Early response to therapy is an independent prognostic factor in childhood acute lymphoblastic leukemia. Although most patients have rapid early responses, as detected by morphology, 15% to 20% of patients have relapses. The authors evaluated residual disease by molecular methods on day 15 of minimal residual disease (MRD) therapy and compared these data with their recently established MRD-based risk stratification, defined by MRD levels 5 weeks after induction treatment and before consolidation. All 68 children treated according to current Berlin-Frankfurt-Münster (BFM) protocols went into morphologically complete remission after induction. There was a significant difference in outcome between children with rapid disease clearance and those with high levels of day-15 MRD (P = .035). Among patients with high levels of day-15 MRD, only the MRD-based risk stratification was predictive of the outcome. All patients with negative or low day-15 MRD had excellent prognoses and were in the MRD-based low-risk group. Thus, after only 2 weeks of treatment, the authors were able to identify a patient population of 20% who may benefit from the least intensive treatment.
Current treatment strategies use clinical and biologic features at the time of diagnosis for risk-adapted therapy in childhood acute lymphoblastic leukemia (ALL), resulting in overall relapse-free survival rates of approximately 75%.1 Early response to chemotherapy was found to add to the definition of a patient's individual risk.2 Early response has been measured by morphologic evaluation of blasts in peripheral blood after 1 week of prednisone therapy and of multiagent chemotherapy.3,4 An adverse prognosis was preceded by a slow response. However, even 20% of patients with rapid early responses to either of those treatments undergo relapse.3,4 In another approach, bone marrow rating after 1 or 2 weeks of therapy was investigated for its predictive value. Again, patients with high blast cell counts were at higher risk for relapse than were patients with counts smaller than 5%. Relapses, however, primarily occur in the latter group, to which most patients belong.2 Molecular evaluation has shown that the amount of residual disease a patient has at the time of conventional morphologic remission and after treatment with induction chemotherapy is of prognostic significance.5-8 By using 2 time points—after 5 weeks of induction chemotherapy and before consolidation—we identified 3 MRD-based risk groups.8
We previously observed that some patients undergo very rapid clearance of residual disease, as evaluated by antigen-receptor gene rearrangements (E.R.P.-G., unpublished data). Therefore, we sought to gain information in a larger group of patients on the relationship between residual disease detected by morphologic marrow rating and molecular analysis on day-15 MRD with the MRD-based risk groups. We hypothesized that by day-15 MRD, a subgroup of patients with less than 5% blasts in the bone marrow on day 15 could be identified as part of the MRD-based low-risk group.
Patients and methods
Patient selection
Sixty-eight children with ALL treated according to current BFM protocols (ALL-BFM 90 and 95) were included in this study. The patients were selected on the availability of day 15 marrow samples for morphologic and molecular analysis and of bone marrow cells during follow-up for MRD analysis. Clinical characteristics of the patients did not deviate from those of the additional 223 children enrolled in the MRD studies in Austria between January 1, 1992 and April 30, 1997 regarding age, sex, white blood cell count, immunophenotype of the leukemia, prednisone response, distribution among BFM risk groups, and incidence and site of relapse (Table 1;P > .1). Of these 68 children, 33 were girls and 35 were boys. Age at diagnosis ranged from 2 months to 17 years (median, 4.4 years). The diagnosis of ALL was based on standard morphologic criteria and cytochemical staining of leukemia cells. Immunophenotyping of the leukemia cells was performed according to standard procedures, and it revealed B-lineage type leukemia in 58 children (3 pre-pre-B ALL, 45 common ALL, 10 pre-B ALL) and T-lineage phenotype leukemia in 10 children (4 early T-ALL, 5 intermediate T-ALL, 1 late T-ALL). Cytogenetic analysis was performed in all children; t(4;11) and t(9;22) were identified in 3 and 2 children, respectively.
Clinical characteristics of 68 patients with ALL at diagnosis
| . | Median (range) . |
|---|---|
| Age (y) | 4.4 (0.2-17.0) |
| Sex (M/F) | 1.1:1 |
| Leukocyte count (×109/L) | 14.0 (1.4-413.6) |
| Immunophenotype (%) | BCP 85, T-cell 15 |
| Prednisone poor response (%) | 11 |
| BFM RG: SRG, MRG, HRG (%) | 23, 59, 18 |
| Relapse rate (%) | 24.5 |
| . | Median (range) . |
|---|---|
| Age (y) | 4.4 (0.2-17.0) |
| Sex (M/F) | 1.1:1 |
| Leukocyte count (×109/L) | 14.0 (1.4-413.6) |
| Immunophenotype (%) | BCP 85, T-cell 15 |
| Prednisone poor response (%) | 11 |
| BFM RG: SRG, MRG, HRG (%) | 23, 59, 18 |
| Relapse rate (%) | 24.5 |
BCP, B-cell precursor.
Criteria for inclusion in this study were morphologic and molecular evaluations of bone marrow day 15 and MRD analyses after induction and before consolidation treatment. Only patients with representative day 15 bone marrow smears, including autochthonous bone marrow structures, were included. Each institution's ethics committee approved the study design, and informed consent was obtained from the patients or their parents.
Treatment protocol
ALL-BFM 90 and 95 study designs, treatment regimens, and preliminary results have been published elsewhere.9 In brief, patients were stratified according to the 3 groups—the standard-risk group (SRG), the medium-risk group (MRG), and the high-risk group (HRG)—on the basis of risk factor 1) (risk factor was substituted in the BFM-ALL 95 study by age at diagnosis and initial WBC count, 2) prednisone response in peripheral blood, 3) translocation t(9;22) and t(4;11) in the BFM- ALL 95 study, 4) by initial central nervous system disease, and 5) blast-cell immunophenotype.
Induction treatment (protocol 1) started with 1 week of prednisone and intrathecal methotrexate (protocol 1/phase 1) and continued with a 5-drug regimen over a period of 4 weeks with daily prednisone, intrathecal methotrexate on days 15 and 29, weekly vincristine and daunorubicin, and 3×/week l-asparaginase. Further therapy (protocol 1/phase 2) for SRG and MRG patients consisted of daily 6-mercaptopurine for 4 weeks, cyclophosphamide on days 36 and 64, cytosine arabinoside (ARA-C) 4×/week, and intrathecal methotrexate on days 45 and 59. The following consolidation treatment (protocol M) consisted of an 8-week course of 6-mercaptopurine, high-dose methotrexate, and intrathecal methotrexate. For HRG patients induction therapy was followed by intensive consolidation therapy with 9 (6 in BFM-ALL 95 protocol plus protocol 2) 6-day cycles containing combinations of dexamethasone, vincristine/vindesine, 6-thioguanine/6-mecaptopurine, ifosfamide, etoposide, intrathecal methotrexate/ARA-C/PRED and HD-methotrexate, or HD-ARA-C. Prophylactic cranial irradiation was applied after intensive consolidation. Most patients underwent maintenance chemotherapy for a total treatment duration of 24 months; SRG boys in the BFM-ALL 95 protocol underwent chemotherapy for 36 months. The local institutional review boards approved the study design, and informed consent of the parents was obtained.
Evaluation of response to chemotherapy
Bone marrow smears by light microscopy.
Bone marrow aspirates were analyzed by cytomorphologic criteria and were rated M1, M2, and M3 based on the percentage of blasts (less than 5%, 5% to 25%, and more than 25%, respectively). The rating was performed centrally.
Bone marrow mononuclear cells by MRD analysis.
After the identification of leukemia clone-specific antigen receptor gene rearrangements (IgH, Igκ, TCRD, TCRG) in DNA from diagnostic bone marrow using single or multiplex polymerase chain reaction (PCR) as described previously,10-12 junctional region-specific oligonucleotides were designed. The oligonucleotides were then used for dot blot hybridization of PCR-amplified DNA from follow-up samples.8,12,13 The amount of residual leukemia was estimated by comparing the signal of the follow-up sample with a log dilution of bone marrow cells from diagnosis. High tumor loads corresponding to greater than 10−2 were summarized as greater than or equal to 10−2. MRD results of 10−5 were clustered with the 10−4results. A sensitivity of at least 10−4 of 1 target was required for inclusion in this study. In accordance with our previous study,8 we used MRD levels of greater than or equal to 10−2, 10−3, and less than or equal to 10−4 for the day 15 evaluations. Classifying the children into 3 different MRD-based risk groups was based on the combined information of MRD levels after induction protocol 1/phase1 (time point 1) and before the start of consolidation 2 weeks after the end of protocol 1/phase 2 (time point 2), as follows: low risk (LR), MRD negative at time point 1; intermediate risk (IR), < 10−3 at time point 2; high risk (HR), persistent high tumor loads greater than or equal to 10−3 at time point 2.8
Statistical analysis
For univariate analysis, the log-rank test14 was used to explore prognostic impact on relapse-free survival of day-15 marrow response and MRD-based risk group stratification. Life-table estimates of relapse-free survival (RFS) at 5 years were derived by the method of Kaplan and Meier15 and were compared using the stratified log-rank test. The influence of potential prognostic factors on RFS was estimated with the Cox proportional hazard model.16 The factors tested included BFM risk group stratification (SRG, MRG, and HRG), marrow rating on day 15, day-15 MRD levels (MRD less than or equal to 10−4, 10−3, and greater than or equal to 10−2), and MRD-based risk groups (MRD-LR, MRD-IR, and MRD-HR).4 Each factor was first tested as a single variable in the Cox regression model (univariate analysis). The significance of these variables was then tested in a multivariate analysis after removing each from the model that has all 4 factors included.
Results
Clinical course of the disease
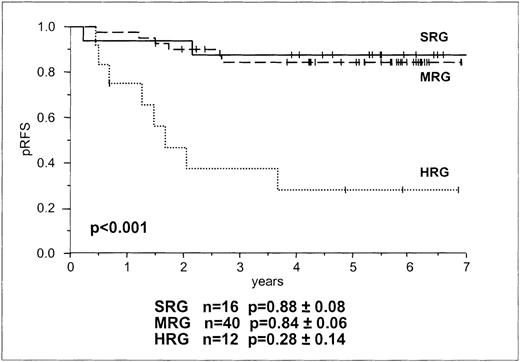
Based on their symptoms and their responses to prednisone therapy, children were stratified for treatment according to BFM risk groups (Table 1). Sixteen children were in SRG, 40 were in MRG, and 12 were in HRG (Tables 1 and 2). All 68 children enrolled in this study went into morphologic remission after induction chemotherapy. Currently, the median observation time is 5 years 6 months (range, 1 year 7 months to 8 years 11 months). Sixteen patients had relapses. Fourteen patients had isolated relapses in the bone marrow; 1 was in the SRG, 6 were in the MRG, and 7 were in the HRG. One SRG patient suffered a combined central nervous system/bone marrow relapse and 1 HRG patient had an isolated relapse in the testis. The probability for RFS at 5 years for SRG, MRG, and HRG was 88.8%, 84.6%, and 28.14%, respectively. Log-rank tests for BFM risk group stratification showed no differences between SRG and MRG in RFS but a significant difference in RFS between these 2 groups and HRG (P < .001, Figure 1). Relative risk for relapse of HRG and MRG patients compared with SRG patients was 8.9 (95% CI, 1.8-42.4) and 1.2 (95% CI, 0.3-6.1), respectively, and of HRG patients compared with MRG patients it was 7.2 (95% CI, 2.5-20.8).
Comparison of day-15 marrow rating, day-15 MRD, and MRD-based risk groups with BFM risk groups
| BFM RG . | Marrow Rating . | Day-15 MRD . | MRD RG . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 . | M2 . | M3 . | ≤ 10−4 . | 10−3 . | ≥ 10−2 . | LR . | IR . | HR . | |
| All patients/relapses | |||||||||
| SRG | 13/1 | 3/1 | 0/0 | 3/0 | 4/2 | 9/0 | 9/0 | 6/1 | 1/1 |
| MRD | 24/2 | 13/2 | 3/2 | 10/0 | 12/0 | 18/6 | 21/0 | 17/5 | 2/1 |
| HRG | 0/0 | 4/2 | 8/6 | 1/0 | 1/1 | 10/7 | 1/0 | 6/3 | 5/5 |
| Total | 37/3 | 20/5 | 11/8 | 14/0 | 17/3 | 37/13 | 31/0 | 29/9 | 8/7 |
| BFM RG . | Marrow Rating . | Day-15 MRD . | MRD RG . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M1 . | M2 . | M3 . | ≤ 10−4 . | 10−3 . | ≥ 10−2 . | LR . | IR . | HR . | |
| All patients/relapses | |||||||||
| SRG | 13/1 | 3/1 | 0/0 | 3/0 | 4/2 | 9/0 | 9/0 | 6/1 | 1/1 |
| MRD | 24/2 | 13/2 | 3/2 | 10/0 | 12/0 | 18/6 | 21/0 | 17/5 | 2/1 |
| HRG | 0/0 | 4/2 | 8/6 | 1/0 | 1/1 | 10/7 | 1/0 | 6/3 | 5/5 |
| Total | 37/3 | 20/5 | 11/8 | 14/0 | 17/3 | 37/13 | 31/0 | 29/9 | 8/7 |
MRD RG, MRD-based risk groups; LR, low risk; IR, intermediate risk; HR, high risk; BFM RG, BFM risk groups; SRG, standard-risk group; MRG, medium-risk group; HRG, high-risk group.
Probability of RFS in children with B and T lineage ALL according to BFM risk group stratification.
Probability of RFS in children with B and T lineage ALL according to BFM risk group stratification.
Day-15 marrow blasts and day-15 MRD analysis
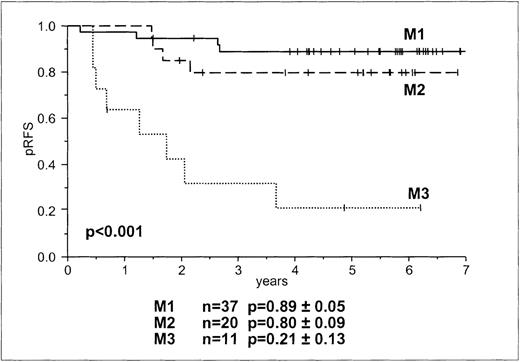
Overall, and in line with previous reports,2 the predictive value of marrow rating on day 15 on RFS was significant (P < .001, Figure 2). A higher rating (M2 and M3) was associated with an increased incidence of relapse. However, even an M1 rating was found in patients who later had relapses (Table 2). The relative risk for relapse of patients with M3 and M2 compared to M1 was 12.8 (95% CI, 3.8-43.2) and 2.0 (95% CI, 0.5-7.9), respectively, and of patients with M3 compared to M2 it was 6.5 (95% CI, 3.8-21.7). As was already assumed from the BFM risk group stratification, a higher rating was seen in HRG patients. No patient in the BFM HRG had an M1 rating, whereas several had M3 ratings. Although M2 and M3 ratings did not discriminate between children in the BFM HRG who had relapses and those who remained in continuous complete remission, day-15 M3 morphology was a prognostic parameter in the remaining patients. The trend for day-15 marrow rating as a prognostic factor occurred only after BFM risk group stratification (P = .072).
Probability of RFS according to morphologic bone marrow rating on day 15.
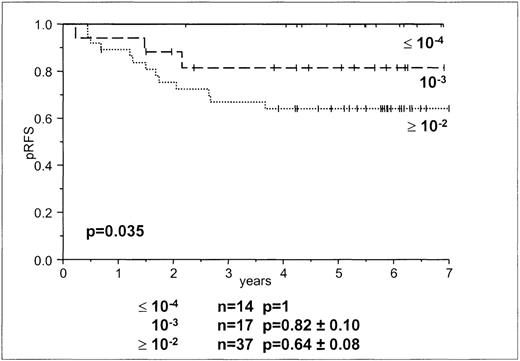
In accordance with the MRD levels in our previous study,8we used MRD loads of greater than or equal to10−2, 10−3, and less than or equal to10−4. There was a significant difference between patients with tumor loads of greater than or equal to 10−2 and those with less than or equal to 10−4 (P = .014), and a trend between patients with MRD levels of less than or equal to 10−4 and 10−3 (P = .096; Table 2, Figure 3). The levels allowed discrimination among 3 groups of patients—1 group in which all children remained in continuous complete remission (day-15 MRD less than or equal to 10−4) and 2 in which children went into relapse (day-15 MRD 10−3 and greater than or equal to 10−2) (Table 2, Figure 3). Day-15 MRD levels of 10−3 and greater than or equal to 10−2 were found in patients who remained in continuous complete remission as well as in patients who had relapses. In 37 patients with M1 marrow, day-15 MRD levels were less than or equal to 10−2, and in 31 children with M2 or M3 ratings, day-15 MRD levels were greater than or equal to 10−2. We used Cox regression analysis to determine whether day-15 MRD data added to the information gained by the BFM risk group stratification. Day-15 MRD and BFM stratification were significant prognostic factors (P < .023).
MRD-based risk group stratification
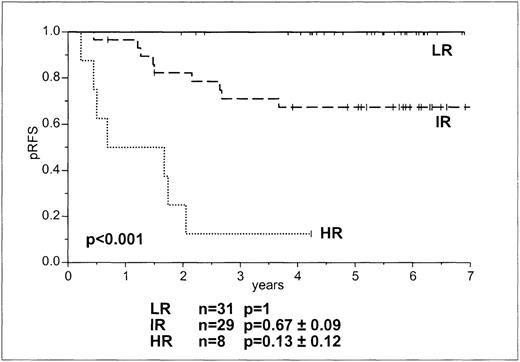
There was no relapse in the MRD-based LR group, but relapses in the IR and HR groups were 33% and 88%, respectively (Table 2, Figure4). There was a significant difference for the probability of RFS in the 3 groups at 5 years (P < .001). Approximately 50% of patients in the BFM SRG and MRG were in the MRD-based LR group (Table 2).
Comparison between BFM risk groups, morphologic and molecular analysis on day 15, and MRD-based risk group stratification
All 14 patients with low (less than or equal to 10−4) or undetectable levels of day-15 MRD were in the MRD-based LR group. Seventeen patients had high tumor loads (greater than or equal to 10−3) on day 15 but subsequently achieved MRD-based LR criteria. Twenty-nine patients with high day-15 MRD levels were in the MRD-based IR group, and 9 of them had relapses. Eight children had high day-15 MRD levels and retained MRD-based HR criteria; 7 had relapses. We used Cox regression analysis to test the significance for each of the factors (BFM risk group, day-15 marrow rating, day-15 MRD, MRD-based risk group). The MRD-based risk group stratification was the only independent prognostic factor for outcome (P < .001). Although day-15 MRD was not significant in this model, low levels of day-15 MRD (less than or equal to 10−4) identified a group of patients with very rapid responses to therapy who had good outcomes. Their low risk was substantiated by the MRD-based LR group assignment, which was based on MRD levels obtained at later time points.
Discussion
By analyzing MRD in bone marrow on day 15, we were able to identify a group of patients who responded rapidly to chemotherapy and had excellent prognoses. This prediction of a good outcome was substantiated by the findings that these patients belonged to the MRD-based low-risk group, which was based on MRD levels at later time points.
Currently, approximately 30% of patients who will have relapses of leukemia can be identified by clinical and biologic features at diagnosis.1 Early response to therapy is of additional importance.2 In the past, early response to chemotherapy was evaluated by morphology. It is recognized that approximately10% of all patients have more than 25% blasts in the bone marrow after 2 weeks of chemotherapy and that they are at increased risk for relapse.2 It is also recognized that relapses even occur in patients with good early responses (M1) and favorable features at diagnosis.17 Moreover, according to Children's Cancer Group (CCG) studies, more than 20% of patients at higher risk have relapses despite rapid early responses.18 19 In line with these data, we observed that 10% of patients in BFM MRG had relapses even though they had M1 marrow at day 15. We therefore hypothesized that by day-15 MRD, differentiation could be made between patients who would remain in complete remission and those who would have relapses. Approximately 20% of patients with less than 5% blasts in the bone marrow had low (less than or equal to 10−4) or undetectable amounts of day-15 MRD and did not have relapses. When the results from the MRD-based risk group stratification became available, these patients were in the MRD-based low risk group, confirming their excellent prognoses. Thus, we identified by day-15 MRD analysis a subgroup of patients with favorable prognoses who may comprise the target population for the least intensive form of chemotherapy. By life table estimates, patients with MRD levels of less than or equal to 10−4 had RFS probability levels of 100% projected at 5 years. One patient, however, had an observation time of < 3.5 years. This was the latest time point at which a patient with MRD levels of greater than or equal to 10−3 had a relapse. All relapses occurred in patients with MRD levels of greater than or equal to 10−3. There was no difference regarding risk for a relapse whether the day-15 MRD level was greater than or equal to 10−2 or 10−3. Therefore, those high MRD levels did not predict risk for relapse. For these patients only the MRD-based risk groups, as defined at later time points, were predictive.
Prednisone response, measured by the number of persistent blasts in the peripheral blood on day 8, has been used for treatment stratification in BFM protocols since 1986.9 Therefore, we appointed prednisone-poor responders to the BFM HRG. Day-15 M3 marrow, found in 67% of our BFM HRG patients, did not provide additional prognostic information. In contrast, day-15 M3 marrow indicated a poor prognosis for the patient with a good response to prednisone. Similar observations in a larger group of patients have been reported.3 9 However, according to MRD-based risk group, patients with day-15 M3 marrow can be differentiated into those who will remain in complete remission and those who will have relapses. Thus, MRD-based risk groups abolish the prognostic impact of day-15 morphology. This finding is of particular interest because in the next treatment protocol we will stratify patients according to the recently established MRD-based risk groups, prognostic factors at diagnosis, and prednisone responses.
In conclusion, with low levels of day-15 MRD, we were able to identify patients with rapid molecular responses to therapy. These patients were derived from groups with good responses to prednisone therapy and those with rapid morphological responses on day 15. They comprised half the patients in the MRD-based low-risk group and were the most likely to be cured with the least intenive therapy.
Acknowledgments
We thank S. Fischer and R. Kornmüller for excellent technical assistance, U. Pötschger for statistical analysis, and O. A. Haas for providing the data on day-15 marrow rating and cytogenetic analysis. We also thank Drs B. Ausserer, F. M. Fink, R. Jones, G. Mann, G. Müller, I. Mutz, R. Ployer, N. Pobegen, K. Schmitt, and O. Stöllinger for providing bone marrow samples.
Supported in part by the Österreichische Kinderkrebshilfe and by private donations to the Children's Cancer Research Institute.
Reprints:E. Renate Panzer-Grümayer, Children's Cancer Research Institute/St. Anna Kinderspital, Kinderspitalg, 6 A-1090 Vienna, Austria; e-mail: panzer@ccri.univie.ac.at.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal