Juvenile myelomonocytic leukemia (JMML) is an early childhood disease for which there is no effective therapy. Therapy with 13-cis retinoic acid or low-dose chemotherapy can induce some responses, but neither mode is curative. Stem cell transplantation can produce lasting remissions but is hampered by high rates of relapse. The pathogenesis of JMML involves deregulated cytokine signal transduction through the Ras signaling pathway, with resultant selective hypersensitivity of JMML cells to granulocyte-macrophage colony-stimulating factor (GM-CSF). A JMML mouse model, achieved through homozygous deletion of the neurofibromatosis gene, confirmed the involvement of deregulated Ras in JMML pathogenesis. With this pathogenetic knowledge, mechanism-based treatments are now being developed and tested. Ras is critically dependent on a prenylation reaction for its signal transduction abilities. Farnesyltransferase inhibitors are compounds that were developed specifically to block the prenylation of Ras. Two of these compounds, L-739,749 and L-744,832, were tested for their ability to inhibit spontaneous JMML granulocyte-macrophage colony growth. Within a dose range of 1 to 10 μmol/L, each compound demonstrated dose-dependent inhibition of JMML colony growth. An age-matched patient with a different disease and GM-CSF–stimulated normal adult marrow cells also demonstrated dose-dependent inhibitory effects on colony growth, but they were far less sensitive to these compounds than JMML hematopoietic progenitors. Even if the addition of L-739,749 were delayed for 5 days, significant inhibitory effects would still show in JMML cultures. These results demonstrate that a putative Ras-blocking compound can have significant growth inhibitory effects in vitro, perhaps indicating a potential treatment for JMML.
Juvenile myelomonocytic leukemia (JMML), previously termed juvenile chronic myelogenous leukemia, is a rare, clonal myeloproliferative/myelodysplastic disorder of infancy and early childhood.1-5 It converts to an acute leukemia-type blast crisis in only a few patients. Nevertheless the mortality rate is high because of the infiltration of monocytic cells into nonhematopoietic organs such as the lungs and the intestines, leading to organ failure, bleeding, and infection. Intensive chemotherapeutic regimens have largely proved futile in inducing durable remissions.6-9Low-to-intermediate dose chemotherapy may be temporarily effective in a proportion of patients, but it has generally not been shown to result in long-term disease control.10,11 Although 13-cis retinoic acid has an overall response rate of 40% to 50% and minimal toxicity, it is associated with extended responses in <10% of patients.12,13 Stem cell transplantation is the only therapy capable of producing durable remissions.14-17Unfortunately, the relapse rate remains high, and the overall survival rate is approximately 25%. Clearly, more effective therapy is sorely needed for this disease.
The pathogenesis of JMML has been linked to deregulated signal transduction through the Ras signaling pathway. This deregulation results in JMML cells demonstrating hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF) in in vitro dose-response assays.18-20 This hypersensitivity is selective because the responsiveness of JMML cells to IL-3 and G-CSF is normal.18 The family of Ras proteins acts as the master switch in transducing signals from the cell surface to the nucleus.21-24 Activating mutations of the RAS gene are observed in 15% to 30% of patients with JMML.25-28One of the major inactivators of Ras within cells is the neurofibromin protein, encoded by the neurofibromatosis type 1 tumor suppressor gene (NF1).29-31 Neurofibromin is a GTPase-activating protein, and it serves to hydrolyze Ras from its active GTP-bound state to its inactive GDP-bound state. The incidence of clinically apparent neurofibromatosis in patients with JMML is a striking 10% to 15%,4,32-34 compared with a general incidence of 1 in 3500. Many patients with JMML and neurofibromatosis demonstrate loss of heterozygosity at the NF1 locus.35-38 In addition to the 10% to 15% of patients with clinical evidence of neurofibromatosis, another 15% with JMML harbor NF1 mutations within their leukemic cells but do not have outward clinical manifestations.39 Although a causal relationship between the activating RAS mutations and the pathogenesis of JMML has yet to be established, RAS mutations and NF1abnormalities do appear to be mutually exclusive.28,39Conversely, Nf1 mutations have been proven causal in a mouse model of JMML. Homozygous deletion of Nf1 in mice leads to embryonic death.40 However, the hematopoietic fetal liver cells from these embryos demonstrate the same selective hypersensitivity to GM-CSF as do JMML cells, and transplantation of these cells into irradiated recipient mice results in the development of a myeloproliferative disorder similar to the human JMML syndrome41,42 and characterized by activated Ras-MAP kinase signaling in hematopoietic cells.43 Furthermore, recent studies show that murine cells lacking Nf1 are critically dependent on GM-CSF for growth.44
Given these compelling data linking JMML pathogenesis to deregulated GM-CSF signal transduction through the Ras intracellular pathway, it seems reasonable to begin to explore mechanism-based therapy for JMML. Because JMML hematopoietic progenitor cells do not produce sufficient GM-CSF themselves to sustain in vitro colony growth, JMML is not an autocrine-driven disease.45 Rather, because of their inherent hypersensitivity to GM-CSF, JMML progenitors are dependent on the basal production of GM-CSF from monocytes.45 IL-10 has been shown to inhibit the monocytic production of GM-CSF and specifically to inhibit JMML cell growth.46 The GM-CSF antagonist and apoptotic agent, E21R, has also been shown to inhibit JMML in vitro cell growth and JMML cell engraftment in immunodeficient mice.47-49 Finally, 1 of 2 recently developed50,51 GM-CSF/diphtheria toxin fusion proteins has been shown to inhibit JMML cell growth in vitro.52 It is hypothesized that most of these potential therapies interfere with GM-CSF–cell interactions at the JMML cell surface. Whether any of these potential therapies can actually abolish the entire malignant clone is a matter of ongoing investigation.
Another feasible way to block the GM-CSF hypersensitive growth of JMML cells is to block the intracellular signaling pathway. For Ras to be active as a master switch for signal transduction, it must localize to the inner surface of the plasma membrane; this occurs after a series of posttranslational modifications. The first obligatory step in this series, which is essential for Ras cell-transforming activity, is the addition of a 15-carbon isoprenyl (farnesyl) group to Ras through a covalent link.53-58 The addition of the farnesyl moiety to the cysteine residue of the COOH-terminal CAAX motif (C, cysteine; A, usually an aliphatic residue; X, any other amino acid) is catalyzed by the enzyme farnesyl-protein transferase (FPTase). Several inhibitors of FPTase, representing broad structural diversity, have been synthesized.59,60 Some of these compounds, now termed farnesyltransferase inhibitors (FTIs), have been evaluated in several different in vitro and in vivo preclinical systems and have demonstrated significant antitumor effects.61-68 They have demonstrated an ability to inhibit the Ras-induced transformation of tissue culture cells and several cancer cell lines (primarily solid tumor types) and to block the proliferation of Ras-activated xenografts in nude mice. Further, FTIs have shown efficacy in RAS-driven transgenic mouse models of mammary and salivary carcinomas in which theRAS expression was forced by a mammary tumor virus. Finally, FTIs have demonstrated efficacy in blocking some of the phenotypic changes in NF1-deficient cells.69 70 Given this background of the developmental design of the FTIs and the pathogenetic mechanisms involving RAS and NF1 in JMML, the goal of this study was to determine the effectiveness of the CAAX peptidomimetic FPTase inhibitors L-739,749 and L-744,832 to abrogate JMML cell growth in vitro.
Materials and methods
Acquisition of donor samples
With the approval of the respective institutional review boards and after obtaining parental consent, peripheral blood samples, bone marrow samples, or both were obtained from children with JMML and from a child with another disorder to serve as an age-matched control. The diagnosis of JMML was based on uniform criteria as agreed on by the International JMML Working Group71 and confirmed by the demonstration of selective hypersensitivity to GM-CSF in all children.
Normal controls were volunteer adults who donated bone marrow samples after informed consent and with the approval of the Institutional Review Board of the University of Alabama at Birmingham.
Mononuclear cell isolation and colony assays
Peripheral blood or marrow mononuclear cells for patient and control samples were isolated by density gradient centrifugation as described previously.18 Soft agar assays for granulocyte-macrophage colonies (CFU-GM) were established in 1-mL cultures of 0.3% agarose with McCoys' 5A medium plus nutrients and 15% fetal bovine serum as described previously.18,45,72 73 Cultures were incubated for 14 days at 37°C in a 5% CO2 atmosphere. CFU-GM colonies (≥40 cells/colony) were scored at day 14 using a dissecting microscope. GM-CSF (R & D, Minneapolis, MN) was added to the control cultures from the normal adult volunteer samples to stimulate growth at final concentrations of either 0.32 ng/mL or 2 ng/mL.
Addition of farnesyltransferase inhibitor to CFU-GM assays
The FTIs, L-739,749 and L-744,832, were supplied by Drs Allen Oliff and Jackson Gibbs of Merck Research Laboratories (West Point, PA) and were dissolved in a stock solution of 50% methanol at a concentration of 100 mmol/L and stored at −20°C. Three methods of adding the FTI to the CFU-GM assays were evaluated: (1) L-739,749 or L-744,832 was added only once, 24 hours after the cultures were established, duplicating the type of in vitro assay that established the effectiveness of 13-cis retinoic acid12 13; (2) the one-time dosing of FTI was delayed and was added at either day 3, day 5, or day 7 after the cultures were established; or (3) the cells were exposed to FTI before the establishment of the semisolid agar cultures. In the latter experiment, the mononuclear cells were placed in liquid suspension in McCoys' 5A medium with nutrients and 15% fetal bovine serum and then the FTI inhibitor was mixed in. Cells were exposed to the FTI inhibitor for 1, 3, or 5 days, then washed twice and placed in agar assays without any further addition of FTI inhibitor. In all types of cultures, the appropriate methanol dilutions for the respective FTI concentrations were simultaneously established to control for any effects on CFU-GM growth imposed by the methanol itself. Appropriate dilutions of the FTI or methanol control were made such that, for each dose, 100 μL of volume was spread uniformly over the agarose surface.
Results and discussion
Samples from 12 patients with JMML were evaluated. All patients fulfilled the diagnostic criteria for JMML71 and demonstrated selective GM-CSF hypersensitivity of hematopoietic progenitor cells in vitro. Only 5 of 12 patient samples have been fully evaluated for NF1 or RAS abnormalities. Of the 5 fully studied, 2 had RAS mutations (both KRAS point mutations) and the other 3 had NF1 abnormalities (unpublished observations, Snyder RC, Emanuel PD and ref. 39).
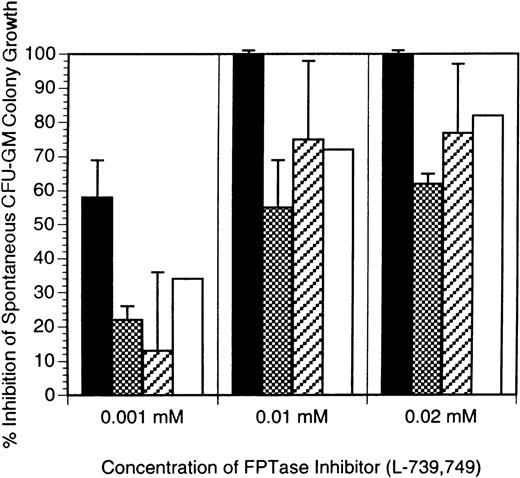
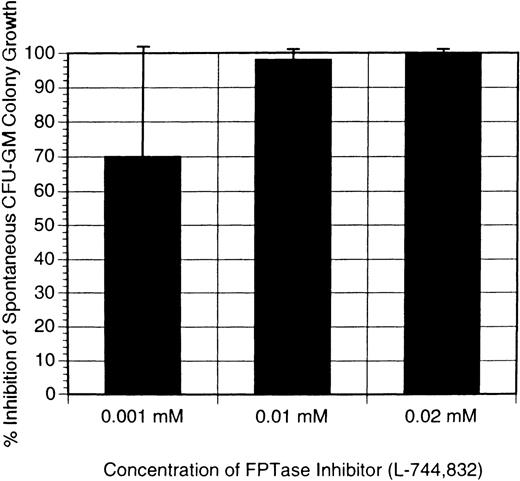
In the first series of experiments, the FTI was added once 24 hours after the establishment of the cultures. Numbers of CFU-GM (>40 cells/colony) were counted, and the amount of inhibition by the FTI was calculated as a percentage of the maximal colony growth. As depicted in Figures 1 and 2, there was inhibition of JMML spontaneous CFU-GM colony growth at all concentrations of either FTI, L-739,749, or L-744,832. At concentrations ≥10 μmol/L FTI, there was complete abrogation of growth, and virtually no colonies were present in any patient sample. At a concentration of 1 μmol/L FTI, significant inhibition of CFU-GM colony growth was noted, and the colonies were smaller (fewer cells) than in the no addition controls. Because the FTI was in a methanol stock solution, appropriate methanol controls were also established, and these showed no significant effect on CFU-GM growth (data not shown).
Because of the nature of JMML, finding suitable controls for these experiments was problematic. We have demonstrated that JMML peripheral blood-derived or marrow-derived progenitor cells are essentially equivalent with regard to spontaneous growth patterns and to GM-CSF hypersensitivity patterns.18,45 Normal peripheral blood mononuclear cells did not show spontaneous colony growth, and normal marrow mononuclear cells did so only sporadically. Numerous normal age-matched marrow samples were not obtainable because of ethical concerns. Therefore, as controls for these experiments, we used normal adult marrow mononuclear cells stimulated with GM-CSF to simulate colony growth similar to that observed in JMML. We examined the inhibitory effects of the FTI on normal CFU-GM colony growth under several conditions: freshly obtained specimens stimulated with maximal concentrations of GM-CSF (2 ng/mL), freshly obtained specimens stimulated with threshold concentrations of GM-CSF (0.32 ng/mL), and specimens that had been stored in a liquid nitrogen environment for a period of time. The latter 2 conditions were attempted to simulate JMML conditions as much as possible. Because JMML cells are hypersensitive to GM-CSF, we stimulated normal cells with a GM-CSF concentration obtained from our dose-response curve data that represents a threshold concentration (0.32 ng/mL GM-CSF) at which JMML cells show an initial growth response.3 This was compared with effects at 2 ng/mL, which represents a maximal stimulation situation. Therefore, both physiologic and pharmacologic situations were simulated. No significant difference was noted in normal adult marrow cultures between the 2 GM-CSF concentrations (Figure1). In each situation the amount of colony growth inhibition by the FTI was always less than that seen in the JMML samples. The third situation simulated the JMML conditions in that several of our JMML samples had been stored in liquid nitrogen for various periods of time before evaluation. The storage conditions did have some increased effects on the amount of colony growth inhibition in the normal adult control samples (Figure 1), but these effects were still less than those seen in JMML samples. Taking into account the hypersensitivity to GM-CSF in JMML cells, these results are predictable. Finally, we were able to evaluate an age-matched marrow sample from a patient whose hematopoietic progenitor cells did in fact show spontaneous CFU-GM colony growth. This patient was ultimately diagnosed with thrombocytopenia with absent radii (TAR) syndrome and did not meet the clinical or culture criteria for JMML. The effect of L-739,749 on this patient's spontaneous colony growth was more similar to the effect of the FTI on the normal adult controls than to the effect of the FTI on JMML cells (Figure 1).
Inhibition of CFU-GM colony growth by the addition of L-739,749.
L-739,749 was a one-time addition 24 hours after establishment of the cultures, with respective concentrations of L-739,749 as indicated. Results in all cases are the mean of experiments performed in triplicate and are expressed as the percentage inhibition of maximal spontaneous CFU-GM colony growth. Error bars indicate the standard error of margin. Solid bars: Inhibition of JMML spontaneous CFU-GM colony growth. Results represent the mean of experiments performed in triplicate from 10 different patients with JMML. Hatched bars: Inhibition of GM-CSF–stimulated normal donor CFU-GM colony growth from marrow mononuclear cells obtained fresh from 5 different normal adult marrow donors. Results are expressed as the percentage inhibition of maximal CFU-GM colony growth stimulated by GM-CSF (either 2 ng/mL or 0.32 ng/mL). Diagonal bars: Inhibition of GM-CSF stimulated normal donor CFU-GM colony growth from marrow mononuclear cells that had been previously obtained from 3 different normal adult marrow donors and had been put in liquid nitrogen for long-term storage. Results are expressed as the percentage inhibition of maximal CFU-GM colony growth stimulated by GM-CSF (2 ng/mL). Open bars: Inhibition of spontaneous CFU-GM colony growth from the bone marrow mononuclear cells of an age-matched patient eventually diagnosed with thrombocytopenia with absent radii (TAR syndrome). The child did not meet the diagnostic criteria for JMML. CFU, colony-forming unit; CSF, colony-stimulating factor; GM, granulocyte macrophage; JMML, juvenile myelomonocytic leukemia.
Inhibition of CFU-GM colony growth by the addition of L-739,749.
L-739,749 was a one-time addition 24 hours after establishment of the cultures, with respective concentrations of L-739,749 as indicated. Results in all cases are the mean of experiments performed in triplicate and are expressed as the percentage inhibition of maximal spontaneous CFU-GM colony growth. Error bars indicate the standard error of margin. Solid bars: Inhibition of JMML spontaneous CFU-GM colony growth. Results represent the mean of experiments performed in triplicate from 10 different patients with JMML. Hatched bars: Inhibition of GM-CSF–stimulated normal donor CFU-GM colony growth from marrow mononuclear cells obtained fresh from 5 different normal adult marrow donors. Results are expressed as the percentage inhibition of maximal CFU-GM colony growth stimulated by GM-CSF (either 2 ng/mL or 0.32 ng/mL). Diagonal bars: Inhibition of GM-CSF stimulated normal donor CFU-GM colony growth from marrow mononuclear cells that had been previously obtained from 3 different normal adult marrow donors and had been put in liquid nitrogen for long-term storage. Results are expressed as the percentage inhibition of maximal CFU-GM colony growth stimulated by GM-CSF (2 ng/mL). Open bars: Inhibition of spontaneous CFU-GM colony growth from the bone marrow mononuclear cells of an age-matched patient eventually diagnosed with thrombocytopenia with absent radii (TAR syndrome). The child did not meet the diagnostic criteria for JMML. CFU, colony-forming unit; CSF, colony-stimulating factor; GM, granulocyte macrophage; JMML, juvenile myelomonocytic leukemia.
Because the inhibitory effect of L-739,749 could be a nonspecific toxicity, we next sought to determine whether a second FTI would produce similar inhibitory effects on JMML spontaneous CFU-GM growth. L-744,832, which has been explored in the Nf1 mouse model of JMML, as discussed below, was also used in these human JMML sample experiments. As shown in Figure 2, L-744-832 had inhibitory effects on JMML CFU-GM growth similar to those of L-739,749. Samples from 4 patients were tested with this compound. One was tested with both L-739,749 and L-744,832, and the inhibitory results were almost identical (Figures 1 and 2).
Inhibition of CFU-GM colony growth by addition of L-744,832.
L-744,832 was a one-time addition 24 hours after establishment of the cultures, with respective concentrations of L-744,832 as indicated. Results are the mean of experiments performed in triplicate and expressed as the percentage inhibition of maximal spontaneous CFU-GM colony growth. Error bars indicate the standard error of margin. The solid bars show the inhibition of JMML spontaneous CFU-GM colony growth. Results represent the mean of experiments performed in triplicate from 4 different patients with JMML. CFU, colony-forming unit; GM, granulocyte macrophage; JMML, juvenile myelomonocytic leukemia.
Inhibition of CFU-GM colony growth by addition of L-744,832.
L-744,832 was a one-time addition 24 hours after establishment of the cultures, with respective concentrations of L-744,832 as indicated. Results are the mean of experiments performed in triplicate and expressed as the percentage inhibition of maximal spontaneous CFU-GM colony growth. Error bars indicate the standard error of margin. The solid bars show the inhibition of JMML spontaneous CFU-GM colony growth. Results represent the mean of experiments performed in triplicate from 4 different patients with JMML. CFU, colony-forming unit; GM, granulocyte macrophage; JMML, juvenile myelomonocytic leukemia.
Given that either FTI was able to inhibit 100% of spontaneous CFU-GM growth in virtually all patients with JMML at concentrations of 10 μmol/L, in the next series of experiments we sought to determine whether variations in time of exposure to the FTI would affect the degree of growth inhibition. Therefore, L-739,749 was added at either days 1, 3, 5, or 7 after the establishment of cultures. Because of limited supplies of L-739,749, this type of multiple-dosing experiment could be performed on only 1 patient sample. Figure3 demonstrates a clear-cut, time-dependent loss of inhibitory effect of L-739,749 on spontaneous JMML CFU-GM colony growth.
Inhibition of JMML spontaneous CFU-GM colony growth by addition of L-739,749.
L-739,749 was a one-time addition at either 1, 3, 5, or 7 days after establishment of the cultures. Results, expressed as the percentage inhibition of maximal spontaneous CFU-GM colony growth, are the mean of experiments performed in triplicate from patient J97 with JMML. Concentrations of L-739,749 added are 1μmol/L (solid bars) and 10μmol/L (hatched bars). CFU, colony-forming unit; GM, granulocyte macrophage; JMML, juvenile myelomonocytic leukemia.
Inhibition of JMML spontaneous CFU-GM colony growth by addition of L-739,749.
L-739,749 was a one-time addition at either 1, 3, 5, or 7 days after establishment of the cultures. Results, expressed as the percentage inhibition of maximal spontaneous CFU-GM colony growth, are the mean of experiments performed in triplicate from patient J97 with JMML. Concentrations of L-739,749 added are 1μmol/L (solid bars) and 10μmol/L (hatched bars). CFU, colony-forming unit; GM, granulocyte macrophage; JMML, juvenile myelomonocytic leukemia.
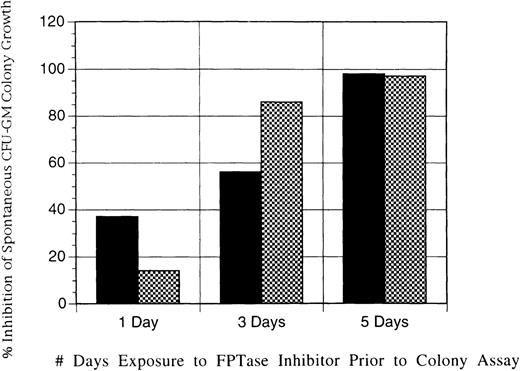
In a final series of experiments to determine the effectiveness of L-739,749 at inhibiting JMML CFU-GM growth, the JMML mononuclear cells from 1 patient were placed in liquid suspension in the presence of L-739,749 and in McCoys' 5A medium with nutrients and 15% fetal bovine serum. After either 1, 3, or 5 days of liquid suspension culture incubation with exposure to the FTI, the cells were removed, washed twice to remove any extracellular FTI, and placed in soft agar colony assay for 14 days. Trypan blue exclusion viability assays performed at all time points showed 95% to 100% viability, indicating that L-739,749 was not exerting any effect of cell necrosis on the cells (data not shown). Figure 4 shows that even as little as 24 hours of exposure to the FTI before a 14-day colony assay was sufficient to produce some minimal inhibition. More significant inhibition was obtained with 3 days of exposure before washout, and 5 days of exposure at doses of either 1 or 10 μmol/L of L-739,749 was sufficient to inhibit virtually all spontaneous CFU-GM colony growth in JMML.
Inhibition of JMML spontaneous CFU-GM colony growth by exposure of JMML mononuclear cells to L-739,749.
Exposure was in liquid suspension for either 1, 3, or 5 days before the establishment of colony assays. Cells were washed twice before the establishment of soft agar cultures to remove any extracellular L-739,749 in the liquid suspension. Results expressed are the percentage inhibition of maximal spontaneous CFU-GM colony growth, and they are the mean of experiments performed in triplicate from patient J49 with JMML. Concentrations of L-739,749 added are 1μmol/L (solid bars) and 10μmol/L (hatched bars).
Inhibition of JMML spontaneous CFU-GM colony growth by exposure of JMML mononuclear cells to L-739,749.
Exposure was in liquid suspension for either 1, 3, or 5 days before the establishment of colony assays. Cells were washed twice before the establishment of soft agar cultures to remove any extracellular L-739,749 in the liquid suspension. Results expressed are the percentage inhibition of maximal spontaneous CFU-GM colony growth, and they are the mean of experiments performed in triplicate from patient J49 with JMML. Concentrations of L-739,749 added are 1μmol/L (solid bars) and 10μmol/L (hatched bars).
These experiments demonstrate that farnesyl-protein transferase inhibitors, initially developed for their potential to block Ras signal transduction, profoundly inhibited JMML cell growth in vitro. The value of this study stems from the fact that these cell culture studies were performed using primary cells from patient samples obtained from 12 different patients with confirmed diagnoses of JMML. These patients accurately represented a spectrum of JMML; some had NF1abnormalities, some had RAS abnormalities, and some probably had neither. The pathogenesis of JMML is intricately linked with the deregulation of signal transduction through the Ras signaling pathway.3,41,42 This deregulation, regardless of where in the Ras pathway the causative mutation(s) may occur, ultimately results in JMML cells demonstrating in vitro selective hypersensitivity to GM-CSF in granulocyte-macrophage colony-forming proliferation assays (CFU-GM).18-20 Activating RAS mutations are found in 15% to 30% of patients with JMML,25-28,39 andNF1 gene abnormalities are found in as many as 30% more.35-39NF1 is a tumor-suppressor gene that encodes for the protein neurofibromin, which serves to inactivate Ras by hydrolyzing it from its active guanosine triphosphate (GTP)-bound state to an inactive guanosine diphosphate (GDP)-bound state.29-31 Loss of heterozygosity or other loss of function mutations of NF1, therefore, are essentially equivalent to activating RAS point mutations. All JMML samples examined in this study demonstrated similar growth inhibition by FTIs regardless of the identification within individual samples ofRAS mutations, NF1 abnormalities, or other as yet undefined mutations.
FTIs were developed to exploit Ras signal transduction physiology. For Ras proteins to serve as molecular master switches in mitogenic signal transduction, they must be in a membrane-bound, GTP-bound state. Obligatory for Ras cell-transforming activity is the prenylation reaction that attaches a farnesyl group (a 15-carbon isoprenyl group) to Ras to allow its association to the outer membrane of the cell, thus becoming a fully mature protein. Prenylated proteins share characteristic C-terminal sequences such as the CAAX motif. Farnesyl-protein transferase is 1 of 3 enzymes that catalyze protein prenylation. The others are geranylgeranyl protein transferase (GGPTase) types I and II. Selective inhibition of FPTase was explored and developed because geranylgeranylation of normal cellular proteins is 5 to 10 times more prevalent than farnesylation. Several farnesylated proteins play important roles in normal cells, including nuclear lamins essential for nuclear structural integrity, proteins of the retinal visual signal transduction system, the human homologue of the yeast molecular chaperone YDJ1, the skeletal muscle phosphorylase kinase, and others. The question that arises is how can the FTIs exert selective effects on tumor cells when so many normal cells also depend on farnesylation. The answer is likely not simple, but it may in part relate to the sensitivity of particular proteins to the FTIs, the ability of some proteins also to use GGPTase-I, and the dependency of the tumor cell on the Ras signal pathway rather than on the other redundant pathways in normal cells. Reports published recently74-77 indicate that KRAS-transformed cells may not be nearly as sensitive to FTIs as HRAS-transformed cells in which much of the preliminary testing of the FTIs was performed.60,61These reports demonstrate that KRAS proteins can be prenylated by geranylgeranyl protein transferase if the farnesylation pathway is blocked by an FTI.75,76 KRAS is the gene form most often mutated in human tumors. However, the specific RAS mutational status of human tumor cells may not necessarily correlate with their sensitivity to FTIs.63 In these cases the sensitivity to FTIs may lie in the dependency of the tumor cells on the Ras pathway, or it may raise the possibility that other farnesylated proteins besides Ras contribute to their biologic phenotypes. In this regard, recent studies78-81 implicate RhoB as a potential alternative or an additional target for the block of farnesylation. Therefore, though FTIs were developed to be specific compounds to block Ras signal transduction, it is becoming evident that inhibiting the farnesylation of proteins other than Ras may play a major role in their mechanism of action. In addition, since the initial development of the FTIs, more knowledge is emerging about the trafficking of Ras. A recent report82 shows that prenylated CAAX proteins do not, in fact, associate directly with the plasma membrane; rather, they associate with the endomembrane and are subsequently transported to the plasma membrane. Therefore, it is clear that the full function and mechanism of action of the FTIs remain to be elucidated.
The first FTI used in this study, L-739,749, is the methyl ester of the prodrug L-739,750. In the initial report61 on the development of these compounds, L-739,750 was shown to have profound effects on blocking prenylation in vitro, and this was highly selective for farnesylation over geranylgeranylation, as studied by prenylation assays. However, though L-739,750 could block prenylation on a subcellular level, it demonstrated minimal effectiveness in inhibiting whole-cell growth. Conversely, the methyl ester L-739,749 demonstrated less effectiveness in prenylation experiments (though selectively was maintained), but it did have profound effects specifically blocking Ras-transformed growth in whole-cell experiments. The inhibition of Ras-transformed Rat1 cell growth displayed a clear dose dependency of L-739,749 in the range from 1 μmol/L to 20 μmol/L.61Similarly, in the current study, we observed a dose-dependent inhibition of JMML spontaneous CFU-GM growth over the same drug concentration range of 1 to 20 μmol/L. The second FTI used in this study was L-744,832, the isopropyl ester of the prodrug L-739,750.62 It was employed to demonstrate that the effect of L-739,749 on JMML hematopoietic progenitor cells was not simply the result of a nonspecific toxicity event. The isopropyl ester and the methyl ester are similar compounds, and it is not unexpected that the results obtained in the JMML cell cultures showed similar levels of inhibition. L-744,832 has also been evaluated recently in theNf1-deficient mouse model by Shannon et al.83 They demonstrated dose-dependent inhibition of CFU-GM colony growth in the murine system at dose ranges of 1 to 20 μmol/L L-744,832, similar to the human system in this study and in the cell line testing previously reported. They were also able to demonstrate that L-744,832 could block H-Ras but not N-Ras farnesylation. GM-CSF-induced MAP kinase activation was blunted as well. Disappointingly, L-744,832 did not produce responses when used in the in vivo whole mouse with the myeloproliferative syndrome. This potential discrepancy between the in vitro human cell testing and the in vivo whole mouse testing is as yet unexplained. The answer may ultimately lie in the effect of FTIs on cellular events other than activating mutations of RAS, such as the recently reported FTI-induced activation of suppressed apoptotic pathways.67 In this regard, a report84describes the enhanced cell survival of JMML cells with a reversal of this effect using the GM-CSF antagonist analogue E21R. We recently described altered levels of the p85 regulatory subunit of phosphatidylinositol 3′-kinase in JMML cells,85 which has been linked to apoptotic pathways in other hematopoietic cells. Similar to the apoptosis-inducing effects seen with the GM-CSF antagonist analogue E21R, the possibility exists that some of the effectiveness of FTIs in JMML may result from their influences on the cell survival pathways. The possibility exists that theNf1-deficient mouse may not harbor the same alterations in the cell survival pathways as in the primary human JMML cells.
In summary, farnesyltransferase inhibitors demonstrate profound in vitro inhibitory effects on the cell growth of primary cells obtained from humans with JMML. Determining whether this is because of a specific Ras-related inhibition, other cellular effects, or multifactorial events will be the subject of ongoing investigations as the search for effective FTIs, and potentially other new therapeutic strategies for JMML, continues.
Acknowledgments
The authors thank Allen Oliff and Jackson Gibbs of Merck Research Laboratories for supplying the farnesyltransferase inhibitors evaluated in this study and for their helpful discussions and criticisms.
Supported in part by Public Health Service grants CA60407, CA25408, and CA80916; the J. L. Griffin Foundation; and the Cancer Research and Youth Outreach Network.
Reprints:Peter D. Emanuel, Division of Hematology/Oncology, Wallace Tumor Institute, Suite 520, University of Alabama at Birmingham, Birmingham, AL 35294-3300; e-mail:peter.emanuel@ccc.uab.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal