Gastric mucosa-associated lymphoid tissue (MALT) lymphoma originates from reactive lymphocytic infiltrates during chronic gastritis, closely associated with Helicobacter pylori infection. MALT lymphomas may be either “low grade” or “high grade,” and transformation from low grade to high grade can occur. To obtain information on the maturational state of MALT lymphoma cells, we investigated their ability to undergo isotype switch recombination, which together with immunoglobulin variable gene somatic mutation, contributes to normal B-cell maturation. Using specific probes for the immunoglobulin heavy-chain (IgH) switch regions, we found by Southern blot that 3 out of 5 low-grade cases and 2 out of 2 high-grade cases showed rearrangements within IgH switch regions, which appeared aberrant in 4 of the 5 cases. The cloning of two rearranged fragments from one low-grade and one high-grade case confirmed the aberrant nature of the rearranged fragments. A deletion from the switch μ region (Sμ) to the first constant μ exon (Cμ 1) and a second deletion from the second constant μ exon (Cμ 2) to the gamma 3 region (γ 3) was detected in the low-grade case. In the high-grade case, there was a deletion of the IgH intronic enhancer (Eμ) and a 336–base pair (bp) insertion into the Sμ region of a gene (KIAA0307) normally located at 15q24. These data demonstrate for the first time the ability of MALT lymphoma cells to undergo aberrant isotype switch recombinations, which might be directly involved in the development or progression of malignancy.
Mucosa-associated lymphoid tissue (MALT) lymphomas can develop in a variety of extranodal locations.1-3 Most commonly, however, they develop within the gastrointestinal tract.4 They may be classified either as low or high grade, and transformation from low grade to high grade can occur.5Sites where MALT lymphomas develop are usually associated with autoimmune disease or an infection preceding the lymphoma.6For example, MALT lymphomas of the stomach are associated withHelicobacter pylori infection, while MALT lymphomas of the thyroid are associated with Hashimoto's thyroiditis.7-9The etiologic link between low-grade gastric MALT lymphomas and lymphoid reaction to H pylori infection has been demonstrated by the regression of some cases with antibiotic therapy alone.10 It has been suggested for 1 case that the progression from H pylori–dependent to H pylori–independent low-grade MALT lymphoma is determined by the survival benefit induced by a t(1;14)(p22;q32) that truncates the novel gene Bcl10.11 Further progression to a high-grade MALT lymphoma may be determined by subsequent genetic changes.12
MALT lymphoma cells are thought to derive from marginal zone B cells that are typically CD5−, CD10−, and IgM+IgD−.13,14 To define the stage of MALT lymphoma differentiation, numerous investigators have sequenced immunoglobulin (Ig) variable heavy-chain genes and have found frequent somatic mutations that can be ongoing.15 16Somatic hypermutations, together with isotype switch recombination, contribute to the process of B-cell maturation.
Intrigued by the ability of MALT lymphoma cells to undergo somatic hypermutation and in most cases maintain an “M” isotype, we investigated their ability to undergo DNA rearrangements within or near IgH switch regions. We analyzed 7 cases of gastric MALT lymphoma, 5 low grade and 2 high grade, using a unique Southern blot assay that could distinguish between a “legitimate” or an “illegitimate” form of IgH switch recombination.17 Legitimate switch recombination involves 2 switch regions and may be a productive switch, downstream switch, or an inversion. Illegitimate switches are aberrant recombinations that involve only 1 switch region and may be chromosomal translocations, deletions, insertions, or inversions. It was found that 3 out of the 5 low-grade and 2 out of the 2 high-grade MALT lymphomas underwent single or multiple switch recombination events, all but 1 of them aberrant.
Materials and methods
Tumor samples
Frozen and paraffin-embedded tissue samples obtained from MALT lymphoma gastrectomies were retrieved from our Department of Pathology. For each tumor sample, a Giemsa-stained paraffin section was histopathologically analyzed and classified either as low or high grade according to the criteria described by Isaacson and Norton.1
Immunohistochemistry
The isotype of surface immunoglobulins was determined immunohistochemically on cryostat or paraffin sections using monoclonal antibodies specific for human Ig isotypes (DAKO, Glostrop, Denmark), according to the streptavidinbiotin method.
Polymerase chain reaction to determine clonality
To determine tumor clonality, a seminested amplification of the variable region from framework 2 (FR2) or framework 3 (FR3) to the joining segment (JH) in the IgH locus was performed as previously described.18 Cases that did not show monoclonality by FR2/JH or FR3/JH amplification were then amplified by polymerase chain reaction (PCR) from framework 1 (FR1) to the JH region as previously reported.19 Resulting PCR products from FR1/FR2 and FR3 amplification were analyzed on a 6% or 10% acrylamide gel, respectively. Single or double PCR bands indicated monoclonality.
Nucleic acid extraction
Selected tissue specimens for nucleic acid extractions showed a percentage of lymphoid tumor cells ranging from 40% to 80%. High molecular weight DNA was extracted following a “salting out” method. The sample was lysed by incubating overnight at 56°C with agitation in 3 mL of lysis buffer (0.01 mol/L Tris-HCl, pH 8.2; 0.4 mol/L NaCl, 0.002 mol/L Na2 EDTA), adding fresh 0.5% SDS and 0.5 mL of proteinase K solution (1 mg proteinase K in 0.5 mL 1% SDS, 0.002 mol/L Na2 EDTA). The following day, 1 mL of 6 mol/L NaCl was added to the samples, shaken for few seconds, and centrifuged at 2500 rpm for 15 minutes. The supernatant was then transferred to a new tube, and 2 volumes of 95% ethanol were added. After nucleic acid precipitation, the pellet was washed 3 times in 70% ethanol and finally resuspended in 50 to 200 μL of TE solution.
Enzymatic digest
A total of 8 μg of genomic DNA was digested with the appropriate restriction enzyme (50 U/reaction) in a 100-μL digestion mix containing bovine serum albumin (100 μg/mL), spermidine (1 mM), and ribonuclease A (50 μg/mL).
Southern blot
Digested genomic DNA was fractionated by gel electrophoresis on a 0.8% agarose gel, denatured with 0.5 N NaOH/1.5 mol/L NaCl, neutralized with 1 mol/L Tris-HCl/1.5 mol/L NaCl, and transferred overnight to nylon filters with 20 × SSC. The following day, filters were air-dried and fixed with ultraviolet light (UV Stratalinker, Stratagene, La Jolla, CA).
Probes
Hybridization conditions
Filters were hybridized overnight at 42°C with 1 × 106 cpm/mL of probe in a hybridization buffer containing 1 mol/L NaCl; 50mM Tris-HCl, pH 7.4; 40% formamide; 10% dextran sulfate; 1% SDS; and 100 μg/mL salmon sperm DNA. The following day, filters were washed sequentially in solutions containing 2 × SSC/0.1%SDS (20°C), 1 × SSC/0.1%SDS (40°C), and 0.1 × SSC/0.1% SDS (65°C). They were finally exposed to autoradiogram films at −80°C or scanned on a Phosphorimager.
Long-distance inverse PCR cloning
Genomic DNA, digested with the appropriate restriction enzyme, was purified using the Wizard DNA Clean-Up System (Promega, Madison, WI), and 400 ng was self-ligated at 14°C overnight in a total volume of 500 μL with 5 U of T4 DNA ligase (Promega). The ligated DNA was again purified using the Wizard DNA clean-up system and eluted in a final volume of 50 μL. A total of 5 μL was used as a template for the PCR reaction, which contained 200 μmol/L deoxyribonucleotide triphosphate, primers at 0.2 μmol/L, 1 U Thermus thermophilus polymerase (Perkin Elmer, Foster City, CA), and 1.2 mmol/L Mg(OAc)2 in a final volume of 50 μL. The enzyme was added after the first cycle (hot start procedure). PCR cycles were as follows: 1 cycle 94°C 3 minutes, 30 cycles 94°C 1 minutes, 60°C 1 minute, 72°C 6 minutes, and 1 cycle 72°C 10 minutes. A total of 5 μL of the PCR reaction was reamplified for 30 cycles using identical conditions with nested primers. After PCR amplification, the products were gel-purified and cloned using the PCR-Script Cloning Kit (Stratagene). Clones were sequenced using an ABI Prism automated sequencer. (Modified from Willis et al21.)
Results
Five low-grade and 2 high-grade gastric MALT lymphomas were analyzed both immunohistochemically and by PCR to respectively determine IgH isotype and clonality. Five cases were found to be IgM-producing and 2 cases nonproducing. All cases showed PCR monoclonality by amplification from FR1, FR2, or FR3 to JH (Table 1).
Description of MALT lymphomas analyzed in this study
| Case No. . | Diagnosis . | Sex . | Age (y) . | Ig Isotype . | Clonality . |
|---|---|---|---|---|---|
| 1 | Low grade | M | 47 | M | MON |
| 2 | Low grade | F | 59 | M | MON |
| 3 | Low grade | F | 73 | M | MON |
| 4 | Low grade | M | 53 | M | MON |
| 5 | Low grade | M | 65 | NP | MON |
| 6 | High grade | F | 31 | M | MON |
| 7 | High grade | F | 59 | NP | MON |
| Case No. . | Diagnosis . | Sex . | Age (y) . | Ig Isotype . | Clonality . |
|---|---|---|---|---|---|
| 1 | Low grade | M | 47 | M | MON |
| 2 | Low grade | F | 59 | M | MON |
| 3 | Low grade | F | 73 | M | MON |
| 4 | Low grade | M | 53 | M | MON |
| 5 | Low grade | M | 65 | NP | MON |
| 6 | High grade | F | 31 | M | MON |
| 7 | High grade | F | 59 | NP | MON |
NP indicates nonproducing; MON, monoclonal.
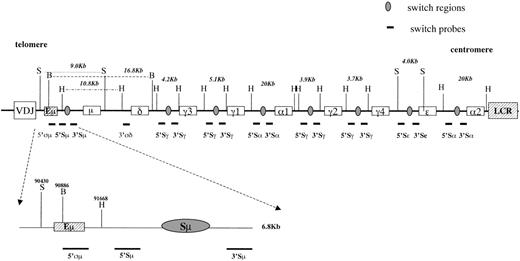
To study rearrangements within the IgH switch regions, 5 pairs of probes (1 pair for each isotype) that hybridize upstream (5′) and downstream (3′) of each switch region (Figure1) were synthesized by PCR.17Using these probes and restriction enzymes with digestion sites outside the pair of switch probes, it was possible to classify each switch region as germline, or having undergone either a legitimate or illegitimate switch recombination. Upstream and downstream switch probes detect the same restriction fragment for germline switch regions. Legitimate switch recombinations between any 2 switch regions result in the generation of a restriction fragment detected by 2 probes from different switch regions. In contrast, illegitimate switch recombination generates restriction fragments uniquely detected by only 1 switch probe. Genomic DNA from 7 cases of gastric MALT lymphoma was digested with different restriction enzymes and screened by Southern blot using the set of probes specific for the switch regions.
IgH locus at 14q32.
Top, IgH locus organization showing the localization of the switch probes and of the restriction enzymes (vertical lines) used for the Southern blot assay. Because there is no conventional switch region upstream of Cδ, the 5′ςμ and 3′ςδ probes were used to detect δ rearrangements. Distances between restriction sites in germline configuration are also represented. H = HindIII, B = BglII, S = SphI, LCR = locus control region. Bottom, Enlargement of the intronic enhancer region (Eμ). Numbers above the restriction sites indicate their postions in the GenBank sequence HSIMMDL.
IgH locus at 14q32.
Top, IgH locus organization showing the localization of the switch probes and of the restriction enzymes (vertical lines) used for the Southern blot assay. Because there is no conventional switch region upstream of Cδ, the 5′ςμ and 3′ςδ probes were used to detect δ rearrangements. Distances between restriction sites in germline configuration are also represented. H = HindIII, B = BglII, S = SphI, LCR = locus control region. Bottom, Enlargement of the intronic enhancer region (Eμ). Numbers above the restriction sites indicate their postions in the GenBank sequence HSIMMDL.
Two cases of low-grade MALT lymphoma have aberrant switch μ rearrangements
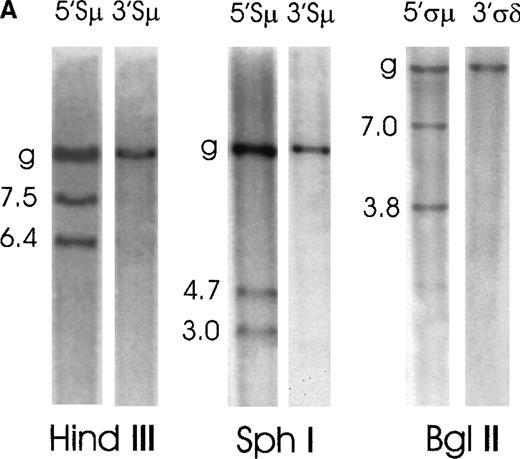
Case No. 2 was positive for IgM production. Genomic DNA from this case was digested with 3 restriction enzymes (HindIII, SphI, and BglII) and hybridized with the switch probes (Figure2A). The 5′Sμ probe showed the germline fragment and 2 rearranged fragments of 7.5 kilobases (kb) and 6.4 kb on HindIII digest and of 4.7 kb and 3.0 kb on SphI. The 3′Sμ probe hybridized to the germline fragment only. As far as the δ region is concerned, the 5′ςμ probe identified the germline fragment and 2 rearranged fragments of 7.0 kb and 3.8 kb on a BglII digest, while the 3′ςδ probe hybridized to the germline fragment only. All rearranged fragments were illegitimate because they did not hybridize with any other switch region probe (data not shown). To amplify the 3 Kb 5′Sμ rearranged fragment observed on SphI digest, genomic DNA from case No. 2 was digested with SphI, was self-ligated, and long-distance inverse PCR was performed using 4 primers (5MFA, 5MRA, 5MFB, 5MRB) internal to the 5′Sμ region (Table2). The fragment was then cloned and sequenced. We found a deletion from the Sμ region at nucleotide 628 in HSJHCMU (GenBank nucleotide sequence of the IgH Sμ region) to just upstream of Cμ exon 1 (Cμ exon 1 is from 145 to 456, Cμ exon 2 is from 547 to 882, Cμ exon 3 is from 1128 to 1445) at nucleotide 124 in HSIGCMUDE (GenBank nucleotide sequence of the human IgH Cμ and Cδ genes). The sequence continued through Cμ exon 2, and it was once again interrupted upstream of Cμ exon 3 (position 1018 in HSIGCMUDE) with γ 3 sequences upstream of the 2 membrane exons starting at 10 541 in D78 345 (GenBank nucleotide sequence of the human Ig γ heavy chain) and ending at the SphI site at position 10 933. (Figure3). Regions upstream of Sμ to the SphI site at 90 430 in HSIMMDL (GenBank DNA sequence of the human immunoglobulin D segment locus) are in the germline configuration.
Southern blot analysis of switch μ/δ regions in MALT lymphoma cases No. 2 and No. 1.
Genomic DNA was digested with the indicated restriction enzymes, electrophoresed, blotted, and probed sequentially with various switch probes shown above each lane. Illegitimate switch recombination fragments are indicated by the molecular weight and germline fragments by a “g.” Seizes of the expected germline bands are reported in Figure 1. (A) Case No. 2 shows 2 illegitimate switch fragments identified by the 5′Sμ probe on a HindIII and SphI digest and 2 fragments identified by the 5′ςμ probe on a BglII digest. (B) Case No. 1 shows 1 illegitimate switch fragment identified by the 5′Sμ probe on a HindIII digest and 1 illegitimate fragment on an SphI digest, which is identified also by the 3′Sμ probe. This case shows other 2 illegitimate switch fragments with a 5′ςμ probe on a BglI digest.
Southern blot analysis of switch μ/δ regions in MALT lymphoma cases No. 2 and No. 1.
Genomic DNA was digested with the indicated restriction enzymes, electrophoresed, blotted, and probed sequentially with various switch probes shown above each lane. Illegitimate switch recombination fragments are indicated by the molecular weight and germline fragments by a “g.” Seizes of the expected germline bands are reported in Figure 1. (A) Case No. 2 shows 2 illegitimate switch fragments identified by the 5′Sμ probe on a HindIII and SphI digest and 2 fragments identified by the 5′ςμ probe on a BglII digest. (B) Case No. 1 shows 1 illegitimate switch fragment identified by the 5′Sμ probe on a HindIII digest and 1 illegitimate fragment on an SphI digest, which is identified also by the 3′Sμ probe. This case shows other 2 illegitimate switch fragments with a 5′ςμ probe on a BglI digest.
Oligonucleotides used for PCR amplification
| Name . | Sequence 5′-3′ . | Position . |
|---|---|---|
| 5MFA | GGC TCC TAA ATT CTT GGT CTC A | 92291-92312 HSIMMDL |
| 5MRA | TCC CTC TAG ATA ACA GTC ATC A | 92140-92118 HSIMMDL |
| 5MFB | GGC AAT GAG ATG GCT TTA GCT GA | 92498-92520 HSIMMDL |
| 5MRB | CTC GTG CGA CCT CTC CTT CAA A | 91910-91888 HSIMMDL |
| 3MFA | CAG TCA GGC CTC AGA GTG CA | 4095-4115 HSJHCMU |
| 3MRA | CCG CAG GCA GCC AAT AGA GT | 3931-3911 HSJHCMU |
| 3MFB | CAA GTA GTG AGG GCA CTC AGA ACG | 4353-4377 HSJHCMU |
| 3MRB | GTG ATG GGG AAC GCA GTG TAG A | 3803-3781 HSJHCMU |
| JHF | TCT CCT GCC AAG AGA GCC CC | 88884-88903 HSIMMDL |
| Name . | Sequence 5′-3′ . | Position . |
|---|---|---|
| 5MFA | GGC TCC TAA ATT CTT GGT CTC A | 92291-92312 HSIMMDL |
| 5MRA | TCC CTC TAG ATA ACA GTC ATC A | 92140-92118 HSIMMDL |
| 5MFB | GGC AAT GAG ATG GCT TTA GCT GA | 92498-92520 HSIMMDL |
| 5MRB | CTC GTG CGA CCT CTC CTT CAA A | 91910-91888 HSIMMDL |
| 3MFA | CAG TCA GGC CTC AGA GTG CA | 4095-4115 HSJHCMU |
| 3MRA | CCG CAG GCA GCC AAT AGA GT | 3931-3911 HSJHCMU |
| 3MFB | CAA GTA GTG AGG GCA CTC AGA ACG | 4353-4377 HSJHCMU |
| 3MRB | GTG ATG GGG AAC GCA GTG TAG A | 3803-3781 HSJHCMU |
| JHF | TCT CCT GCC AAG AGA GCC CC | 88884-88903 HSIMMDL |
HSIMMDL indicates GenBank sequence of the human immunoglobulin D segment locus; HSJHCMU, GenBank sequence of the human IgM heavy chain switch region.
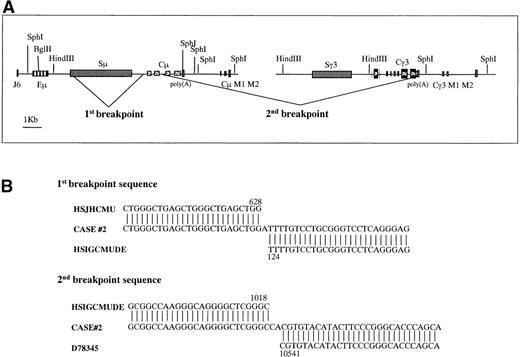
Graphic representation of the 2 breakpoints in case No. 2.
(A) The 3kb 5′Sμ aberrant fragment was amplified by long-distance inverse PCR. Genomic DNA from case No. 2 was digested with SphI, was self-ligated, and long-distance inverse PCR was performed using 4 primers (5MFA, 5MRA, 5MFB, 5MRB) internal to the 5′Sμ region. The 2 breakpoints were generated by a deletion from the Sμ region to just upstream of Cμ exon 1 and a second deletion from Cμ exon 3 to γ 3 sequences, upstream of the 2 membrane exons. Eμ = intronic enhancer, Sμ = switch μ region, Cμ = constant μ exons, M1, M2 = exons for membrane form, Sγ = switch γ region, Cγ3 = constant γ3 exons. (B) Alignment of the breakpoint regions of case No. 2 with germline GenBank sequences. Numbers indicate the nucleotide position at which the breakpoint occurred within the corresponding GenBank sequence. These breakpoint sequences are available from GenBank under accession numbers AF156 531 and AF156 532. HSJHCMU = GenBank nucleotide sequence of the IgH Sμ region, HSIGCMUDE = GenBank nucleotide sequence of the human IgH Cμ and Cδ genes, D78 345 = GenBank nucleotide sequence of the human Ig γ heavy chain.
Graphic representation of the 2 breakpoints in case No. 2.
(A) The 3kb 5′Sμ aberrant fragment was amplified by long-distance inverse PCR. Genomic DNA from case No. 2 was digested with SphI, was self-ligated, and long-distance inverse PCR was performed using 4 primers (5MFA, 5MRA, 5MFB, 5MRB) internal to the 5′Sμ region. The 2 breakpoints were generated by a deletion from the Sμ region to just upstream of Cμ exon 1 and a second deletion from Cμ exon 3 to γ 3 sequences, upstream of the 2 membrane exons. Eμ = intronic enhancer, Sμ = switch μ region, Cμ = constant μ exons, M1, M2 = exons for membrane form, Sγ = switch γ region, Cγ3 = constant γ3 exons. (B) Alignment of the breakpoint regions of case No. 2 with germline GenBank sequences. Numbers indicate the nucleotide position at which the breakpoint occurred within the corresponding GenBank sequence. These breakpoint sequences are available from GenBank under accession numbers AF156 531 and AF156 532. HSJHCMU = GenBank nucleotide sequence of the IgH Sμ region, HSIGCMUDE = GenBank nucleotide sequence of the human IgH Cμ and Cδ genes, D78 345 = GenBank nucleotide sequence of the human Ig γ heavy chain.
If we assume that the IgH region downstream of the SphI site at 10 933 in D78 345 is in germline configuration, it is plausible that the 6.4-kb fragment on the HindIII blot and the 7.0-kb fragment on the BglII blot correspond to the sequenced fragment.
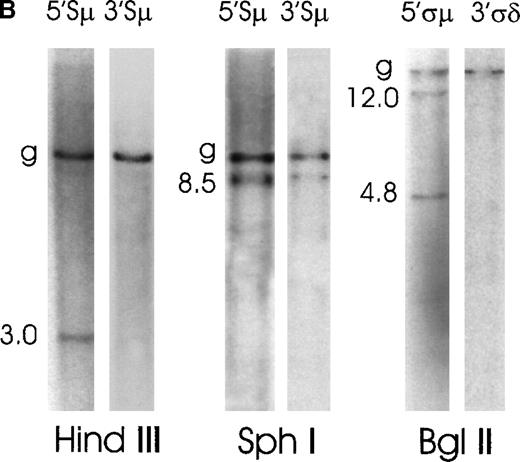
Case No. 1 produced IgM and showed a small rearranged 5′Sμ fragment (3.0 kb) on a HindIII digest that did not cohybridize with any 3′Sμ fragment. (Figure 2B). On an SphI digest, the 5′Sμ probe hybridized to an 8.5-kb aberrant fragment, which was also identified by the 3′Sμ probe. One possible explanation for the presence of a 3′Sμ aberrant fragment on an SphI but not on a HindIII digest could be that the aberrant HindIII digest fragment is either too big or too small to be seen by Southern blot. This patient sample showed 2 rearranged bands (12.0 kb and 4.8 kb) with the 5′ςμ probe on a BglII digest, while the 3′ςδ probe only detected the germline fragment. No rearranged fragment cohybridized with any of the fragments identified by downstream probes, which only identified germline fragments (data not shown). Considering the hybridization pattern of the HindIII and SphI blots, it appears that in this case only 1 IgH allele underwent aberrant recombination in the Sμ region, because only 1 rearranged fragment is present in each lane. On the contrary, the BglII hybridization pattern suggests that the aberrant switch recombination involved both IgH alleles (or that the breakpoint split the probe). It is possible to suppose that a “downstream” recombination starting at ςδ 20deleted on 1-allele sequences downstream of ςδ but maintained a germline configuration upstream of ςδ. In this situation, the 5′Sμ and the 3′Sμ probes would be able to detect only 1 rearranged allele, and the δ probes would detect the rearrangements on both alleles. Rearrangements downstream of the Cμ-Cδ genes were recently reported for IgM-expressing follicular lymphoma, and it was suggested they may reflect a tumor-specific deregulation of the class-switch machinery.22
One case of low-grade MALT lymphoma has a legitimate recombination resulting in a partial deletion of the Sμ region
In the IgM-producing case No. 3 (Figure4), the 5′Sμ and 3′Sμ probes coybridized to the germline fragment and to a fragment of 8.0 kb on a HindIII digest and of 7.6 kb on an SphI digest. 5′ςμ and 3′ςδ cohybridized to the germline and to a 15.8-kb fragment on a BglII digest. We amplified the Sμ region from tumoral genomic DNA by PCR using primers 5′MFB and 3′MRB (Table 2). As expected, we found in this sample a smaller Sμ region compared to the germline band (1200 base pairs [bp] versus 3557 bp in HSJHCMU), indicating a deletion of 2350 bp internal to the switch region. We cloned and sequenced the 1200-bp fragment and found 2 regions in germline configuration (from nucleotide 246 to 521 and from 3300 to 3803 in HSJHCMU). Therefore, the breakpoint occurred somewhere between the 2 sequenced germline regions. It has been reported that an internal deletion or rearrangement of the switch region can occur in IgM-producing B cells to stabilize the isotype.23 This might explain the Sμ deletion observed in this case.
Southern blot analysis of switch μ/δ regions in case No. 3.
Probes for the μ and δ switch regions all cohybridized to germline fragments and to smaller rearranged fragments.
Southern blot analysis of switch μ/δ regions in case No. 3.
Probes for the μ and δ switch regions all cohybridized to germline fragments and to smaller rearranged fragments.
Two cases of low-grade MALT lymphoma do not show switch rearrangements
Cases No. 4 and No. 5 showed a hybridization pattern identical to that of placental DNA (data not shown).
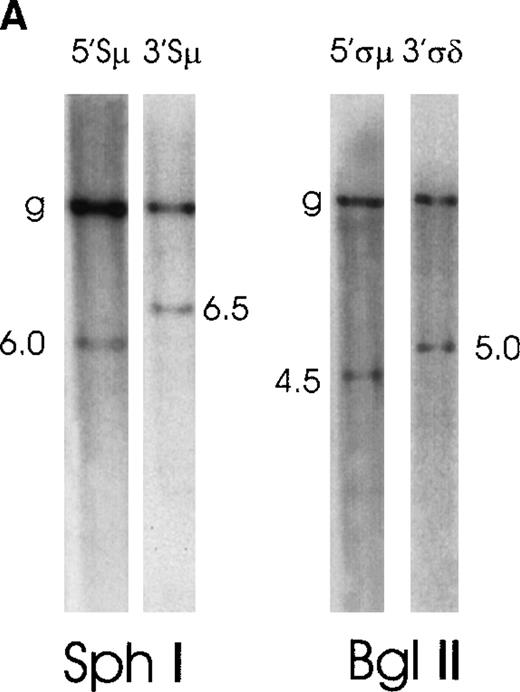
The 2 cases of high-grade MALT lymphoma have rearrangements in Sμ and 1 of them also in downstream switch regions
Case No. 6, positive for IgM production, showed on an SphI digest, in addition to the germline fragment, a 5′Sμ rearranged fragment of 6.0 kb and a 3′Sμ rearranged fragment of 6.5 kb (Figure 5A). Furthermore, on a BglII digest, the 5′ςμ and 3′ςδ probes respectively hybridized to a 4.5-kb and a 5.0-kb rearranged fragment. Downstream probes showed no switch rearrangements. This illegitimate switch recombination may possibly be a chromosomal translocation involving the Sμ region. The 5′Sμ probe would hybridize to the telomeric 14q32, which moved to an unknown chromosome, and the 3′Sμ probe would hybridize to the der(14q32).
Southern blot analysis of switch regions in 2 patients with “high-grade” MALT lymphoma.
(A) Case No. 6 shows 2 illegitimate switch fragments identified by the 5′Sμ probe and by the 3′Sμ probe on an SphI digest. Two additional illegitimate switch fragments are identified by the 5′ςμ and the 3′ςδ probes on a BglII digest. (B) Case No. 7 presents 1 illegitimate 3′Sμ fragment, 3 5′Sγ fragments (2 of them cohybridize with the 3γ probe), and 1 3′Sα fragment, all on HindIII digest.
Southern blot analysis of switch regions in 2 patients with “high-grade” MALT lymphoma.
(A) Case No. 6 shows 2 illegitimate switch fragments identified by the 5′Sμ probe and by the 3′Sμ probe on an SphI digest. Two additional illegitimate switch fragments are identified by the 5′ςμ and the 3′ςδ probes on a BglII digest. (B) Case No. 7 presents 1 illegitimate 3′Sμ fragment, 3 5′Sγ fragments (2 of them cohybridize with the 3γ probe), and 1 3′Sα fragment, all on HindIII digest.
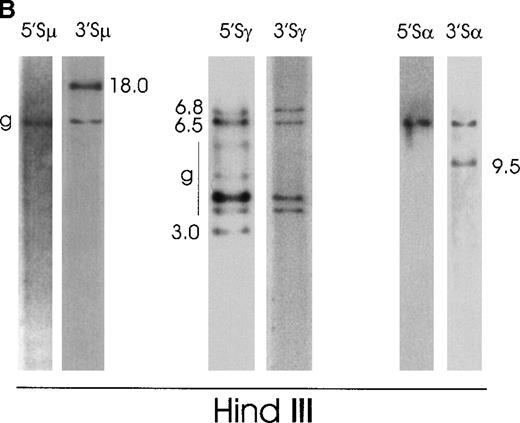
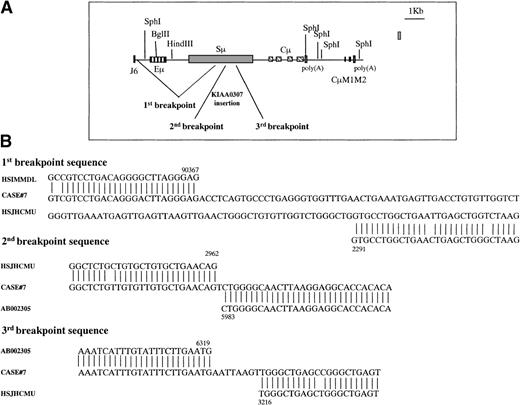
Case No. 7 (Figure 5B) showed on a HindIII digest multiple rearranged fragments: one 3′Sμ fragment of 18.0 kb, one 3′Sα fragment of 9.5 kb, and three 5′Sγ fragments of 6.8 kb, 6.5 kb, and 3.0 kb, only 2 of which (6.8 kb and 6.5 kb) cohybridized with 3′Sγ fragments. We decided to clone the 18.0-kb 3′Sμ aberrant fragment. Because this fragment was very large, we performed additional blots with numerous restriction enzymes and identified, on an EcoRI digest, a 3′Sμ rearranged fragment of 5.0 kb, which was a good candidate to be cloned. We amplified this fragment by long-distance inverse PCR using primers (3MFA, 3MRA, 3MFB, 3MRB) (Table2) in the 3′Sμ region. We obtained a 4.5-kb band, which was cloned and sequenced. The sequence showed a V3-43/D/JH3 rearrangement followed by a deletion of the IgH intronic enhancer (Eμ) starting from position 90 367 in HSIMMDL (downstream of JH6) to the Sμ region (2291 in HSJHCMU) (Figure 6A). Moreover, there was an insertion of a 336-bp region from the recently cloned gene KIAA0307 (5983-6319) into the Sμ region with subsequent deletion of the Sμ sequence from position 2962 to 3216 in HSJHCMU. Sequences of the 3 breakpoints are reported in Figure 6B. To confirm this IgH enhancer deletion, we performed a PCR on tumoral and placental genomic DNA with a forward primer in the JH region (JHF) and a reverse primer (3MRB) in the Sμ region (Table 2). This PCR produced a 3.0-kb band in the tumor versus a 6.0-kb product in placenta. It is possible to assume that the IgH enhancer (90 650-91 231 in HSIMMDL) was deleted from the cell or that it moved somewhere else in the genome, where, as previously reported for mouse plasmacytoma,24 it might dysregulate an oncogene. As far as the 3 Sγ illegitimate switch fragments (6.8 kb, 6.5 kb, and 3.0 kb) are concerned, it is likely that they are extra Cγ polymorphic alleles.17 22 The 3.0-kb band that is identified only with the 5′Sγ probe but not with the 3′Sγ probe could be an extra polymorphic Cγ allele in which HindIII cuts within the 5′Sγ probe, thus giving rise to 2 germline bands.
Graphic representation of the 3 breakpoints in case No. 7.
(A) The 3′Sμ aberrant fragment was amplified by long-distance inverse PCR. Genomic DNA from case No. 7 was digested with EcoRI, was self-ligated, and long-distance inverse PCR was performed using 4 primers (3MFA, 3MRA, 3MFB, 3MRB) internal to the 3′Sμ region. The 3 breakpoints are generated by a deletion from downstream JH6 to the middle of the Sμ region and by an insertion of a chromosome 15 gene (KIAA0307) into the Sμ region. Eμ = intronic enhancer, Sμ = switch μ region, Cμ = constant μ exons, M1, M2 = exons for membrane form. (B) Alignment of the breakpoint regions of case No. 7 with germline GenBank sequences. Numbers indicate the nucleotide position at which the breakpoint occurred within the corresponding GenBank sequence. These sequences are available from GenBank under accession numbers AF156 533, AF156 534, and AF156 535. HSIMMDL = GenBank DNA sequence of the human immunoglobulin D segment locus, HSJHCMU = GenBank nucleotide sequence of the IgH Sμ region, AB002 305 = GenBank nucleotide sequence of human mRNA for KIAA0307 gene.
Graphic representation of the 3 breakpoints in case No. 7.
(A) The 3′Sμ aberrant fragment was amplified by long-distance inverse PCR. Genomic DNA from case No. 7 was digested with EcoRI, was self-ligated, and long-distance inverse PCR was performed using 4 primers (3MFA, 3MRA, 3MFB, 3MRB) internal to the 3′Sμ region. The 3 breakpoints are generated by a deletion from downstream JH6 to the middle of the Sμ region and by an insertion of a chromosome 15 gene (KIAA0307) into the Sμ region. Eμ = intronic enhancer, Sμ = switch μ region, Cμ = constant μ exons, M1, M2 = exons for membrane form. (B) Alignment of the breakpoint regions of case No. 7 with germline GenBank sequences. Numbers indicate the nucleotide position at which the breakpoint occurred within the corresponding GenBank sequence. These sequences are available from GenBank under accession numbers AF156 533, AF156 534, and AF156 535. HSIMMDL = GenBank DNA sequence of the human immunoglobulin D segment locus, HSJHCMU = GenBank nucleotide sequence of the IgH Sμ region, AB002 305 = GenBank nucleotide sequence of human mRNA for KIAA0307 gene.
Discussion
In this study, 7 cases of gastric MALT lymphoma, 5 low grade and 2 high grade, were analyzed for the presence of genomic rearrangements within IgH switch regions. Using a Southern blot assay capable of distinguishing between legitimate and illegitimate switch recombinations, it was found that 3 out of 5 low-grade and 2 out of 2 high-grade gastric MALT lymphomas displayed single or multiple aberrant recombination within or near the switch regions. Two cases of low grade (Nos. 1 and 2) and 1 of high grade (No. 6) had illegitimate switch recombination events involving the Sμ region. One case of high grade (No. 7) had illegitimate switch recombination events involving Sμ and the downstream switch regions. One case of low grade (No. 3) showed a partial deletion of the Sμ region. The molecular cloning of the rearranged switch fragments confirmed the aberrant nature of the recombination event that generated the fragments. In case No. 2, we found a deletion from the Sμ region to the first Cμ exon and a second deletion from the second Cμ exon to γ3 sequences. Therefore, because there is clear involvement of the Sμ region, the first breakpoint was generated by an aberrant switch recombination, while the second breakpoint, which does not involve any switch region, might have been generated by an aberrant recombination other than a switch recombination. In case No. 7, we found a deletion of the intronic enhancer (Eμ) from the JH region to the Sμ region and an insertion of a chromosome 15 gene into the Sμ region. This double event, involving the Sμ region, has been clearly mediated by an aberrant switch recombination. We believe that these aberrant fragments were generated by a mistake of the cell during an attempt to undergo a physiologic isotype switch recombination, but we are not able to define if this aberrant recombination occurred before or after the onset of malignancy. In the first case, it would be involved in the development of the tumor, in the second case in the progression.
The presence of illegitimate switch recombination events in IgM-producing lymphoma cells indicates that MALT lymphoma cells reached a maturation stage where isotype switch recombination could occur. Many B-cell malignancies produce errors during the developmentally regulated processes of VDJ recombination and isotype switch recombination.25,26 By analyzing IgH locus rearrangements within the VDJ or switch regions and by considering the status of VDJ hypermutation, it is possible to estimate the stage of B-cell development at which malignant transformation occurred.27For example, mantle cell lymphoma, a tumor of virgin surface IgM+ B cells, does not show VDJ hypermutation or switch recombination and is characterized by a recurrent t(11;14)(q13;q32) occurring within JH regions adjacent to heptamer-nonamer recognition sequences.28,29 Follicular lymphoma, deriving from surface IgM+ B cells, shows ongoing VDJ hypermutations and is characterized by a typical t(14;18)(q32;q21) occurring within JH exons.30-32 In Burkitt's lymphoma, there is evidence of ongoing VDJ somatic mutations. Furthermore, these tumors are characterized by a t(8;14)(q24;q32) with breakpoints within JH exons (endemic form) or within Sμ regions (sporadic form).33-35 Finally, multiple myeloma is a tumor of mature plasma cells that clearly undergo VDJ somatic mutations and isotype switch recombination. Typical transclocations are promiscuous and may involve all switch regions.17 MALT lymphoma cells show VDJ hypermutations that can be ongoing15 16 and, as we have demonstrated in this work, can undergo IgH switch recombination. We suggest that IgM+ MALT lymphoma cells that do not have evidence of a switch recombination possibly originate from a less-differentiated B cell.
Our experiments additionally demonstrate that the frequency of IgH switch rearrangements, other than normal class switching, has been grossly underestimated by other studies. The Southern blot assay allowed us to estimate that illegitimate switch recombination is a frequent event in MALT lymphomas.
Moreover, despite the limited number of patients, the presence of illegitimate switch recombination in low-grade lymphomas appeared to be associated with clinical outcome. Cases without evidence of illegitimate switch recombination had a shorter median disease-free time after gastrectomy (6 months) than those with illegitimate switch recombinations (up to 56 months).
As recently shown by Willis et al,11 cloning of a t(1;14)(p22;q32) translocation breakpoint from 1 case of MALT lymphoma allowed for the identification of a new gene, Bcl10, which is also altered in other tumor types. It was later reported by Zhang et al36 that Bcl10, a novel apoptotic signaling gene that encodes an amino-terminal caspase recruitment domain, is overexpressed and truncated in MALT tumors carrying the t(1;14)(p22;q32). Mutant Bcl10 overexpression would provide anti-apoptotic and proliferative signals and confer a survival advantage to MALT B cells. It is likely that, as for the investigation of the t(1;14), the identification of illegitimate switch recombination fragments in MALT lymphoma will give insights into the potential pathogenic role of new dysregulated genes. Then, based on the presence or absence of illegitimate switch recombination events, it might be possible in the future to divide MALT lymphomas into subtypes that may have different clinical courses or therapeutic sensitivities.
Acknowledgment
We thank Dr P. L. Bergsagel for manuscript revision, Drs A. Parazzoli and M. Du for technical support, Drs T. Dragani, R. G. Nador, and A. Cerutti for helpful suggestions, Mrs L. Mameli for manuscript preparation, and Mr M. Azzini for photographic reproduction.
Partially supported by Associazione Italiana Ricerca Cancro (AIRC).
Reprints:Andrea Balsari, Chair of Immunology, c/o Molecular Targeting Unit, Department of Experimental Oncology, National Cancer Institute, Via Venezian 1, 20 133 Milan, Italy; e-mail:balsari@istitutotumori.mi.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal