Loxosceles is the most venomous spider in Brazil, and envenomation causes dermonecrosis and complement (C)-dependent intravascular hemolysis. The authors studied the mechanism of induction of C-induced hemolysis. Purified Loxosceles toxins rendered human erythrocytes susceptible to lysis by human C but did not have an effect on the E-bound C-regulators DAF, CR1, or CD59. However, incubation with venom toxins caused cleavage of glycophorin from the erythrocyte (E) surface, facilitating C activation and hemolysis. The results suggest that glycophorin is an important factor in the protection of E against homologous C. Cleavage of glycophorin (GP) A, GPB, and GPC occurred at sites close to the membrane but could not be accomplished using purified GPA and purified toxins, demonstrating that cleavage was not an effect of a direct proteolytic action of theLoxosceles toxins on the glycophorins. Inhibition of the cleavage of glycophorins induced by Loxosceles venom was achieved with 1,10-phenanthroline. The authors propose that the sphingomyelinase activity of the toxins induces activation of an endogenous metalloproteinase, which then cleaves glycophorins. They observed the transfer of C-dependent hemolysis to other cells, suggesting that the Loxosceles toxins can act on multiple cells. This observation can explain the extent of hemolysis observed in patients after envenomation. Identification of the mechanism of induction of susceptibility to C-mediated lysis afterLoxosceles envenomation opens up the possibility of the development of an effective therapeutic strategy.
Envenomation by spiders of the genusLoxosceles, found in temperate and tropical regions of North and South America, Africa, and Europe, commonly results in impressive local necrotic skin lesions and, more rarely, causes systemic effects, including profound intravascular hemolysis .1-8 The predominant clinical sign is a cutaneous reaction characterized by the appearance of necrosis around the bite, resulting in ulceration. Healing of the ulcer often requires months. The scale of these lesions is remarkable considering that the spider injects only a few tenths of a microliter of venom containing no more than 30 μg protein. Mild systemic effects induced by envenomation, such as fever, malaise, pruritus, and exanthema are common, whereas intravascular hemolysis and coagulation, sometimes accompanied by thrombocytopenia and renal failure, occur in approximately 16% of those bitten.1-8Loxosceles is the most poisonous spider in Brazil, and children who have the more severe systemic effects after envenomation nearly always die. At least 3 different Loxosceles species of medical importance are known in Brazil (L. intermedia, L. gaucho, L. laeta), and more than 1500 cases of envenomation by L. intermedia alone are reported each year. In the United States, at least 6 Loxosceles species (including L. reclusa, the brown recluse) are known to cause numerous incidents.4,6 8Because of a lack of understanding of the venom's mechanism of action, effective treatment is unavailable. Biochemical and functional characterization of the active components in the venom may aid the development of a suitable therapy.
We have recently identified and characterized the toxins from L. intermedia venom that are responsible for all the local and systemic effects induced by whole venom.9,10 Two highly homologous proteins with Mr 35 kd were purified to homogeneity and were shown to be endowed with sphingomyelinase activity. These proteins, termed P1 and P2, induced dermonecrosis in experimental animals and rendered human erythrocytes susceptible to lysis by C in vitro. In a mouse model of Loxosceles envenomation,11 we showed that the toxins also induced intravascular hemolysis and provoked a cytokine response resembling that seen in endotoxic shock.8The spider toxins P1 and P2, which probably originated by gene duplication, showed a high level of homology with two other molecules in the spider venom. The latter molecules, named P3 and P4—despite the high degree of homology in N-terminal amino acid sequence with P1 and P2—were ineffective in all assays of activity.10 The mechanism by which a single molecule displaying only sphingomyelinase activity can induce such a wide variety of local and systemic effects is the subject of this study.
We have shown that the E lysis induced by venom is dependent on the activation of C by an alternative pathway.9 The toxins P1 and P2, unlike some other sphingomyelinases, do not directly induce E lysis, but they do render cells susceptible to C lysis. Acquisition of C-activating capacity or loss of C regulation by treated E leads to the deposition of C fragments, including C3b and factor B, C3-convertase assembly, and membrane attack complex (MAC) formation, with hemolysis as the final outcome.9 Erythrocytes are protected against lysis by their own C by a number of specialized regulators of C (CR).12,13 A high level of expression of the regulators of the C3/C5 convertases decay accelerating factor (DAF) and C receptor 1 (CR1) and the regulator of the MAC, CD59 ensures the survival of E in vivo. The importance of CR is illustrated by the spontaneous occurrence of hemolysis in patients with paroxysmal nocturnal hemoglobinuria (PNH).14-17 In PNH, because of a clonal defect in the anchorage of glycosyl phosphatidylinositol-anchored molecules, DAF and CD59 are not expressed on the surface of erythrocytes.14Although CR1 is expressed in normal amounts, DAF and CD59 deficiencies render these PNH E susceptible to C-dependent lysis. The importance of DAF, and of CD59 in particular, in the protection against C is also demonstrated by the sensitivity to C lysis of human E after the blocking of DAF and CD59 by monoclonal antibodies (mAb) or biotin–avidin cross-linking.18 Other factors have been shown to protect erythrocytes against lysis by homologous C, including surface carbohydrates.19-25 Glycophorins, heavily glycosylated proteins that are the most abundantly expressed molecules on E, have been shown to act as inhibitors of C-deposition, a consequence of the presence of high amounts of sialic acid in the structures.20,21,22 Removal of sialic acid by neuraminidase results in an enhanced susceptibility of E to C lysis as a consequence of the reduction of binding of factor H (fH; cofactor for factor I in the degradation of C3b) to surface-bound C3b.23,24Furthermore, it has been shown that alteration in the lipid composition of membranes can affect the susceptibility to C.25
The aim of this study was to elucidate the precise mechanism by which P1 and P2 toxins from Loxosceles intermedia venom induce C-susceptibility in E. Human E, treated with toxins, were examined for the expression of CR, DAF, CD59, and CR1. No change in expression was observed, which eliminated the possibility that C-susceptibility was induced by the removal of these proteins by the spider toxins. However, toxin treatment of E caused cleavage of the extracellular portions of glycophorins (GP) A, GPB, and GPC. As a consequence, C3b deposition was enhanced and was followed by the activation of terminal pathway and C5b-C9 lytic complex formation, with hemolysis as the final outcome. Indeed, the removal of sialic acid by neuraminidase had the same effect on C susceptibility as treatment of E with the spider toxins.
Materials and methods
Chemicals, reagents, and buffers
Tween 20, bovine serum albumin (BSA), human GPA, neuraminidase, dimethylsulfoxide, 1,10 phenanthroline, and phenylmethylsulfonyl fluoride (PMSF) were purchased from Sigma (St. Louis, MO). Calcein-AM was from Molecular Probes (Cambridge, UK). Supersignal chemiluminescent developer was from Pierce Chemical (Rockford, IL). Buffers were veronal-buffered saline (VBS2+), pH 7.4, containing 10 mmol/L Na barbitone, 0.15 mmol/L CaCl2, and 0.5 mmol/L MgCl2; PBS containing 10 mmol/L Na phosphate and 150 mmol/L NaCl, pH 7.2; and FACS buffer containing 1% PBS, 0.01% BSA, and sodium azide.
Antibodies
The following mAbs were all from International Blood Group Reference Laboratory (IBGRL, Bristol, UK): mAb against DAF (Bric216), against CD59 (Bric229), against GPA (Bric256, recognize extracellular part epitope aa 41-58; Bric163, recognize an intracellular epitope; TM region aa 73-95), and against GPC (Bric4, extracellular epitope aa 16-23; Bric10, extracellular epitope aa 1-6; BGRL 100, intracellular epitope aa 112-128; TM region, 59-81).26 Anti-GPB was from Sigma Chemical. Monospecific rabbit polyclonal sera against CR1, C3, and C8 were produced in-house. RAM/IgG–fluorescein isothiocyanate (FITC) was from Dako (High Wycombe, Bucks, UK), GAM/IgG-HRP was from Bio-Rad (Hemel, Hempstead, UK), and GAR/IgG-FITC was from Sigma Chemical.
Venom
Laboratório de Imunoquı́mica (Instituto Butantan, São Paulo, Brazil) provided Loxosceles intermediaMello-Leitão spiders. The venom was obtained by electrostimulation by the method of Bucherl,27 with slight modifications. Briefly, electrical stimuli of 15 to 20 V were repeatedly applied to the spider sternum, and the venom drops were collected with a micropipette, vacuum dried, and stored at −20°C. Stock solutions were prepared in PBS at 1 mg/mL. Toxins (P1, P2, and P3) from L. intermedia were purified by Superose 12-gel filtration followed by reverse-phase high-performance liquid chromatography using a Wide-Pore Butyl C4 column (Pharmacia, Uppsala, Sweden) as described.10 The protein content of the samples was evaluated by the Lowry method.28
Production of rabbit antiserum against F35
Adult rabbits were injected intradermally with 500 ng of F35 (unfractionated P1, P2, P3)9 absorbed to Al(OH)3. Injections were repeated 4 times at weekly intervals. Blood samples were collected 1 week after the last injection, and the serum was stored at −20°C.
Normal human serum and erythrocytes
Human blood was obtained from healthy donors. Blood samples drawn to obtain sera were collected without anticoagulant and allowed to clot for 2 hours at room temperature, and the normal human serum (NHS) was stored at −80°C. C8-depleted human serum (C8d-HS) was obtained by the passage of NHS over an mAb anti-C8 Sepharose 4B column. Blood samples drawn to obtain E for subsequent use as target cells were collected in anticoagulant (Alsever old solution: 114 mmol/L citrate, 27 mmol/L glucose, 72 mmol/L NaCl, pH 6.1).
Treatment of E with Loxosceles venom proteins
E were washed and resuspended at 2% in VBS2+ and incubated with whole venom or purified fractions for 30 minutes at 37°C. Control samples were incubated with VBS2+. Purified fractions did not induce spontaneous lysis of the cells. The cells were washed 5 times, resuspended to the original volume in VBS2+, analyzed in a hemolysis assay, and prepared for flow cytometry or Western blot analysis. For Western blotting, E ghosts were prepared by lysis of E in water. Ghosts were pelleted by centrifugation (14 000g for 20 minutes at 4°C) and washed with water.
Treatment of E with neuraminidase
One milliliter 2% HuE suspension was incubated with 0.2 U of neuraminidase for 1 hour at 37°C. Cells were washed 5 times and resuspended to the original volume in VBS2+ and assayed as described.
Treatment of purified glycophorin A with L. intermedia venom toxins
Purified GPA (2 μg) was incubated with VBS2+, theL. intermedia venom, or the purified toxins P1, P2, and P3 (2 μg each) in a total volume of 20 μL in VBS2+ at 37°C for 60 minutes. Samples were run on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Western blotting.
Hemolysis assays
One hundred microliters 2% E pretreated with Loxosceles venom, purified toxin P1, P2, or P3, and neuraminidase or VBS2+were mixed with 100 μL NHS (1/2 in VBS2+). Background or total cell lysis was evaluated by incubation of E with VBS2+ or H2O respectively. After incubation for 1 hour at 37°C, unlysed cells were spun down; the absorbance of the supernatant was measured at 541 nm and expressed as a percentage of lysis. Mean and SD were determined from duplicate samples. E and NHS were always from the same donor.
Calcein-AM loading of E
E were washed, resuspended at 2% in VBS2+ containing 1/200 dilution of calcein-AM (1 mg/mL stock in dimethyl sulfoxide), and incubated for 30 minutes at 37°C. The cells were washed twice and resuspended to the original volume in VBS2+.
Transfer of hemolysis-inducing activity
Samples of buffer-, venom-, and toxin-treated E were mixed with the same volume of calcein-loaded E suspension or with buffer and incubated for 30 minutes at 37°C. After this period, cells were washed once, resuspended with VBS2+, and analyzed for autologous C lysis susceptibility as described above. Final lysis was measured spectrophotometrically at 541 nm and fluorometrically by measuring the calcein fluorescence of the supernatants in a Denley-Wellfluor fluorometer with the excitation filter at 488 nm and the emission filter at 530 nm. Percentage lysis for each sample was calculated as specific hemoglobin release/total hemoglobin and as specific calcein release/total calcein loading. Mean and SD were determined from duplicate samples.
Nucleated cells
The K562 (erythroblast), U937 (promonocyte), and Jurkat (T-cell) cell lines were obtained from the European Collection of Animal Cell Cultures (Porton Down, Salisbury, UK). Cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum, 4 mmol/L glutamine, 2 mmol/L sodium pyruvate, 100 IU/mL penicillin, and 100 IU/mL streptomycin at 37°C and 5% CO2.
Treatment of nucleated cells with P2 toxin
Log-phase nucleated cells were harvested, washed 3 times in PBS, and resuspended in VSB2+ at 107/mL. The cells were treated with 10 μg/mL P2 toxin for 30 minutes at 37°C. Control samples were incubated with VSB2+. The cells were washed 5 times, resuspended to the original volume in VSB2+,and prepared for analysis by flow cytometry.
Flow cytometry
E (50 μL 2%) or 50 μL 106 nucleated cells were incubated for 30 minutes at 4°C with 50 μL of 1 μg/mL anti-C regulators or anti-glycophorin mAb or with rabbit antiserum recognizing P1, P2, and P3 (F35; diluted 1:250) in FACS buffer. After they were washed, cells were incubated with RAM/IgG-FITC or GAR/IgG-FITC for 30 minutes at 4°C. Cells were washed and fixed in FACS buffer containing 1% paraformaldehyde and were analyzed by flow cytometry (FACScalibur; Becton Dickinson, San Jose, CA).
Analysis of deposition of C components
E treated with spider toxins was incubated with C8-depleted serum (1/10 in VBS2+, 30 minutes, 37°C), washed, and processed for flow cytometry using rabbit polyclonal anti-C component sera diluted 1/100 and was followed by GAR/IgG-FITC as described above.
Electrophoresis and Western blot analysis
E ghosts (10 μL) or purified GPA samples (1 μg) were solubilized in a nonreducing sample buffer and run on 12% SDS-PAGE.29Gels were stained with silver30 or blotted onto nitrocellulose. After transfer, the membranes were blocked with PBS/BSA 1% and incubated with anti-glycophorin mAb (1 μg/mL) for 1 hour at room temperature. Membranes were washed 3 times with PBS/0.05% Tween 20 for 10 minutes and incubated with GAM/IgG-HRP 1/3000) in PBS/BSA 1% for 1 hour at room temperature. After they were washed 3 times with PBS/0.05% Tween 20 for 10 minutes and twice with PBS, blots were developed using Supersignal chemiluminescent substrate (Pierce) and Kodak X-ray film (Eastman Kodak, Rochester, NY).
Pretreatment of E with protease inhibitors
E were incubated with 10 mmol/L EDTA, 5 mmol/L, 1,10-phenanthroline, 1 mmol/L PMSF, or buffer for 30 minutes on ice. Venom or toxins were added and incubated for 30 minutes at 37°C. Cells were washed 3 times and analyzed for C susceptibility or GP expression by flow cytometry.
Results
C activation induced by L. intermedia venom toxins
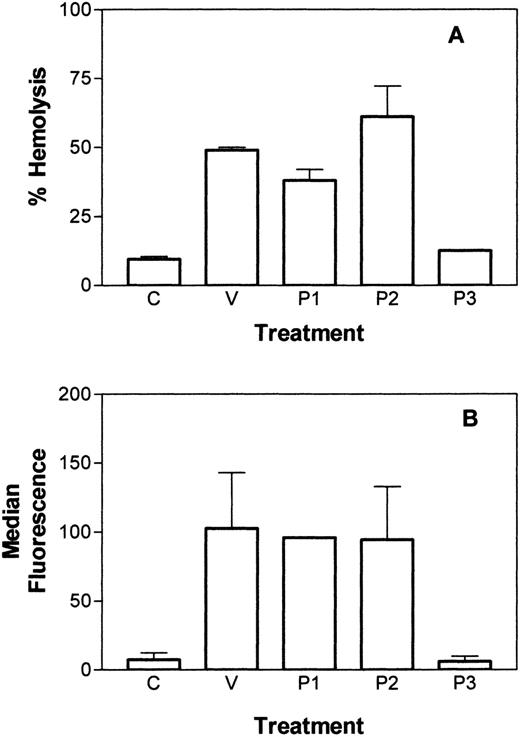
To assess the ability of L. intermedia venom to induce C-dependent hemolysis, E were incubated with 10 μg/mL L. intermedia venom or purified P1, P2, or P3 toxin and were assessed for the susceptibility to lysis by human C. As shown in Figure1A, the L. intermedia venom and the pure proteins P1 and P2, but not P3, were able to render E susceptible to lysis by autologous C. A similar level of C susceptibility was obtained after incubation of the cells with neuraminidase (data not shown). To assess the effect of the toxins on C3 deposition, toxin-treated E were incubated with C8-depleted human serum and analyzed by flow cytometry for the deposition of C3b. Figure 1B shows an increased deposition of C3b on the E treated with venom, P1, or P2, but not with P3 or buffer.
Induction of C-susceptibility by L. intermediavenom toxins.
(A) C-dependent hemolysis of E after incubation with VBS2+(C), with 10 μg/mL of L. intermedia venom (V), or with purified toxin P1, P2, or P3. (B) C3b deposition after treatment of E, followed by incubation with C8-depleted serum. Deposition was measured using polyclonal sera against C3 and analysis by flow cytometry. Results are representative of 3 different experiments carried out in duplicate and represented as mean ± SD.
Induction of C-susceptibility by L. intermediavenom toxins.
(A) C-dependent hemolysis of E after incubation with VBS2+(C), with 10 μg/mL of L. intermedia venom (V), or with purified toxin P1, P2, or P3. (B) C3b deposition after treatment of E, followed by incubation with C8-depleted serum. Deposition was measured using polyclonal sera against C3 and analysis by flow cytometry. Results are representative of 3 different experiments carried out in duplicate and represented as mean ± SD.
Effect of L. intermedia toxins on membrane-bound regulators of C
To assess whether the increased susceptibility to human C was caused by interference of the toxins with membrane regulators of C, E were analyzed for the expression of DAF, CR1, and CD59 by flow cytometry. No change in expression of any of the regulators was observed after incubation of E with whole venom or any of the purified toxins (data not shown).
Removal of glycophorin from E induced by L. intermedia venom toxins
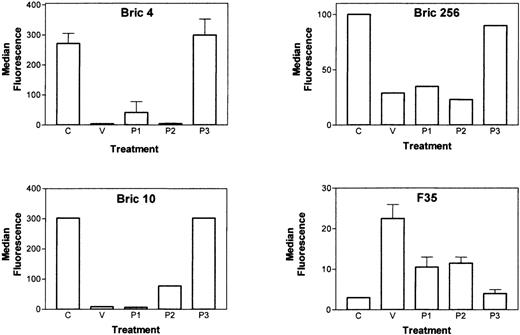
Although DAF, CR1, and CD59 are powerful inhibitors of C-mediated lysis, the abundantly expressed, heavily glycosylated E-membrane proteins known as glycophorins also contribute substantially to C resistance.19-24 E, incubated with L. intermediatoxins, were analyzed for the expression of GPA and GPC by flow cytometry. A large reduction in the binding of anti-GPA (Bric256) and anti-GPC (Bric4, Bric10) antibodies recognizing extracellular epitopes close to the membrane was observed after treatment of E with venom, P1, or P2 (Figure 2). The disappearance of these epitopes was associated with the incorporation of the toxins into E, as detected by antibody F35 (Figure 2). These data show also that P3 does not incorporate into E, which may account for its lack of toxicity.
Loxosceles venom components incorporate into E and cause loss of expression of glycophorins.
E were treated with buffer (C), L. intermedia venom, P1, P2, or P3 toxins (10 μg/mL each), and analyzed for the expression of GPA and GPC by flow cytometry using the antibodies Bric256 (GPA) and Bric4 and Bric10 (GPC). The ability of the toxins P1, P2, and P3 to insert into the E surface was analyzed using a monospecific polyclonal rabbit serum (F35) against the toxins. Results are representative of 3 different experiments and are expressed as mean of triplicates ± SD.
Loxosceles venom components incorporate into E and cause loss of expression of glycophorins.
E were treated with buffer (C), L. intermedia venom, P1, P2, or P3 toxins (10 μg/mL each), and analyzed for the expression of GPA and GPC by flow cytometry using the antibodies Bric256 (GPA) and Bric4 and Bric10 (GPC). The ability of the toxins P1, P2, and P3 to insert into the E surface was analyzed using a monospecific polyclonal rabbit serum (F35) against the toxins. Results are representative of 3 different experiments and are expressed as mean of triplicates ± SD.
Analysis of the cleavage of glycophorins induced by active venom toxins
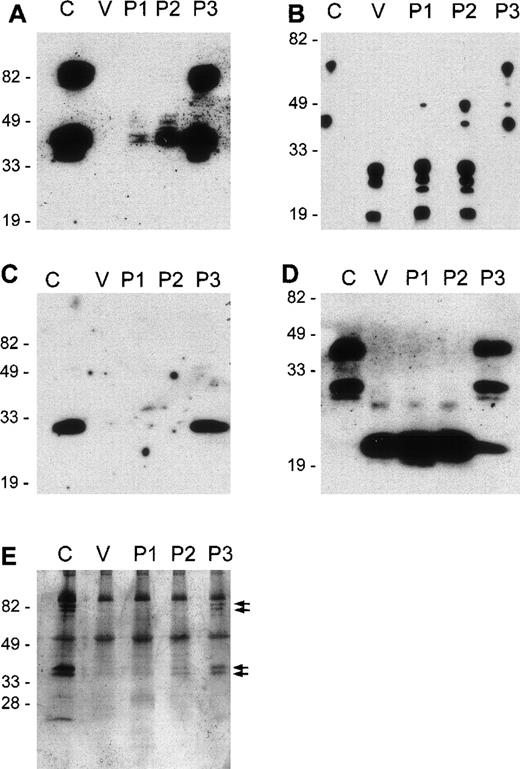
To assess whether removal of the GP epitopes resulted from the cleavage of GP or from complete extraction, E were treated with venom toxins, and, after washing, ghosts were prepared by hypotonic lysis. E ghosts were analyzed by Western blot using mAb recognizing intracellular and extracellular epitopes of GPA, GPB, and GPC. As shown in Figure 3, incubation of E with whole venom or toxins P1 and P2 resulted in the cleavage of all glycophorins.
Cleavage fragments of GPA, GPB, and GPC remain on E ghosts after treatment with L. intermedia venom toxins.
E were treated with VBS2+ (C) or with 10 μg/mL of L. intermedia venom (V) or purified toxins P1, P2, or P3. E ghosts were prepared and run on 12% SDS-PAGE under nonreducing conditions (A to D) or were stained with silver (E). Western blots: A, GPA extracellular epitope; B, GPA intracellular epitope; C, GPB extracellular epitope; D, GPC intracellular epitope.
Cleavage fragments of GPA, GPB, and GPC remain on E ghosts after treatment with L. intermedia venom toxins.
E were treated with VBS2+ (C) or with 10 μg/mL of L. intermedia venom (V) or purified toxins P1, P2, or P3. E ghosts were prepared and run on 12% SDS-PAGE under nonreducing conditions (A to D) or were stained with silver (E). Western blots: A, GPA extracellular epitope; B, GPA intracellular epitope; C, GPB extracellular epitope; D, GPC intracellular epitope.
Western blot analysis revealed 2 bands in GPA, a monomeric form of 41 kd and a dimer with an Mr of 82 kd. Using antibodies that recognize an extracellular epitope in GPA, Western blotting showed that on incubation with venom toxins, a nearly complete loss of this epitope was induced (Figure 3A). Using an mAb recognizing an intracellular epitope of GPA, 2 bands again were observed, but on incubation of the E with venom toxins, a large reduction in Mr of the bands was observed (Figure 3B). This shows that the intracellular epitope was retained in the E and that the loss of the extracellular epitope was caused by the cleavage of GPA rather than by extraction of the whole molecule. In this case, venom or P1 and P2 treatment resulted in multiple GPA fragments of GPA ranging from 19 to 27 kd. The occurrence of multiple bands may have been caused by the cleavage of GPA at multiple sites. Some bands may also have represented dimers of cleaved GPA fragments.
GPB ran as a single band on Western blotting and was detected using an mAb against an extracellular epitope (Figure 3C). This epitope completely disappeared on incubation of E with venom toxins. An mAb against an intracellular epitope of GPB was unavailable.
GPC runs as a monomer (Mr, 44 kd) on SDS-PAGE (Figure 3D). The N-terminal truncated form of GPC, GPD is also detected as a monomer of 27 kd in the gel. Using an mAb recognizing an intracellular epitope of GPC, it was shown that on the incubation of E with venom, P1, or P2, but not P3, these bands disappeared and a single band with an Mr of 20 kd was observed. This band represented the transmembrane and cytoplasmic fragment of GPC/GPD retained in the ghosts (Figure 3D). These data demonstrate that glycophorins are cleaved and are not extracted on venom toxin incubation.
Silver staining of the gels showed equal loading in all lanes, indicating that the absence of GP bands on Western blots did not result from the loss of venom-treated cells during ghost preparation (Figure3E). Silver staining also showed the loss of some bands with Mr 40 to 42 kd and 80 to 82 kd on venom toxin-treated E (indicated by arrows), which most likely represented GPA (Figure 3E). Because of their abundance in E and of heavy glycosylation, GPs are strongly stained by silver on SDS-PAGE.
GPA cleavage is not caused by a direct proteolytic action of L. intermedia toxins
Toxins P1 and P2 have been shown only to have sphingomyelinase activity.10 To analyze whether GP cleavage resulted from direct proteolytic action of toxins on glycophorins, purified GPA was incubated with whole venom or purified toxins. Samples were submitted to nonreducing SDS-PAGE, followed by Western blot analysis, using the mAb recognizing the intracellular domain of GPA. Figure4 shows that cleavage of GPA was not induced. Increases in incubation time or toxin content of the samples did not result in any alteration in the mobility and banding patterns of GPA (data not shown). These results show that P1 and P2 are not specific glycophorin proteases, and they suggest that the GP cleavage process is caused by the activation of an endogenous E protease induced by P1 and P2 toxins.
Loxosceles venom and purified toxins do not cleave purified glycophorin A.
Purified GPA (2 μg) was incubated with buffer (C) or with 2 μg ofL. intermedia venom (V), P1, P2, or P3 toxins for 60 minutes at 37°C. Samples were separated by SDS-PAGE under nonreducing conditions (12% gel) and analyzed by Western blot using the monoclonal antibody Bric163 .
Loxosceles venom and purified toxins do not cleave purified glycophorin A.
Purified GPA (2 μg) was incubated with buffer (C) or with 2 μg ofL. intermedia venom (V), P1, P2, or P3 toxins for 60 minutes at 37°C. Samples were separated by SDS-PAGE under nonreducing conditions (12% gel) and analyzed by Western blot using the monoclonal antibody Bric163 .
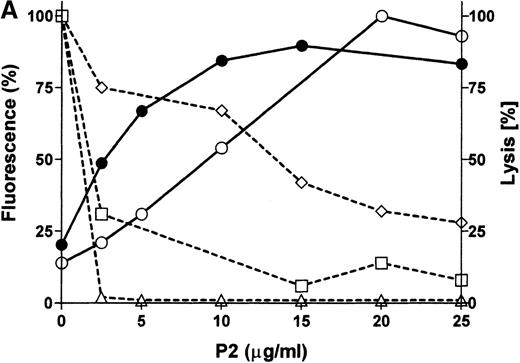
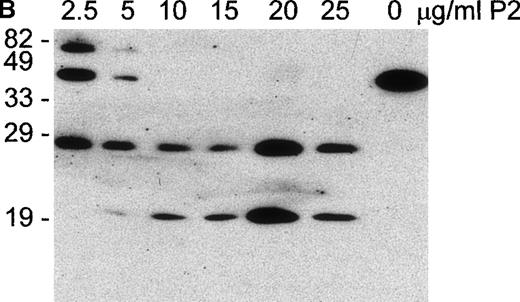
P2 incorporation correlates with glycophorin cleavage and C susceptibility
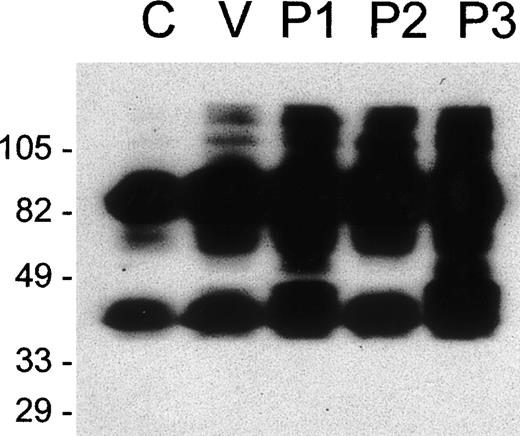
E were treated with increasing concentrations of P2 toxin and were analyzed by flow cytometry for the expression of GPA, GPB, and GPC, for the incorporation of P2 into the E membrane, and for C susceptibility. P2 toxin became incorporated into E in a dose-dependent manner and reached a maximal incorporation at approximately 20 μg/mL (equivalent to the addition of 3.4 × 106 molecules per cell; Figure 5A). Concomitant with the incorporation of P2, a decrease in the expression of GPA, GPB, and GPC and an increase in C susceptibility were observed. The disappearance of the GPB epitope was slow, possibly reflecting a relative resistance of GPB to cleavage. Western blot analysis of E ghosts showed that GPA was already hydrolyzed in the presence of 2.5 μg/mL of the toxin (Figure5B). Complete fragmentation of GPA was achieved within 30 minutes in E preparations treated with 10 μg/mL of the toxin P2 (Figure 5B). In this experiment, only 2 fragments of GPA were obtained, likely representing monomeric and dimeric forms of the transmembrane portion and cytoplasmic tail, suggesting that in this experiment cleavage was induced at only 1 site. These experiments showed a positive correlation between the cleavage of GPA, GPB, and GPC and increased C susceptibility induced by increasing amounts of E-bound P2 toxin.
P2 incorporates into E and causes dose-dependent cleavage in glycophorin expression and hemolytic susceptibility.
(A) E were treated with various concentrations of P2 and analyzed by flow cytometry using the mAb for GPA (□), GPB (⋄), and GPC (▵) or the polyclonal serum F35 against P2 (○). Cells were also subjected to C in a hemolysis assay (•). (B) E ghosts obtained from samples described above and were prepared and analyzed by Western blot using the monoclonal antibody against the intracellular portion of GPA (Bric163).
P2 incorporates into E and causes dose-dependent cleavage in glycophorin expression and hemolytic susceptibility.
(A) E were treated with various concentrations of P2 and analyzed by flow cytometry using the mAb for GPA (□), GPB (⋄), and GPC (▵) or the polyclonal serum F35 against P2 (○). Cells were also subjected to C in a hemolysis assay (•). (B) E ghosts obtained from samples described above and were prepared and analyzed by Western blot using the monoclonal antibody against the intracellular portion of GPA (Bric163).
Transfer of hemolysis-inducing activity
Although the bite of the L. intermedia spider results in the secretion of only a fraction of a microliter of venom containing not more than 30 μg toxin, in incidents of systemic effects extensive intravascular hemolysis is observed.1-8 The low amount of toxin injected could not account for the large number of erythrocytes lysed unless the toxins can transfer from 1 cell to the other in vivo and hence have an effect on many erythrocytes. To test this hypothesis, venom toxin-treated E were mixed with untreated E. After incubation, the mixtures were assessed for their susceptibility to C. To distinguish between lysis of E incubated with venom toxins and the freshly added untreated E, the untreated E were labeled with the fluorescent dye calcein. Lysis of the untreated E could then be measured as the release of the entrapped calcein, whereas lysis of the treated and untreated E was measured as the release of hemoglobin (Hb). Venom-treated and untreated E were mixed 1:1 so that an Hb release of more than 50% would indicate that untreated E were also lysed. Figure6A shows the hemolysis of the total E. Nearly 100% release of hemoglobin was obtained at the highest dose of P2, demonstrating that the erythrocytes that had not been incubated with toxins were lysed. This was confirmed by the observation that nearly 100% of the entrapped calcein could be released from the E that had not been treated with toxins (Figure 6B). The cells were also analyzed by flow cytometry for the cleavage of GPA, and, as shown in Figure 6C, in the P2-treated E and in the mixture of P2-treated and untreated E, a similar pattern of reduction of GPA expression was induced. These results show that hemolysis-inducing activity can be transferred to a new erythrocyte population and that this phenomenon can explain the extent of the systemic hemolysis observed after envenomation. The sensitivity of detection of P2 by flow cytometry was not adequate to observe the actual transfer of the toxin.
Transfer of hemolysis-inducing and glycophorin-cleaving activity.
E were treated with increasing concentrations of P1 (•), P2 (○), orL. intermedia venom (*), washed, and incubated with the same volume and number of calcein-AM loaded E or buffer. After 30 minutes of incubation at 37°C, cells were analyzed for autologous C lysis (A, B) or for the expression of GPC (C). (A) Total hemoglobin release induced by NHS determined spectrophotometrically at 541 nm. (B) Release of entrapped calcein determined fluorometrically. [C] E samples incubated with buffer or with P2 (2.5 and 5.0 μg/mL) were incubated with the same volume of untreated E (□) or with buffer (▪) for 30 minutes at 37 °C and analyzed by flow cytometry for expression of GPC using the monoclonal antibody Bric4. Results are expressed as the percentage of fluorescence of untreated E.
Transfer of hemolysis-inducing and glycophorin-cleaving activity.
E were treated with increasing concentrations of P1 (•), P2 (○), orL. intermedia venom (*), washed, and incubated with the same volume and number of calcein-AM loaded E or buffer. After 30 minutes of incubation at 37°C, cells were analyzed for autologous C lysis (A, B) or for the expression of GPC (C). (A) Total hemoglobin release induced by NHS determined spectrophotometrically at 541 nm. (B) Release of entrapped calcein determined fluorometrically. [C] E samples incubated with buffer or with P2 (2.5 and 5.0 μg/mL) were incubated with the same volume of untreated E (□) or with buffer (▪) for 30 minutes at 37 °C and analyzed by flow cytometry for expression of GPC using the monoclonal antibody Bric4. Results are expressed as the percentage of fluorescence of untreated E.
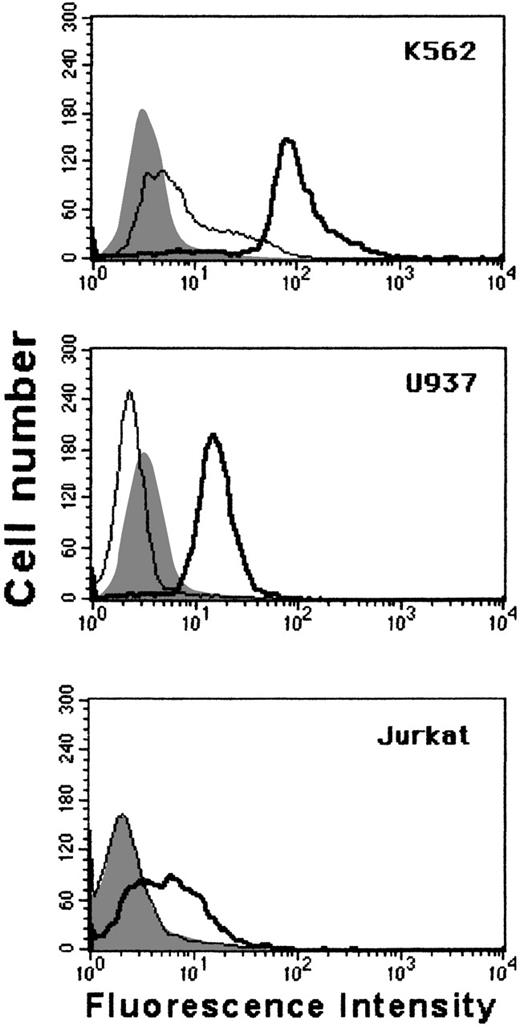
Removal of extracellular domains of GPC from the surface of nucleated cells
Although GPA is predominantly expressed on cells of the erythrocyte lineage, GPC is expressed on a wide range of cells. To establish whether glycophorin cleavage could occur in cells other than E, 3 different nucleated cell types—K562 (erythroid), U937 (myeloid), and Jurkat (lymphoid)—were incubated with L. intermedia toxins and analyzed for the expression of GPC by flow cytometry. Figure7 shows that venom treatment induced the loss of GPC from all these cells.
P2 toxin induces cleavage of GPC from nucleated cells.
K562, U937, and Jurkat cells were incubated for 30 minutes with buffer (thick line) or with 10 μg of P2 toxin (thin line), washed and stained for flow cytometry using the monoclonal antibody Bric4 against GPC. Background fluorescence (shaded histogram). Results are representative for 2 different experiments.
P2 toxin induces cleavage of GPC from nucleated cells.
K562, U937, and Jurkat cells were incubated for 30 minutes with buffer (thick line) or with 10 μg of P2 toxin (thin line), washed and stained for flow cytometry using the monoclonal antibody Bric4 against GPC. Background fluorescence (shaded histogram). Results are representative for 2 different experiments.
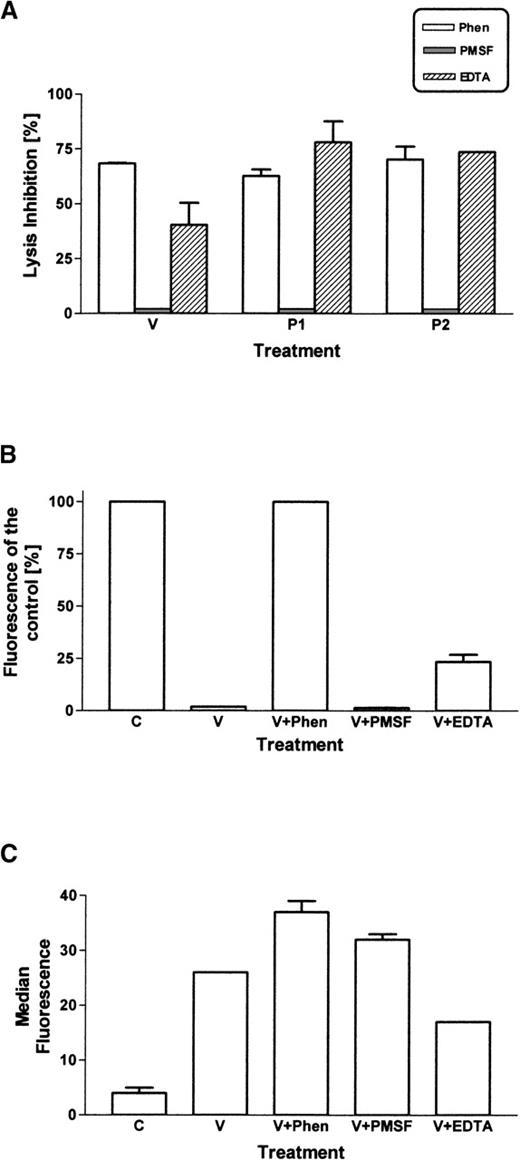
Inhibition of venom and toxins
Cleavage of glycophorins was shown not to be a direct action of the Loxosceles toxins (Figure 4). The hypothesis that a membrane-bound protease was involved was investigated. Many of the known membrane-bound proteases that release cell-bound molecules are metalloproteinases. The effect of EDTA (binding of divalent cations), 1,10 phenanthroline (specific for metalloproteinase), and PMSF (inhibitor of serine proteases) on the ability of theLoxosceles venom and purified toxins to cleave glycophorins and induce C susceptibility was assayed. Both EDTA and 1,10 phenanthroline inhibited the cleavage and induction of C susceptibility by P2 (Figure8). Previously, we showed that Ca++ is necessary for the sphingomyelinase activity of theLoxosceles toxins and that it can be inhibited by EDTA.10 In this study, we showed that toxin binding to the E membrane is also partially blocked by EDTA. However, 1,10 phenanthroline did not have an effect on toxin binding but only prevented glycophorin cleavage and the induction of C susceptibility. Given the known properties of 1,10 phenanthroline, these data suggest that a metalloproteinase is activated that is responsible for the cleavage of glycophorin. Results obtained with whole venom or P1 were similar to that obtained with P2 (data not shown).
Chelating agents inhibit Loxosceles venom-induced cleavage of glycophorins and induction of C susceptibility.
E were incubated with EDTA (10 mmol/L), 1,10-phenanthroline (5 mmol/L), or PMSF (1 mmol/l). Loxosceles venom or toxins were added and incubated 30 minutes 37°C. Cells were washed and analyzed for C susceptibility (A), expression of GPC using mAb Bric4 (B), incorporation of toxins using Ab F35 (C). Results are mean ± SEM of experiments carried out in triplicate.
Chelating agents inhibit Loxosceles venom-induced cleavage of glycophorins and induction of C susceptibility.
E were incubated with EDTA (10 mmol/L), 1,10-phenanthroline (5 mmol/L), or PMSF (1 mmol/l). Loxosceles venom or toxins were added and incubated 30 minutes 37°C. Cells were washed and analyzed for C susceptibility (A), expression of GPC using mAb Bric4 (B), incorporation of toxins using Ab F35 (C). Results are mean ± SEM of experiments carried out in triplicate.
Discussion
The bite of spiders of the genus Loxosceles can induce various biologic effects, including dermonecrosis and C-dependent hemolysis.1-8 The aim of this study was to elucidate the mechanism of action of L. intermedia venom, its active toxins P1 and P2, and in particular the mechanism of induction of C susceptibility. We have previously shown that in vitro hemolysis of erythrocytes, induced by Loxosceles venom, is accomplished by activation of the alternative pathway of C.9 In this study we did not observe an effect of Loxosceles venom and purified toxins on the expression of C-regulators CD59, DAF, or CR1, eliminating loss of C regulators as a cause of hemolysis. However,Loxosceles venom and purified toxins P1 and P2 efficiently induced the loss of GPA, GPB and GPC (Figures 2 and 3). Using mAbs specific for intracellular and extracellular epitopes of GPA, GPB, and GPC, we showed that glycophorins are cleaved extracellularly (Figure3). Glycophorin cleavage was accompanied by the induction of C susceptibility. Glycophorin cleavage was only observed when the active component of the venom or the purified toxins P1 and P2 bound to the membrane. The inability of the venom component P3, despite its high homology with P1 and P2, to induce C-dependent hemolysis is here shown to result from its inability to bind the erythrocyte membrane.
GPA is the major membrane sialoglycoprotein of human E, and it represents a typical example of a transmembrane glycoprotein. The main functional role of GPA is thought to be in retaining E structural integrity. Purified glycophorins inhibit C-mediated lysis,19,21,22 and the removal of sialic acid makes E activators of the alternative pathway of C.23,24 Our results suggest that a major functional role of glycophorin is in protection from homologous C and that glycophorin may be more important in this respect than DAF or CD59. In support of this suggestion, PNH E, which lack both DAF and CD59, exhibit only low-grade hemolysis in vivo.14-17 PNH E are not spontaneous activators of C, and marked lysis is only observed on in vitro activation of C by acidification of the serum. In contrast, on Loxoscelesenvenomation, E become spontaneous activators of C, resulting in massive hemolysis often followed by kidney failure.1-8Erythrocytes express 1 to 5 × 104 copies of CD59,31,32 4 × 103 copies of DAF,33 and 1 to 8 × 103 copies of CR1.34 Glycophorins are more abundant (molecules per E: GPA, 106; GPB, 2.5 × 105; GPC, 5-12 × 104),20 and on a mole-to-mole basis the C-regulatory molecules are likely to be more effective than the glycophorins.
Deficiencies of glycophorin have been described, but hemolytic syndromes have not been observed in patients,20,35-37probably because not all the glycophorin species are missing in these syndromes. However, it cannot be excluded that in Loxoscelesenvenomation, factors other than glycophorin removal affect the E susceptibility to C. Change in lipid composition has been shown to have an effect on C susceptibility, probably because of a change in efficiency of MAC binding.25 Increased accessibility of the membrane because of removal of the bulky glycophorin molecules might also contribute to the increase in complement susceptibility. However,Loxosceles toxins do not increase the susceptibility to lysis by other pore formers such as perforin or melittin (Tambourgi et al, unpublished observations). Extensive hemolysis of E also occurs in the hemolytic uremic syndrome.38-40 In this disease various factors have been identified, including a deficiency in factor H40 and desialylation of glycophorin by bacterial neuraminidases.41 Another common form of HUS occurs in the presence of fH, which is induced by verotoxins produced by certain bacteria.42 These toxins bind to a glycoceramide43 and have a substrate specificity similar to that of Loxosceles toxins. It would be of interest to examine the effects of these toxins on glycophorin expression. The only sphingomyelinases functionally similar to Loxosceles toxins are some bacterial phospholipases D (PLD) from Corynebacterium pseudotuberculosis44 and Arcanobaterium haemolyticum. This PLD also shows 24% to 30% homology with the first 30 amino acids of the Loxosceles toxins,10and it has a similar molecular weight, pI, and substrate specificity.44 The purified PLD from C. pseudotuberculosis can also cause hemolysis and kidney failure in lambs.45 It is unknown whether complement is involved in this process. Phospholipase D from, for example, cabbage andStreptomyces pyogenes does not induce C susceptibility (data not shown), whereas sphingomyelinase C causes E lysis in the absence of C.
Cleavage of glycophorin could not be reproduced using purified toxins and GPA, inducing us to investigate whether hydrolysis of sphingomyelin by the spider toxins activate an endogenous protease responsible for the cleavage of glycophorins. Membrane-bound secretases, also called sheddases or membrane-protein convertases, are responsible for the cleavage of many membrane-bound proteins.46 Most secretases depend on metal ions, and we show that the cleavage of glycophorin and the induction of C susceptibility were inhibited by EDTA and 1,10-phenanthroline. Although EDTA also inhibited the binding of the toxins to the cells, in the presence of 1,10-phenanthroline binding occurred but the toxin-induced glycophorin cleavage was inhibited. 1,10 Phenanthroline binds Zn2+ and is a powerful inhibitor of metalloproteinase, including those of the ADAMs family.47We suggest that venom proteins, through their sphingomyelinase activities, alter the membrane environment and membrane fluidity, causing activation of an as yet unknown metalloproteinase. Recently metalloproteinase activity has been detected in Loxoscelesvenom.48 This metalloproteinase with Mr 35 kd had substrate specificity for fibronectin as shown in a zymogram assay, but it has not been purified and characterized in more detail. It is unlikely that this protein is the same molecule as our purified toxins P1 and P2, which lack direct proteolytic activity toward glycophorin. A few E metalloproteinase have been characterized, but no natural substrates have been identified.49-51 The identity of the metalloproteinase involved in glycophorin cleavage remains to be elucidated.
The glycophorins are all similar in topology, but they are distinct in sequence.20 Although GPA and GPC are cleaved on treatment with venom toxins, no obvious sequence homology in the extracellular regions of GPA and GPC is observed. Sheddases often have a broad specificity and require only 1 or 2 specific amino acids at the site of cleavage. We did not observe a change in expression of any of the C regulators or on band 3 (data not shown), and there were no gross changes in E-protein pattern on SDS-PAGE, but it remains possible that other membrane-bound molecules are also cleaved.
The hemolysis-inducing and glycophorin-cleavage activity was transferred from treated E to a new E population (Figure 6). This transfer phenomenon explains the extent of the systemic hemolysis observed after envenomation. This situation is not unique in that the transfer of sphingomyelinase activity between cells has been reported.52
In contrast to GPA and GPB, which appear late during erythroid development, at the proerythroblast stage, GPC is already present on erythroid progenitors and can be detected on leukocytes. Venom toxins caused a nearly complete removal of GPC expressed on the human cell lines K562 (erythroblast), U937 (promonocyte), and Jurkat (T cell), demonstrating that the toxins can induce activation of proteases in nucleated cells. The observation that metalloproteinase in nucleated cells is activated by venom proteins may help explain the local effects of dermonecrosis.
In conclusion, we present a mechanism through which Loxoscelesvenom renders E susceptible to lysis by C, which explains the extent of hemolysis observed in patients after envenomation and shows that it is possible to inhibit a biologic effect of this harmful venom. The discovery of the involvement of metalloproteinase in the toxicity of the Loxosceles venoms is novel and of obvious therapeutic significance given the availability of metalloproteinase inhibitor drugs. Our unpublished data show a similar activity of purified toxins from at least 2 other Loxosceles species. The mechanism by which the spider toxins induce activation of metalloproteinase on E—identification of the metalloproteinase responsible for glycophorin cleavage and testing of a therapeutic strategy—are the subjects of further study.
Supported by FAPESP, CNPq and by a Senior Fellowship from The Wellcome Trust.
Reprints:Denise V. Tambourgi, Laboratório de Imunoquı́mica, Instituto Butantan, Avenida Prof. Vital Brazil, 1500, CEP 05508-900 São Paulo, Brazil; e-mail:butlim@eu.ansp.br.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 6. Transfer of hemolysis-inducing and glycophorin-cleaving activity. / E were treated with increasing concentrations of P1 (•), P2 (○), orL. intermedia venom (*), washed, and incubated with the same volume and number of calcein-AM loaded E or buffer. After 30 minutes of incubation at 37°C, cells were analyzed for autologous C lysis (A, B) or for the expression of GPC (C). (A) Total hemoglobin release induced by NHS determined spectrophotometrically at 541 nm. (B) Release of entrapped calcein determined fluorometrically. [C] E samples incubated with buffer or with P2 (2.5 and 5.0 μg/mL) were incubated with the same volume of untreated E (□) or with buffer (▪) for 30 minutes at 37 °C and analyzed by flow cytometry for expression of GPC using the monoclonal antibody Bric4. Results are expressed as the percentage of fluorescence of untreated E.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.683/6/m_bloo00248006x.jpeg?Expires=1769152109&Signature=3IlgsKKVHvjtcj1HDeckQ91Hj0THjmotahCVt~qx2iSOrG50zsPcqJjfnPU-h361mCnXYaixaLfKRbnF2MkvxXXO1aXppyzt8ie~3oDGXyenO62U-9F6v5zui6OpSs8R9QPiwUhE6OAmi-O2TpWWYgD2NNZHXzHWcNSX6Qy-qrTGEjxrZS-z6TlGo-1ZohaMJ2VwU3h-iOY-t6sdkw6VYif8NiIR82a857sQ9pHrdUe6qfwzao9ExHJjYUgBNDyueENhsUNjdGL0ZxZnzgyeXYusuaK~Mf4Z6HhQOg82p0ESyXH9xEiwnnG2eUqiSgBgu-fHto61L0Hu7bqeec7pWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal