Stromal cell-derived factor-1 (SDF-1) is a CXC chemokine that acts as a stimulator of pre-B lymphocyte cell growth and as a chemoattractant for T cells, monocytes, and hematopoietic stem cells. More recent studies also suggest that megakaryocytes migrate in response to SDF-1. Because genetic elimination of SDF-1 or its receptor lead to marrow aplasia, we investigated the effect of SDF-1 on megakaryocyte progenitors (colony-forming units-megakaryocyte [CFU-MK]). We report that SDF-1 augments the growth of CFU-MK from whole murine bone marrow cells when combined with thrombopoietin (TPO). The addition of SDF-1 to interleukin-3 (IL-3) or stem cell factor (SCF) had no effect. Specific antagonists for CXCR4 (the sole receptor for SDF-1), T22, and 1-9 (P2G) SDF-1 reduced megakaryocyte colony growth induced by TPO alone, suggesting that many culture systems contain endogenous levels of the chemokine that contributes to the TPO effect. To examine whether SDF-1 has direct effects on CFU-MK, we developed a new protocol to purify megakaryocyte progenitors. CFU-MK were highly enriched in CD41high c-kithigh cells generated from lineage-depleted TPO-primed marrow cells. Because the growth-promoting effects of SDF-1 were also observed when highly purified populations of CFU-MK were tested in serum-free cultures, these results suggest that SDF-1 directly promotes the proliferation of megakaryocytic progenitors in the presence of TPO, and in this way contributes to the favorable effects of the bone marrow microenvironment on megakaryocyte development.
Blood cell development is a complex process dependent on hematopoietic stem cells, a family of endocrine and paracrine growth factors and the stromal cells of the marrow microenvironment. The microenvironment, composed of endothelial cells, fibroblasts, adipocytes, monocytes, and interstitial cells, acts to provide extracellular matrix molecules such as VCAM-1 or the cell-bound form of steel factor (SF), and secreted growth factors and chemokines, including thrombopoietin (TPO), all of which help regulate stem and progenitor cell growth and trafficking.
Stromal-derived factor-1 (SDF-1) is a member of the CXC family of chemokines constitutively secreted from the bone marrow stroma and several other cell types,1 initially characterized as a pre-B cell-stimulating factor and as a highly efficient chemotactic factor for T cells and monocytes.2 The biologic effects of SDF-1 are mediated by the chemokine receptor CXCR4 (fusin, LESTR), which is expressed on mononuclear leukocytes including hematopoietic stem cells.3,4 Unlike most chemokines and chemokine receptors, SDF-1 is the only known ligand for CXCR4, and CXCR4 is the only known receptor for SDF-1.5,6 Genetic elimination of SDF-1 is associated with perinatal lethality, due to severe abnormalities in cardiac development, B-cell lymphopoiesis, and bone marrow myelopoiesis.7 Consistent with this latter finding, SDF-1 was reported to mediate chemotaxis of CD34+ stem cells and might play an important role in migration and homing of circulating hematopoietic progenitors to the bone marrow.4,8 9
Recently, it was reported that megakaryocytic progenitors, megakaryocytes, and platelets express CXCR4 and that megakaryocytes can migrate through endothelial cell monolayers in response to an SDF-1 concentration gradient.10,11 Although other chemokines, such as platelet factor 4 (PF4), neutrophil activating peptide-2 (NAP-2), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, or C10 inhibit megakaryocyte colony growth,12-15 SDF-1 was reported to augment megakaryocyte development from CD34+ cells when combined with TPO in suspension culture.10 However, the direct effect of SDF-1 on megakaryocyte progenitors has not been reported. In this study, using highly purified murine megakaryocyte progenitors, we found that SDF-1 displays a direct growth-promoting effect on megakaryocyte colony formation when added to cultures containing TPO. Previous studies from our and other laboratories established that the marrow stroma can produce TPO, particularly in thrombocytopenic states.16 17 Our present results indicate that marrow stromal cell production of SDF-1 is another mechanism by which the hematopoietic microenvironment contributes to megakaryopoiesis.
Materials and methods
Cytokines and chemokines
Recombinant human TPO was provided by ZymoGenetics, Inc (Seattle, WA). Recombinant murine TPO, interleukin (IL)-3, SCF, and human IL-6 were provided by Kirin Brewery Co. (Gumma, Japan). The murine SDF-1 used was chemically synthesized as previously described.18
Preparation and initial cell fractionation
Recombinant human TPO (2 μg/mouse/d) was administered daily to 8- to 10-week-old B6D2F1 mice (Jackson Laboratories, Bar Harbor, ME), and the animals humanely killed by cervical dislocation on day 6. Marrow cells were harvested by flushing the femurs and tibias with Iscove's modified Dulbecco's medium (IMDM; Gibco, Grand Island, NY) supplemented with 2% fetal calf serum (FCS; Sigma, St. Louis, MO). The cell suspension was passed 3 times through a 25-gauge needle and finally passed through a 200-μm nylon mesh to obtain a single cell suspension. The low-density bone marrow cells were isolated on a gradient by layering 5-mL aliquots of 107 cells/mL over 3 mL of Optiprep (density = 1.080 g/mL; Nycomed, Oslo, Norway). After centrifugation at 400g for 20 minutes, interface cells were collected, washed twice, and resuspended in phosphate-buffered saline (PBS) containing 5% FCS.
Lineage committed cell depletion
Rat antimouse monoclonal antibodies were used in a titrated mixture. Anti-7/4 (neutrophils and activated macrophages) was purchased from Serotec (Raleigh, NC). Anti-B220 (B cells and pre-B cells), anti-CD5 (T cells), anti-TER119 (erythroid cells), anti-Gr-1 (myeloid cells), and anti-Mac-1 (macrophages) were purchased from Pharmingen (San Diego, CA). The low-density marrow cells were incubated with the cocktail of monoclonal antibodies for 15 minutes on ice. Labeled cells were then washed and resuspended in PBS supplemented with 5% FCS, mixed with Dynabeads M-450 coated with sheep antirat IgG (Dynal, Great Neck, NY) at a bead-cell ratio of 5:1 and incubated for 30 minutes at room temperature on a rotating platform. The tube containing cells was then placed in a Dynal MPC-1 magnetic particle concentrator for 5 minutes. The nonrosetted cells were harvested, washed, and resuspended in PBS supplemented with 0.1% BSA. The cells recovered following immunomagnetic selection were designated as lineage-negative cells.
FACS (fluorescence-activated cell sorter) purification
The lineage-negative cells were treated with anti-CD16/CD32 antibody (Fc block, Pharmingen) for 5 minutes on ice to prevent nonspecific binding of subsequent monoclonal antibody to Fcreceptors on progenitor cells. The cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated antimouse CD41 and phycoerythrin (PE)-conjugated antimouse c-kit antibody (Pharmingen) for 15 minutes on ice. Both FITC-conjugated rat IgG1 and PE-conjugated IgG2b were used as isotype controls. The cells were washed twice and resuspended in PBS/0.1% BSA and kept on ice for cell sorting. CD41bright and c-kitbright cells were collected by sorting on a single laser FACStar Plus using Lysis II software (Becton Dickinson, Mountain View, CA). In some experiments, the CD41bright and c-kitbright cells were incubated with antimouse CXCR4 (from Dr Jose Carlos Gutierrez-Ramos, Millennium, Cambridge, MA) followed by staining with biotin-conjugated antirabbit IgG antibody (Caltag, Burlingame, CA) and Cy-chrome-labeled streptavidin (Pharmingen).
Analysis of megakaryocyte polyploidy
Sorted CD41bright c-kitbright cells were placed in suspension culture at a concentration of 1 × 104 cells/mL in IMDM supplemented with 10% FCS and murine TPO (25 ng/mL) at 37°C in a humidified atmosphere containing 5% CO2 for 2 days. Cellular DNA content was quantitated by 2 color flow cytometric analyses after labeling for 1 hour at 37°C in IMDM containing 10 μM Hoechst 33342, and following a PBS/BSA wash, then incubated with antiplatelet glycoprotein V monoclonal antibody, 1C2 (kindly provided by Dr Jun Fujimoto)19 for 15 minutes on ice. The cells were then washed twice, incubated with biotin-labeled antihamster IgG, and stained with Cy-chrome-labeled streptavidin. The DNA content of cultured 1C2-positive cells was analyzed by FACStar plus.
Colony-forming assays
Cells were plated in IMDM supplemented with 15% horse serum (HyClone, Logan, UT), 5 × 10−5 mol/L β-mercaptoethanol and penicillin-streptomycin (BioWhittaker, Walkerville, MD), and made semisolid with 0.275% (final concentration) agar (Difco, Detroit, MI). Each experiment was performed in triplicate. The plates were incubated at 37°C in a humidified atmosphere containing 5% CO2. Different marrow fractions were plated at different cell concentrations; whole marrow cells were plated at 1 × 105 cells/mL, low-density cells and Lin−cells were plated at 1 × 104 cells/mL, and CD41/c-kit sorted cells were plated at 1 × 103 cells/mL. Megakaryocyte colonies were enumerated on day 5 using an inverted microscope, defined as aggregates containing at least 3 large refractile cells, and confirmed by staining with acetylcholinesterase. For enumeration of burst-forming unit-erythroid (BFU-E)-derived colonies, cells were incubated with erythropoietin (2 U/mL) and stem cell factor (SCF) (100 ng/mL) in 2.2% methylcellulose supplemented with 30% FCS and 10% BSA. In some experiments, cells were plated in 1 mL serum-depleted medium (ASF-104, Ajino-moto, Tokyo, Japan) supplemented with 1% BSA, 1.2% methylcellulose, 300 μg/mL iron-saturated transferrin (Nakalai Tesque, Kyoto, Japan), 10 μg/mL lecithin (Sigma), 6 μg/mL cholesterol (Sigma),l-glutamine, penicillin-streptomycin, and growth factors in 35-mm culture plates.
Statistical analysis
The significance of differences in mean values was determined using the 2-tailed Student's t-test.
Results
Effect of SDF-1 on megakaryocyte colony formation from whole marrow cells
We examined the ability of SDF-1 alone or in combination with other cytokines to stimulate the growth of megakaryocytic colonies. SDF-1 alone did not support the growth of megakaryocytic colonies from marrow cells in serum-containing semisolid media, but the combination of TPO and SDF-1 increased the number of colony-forming units-megakaryocytes (CFU-MK)-derived colonies in a supra-additive manner over that seen with either cytokine alone (Table1). IL-3, SCF, and TPO all supported CFU-MK-derived colony growth when added as single agents. However, the addition of SDF-1 to SCF or IL-3 had no significant effect on megakaryocyte colony growth (SDF-1: 2 ± 1; SCF: 7 ± 2; SDF-1/SCF: 6 ± 1; IL-3: 13 ± 3, SDF-1/IL-3: 12 ± 1). In addition, SDF-1 had no effect when cells were stimulated with the combination of TPO and IL-3 or TPO and SCF (data not shown).
Effects of SDF-1 on the growth of CFU-MK-derived colonies
| Experiment no. . | Growth Factor . | No. MK Colonies (per 105 cells) . |
|---|---|---|
| 1 | TPO | 21 ± 1 |
| TPO + SDF-1 | 31 ± 1* | |
| 2 | TPO | 19 ± 1 |
| TPO + SDF-1 | 34 ± 5† | |
| 3 | TPO | 25 ± 5 |
| SDF-1 | 2 ± 1 | |
| TPO + SDF-1 | 34 ± 1‡ | |
| 4 | TPO | 17 ± 2 |
| TPO + SDF-1 | 38 ± 2* | |
| Summary | TPO | 21 ± 1.3 |
| TPO + SDF-1 | 34 ± 1.01-153 |
| Experiment no. . | Growth Factor . | No. MK Colonies (per 105 cells) . |
|---|---|---|
| 1 | TPO | 21 ± 1 |
| TPO + SDF-1 | 31 ± 1* | |
| 2 | TPO | 19 ± 1 |
| TPO + SDF-1 | 34 ± 5† | |
| 3 | TPO | 25 ± 5 |
| SDF-1 | 2 ± 1 | |
| TPO + SDF-1 | 34 ± 1‡ | |
| 4 | TPO | 17 ± 2 |
| TPO + SDF-1 | 38 ± 2* | |
| Summary | TPO | 21 ± 1.3 |
| TPO + SDF-1 | 34 ± 1.01-153 |
Murine marrow cells were incubated in 0.275% agar in IMDM supplemented with 15% horse serum, 5 × 10−5 mol/L β-mercaptoethanol, and cytokines with or without 200 ng/mL SDF-1. The number of colonies was enumerated on day 5 using an inverted microscope. The combination of TPO, IL-3, IL-6, and SCF provided a measure of the total number of CFU-MK in the cells plated. The data represent the mean ± SEM of 4 experiments of triplicate plates. Summation of all 12 plates for each condition is shown on the lower two lines.
P < 0.01.
P < 0.02.
P < 0.05.
P < 0.001 (Student's 2-tailed t-test for paired values) compared to cultures containing TPO alone.
To examine the cellular specificity of these effects we determined whether SDF-1 could affect other hematopoietic cells. When whole marrow cells were incubated with IL-3 or SCF, 88 ± 12.0 and 8.3 ± 1.5 colony-forming units-granulocyte-macrophage (CFU-GM)-derived colonies developed, respectively. SDF-1 had no effect on CFU-GM growth in combination with IL-3 (80 ± 6.0, P = 0.55); however, GM colony formation was enhanced slightly when SCF was combined with SDF-1 (15 ± 2.9, P = 0.03). SDF-1 had no effect on BFU-E colony growth in combination with EPO and SCF (8.7 ± 1.2 vs. 8.7 ± 1.5).
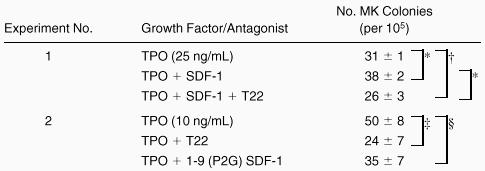
T22 is a small peptide CXCR4 antagonist derived from Limulus polyphemus and specifically inhibits the entry of T-cell line-trophic human immunodeficiency virus (HIV)-1 into target cells and Ca++ mobilization induced by SDF-1 stimulation of CXCR4.18 To demonstrate its capacity to block the SDF-1 effect on megakaryocyte growth, we added the inhibitor to cultures containing TPO and SDF-1. The addition of T22 to marrow cells incubated with TPO and SDF-1 eliminated the increased CFU-MK-derived colony numbers seen by adding SDF-1 to TPO, surprisingly, to levels significantly below that induced by TPO alone (Experiment 1, Table2). This result indicates that T22 effectively neutralized SDF-1 action and suggested that our serum-containing colony-forming cultures contain SDF-1. To test this directly, the inhibitor was added to cultures of whole marrow cells grown in TPO alone. T22 reduced megakaryocyte colony formation from 50% to 70%, depending on the dose of TPO present (Experiment 2, Table2), indicating that the culture-derived chemokine can affect megakaryocyte development in the presence of TPO. Although T22 had no effect on BFU-E or CFU-GM-derived colony growth (data not shown), to be certain our results were not due to nonspecific toxicity, we used a second, distinct CXCR4 inhibitor. A mutant of the N-terminal sequence of SDF-1, 1-9 (P2G) SDF-1 is a nonapeptide shown to competitively block SDF-1 binding to CXCR4.20 The addition of 1-9 (P2G) SDF-1 also tended to inhibit TPO-induced megakaryocyte colony growth; however, the difference did not quite reach statistical significance (see Table 2).
The inhibition of the effect of SDF-1 by T22 and 1-9 (P2G) SDF-1
Marrow cells were incubated with TPO (25 ng/mL) and SDF-1 (200 ng/mL) in the presence or absence of 3 μM T22 or 3 μM 1-9 (P2G) SDF-1. The data represent the mean ± SEM in triplicate plates. Similar results to those shown in experiment 1 were obtained in 3 additional experiments.
P < 0.01.
P < 0.05.
P < 0.02.
P = 0.1 for the comparisons shown.
Purification of CFU-MK
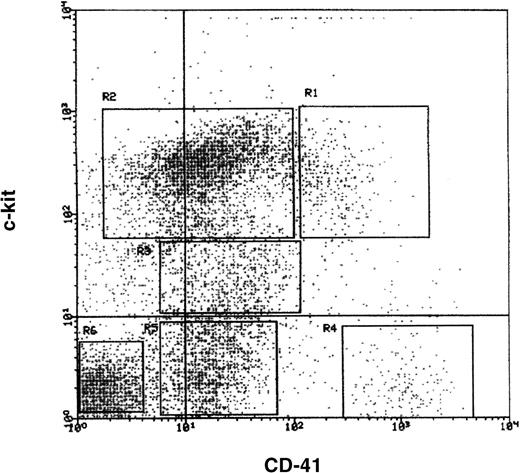
To determine whether SDF-1 would directly affect megakaryocytic progenitor cells, we developed a protocol to purify CFU-MK from TPO-treated mice and examined the effect of SDF-1 alone or in combination with TPO. Bone marrow cells from mice previously treated with TPO were sequentially fractionated by density gradient, lineage depletion, and FACS sorting. Cells obtained after each step of purification were assayed for their total number of megakaryocyte colony-forming cells by the addition of TPO, IL-3, IL-6, and SCF. After separation on a 1.080 Nycodenz density gradient, about 50% of the cells were recovered. Nearly all of the CFU-MK were recovered in this fraction; the degree of enrichment was 2-fold. The low-density cells were then incubated with cocktail of 6 monoclonal antibodies and lineage-positive cells were depleted with magnetic beads. The cloning efficiency of CFU-MK in the lineage-depleted fraction was 760 ± 110/105 cells, representing an 18-fold enrichment over unseparated marrow cells (Table 3). The lineage-negative cells were then stained with anti-CD41 and anti-c-kit antibodies for positive selection by cell sorting. Of the lineage-negative cells, 65% were CD41 positive and 55% were c-kit positive. The expression of CD41 and c-kit is shown in Figure1. To further purify CFU-MK, the lineage-negative cells were further subdivided into CD41bright c-kitbright (R1), CD41dull c-kitbright (R2), CD41dullc-kitdull (R3), CD41bright c-kit negative (R4), CD41dull c-kit negative (R5), and CD41 negative c-kit negative (R6) populations. Megakaryocytic progenitors were enriched in the R1 fraction, and the clonal efficiency of CFU-MK ranged from 11.4% to 15.3%. This fraction contained only 1% CFU-GM and no BFU-E (Table4). In contrast, CFU-GM was enriched in the R2 fraction (Table 4). Colony-forming capacity was not detected in any of other sorted fractions. The fact that most cells in the R1 fraction became megakaryoblasts or polyploid megakaryocytes after 24 to 48 hours of incubation with TPO (see below) indicated the vast majority of cells were committed to the megakaryocytic lineage.
The purification of murine CFU-MK
| Experiment No. . | Purification Step . | Recovery . | No. MK Colonies (per 105 cells) . | Fold Enrichment . | |
|---|---|---|---|---|---|
| Cell . | CFU-MK . | ||||
| 1 | Whole marrow | (100%) | (100%) | 42 ± 3 | 1 |
| Density gradient | 48% | 95% | 110 ± 20 | 2.6 | |
| Lineage depletion | 3.2% | 58% | 760 ± 114 | 18 | |
| FACS sorting | 0.03% | 8.3% | 11400 ± 2030 | 271 | |
| 2 | Whole marrow | (100%) | (100%) | 44 ± 11 | 1 |
| Density gradient | 51% | 100% | 92 ± 15 | 2.1 | |
| Lineage depletion | 3.3% | 63% | 847 ± 116 | 19.3 | |
| FACS product | 0.02% | 6.1% | 11700 ± 3770 | 265 | |
| Experiment No. . | Purification Step . | Recovery . | No. MK Colonies (per 105 cells) . | Fold Enrichment . | |
|---|---|---|---|---|---|
| Cell . | CFU-MK . | ||||
| 1 | Whole marrow | (100%) | (100%) | 42 ± 3 | 1 |
| Density gradient | 48% | 95% | 110 ± 20 | 2.6 | |
| Lineage depletion | 3.2% | 58% | 760 ± 114 | 18 | |
| FACS sorting | 0.03% | 8.3% | 11400 ± 2030 | 271 | |
| 2 | Whole marrow | (100%) | (100%) | 44 ± 11 | 1 |
| Density gradient | 51% | 100% | 92 ± 15 | 2.1 | |
| Lineage depletion | 3.3% | 63% | 847 ± 116 | 19.3 | |
| FACS product | 0.02% | 6.1% | 11700 ± 3770 | 265 | |
Low-density cells were separated from marrow cells by density gradient (density = 1.080), and lineage-committed cells were depleted with magnetic beads. Lineage-negative cells were then stained with FITC-conjugated CD41 and PE-conjugated c-kit monoclonal antibodies. CD41 positive and c-kit positive cells were sorted by FACStar plus. Cells were cultured in semisolid media with murine TPO (25 ng/mL), murine IL-3 (20 ng/mL), murine SCF (50 ng/mL), and human IL-6 (50 ng/mL) for 5 days. Whole marrow cells, low-density cells, lineage-negative cells, and sorted cells were plated at the concentration of 1 × 105, 1 × 104, 1 × 104, and 1 × 103/dish, respectively. The results represent the number of CFU-MK derived colonies (mean ± SEM of triplicate plates).
Expression of CD41 and c-kit on murine lineage-negative marrow cells.
A gate was set on CD41bright and c-kitbrightcells. Six sorting gates were established (R1-R6) and each tested individually for MK and GM colony-forming capacity. The results of these assays are shown in Table 4.
Expression of CD41 and c-kit on murine lineage-negative marrow cells.
A gate was set on CD41bright and c-kitbrightcells. Six sorting gates were established (R1-R6) and each tested individually for MK and GM colony-forming capacity. The results of these assays are shown in Table 4.
The yield of CFU-MK was low after density gradient separation, magnetic bead panning, and flow cytometry (Tables 3 and5). The cloning efficiency was further reduced when the cells were shifted to a serum-free culture system (Table6). Although CFU-MK were not detected in any of the other sorted cell fractions, to determine if the low number of CFU-MK detected after purification or in serum-free culture was due to the removal of beneficial accessory cells during purification, we performed “add-back” experiments. Mixing different sorted cell fractions, singly or all together, with the R1 population shown in Figure 1 failed to increase the number of CFU-MK detected compared to cells from R1 alone (data not shown).
Colony-forming capacity of FACS cell fractions
| Gate . | CFU-MK (per 103 cells) . | CFU-GM (per 103 cells) . |
|---|---|---|
| R1 | 153 ± 5 | 9 ± 1 |
| R2 | 4 ± 1 | 232 ± 21 |
| R3 | 4 ± 2 | 1 ± 1 |
| R4 | 0 ± 0 | 0 ± 0 |
| R5 | 0 ± 0 | 0 ± 0 |
| R6 | 0 ± 0 | 0 ± 0 |
| Gate . | CFU-MK (per 103 cells) . | CFU-GM (per 103 cells) . |
|---|---|---|
| R1 | 153 ± 5 | 9 ± 1 |
| R2 | 4 ± 1 | 232 ± 21 |
| R3 | 4 ± 2 | 1 ± 1 |
| R4 | 0 ± 0 | 0 ± 0 |
| R5 | 0 ± 0 | 0 ± 0 |
| R6 | 0 ± 0 | 0 ± 0 |
The number of CFU-MK-derived colonies was determined on day 5 and GM colonies on day 7.
The effect of SDF-1 on the growth of CFU-MK colonies generated from cells at each purification step
| Experiment No. . | . | Whole Marrow . | Density Gradient . | Lineage Depletion . | FACS Sort . |
|---|---|---|---|---|---|
| 1 | TPO | 19 ± 3 | 32 ± 4 | 310 ± 72 | 5700 ± 129 |
| TPO + SDF-1 | 34 ± 55-151 | 96 ± 165-152 | 420 ± 1005-152 | 7900 ± 4615-152 | |
| 2 | TPO | 24 ± 0.3 | 43 ± 6 | 370 ± 11 | 4000 ± 400 |
| TPO + SDF-1 | 30 ± 0.55-151 | 75 ± 95-150 | 500 ± 405-150 | 7300 ± 1755-151 | |
| Summary | TPO | 22 ± 1.3 | 39 ± 3.3 | 340 ± 22 | 4900 ± 540 |
| TPO + SDF-1 | 32 ± 1.45-150 | 86 ± 6.75-153 | 460 ± 345-152 | 7600 ± 2105-153 |
| Experiment No. . | . | Whole Marrow . | Density Gradient . | Lineage Depletion . | FACS Sort . |
|---|---|---|---|---|---|
| 1 | TPO | 19 ± 3 | 32 ± 4 | 310 ± 72 | 5700 ± 129 |
| TPO + SDF-1 | 34 ± 55-151 | 96 ± 165-152 | 420 ± 1005-152 | 7900 ± 4615-152 | |
| 2 | TPO | 24 ± 0.3 | 43 ± 6 | 370 ± 11 | 4000 ± 400 |
| TPO + SDF-1 | 30 ± 0.55-151 | 75 ± 95-150 | 500 ± 405-150 | 7300 ± 1755-151 | |
| Summary | TPO | 22 ± 1.3 | 39 ± 3.3 | 340 ± 22 | 4900 ± 540 |
| TPO + SDF-1 | 32 ± 1.45-150 | 86 ± 6.75-153 | 460 ± 345-152 | 7600 ± 2105-153 |
Cells separated in each purification step were cultured as described in Table 1 in the presence of TPO (25 ng/mL) with or without SDF-1 (200 ng/mL). CFU-MK-derived colonies were counted on day 5.
P < 0.01.
P < 0.02.
P < 0.05.
P < 0.005 (Student's 2-tailed t-test for paired values) compared to cultures containing TPO alone. The data represent the mean (±SEM) of triplicate plates expressed per 105 cells plated. Summation of the data from both experiments is shown in the last two lines.
The inhibition of the effect of SDF-1 by T22 in serum free cultures
| . | No. MK Colonies (per 105 cells) . | ||
|---|---|---|---|
| TPO . | TPO + SDF-1 . | TPO + T22 . | |
| whole marrow cells | 18 ± 3 | 34 ± 76-150 | 21 ± 3NS |
| Density gradient | 42 ± 6 | 74 ± 126-150 | 50 ± 2NS |
| FACS sorting | |||
| exp. 1 | 500 ± 25 | 800 ± 1006-150 | 525 ± 100NS |
| exp. 2 | 400 ± 100 | 900 ± 756-150 | 425 ± 100NS |
| . | No. MK Colonies (per 105 cells) . | ||
|---|---|---|---|
| TPO . | TPO + SDF-1 . | TPO + T22 . | |
| whole marrow cells | 18 ± 3 | 34 ± 76-150 | 21 ± 3NS |
| Density gradient | 42 ± 6 | 74 ± 126-150 | 50 ± 2NS |
| FACS sorting | |||
| exp. 1 | 500 ± 25 | 800 ± 1006-150 | 525 ± 100NS |
| exp. 2 | 400 ± 100 | 900 ± 756-150 | 425 ± 100NS |
Cells in each purification step were plated in serum-depleted medium with TPO and SDF-1 with or without T22. The results represent the number of CFU-MK-derived colonies (mean ± SEM of triplicate plates).
P < 0.05 compared to TPO alone; NS not significant compared to cultures containing TPO alone.
Characterization of CD41bright c-kitbrightcells
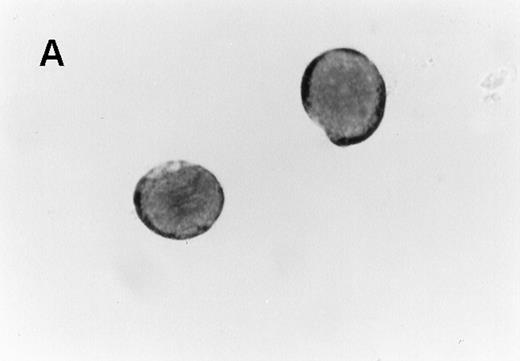
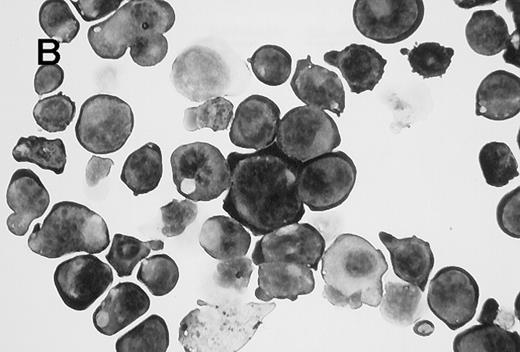
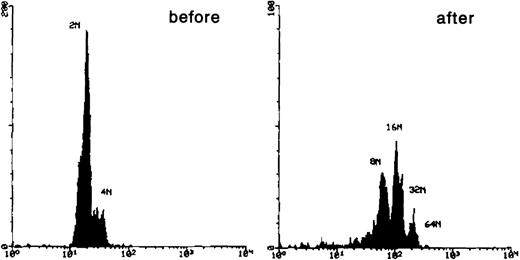
Wright-Giemsa staining of CD41brightc-kitbright cells revealed lymphocyte-sized mononuclear cells with a large nucleus-cytoplasm ratio (Figure2A). After incubation of these cells with TPO for 2 days, they became large cells with complex nuclei as shown in Figure 2B. Acetylcholinesterase staining verified that 77% of the cells were megakaryocytes by this criteria. Flow cytometric analysis showed that the starting CD41bright c-kitbrightcells were diploid; after a 2-day incubation with TPO, the cells became highly polyploid (Figure 3).
Appearance of CD41brightc-kitbright cells.
(A) Cells sorted in the CD41bright c-kitbrightfraction were stained with Giemsa following cytocentrifugation. (B) CD41high c-kithigh cells after incubation with murine TPO (25 ng/mL) for 2 days. The magnification of the two images is similar (×1000).
Appearance of CD41brightc-kitbright cells.
(A) Cells sorted in the CD41bright c-kitbrightfraction were stained with Giemsa following cytocentrifugation. (B) CD41high c-kithigh cells after incubation with murine TPO (25 ng/mL) for 2 days. The magnification of the two images is similar (×1000).
DNA histogram of CD41brightc-kitbright cells before and after incubation with TPO.
Sorted CD41bright c-kitbright cells were labeled with Hoechst 33342 followed by Cy-chrome-conjugated secondary antibody and analyzed by flow cytometry.
DNA histogram of CD41brightc-kitbright cells before and after incubation with TPO.
Sorted CD41bright c-kitbright cells were labeled with Hoechst 33342 followed by Cy-chrome-conjugated secondary antibody and analyzed by flow cytometry.
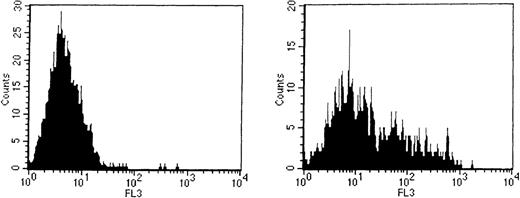
To determine whether CD41bright c-kitbrightcells express CXCR4, they were stained with a polyclonal antimouse CXCR4 antibody (kindly supplied by Dr. Jose Carlos Gutierrez-Ramos). This antibody reacted with human Jurkat but not UT-7 cells (data not shown), cell lines known to be CXCR4 positive and negative, respectively. As shown in Figure 4, the mean fluorescence of the population of CD41brightc-kitbright cells was shifted compared to an isotype-matched control antibody, indicating CXCR4 expression on a sizable fraction of the cells.
Expression of CXCR4 on CD41brightc-kitbright cells.
CD41bright c-kitbright cells were labeled with antimurine CXCR4 polyclonal antibody followed by biotin-conjugated secondary antibody and stained with avidin-conjugated Cy-chrome. The left-hand panel represents the flow histogram obtained with an isotype-matched, irrelevant control antibody. The right-hand panel represents flow results with the anti-CXCR4 antibody.
Expression of CXCR4 on CD41brightc-kitbright cells.
CD41bright c-kitbright cells were labeled with antimurine CXCR4 polyclonal antibody followed by biotin-conjugated secondary antibody and stained with avidin-conjugated Cy-chrome. The left-hand panel represents the flow histogram obtained with an isotype-matched, irrelevant control antibody. The right-hand panel represents flow results with the anti-CXCR4 antibody.
Direct effects of SDF-1 on CFU-MK growth
CXCR4 is expressed on a variety of hematopoietic cells including monocytes and T cells. Although SDF-1 enhanced and T22 inhibited CFU-MK growth from whole marrow cells, it was possible that SDF-1 might affect megakaryocyte formation indirectly by stimulating the release of lymphocyte- or monocyte-derived cytokines (e.g., GM-colony-stimulating factor, IL-3, or IL-6) known to affect CFU-MK. To determine the mechanism by which SDF-1 stimulates CFU-MK growth, we tested the effect of SDF-1 on purified megakaryocyte progenitors. As shown in Table 5, SDF-1 acted together with TPO to enhance megakaryocyte colony formation at each step of progenitor cell purification. We also examined whether neutralizing antibodies against SCF (ACK2)21 or gp130 (RX187)22 could offset the megakaryopoietic effects of SDF-1. Neither of the antibodies neutralized the effect of SDF-1 (data not shown).
To further establish that SDF-1 and TPO are able to stimulate megakaryocytic progenitor cell growth in isolation, we conducted studies using whole marrow, lineage-depleted fractions and purified CFU-MK populations in serum-free culture. As in serum-containing cultures, SDF-1 augmented megakaryocyte colony growth with TPO in all cell fractions including highly purified progenitor cells (Table 6). However, although T22 suppressed the colony growth induced by TPO alone in serum-containing conditions, this effect was not observed in serum-free cultures (contrast lines 4 and 5 of Table 2 with line 1 of Table 6). These experiments may also help to explain the minimal or modest effect of exogenous SDF-1 on megakaryopoiesis in other studies.10 28 The discrepancy in the effects of SDF-1 inhibitors between serum-containing and serum-free cultures argues that serum contributes SDF-1 to standard megakaryocyte cultures and can mask the effects of the exogenous chemokine. We also considered the possibility that megakaryocytes or their progenitors might themselves produce SDF-1. One of two reverse transcriptase-polymerase chain reaction experiments yielded a very low SDF-1-specific signal in mature megakaryocytes, and an even weaker signal in progenitor cells (data not shown). Thus, it is likely that serum is the major source of SDF-1 in standard megakaryocyte cultures. Moreover, these results establish a direct effect of SDF-1 on megakaryocytic progenitor cells.
Discussion
Thrombopoietin, the natural ligand for the c-Mpl receptor, is a potent stimulator of megakaryocytic progenitor cell development. Alone, TPO stimulates the growth of a greater number of CFU-MK that any other cytokine previously described, but we and others have found that the hormone cannot induce all such progenitors to develop into MK colonies.23-25 For example, the addition of IL-3, IL-11, erythropoietin, or SCF to TPO promotes additive or supra-additive colony formation in semisolid cultures of bone marrow cells.26 However, such synergy is not surprising, given the common mechanism of signaling used by all of these growth factor receptors.
In this study we found that CXCR4 is present on megakaryocytic progenitors and that its natural ligand, SDF-1, acts together with TPO to stimulate megakaryocyte colony growth from bone marrow progenitor cells. We have also shown that the chemokine augments megakaryocytic development derived from highly purified CFU-MK in serum-free cultures. Moreover, the favorable effect of the chemokine was not affected by neutralizing antibodies against SCF or the gp130 receptor. Taken together, these results clearly establish a direct effect of SDF-1 on megakaryocytic progenitors, an effect that occurs at chemokine concentrations equivalent to that which induces calcium flux and migration in human hematopoietic stem cells8 and megakaryocytes.10,11 T22 and 1-9 (P2G) SDF-1, specific inhibitors of SDF-1, suppressed megakaryocyte colony growth induced by the combination of TPO and SDF-1, and of TPO alone, in serum-containing marrow cell cultures. However, the inhibitory effect of T22 against TPO-induced CFU-MK was not observed when cells were cultured under serum-free conditions. This result further suggests that endogenous SDF-1 present in serum affects megakaryocyte colony growth in standard cultures. The amino acid sequence of murine SDF-1 is 98% identical to that of human SDF-1 and the chemotactic activity of murine SDF-1 is comparable to that of human SDF-1.27 Thus, it is not surprising that equine SDF-1 might affect the murine cells in our experiments.
One previous report has also commented on the favorable effect of SDF-1 on megakaryocyte production in suspension cultures.10 In contrast, two reports conclude that SDF-1 does not affect the growth of CFU-MK.10 28 In these latter two investigations, marrow cells were stimulated by the combination of TPO and IL-3 or TPO and SCF in serum- or plasma-containing cultures. We also found that SDF-1 had no effect on megakaryocyte colony growth induced by the combination of TPO and IL-3 or TPO and SCF (data not shown). Thus, the stimulatory effects of IL-3 or SCF on TPO-induced megakaryocyte growth, especially in cultures that already contain serum- or plasma-derived SDF-1, may mask the effects of the chemokine on in vitro megakaryopoiesis.
Together with the results of Wang and colleagues,10 our data clearly indicate that one of the ways the hematopoietic microenvironment supports megakaryopoiesis is by the elaboration of SDF-1. Although the marrow of mice nullizygous for either sdf-1 or cxcr4 is devoid of megakaryocytes, the effect of the chemokine on stem cell homing from the fetal liver to the marrow was thought to be responsible for the aplasia characteristic of these animals.29 Assessment of the capacity of nullizygous fetal liver cells or embryonic stem cells might shed further insights into the role of SDF-1 on megakaryopoiesis, studies that are currently underway in our laboratory. The results also suggest that the favorable effects of SDF-1 on marrow hematopoiesis might be broader than previously appreciated.
Very few reports have explored the effect of chemokines on megakaryopoiesis. Megakaryocytes and platelets express chemokine receptors CCR5 (receptors for MIP-1α and MIP-1α), CXCR1, CXCR2 (receptors for IL-8), and CXCR4 (SDF-1).10,11,15 PF4, one of the platelet-specific α-granule CXC chemokines, inhibits megakaryocyte colony formation in a lineage-specific manner.12-14 Other CXC chemokines such as IL-8 or NAP2 and CC chemokines (MIP-1α, MIP-1β, and C10) have also been shown to inhibit megakaryocyte colony growth.15 Thus, chemokines had been considered to play primarily an inhibitory role in megakaryopoiesis. In contrast, SDF-1 promotes the growth of megakaryocytes in combination with TPO. This discrepancy is not completely surprising, given the unique physiology of SDF-1 and CXCR4. Unlike the promiscuity of all other CXC chemokines and chemokine receptors, SDF-1 is the sole mammalian ligand for CXCR4, and CXCR4 is the sole receptor for SDF-1. Moreover, although the genes for all human CXC chemokines localize to the long arm of chromosome 4,30SDF-1 resides on 10q11.1.31 These findings point to a distinct evolutionary history of SDF-1, perhaps making some sense out of the opposing actions of this chemokine and of all the other chemokines tested for megakaryocytic effects.
Stromal cell-derived factor-1 acts as a chemoattractant not only on stem cells but also megakaryocytes. In addition, it has been reported that CFU-MK migrate in response to an SDF-1 concentration gradient, and direct incubation of megakaryocytes with SDF-1 induces a calcium flux.10 28 These results indicate that megakaryocytes of all stages can be affected by SDF-1 in response to signals derived from the CXCR4 receptors. Our report adds megakaryocyte progenitor cell proliferation to the effects of this chemokine on hematopoiesis.
The binding of TPO to its receptor, c-mpl, results in JAK2 activation and the subsequent phosphorylation of STAT3 and STAT5.32JAK2 has been reported to activate the adapter proteins Shc and Grb2, the GTP exchange factors Vav and SOS, and hematopoietic receptor-related phosphatase SHP-2, which activate the Ras/Raf/MEK/MAPK and phosphoinositol 3-kinase (PI3K) pathway in response to various hematopoietic growth factors.33-36 These pathways profoundly affect megakaryocyte differentiation and proliferation. For example, TPO-induced activation of mitogen-activated protein kinases (MAPKs) is associated with megakaryocytic differentiation in a leukemic cell line,37 and inhibition of the MAPK-activating kinase MEK1 blocked the development of polyploidy in normal murine megakaryocytic progenitor cells.38 Unfortunately, the molecular mechanisms by which MAPKs are activated in megakaryocytes are not yet fully understood. One pathway implicated is initiated by the phosphorylation of Shc by JAK2, coupling to Ras and Raf-1 via Grb2 and SOS, and thence to MAPK. However, other pathways appear to operate as the PI3K inhibitor Wortmannin also partially blocks MAPK activation,39 and a truncated Mpl receptor, which cannot activate Shc, can activate MAPK in both cell lines40 and primary megakaryocytes (J. Drachman, unpublished data, October 1999).
CXCR4 is a member of a 7-transmembrane domain, G-protein coupled receptor (GPCR) family.41 It was reported that CXCR4 could activate MAPK in pre-B-cell lines, which was mediated by Gβγ activation of Shc.42 Gβγ can also stimulate PI3K, leading to activation of Shc or a Src-like kinase. Moreover, other GPCRs have been shown to activate JAK and STAT molecules and to phosphorylate growth factor receptors.43-46 Thus, in other cells, pathways have been identified by which chemokine receptors might functionally interact with those derived from Mpl. Taken together, the megakaryopoietic effect of SDF-1 in the presence of TPO might be mediated by known or previously unidentified interacting signaling pathways for the proliferation and differentiation of murine megakaryocytes. To better understand the contributions of the hematopoietic microenvironment and endocrine growth factors in megakaryocyte development, future experiments will need to address the molecular mechanisms by which these two classes of receptors interact in normal megakaryocytes.
Finally, the purified population of MK progenitors we now describe is in many aspects similar to the highly enriched erythroid progenitor cells first obtained by Sawada and colleagues.47 Using a density gradient, antibodies to deplete nonerythroid lineage-committed cells, and expansion cultures these investigators have established a protocol to obtain erythroid colony-forming cells (containing a minor population of BFU-E and a major population of CFU-E) at approximately 80% purity.48 Our protocol yields megakaryocytic progenitors of which 10% to 15% are CFU-MK and 77% of the cells are megakaryoblasts as shown by acetylcholinesterase staining. The reason we consider the 2 populations similar is that in many ways the CFU-E is equivalent to a megakaryoblast, and a BFU-E is equivalent to a CFU-MK. This statement is based on the number of rounds of DNA replication each cell type undergoes before maturation into the following developmental stage. A typical CFU-E-derived colony contains approximately 20 to 50 cells, implying a single CFU-E undergoes 4 to 6 rounds of DNA synthesis and cell division before final maturation. Although a megakaryoblast undergoes DNA synthesis, it does not further divide. These cycles of DNA synthesis without cell division, termed endomitosis, allow the megakaryoblast to accumulate 16 to 64 times the normal chromosomal complement (ie, 4-6 rounds of DNA synthesis), but in a single cell. On this basis one could consider the CFU-E and megakaryoblast equivalent in terms of developmental hierarchy. A similar argument can hold for the analogy of BFU-E and CFU-MK; each cell yields from 20 to 100 progeny (CFU-E and megakaryoblasts, respectively). Thus, the availability of a method to obtain megakaryocytic progenitors of similar developmental potential as Sawada and colleagues have done with the erythroid lineage47 will hopefully allow investigators to advance our understanding of thrombopoiesis to the level presently available for erythropoiesis.
Acknowledgment
The authors wish to thank to Norma Fox and Nancy Lin for excellent technical help; Ewa Sitnicka for advice; Donald Foster for recombinant human TPO; Akihiro Shimosaka for murine IL-3, human IL-6, murine SCF, and murine TPO; Jose Carlos Gutierrez-Ramos for antimurine CXCR4 antibody; Junichiro Fujimoto for the 1C2 antibody; Virginia Broudy for the ACK2 antibody; Tetsuya Taga for the RX187 antibody; and Ian Clark Lewis for 1-9 (P2G) SDF-1.
Performed with the support of the National Institutes of Health grants DK 49855 and CA 31615.
Reprints:Kenneth Kaushansky, Division of Hematology, University of Washington School of Medicine, Box 357710, Seattle, WA 98195-7710; e-mail: kkaushan@u.washington.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal