cGy) or x-irradiated (600 cGy) rhesus monkeys treated with myelopoietin: duration, nadir, time to recovery, and clinical support
| Neutrophil-Related Parameters . | ||||
|---|---|---|---|---|
| Treatment . | Duration (days) Neutropenia . | ANC Nadir (μL−1) . | Time to Recovery (days) . | Antibiotics Required (days) . |
| Series 1 (700 cGy) | ||||
| HSA | 14.8 ± 0.7 | 0 | 19.7 ± 0.5 | 17.4 ± 1.1 |
| MPO, BID (200 μg/kg) | 7.3 ± 3.13-150,3-151 | 390 ± 553-151,3-152 | 12.3 ± 3.13-151 | 7.5 ± 3.33-151 |
| MPO, BID (50 μg/kg)3-153 | 12.0 | 36 | 15.5 | 12.5 |
| Previous data set (700 cGy) | ||||
| HSA | 14.8 ± 0.7 | 0 | 19.7 ± 0.5 | 17.4 ± 1.1 |
| Daniplestim alone, BID | 14.1 ± 1.8 | 92 ± 3.03-151,3-155 | 19.8 ± 0.93-155 | 13.4 ± 1.83-150 |
| G-CSF alone, QD | 12.4 ± 0.53-150 | 5 ± 4 | 16.2 ± 0.63-151 | 13.8 ± 0.63-151 |
| Daniplestim, BID/G-CSF, QD | 10.6 ± 0.73-151 | 124 ± 473-151,3-155 | 15.0 ± 0.63-151,3-155 | 9.2 ± 1.73-151,3-155 |
| Series 2 (600 cGy) | ||||
| 0.1% AS-control | 16.2 ± 0.4 | 8 ± 4 | 21.7 ± 0.4 | 19.3 ± 0.5 |
| MPO, QD | 7.2 ± 2.03-151 | 377 ± 423-151,3-152 | 9.2 ± 3.83-151,3-152 | 10.0 ± 1.93-151 |
| Platelet-related parameters | ||||
| Treatment | Duration (d) Thrombocytopenia | PLT Nadir (μL−1) | Time to Recovery (d) | Transfusions |
| Series 1 (700 cGy) | ||||
| HSA | 11.9 ± 1.6 | 5,000 ± 600 | 20.7 ± 1.4 | 1.7 ± 0.5 |
| MPO (200 μg/kg) | 2.0 ± 1.23-151 | 35,000 ± 10,0003-151 | 7.5 ± 3.33-151 | 1.0 ± 0.7 |
| MPO (50 μg/kg)3-153 | 6.0 | 10,000 | 15.5 | 2.0 |
| Previous data set (700 cGy) | ||||
| HSA | 11.9 ± 1.6 | 5,000 ± 600 | 20.7 ± 1.4 | 1.7 ± 0.5 |
| Daniplestim alone, BID | 3.5 ± 1.23-151,3-155 | 14,400 ± 3,0003-151,3-155 | 13.2 ± 3.33-150 | 0.4 ± 0.23-150 |
| G-CSF alone, QD | 7.2 ± 0.93-150 | 7,400 ± 2,0003-150 | 16.4 ± 0.73-150 | 1.4 ± 0.8 |
| Daniplestim, BID/G-CSF, QD | 1.9 ± 0.83-151,3-155 | 22,200 ± 5,3003-151,3-155 | 8.6 ± 3.53-151,3-155 | 0 ± 03-151 |
| Series 2 (600 cGy) | ||||
| 0.1% AS-control | 10.4 ± 0.8 | 3,000 ± 1,000 | 20.7 ± 0.9 | 2.6 ± 0.4 |
| MPO, QD | 3.2 ± 1.03-151 | 28,000 ± 10,4003-151 | 11.8 ± 2.23-151 | 0 ± 03-151 |
| Neutrophil-Related Parameters . | ||||
|---|---|---|---|---|
| Treatment . | Duration (days) Neutropenia . | ANC Nadir (μL−1) . | Time to Recovery (days) . | Antibiotics Required (days) . |
| Series 1 (700 cGy) | ||||
| HSA | 14.8 ± 0.7 | 0 | 19.7 ± 0.5 | 17.4 ± 1.1 |
| MPO, BID (200 μg/kg) | 7.3 ± 3.13-150,3-151 | 390 ± 553-151,3-152 | 12.3 ± 3.13-151 | 7.5 ± 3.33-151 |
| MPO, BID (50 μg/kg)3-153 | 12.0 | 36 | 15.5 | 12.5 |
| Previous data set (700 cGy) | ||||
| HSA | 14.8 ± 0.7 | 0 | 19.7 ± 0.5 | 17.4 ± 1.1 |
| Daniplestim alone, BID | 14.1 ± 1.8 | 92 ± 3.03-151,3-155 | 19.8 ± 0.93-155 | 13.4 ± 1.83-150 |
| G-CSF alone, QD | 12.4 ± 0.53-150 | 5 ± 4 | 16.2 ± 0.63-151 | 13.8 ± 0.63-151 |
| Daniplestim, BID/G-CSF, QD | 10.6 ± 0.73-151 | 124 ± 473-151,3-155 | 15.0 ± 0.63-151,3-155 | 9.2 ± 1.73-151,3-155 |
| Series 2 (600 cGy) | ||||
| 0.1% AS-control | 16.2 ± 0.4 | 8 ± 4 | 21.7 ± 0.4 | 19.3 ± 0.5 |
| MPO, QD | 7.2 ± 2.03-151 | 377 ± 423-151,3-152 | 9.2 ± 3.83-151,3-152 | 10.0 ± 1.93-151 |
| Platelet-related parameters | ||||
| Treatment | Duration (d) Thrombocytopenia | PLT Nadir (μL−1) | Time to Recovery (d) | Transfusions |
| Series 1 (700 cGy) | ||||
| HSA | 11.9 ± 1.6 | 5,000 ± 600 | 20.7 ± 1.4 | 1.7 ± 0.5 |
| MPO (200 μg/kg) | 2.0 ± 1.23-151 | 35,000 ± 10,0003-151 | 7.5 ± 3.33-151 | 1.0 ± 0.7 |
| MPO (50 μg/kg)3-153 | 6.0 | 10,000 | 15.5 | 2.0 |
| Previous data set (700 cGy) | ||||
| HSA | 11.9 ± 1.6 | 5,000 ± 600 | 20.7 ± 1.4 | 1.7 ± 0.5 |
| Daniplestim alone, BID | 3.5 ± 1.23-151,3-155 | 14,400 ± 3,0003-151,3-155 | 13.2 ± 3.33-150 | 0.4 ± 0.23-150 |
| G-CSF alone, QD | 7.2 ± 0.93-150 | 7,400 ± 2,0003-150 | 16.4 ± 0.73-150 | 1.4 ± 0.8 |
| Daniplestim, BID/G-CSF, QD | 1.9 ± 0.83-151,3-155 | 22,200 ± 5,3003-151,3-155 | 8.6 ± 3.53-151,3-155 | 0 ± 03-151 |
| Series 2 (600 cGy) | ||||
| 0.1% AS-control | 10.4 ± 0.8 | 3,000 ± 1,000 | 20.7 ± 0.9 | 2.6 ± 0.4 |
| MPO, QD | 3.2 ± 1.03-151 | 28,000 ± 10,4003-151 | 11.8 ± 2.23-151 | 0 ± 03-151 |
Series 1. Monkeys were total body irradiated (TBI) to 700 cGy with60Co-γ-radiation and treated with control protein, HSA (n = 10) or MPO at 200 μg/kg/d (n = 4) or 50 μg/kg/d (n = 2), BID, according to protocol (Methods). Data represents mean values ± SEM.
Series 2. Monkeys were total body irradiated (TBI) to 600 cGy with x-radiation and treated with control protein, autologous serum (AS) (n = 11) or MPO at 200 μg/kg/d, QD, (n = 5) according to protocol (Methods). Data represents mean values ± SEM.
Previous Data Set. Published study showing effects of coadministered daniplestim (BID) plus G-CSF (QD) relative to daniplestim and G-CSF monotherapy in the same 700 cGy 60Co-γ-radiation animal model performed in the same approximate time frame as Series 1.33 The control cohort (n = 10) is the same as series 1. The duration of neutropenia is defined as days of ANC <500/μL and thrombocytopenia is defined as days of platelets <20,000/μL. Time to recovery is the number of days required for the ANC to reach ≥500/μL and platelets to reach ≥20,000/μL.
Statistically different from HSA/AS-treated controls (P ≤ .05).
Statistically different from HSA/AS-treated controls (P ≤ .01).
Statistically different from daniplestim(BID)/G-CSF(QD) treated animals (P ≤ .02).
Values for MPO treated with 50 μg/kg/d were not evaluated statistically at n = 2.
Statistically different from G-CSF-treated animals (P ≤ .05).
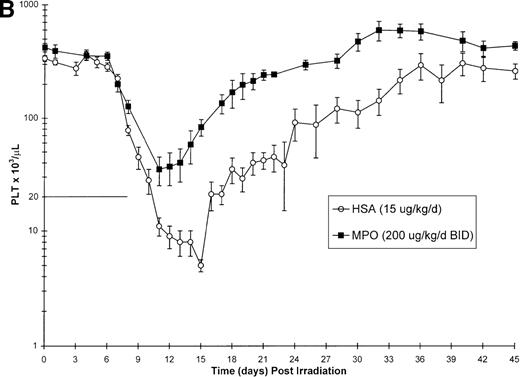
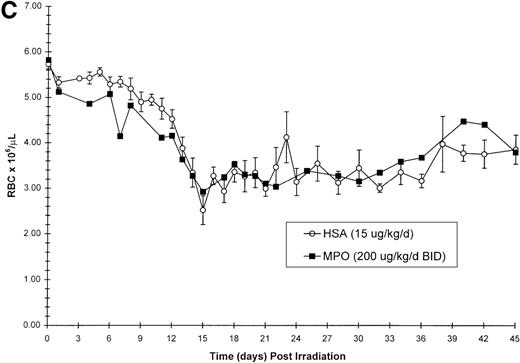
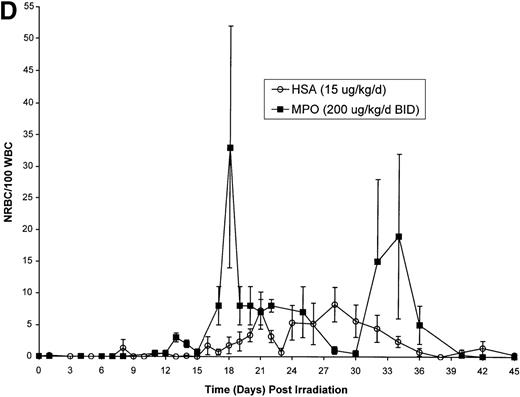
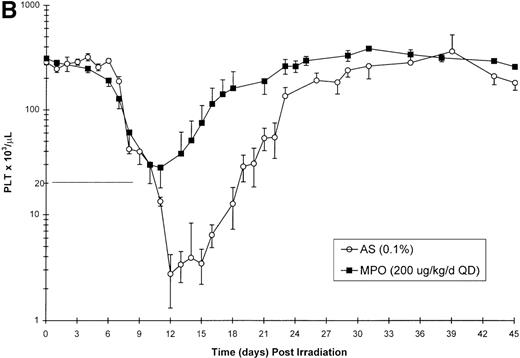
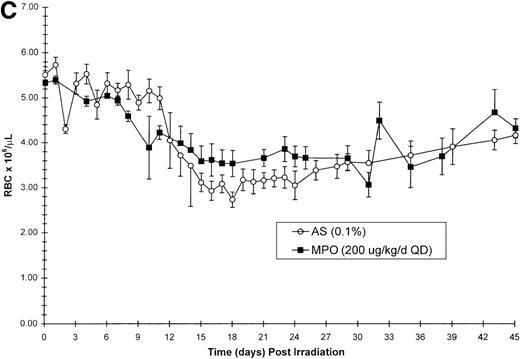
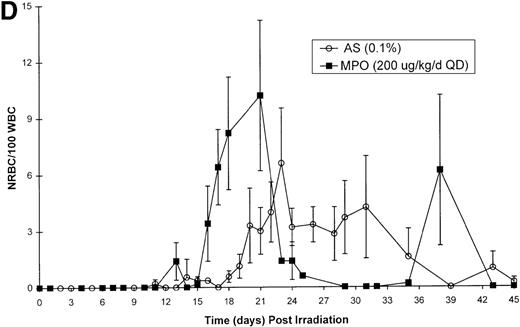
Effect of Myelopoietin (MPO) administration on peripheral blood (A) neutrophils (ANC), (B) platelets (PLT), (C) red blood cells (RBC), and (D) nucleated red blood cells (NRBC) in 700 cGy total body60Co-γ-irradiated animals.
Animals were administered MPO (BID, 200 μg/kg/d total dose, n = 4) or HSA (n = 10) as described in Methods. Average whole blood transfusions per animal: Control = 1.7, MPO = 1.0. Data represents mean values ± SEM.
Effect of Myelopoietin (MPO) administration on peripheral blood (A) neutrophils (ANC), (B) platelets (PLT), (C) red blood cells (RBC), and (D) nucleated red blood cells (NRBC) in 700 cGy total body60Co-γ-irradiated animals.
Animals were administered MPO (BID, 200 μg/kg/d total dose, n = 4) or HSA (n = 10) as described in Methods. Average whole blood transfusions per animal: Control = 1.7, MPO = 1.0. Data represents mean values ± SEM.
Platelet recovery.
The BID administration of MPO at a total daily dose of 200 μg/kg/d significantly enhanced the recovery of platelets after 700 cGy total body γ-irradiation. The 11.9-day duration of thrombocytopenia (PLT < 20 000/μL) noted for control-treated animals was significantly reduced to 2.0 days (P = .001) in the MPO-treated group (200 μg/kg/d) (Table 3, Figure 1B). The mean platelet nadir in MPO-treated animals was also significantly improved (35 000/μL) relative to the control-treated cohort (5 000/μL)(P = .005). Recovery to platelet levels of ≥ 20 000/μL after 700 cGy TBI occurred earlier in the MPO-treated cohort, 7.5 days versus 20.7 days (P = .005) for the control-treated animals. Administration of the lower dose of MPO (50 μg/kg/d), although less effective than the 200 μg/kg dose, improved the duration of thrombocytopenia (6.0 days), platelet nadir (10 000 μL), and recovery of PLT to ≥ 20 000/μL (15.5 days) relative to the control-treated cohorts (Table 3).
Production of red blood cells (RBC), nucleated red blood cells (NRBC) and transfusion requirements.
The recovery of RBC in the MPO-treated cohort (200 μg/kg/d, BID) appears equivalent to the control cohort (Figure 1C). However, the appearance of NRBCs in the peripheral blood occurred earlier after MPO administration (17 to 23 days) than the HSA-treated controls (24 days) (Figure 1D). In addition, the HSA-treated cohort received an average of 1.7 transfusions per animal, whereas the MPO-treated cohort required 1.0 transfusion per animal (Table 3).
Bone marrow-derived clonogenic activity.
Bone marrow-derived GM-CFC, E-BFU, MK-CFC, and GEMM-CFC activity was evaluated at baseline and day 7, 14, 21, 48, and 100 after 700 cGy TBI. In the HSA-treated controls, the concentration of MK-CFC was significantly reduced through 21-day after exposure, whereas GM-CFC and E-BFU were significantly decreased through 48-day after TBI (Figure2). The number of recognizable GEMM-CFC were very low in baseline BM samples. An increase in GEMM-CFC per 105 BM cells was only observed at 48-day after TBI in the control animals (Figure 2). The recovery of clonogenic activity measured in the MPO-treated cohort (200 μg/kg/d, BID) was significantly enhanced relative to that noted for time-matched controls or respective baseline values (Figure 2A through D). All clonogenic activity noted for GM-CFC, MK-CFC, E-BFU, and GEMM-CFC in MPO-treated animals had returned to baseline values within 14 days after TBI.
Effect of Myelopoietin (MPO) administration (200 μg/kg/d) on the bone marrow-derived concentration of GM-CFC, MK-CFC, E-BFU, and GEMM-CFC in 700 cGy total body60Co-γ-irradiated animals.
Clonogenic activity was observed before (baseline [BL]) and on days 7, 14, 21, 48, and 100 after TBI and administration of MPO (n = 4, 200 μg/kg/d, BID) or a control protein (n = 10, HSA or AS) as described in Methods. All animals were assayed at each time point. Clonogenic concentrations (per 105 MNC) are reported as mean values ± SEM. Baseline GEMM-CFC are detectable in low concentration. There is no significant differences between the baseline values of MPO and control-treated animals. Asterisk (*) denotes significantly different from BL (P < .05); Hdenotes significantly greater than control protein, time-matched controls (P < .05); ↓ denotes zero values; ND denotes not done.
Effect of Myelopoietin (MPO) administration (200 μg/kg/d) on the bone marrow-derived concentration of GM-CFC, MK-CFC, E-BFU, and GEMM-CFC in 700 cGy total body60Co-γ-irradiated animals.
Clonogenic activity was observed before (baseline [BL]) and on days 7, 14, 21, 48, and 100 after TBI and administration of MPO (n = 4, 200 μg/kg/d, BID) or a control protein (n = 10, HSA or AS) as described in Methods. All animals were assayed at each time point. Clonogenic concentrations (per 105 MNC) are reported as mean values ± SEM. Baseline GEMM-CFC are detectable in low concentration. There is no significant differences between the baseline values of MPO and control-treated animals. Asterisk (*) denotes significantly different from BL (P < .05); Hdenotes significantly greater than control protein, time-matched controls (P < .05); ↓ denotes zero values; ND denotes not done.
Second series, 600 cGy x-irradiation, MPO QD administration protocol
Neutrophil recovery.
Neutrophil regeneration was profoundly affected by the administration of MPO at 200 μg/kg/d in a QD schedule after TBI. Significant reduction in the duration of neutropenia and improvement in neutrophil nadir were evident in the MPO-treated animals compared with AS-treated controls 7.2 days versus 16.2 days and 377/μL versus 8/μL,P < .001 respectively (Table 3, Figure3A). The recovery of ANC to ≥ 500/μL was also significantly enhanced in MPO-treated animals relative to the controls, 9.2 days versus 21.7 days, P < .001. Consequently, the antibiotic requirements were reduced from 19.3 days for the controls to 10.0 days (P < .001) for MPO-treated animals (Table 3).
Effect of Myelopoietin (MPO) administration on recovery of peripheral blood (A) neutrophils (ANC), (B) platelets (PLT), (C) red blood cells (RBC), and (D) nucleated red blood cells (NRBC) in 600 cGy total body x-irradiated (250 kVp) animals.
Animals were administered MPO (QD, 200 μg/kg/d, n = 5) or control protein (n = 11) as described in Methods. Average whole blood transfusions per animal: Control = 2.6, MPO = 0. Data represents mean values ± SEM.
Effect of Myelopoietin (MPO) administration on recovery of peripheral blood (A) neutrophils (ANC), (B) platelets (PLT), (C) red blood cells (RBC), and (D) nucleated red blood cells (NRBC) in 600 cGy total body x-irradiated (250 kVp) animals.
Animals were administered MPO (QD, 200 μg/kg/d, n = 5) or control protein (n = 11) as described in Methods. Average whole blood transfusions per animal: Control = 2.6, MPO = 0. Data represents mean values ± SEM.
Platelet recovery.
Administration of MPO at 200 μg/kg/d, QD for 18 consecutive days after TBI significantly reduced the duration of thrombocytopenia relative to AS-treated controls 3.2 days versus 10.4 days, respectively, P < .001 (Table 3, Figure 3B). The depth of platelet nadir was also significantly improved by MPO administration from the control value of 3 000/μL to 28 000/μL (Table 3, Figure3B). Recovery times of platelet count to ≥ 20 000/μL were also significantly decreased by QD administration with MPO (11.8 days) versus the control-treated cohort (20.7 days) (P < .001).
Production of red blood cells (RBC), nucleated red blood cells (NRBC) and transfusion requirements.
The MPO-induced recovery of RBCs was modestly improved relative to the control cohort (Figure 3C). However, the RBC recovery observed in the AS-treated animals reflects the effect of whole blood transfusions. The control cohort necessitated an average 2.6 whole blood transfusions per animal, whereas the MPO-treated animals were transfusion independent (P < .001) (Table 3). MPO administration after 600 cGy TBI induced an earlier appearance of NRBC in a fashion similar to that noted for the 700 cGy TBI protocol (Figure 3D).
Bone marrow-derived clonogenic activity.
Bone marrow-derived GM-CFC, MK-CFC, and E-BFU activity (CFC/105 MNCs) were evaluated before (baseline) and at days 7, 14, 21, and 46 after 600 cGy TBI. In the control-treated animals, all clonogenic activity was significantly decreased at least through 21 days after x-irradiation. Administration of MPO-stimulated recovery of GM-CFC and MK-CFC to within preirradiation values by 21 days after irradiation (Figure 4). Interestingly, E-BFU recovery was also enhanced by day 14 after QD administration of MPO.
Effect of Myelopoietin (MPO) administration on the bone marrow-derived concentration of GM-CFC, MK-CFC, and E-BFU in 600 cGy total body x-irradiated (250 kVp) animals.
Clonogenic activity was observed before (baseline [BL]) and on days 7, 14, 21, and 46 after TBI and administration of MPO (n = 5) or a control protein (n = 11, HSA or AS) as described in Methods. Clonogenic concentrations (per 105 MNC) are reported as mean values ± SEM. Asterisk (*) denotes significantly different from BL (P < .05); H denotes significantly greater than control protein, time-matched controls (P < .05); ↓ denotes zero values; ND denotes not done.
Effect of Myelopoietin (MPO) administration on the bone marrow-derived concentration of GM-CFC, MK-CFC, and E-BFU in 600 cGy total body x-irradiated (250 kVp) animals.
Clonogenic activity was observed before (baseline [BL]) and on days 7, 14, 21, and 46 after TBI and administration of MPO (n = 5) or a control protein (n = 11, HSA or AS) as described in Methods. Clonogenic concentrations (per 105 MNC) are reported as mean values ± SEM. Asterisk (*) denotes significantly different from BL (P < .05); H denotes significantly greater than control protein, time-matched controls (P < .05); ↓ denotes zero values; ND denotes not done.
Comparison of MPO-induced hematopoietic recovery to that of coadministered daniplestim plus G-CSF.
We previously investigated the relative efficacy of coadministered daniplestim (BID) plus G-CSF (QD) to monotherapy with daniplestim (BID) or G-CSF (QD) in the equivalent 700 cGy γ-irradiated rhesus monkeys.33 We have added the key parameters in Table 3 for ready comparison to those generated herein. In that study, the coadministration of daniplestim/G-CSF significantly enhanced recovery of all neutrophil and platelet-related parameters relative to the control-treated cohort (Table 3). Furthermore, the coadministration of daniplestim/G-CSF improved all neutrophil and platelet-related parameters relative to the efficacy of daniplestim or G-CSF monotherapy.
Herein, we show that MPO, whether administered in a BID or QD schedule improved all neutrophil-related parameters noted with the coadministered daniplestim/G-CSF (Table 3). Duration of neutropenia, neutrophil nadir, recovery to an ANC ≥ 500/μL and number of days on antibitoics were all improved. Furthermore, significant improvements were noted in the ANC nadir for both BID (P < .001) and QD (P < .01) protocols and in the ANC time to recovery for the MPO, QD (P = .02) protocol relative to the daniplestim/G-CSF cohort. With regard to platelet and RBC-relative parameters, the effect of MPO (BID or QD) was equivalent to that of combined daniplestim/G-CSF (Table 3). In this regard, the QD administration schedule of MPO was as effective in enhancing hematopoietic recovery as the BID schedule.
Discussion
Myelopoietin, a novel, chimeric molecule that binds and activates the IL-3 and G-CSF receptors had marked neutrophil and platelet restorative activity in a nonhuman primate model of radiation-induced myelosuppression. Furthermore, the favorable pharmacodynamic profile exhibited by MPO conferred an equivalent therapeutic efficacy between BID or QD administration protocols. MPO administration in either protocol significantly improved hematopoietic parameters indicative of myelopoiesis, thrombocytopoiesis, and erythropoiesis subsequent to high-dose sublethal radiation exposure. The neutrophil and platelet nadirs were significantly improved, neutropenic and thrombocytopenic durations were significantly reduced, and respective neutrophil and platelet recovery time to ≥ 500/μL and ≥ 20 000/μL were significantly enhanced. RBC recovery was improved subsequent to increased erythroid clonogenic activity. Increased hematopoietic recovery resulted in fewer transfusions and significantly reduced number of days on antibiotics for all MPO-treated animals.
The therapeutic benefit of the chimeric MPO, whether administered BID or QD, compares favorably with the coadministration of daniplestim (BID) and G-CSF(QD) after radiation-induced myelosuppression (Table3).33 MPO administration improved all neutrophil-related parameters relative to the cohort coadministered daniplestim/G-CSF: The duration of neutropenia, recovery time of ANC to ≥ 500/μL and neutrophil nadir were all improved. In fact, significant improvements were noted in neutrophil nadirs and time to recovery. Platelet- and RBC-related parameters, in the MPO-treated groups (BID or QD) were equivalent to that of the combined daniplestim/G-CSF cohort. Low-level immunoreactivity to MPO was only detected at greater than 21 d after initiation of treatment and had no effect on myeloid reconstitution.
The utility of the IL-3 molecule in a combined cytokine protocol has been suggested by a number of in vitro and in vivo studies.25-31,38,39 Specifically, the addition of IL-3 and G-CSF increased in vitro bone marrow-derived myeloid and megakaryocyte colony formation25-28 and in vivo use of IL-3 was noted to prime hematopoietic progenitor cells for subsequent ex vivo stimulation by lineage-dominant cytokines such as G-CSF or GM-CSF.32,38IL-3-induced stimulation of the erythroid lineage has also been noted in normal and myelosuppressed rhesus monkeys.31,40Furthermore, the noted trophic and survival-promoting aspects of IL-3 and G-CSF offer additional support for their use in combination cytokine protocols.41-47 Brandt et al44demonstrated that the presence of IL-3 delayed the induction of apoptosis in highly enriched human hematopoietic progenitor cells. The potential to reduce radiation-induced apoptosis is yet another aspect of the ability of IL-3 to act as a priming agent for the subsequent proliferative and differentiative stimuli of endogenous and/or exogenous cytokines secreted within or added to the postirradiation hematopoietic milieu.41,45 48
Several previous studies evaluated the coadministration of multilineage and lineage dominant cytokines in nonhuman primate models of radiation or drug-induced myelosuppression in an attempt to stimulate multilineage regeneration. Those reported include IL-3/GM-CSF, IL-3/IL-6, IL-6/G-CSF, IL-6/GM-CSF, the fusion protein PIXY321, Tpo/G-CSF, and MGDF/G-CSF.11,12,17,34,39,49-54 Of these protocols, the most efficacious combination for production of both cell lineages, ie, neutrophil and platelet, within their respective models, were MGDF/G-CSF, Tpo/G-CSF, daniplestim (Synthokine)/G-CSF, and IL-3/GM-CSF.11,12,17,33,49 Each of the other combinations showed efficacy in either 1 lineage (IL-3/IL-6) or, if both lineages were enhanced (IL-6/G-CSF, IL-6/GM-CSF), the responses were not significantly greater than the respective cytokines used alone.34,50,51 53
Of particular interest is the combination of the Mpl-L (PEG-rHuMGDF) and G-CSF, which significantly enhanced multilineage, hematopoietic recovery with no evidence of lineage competition in the same 700 cGy γ-radiation-induced myelosuppression model used herein.11The PEG-rHuMGDF plus G-CSF protocol significantly diminished thrombocytopenia, and the severity of platelet nadir relative to controls.11 Neutrophil recovery was also augmented and the combined PEG-rHuMGDF plus G-CSF protocol further decreased the neutropenic duration compared with G-CSF monotherapy. After radiation-induced myelosuppression, efficacy of MPO (BID or QD) compared favorably with regard to all parameters of platelet recovery, and further improved the duration of neutropenia and ANC nadir noted in our previous study with the combined PEG-MGDF/G-CSF protocol.
These results are concordant with the in vitro data suggesting that MPO is a multifunctional agonist of the human IL-3 and G-CSF receptor (R) complexes. Monahan et al55,56 have documented an affinity conversion event when comparing MPO binding to single IL-3R or G-CSFR positive cells versus that observed with double (IL-3 plus G-CSF) R positive cells. Essentially, MPO binds to both IL-3R and G-CSFRs on dual, IL-3R/G-CSFR cells with an affinity closely matching that of IL-3 and G-CSF for their Rs. In contrast, MPO binds with significantly lower affinity to single IL-3R positive cells, whereas MPO binds to G-CSFR positive cells with the same affinity as G-CSF. It is likely that by virtue of its IL-3 component, MPO recruits primitive multilineage HPCs and early myeloid cells into proliferation. IL-3Rs have been detected on unfractionated bone marrow and on purified CD34+ cells.36,57 Further analysis of IL-3R expression on CD34+ subsets purified from normal rhesus bone marrow indicated that Rs are expressed on both CD34bright RhLA-DRdull and CD34bright RhLA-DRbright cells.58Additional studies in the murine system have shown that cells within both primitive and mature hematopoietic stem cells express receptors for both IL-3 and G-CSF.59
In addition to its capacity to stimulate primitive multilineage and early myeloid lineage dominant HPCs, MPO may also augment the hematopoietic milieu in the postirradiation microenvironment. The enhanced production of neutrophils after MPO-administration may be complemented by enhanced megakaryocytopoiesis and thrombopoiesis because of the combined autocrine and paracrine action of endogenously produced IL-1, IL-3, IL-6, IL-11, GM-CSF, c-kitL, and/or thrombopoietin. Marrow-derived megakaryocytes are capable of spontaneously secreting IL-1, IL-3, IL-6, and GM-CSF.60-62IL-3 can also enhance the secretion of IL-3, IL-6, and GM-CSF from marrow-derived megakaryocytes. Thus, the benefit derived from MPO may exceed the direct influence of the chimeric protein on the IL-3 and G-CSF receptor positive target cells.
These results will be of significance in the design considerations for clinical trials particularly because native IL-3 has required BID administration for optimal biologic effect, whether in monotherapy or combined cytokine therapeutic protocols. The equivalent therapeutic efficacy of MPO administered QD relative to that of the BID schedule would also forecast enhanced clinical utility. Based on the animal pharmacokinetic and pharmacodynamic data, effective plasma concentrations of MPO should be easily achieved and sustained for at least 12 to 24 hours after subcutaneous administration to man. The ability to use the most abbreviated and optimal schedules will aid in the design of the most favorable clinical protocols.
The demonstrated efficacy of MPO (SC 68420) in significantly improving the hematopoietic recovery after high-dose, sublethal irradiation in the nonhuman primate forecasts clinical efficacy for this engineered chimeric IL-3 and G-CSF receptor agonist for the treatment of both granulocytopenia and thrombocytopenia in the myelosuppressed patient.
Acknowledgments
We wish to thank Michael Flynn, Nelson Fleming, Lisa B. Lind, Lorelei Dacquel Smith, Daniel Casey, and Heather Webster for their superb technical assistance and William Jackson for assistance with the statistical analysis.
Reprints:Thomas J. MacVittie, Greenebaum Cancer Center, S9D11, University of Maryland, 22 S Greene St, Baltimore, MD 21201.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Effect of Myelopoietin (MPO) administration (200 μg/kg/d) on the bone marrow-derived concentration of GM-CFC, MK-CFC, E-BFU, and GEMM-CFC in 700 cGy total body60Co-γ-irradiated animals. / Clonogenic activity was observed before (baseline [BL]) and on days 7, 14, 21, 48, and 100 after TBI and administration of MPO (n = 4, 200 μg/kg/d, BID) or a control protein (n = 10, HSA or AS) as described in Methods. All animals were assayed at each time point. Clonogenic concentrations (per 105 MNC) are reported as mean values ± SEM. Baseline GEMM-CFC are detectable in low concentration. There is no significant differences between the baseline values of MPO and control-treated animals. Asterisk (*) denotes significantly different from BL (P < .05); Hdenotes significantly greater than control protein, time-matched controls (P < .05); ↓ denotes zero values; ND denotes not done.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.837.003k08_837_845/6/m_bloo00308002x.jpeg?Expires=1765981788&Signature=0Vl12JQYH6IaHbLlDvpOC0v24cyBS901xhyrVh3LYSkekzgQJDMJdmxlHD4WTw4AvWx661KFSo3YEiMM0IkbeJWBXxfz5PxCxOb2Yro~ZQ~A8wdaygNSicX-xm-gxJDuXQ6ZlnSU9MjvYnGLvkuRurhTjreT55hKXRqhTLE2~tidTtwpkpTbn3K9ymwLyMLgGqLq8WVegskDO6U85IlD1WMjI4kKagg4yASwf-VVui64JCcXPDQSyKa5P9FjeEtInUXRmlgMq81U38fdI7FyNrkazcKzw9rLT6KeIG5fJPC4fJ1MSJVUd96FzIUc7igHZKRYn2wSlPkijBoS39az-g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of Myelopoietin (MPO) administration on the bone marrow-derived concentration of GM-CFC, MK-CFC, and E-BFU in 600 cGy total body x-irradiated (250 kVp) animals. / Clonogenic activity was observed before (baseline [BL]) and on days 7, 14, 21, and 46 after TBI and administration of MPO (n = 5) or a control protein (n = 11, HSA or AS) as described in Methods. Clonogenic concentrations (per 105 MNC) are reported as mean values ± SEM. Asterisk (*) denotes significantly different from BL (P < .05); H denotes significantly greater than control protein, time-matched controls (P < .05); ↓ denotes zero values; ND denotes not done.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/3/10.1182_blood.v95.3.837.003k08_837_845/6/m_bloo00308004x.jpeg?Expires=1765981788&Signature=X~9Z8RfStiVo-DnIdX4RiDp1YeGUmUYVXGSIHyTgUH4WSxSzXOyeG7dmuDXJ4eK2BvywvXm7kWEEPkgpczJrZSOJi9mq0ph3o6jskQMNH5oAADOxfK4ebg1uml~9acJ~BKE-02L2hrSL6LwyJpvjV5OEfPA9Hxh1smz7RY8O~k4Szys3x2rRl8oBBQkfChFSngDu1IOWvKkFDrQAK2TN5MFdPfU4kIyWCcYPg1kC47~fra2bzyYsNzUn3vwNgTQl918nyOQ2b3BTWxYe1zjJ05fzPnTShh2JsgJjrMNGWypapy8i71Ws2xQgEZsRrMWGnGzMguoaCdjgvXTIbrrSuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal