Current understanding of the adhesion molecules and mechanisms regulating hematopoietic stem and progenitor cell (HSPC) homing to the bone marrow is limited. In contrast, the process by which mature leukocytes are able to home to and extravasate out of blood vessels at sites of inflammation has been well characterized and invites comparison. We studied the interaction of human HSPC from adult bone marrow (ABM) and fetal liver (FL) with E-, P-, and L-selectin immobilized in a flow chamber. CD34+ HSPC from both ABM and FL rolled avidly on E-, P-, and L-selectin across a range of physiologic shear rates, indicating the presence of ligands for all three selectins on HSPC. Results indicate that CD34+ ABM and FL cells roll more efficiently (to a greater extent and more slowly) than more differentiated CD34− cells, especially on P- and L-selectin. In a similar fashion, increased rolling efficiency was seen with CD34+CD38− ABM cells when compared with committed progenitor cells of the CD34+CD38+ phenotype. Rolling of CD34+ ABM cells on P-selectin could be partially inhibited by monoclonal antibody (mAb) against PSGL-1, and was not inhibited by a mAb against CD34, suggesting that HSPC have unique carbohydrate repertoires that facilitate selectin-mediated rolling. Our results provide direct evidence of selectin ligands on HSPC under physiologic flow conditions and are the first to show a correlation between the maturity of HSPC during development and rolling efficiency on selectins, suggesting a mechanism by which HSPC subsets may differentially home to the extravascular spaces of the bone marrow.
The objective in bone marrow (BM) transplantation is to seed a damaged or destroyed host BM with pluripotent hematopoietic stem and progenitor cells (HSPC) to reestablish the host immune response and erythroid and myeloid cell lineages. The mechanism of HSPC transplantation, where donated HSPC are injected into the blood stream and “home” to the BM, suggests HSPC readily traffic into tissues. Further evidence exists from other physiologic contexts that HSPC traffic avidly.1 During embryonic development, HSPC migrate from the yolk sac to the fetal liver and spleen and then to the BM. After birth and under normal physiologic conditions, HSPC can intravasate into the blood circulation from the BM. This process can be expanded by stem cell mobilization in which cytokines such as granulocyte colony-stimulating factor (G-CSF) increase intravasation from the BM.
Our current understanding about the adhesion molecules and mechanisms regulating HSPC trafficking into and out of the BM is limited. This is in stark contrast to the detailed characterization of leukocyte trafficking in the inflammatory response, in which mature leukocytes are able to home to and extravasate out of blood vessels at sites of inflammation.2 Similarities in the trafficking ability of leukocytes and HSPC to home to particular tissue sites in vivo suggest that we examine whether HSPC trafficking shares common molecular mechanisms with leukocyte trafficking. The selectin family of adhesion molecules includes E-selectin (CD62E, endothelial selectin), P-selectin (CD62P, platelet selectin), and L-selectin (CD62L, leukocyte selectin). Selectins are involved in the initial steps of the inflammation process by mediating the rolling of leukocytes on vascular endothelium that has been activated as a result of inflammatory response. Subsequent steps involve the up-regulation of integrins on the leukocyte surface by chemoattractants that bind to cell surface receptors, integrin binding to endothelial ligands, causing firm arrest of the rolling leukocyte, and leukocyte migration through the blood vessel wall to enter the extravascular tissue. It has not been established whether a similar process occurs for HSPC that home to and extravasate out of BM microvessels.
Very few studies have looked at the interaction of HSPC with selectins under physiologic flow conditions. CD34 isolated from KG1a cells, a CD34+ human hematopoietic progenitor cell line, was shown to support lymphocyte rolling and tethering through interactions with L-selectin on the lymphocyte surface.3 Because of anticipated differences between CD34+ BM cells and KG1a cells, especially in their carbohydrate repertoires, it is not clear whether these results fully translate to CD34+ BM cells. Another study found that rolling of peripheral blood CD34+cells on primary and transformed human BM endothelial cells was E-selectin dependent.4
Recently, in vivo rolling of HSPC in murine BM has been observed using intravital microscopy.5 Rolling was found not to involve L-selectin, but was reduced in wild-type mice treated with antibodies to E- and P- selectin and in knockout mice deficient in these selectins. In vivo experiments also provide evidence of the role of selectins in regulating the circulation and homing of HSPC. Knockout mice deficient in E- and P-selectin were found to have abnormalities in hematopoiesis6 and defective homing of HSPC to the BM.7
HSPC are heterogeneous and it is possible that distinct subpopulations of HSPC may have different homing activity. The presence or lack of various cell surface markers and combinations of these markers on HSPC have been shown to be associated with increased stem cell activity, as measured by long-term multilineage hematopoietic engraftment ability.8,9 CD34 is a surface antigen present on 1% to 3% of human BM cells that serves as a marker for the identification and separation of HSPC, because it is not found on fully differentiated, or mature, hematopoietic cells.10,11 The cell population expressing CD34 is heterogeneous, with CD34 antigen density highest on early progenitors and its density progressively decreasing to undetectable levels as cells mature.12 CD38 is a surface glycoprotein that is absent from the most primitive adult and fetal BM CD34+ HSPC but is expressed by their differentiation-committed immediate progeny.13,14 The CD34+CD38− population comprises approximately 10% to 20% of the total CD34+ population and is highly enriched for multiprogenitor and stem cell activity, including engraftment ability.8,9 14
We studied the interaction of human HSPC from adult bone marrow (ABM) and fetal liver (FL) with E-, P-, and L-selectin surfaces in a flow chamber to gain insight into the mechanisms behind stem cell trafficking. CD34+ HSPC from both ABM and FL rolled on E-, P-, and L-selectin when subjected to physiologic shear rates, indicating the presence of ligands for all the selectins on HSPC. Our results indicate that CD34+ and CD34+CD38−ABM and FL cells roll more efficiently (to a greater extent and more slowly) than the corresponding more differentiated CD34− and CD34+CD38+ cells. These results suggest the existence and differential expression of selectin ligands on HSPC under physiologic flow conditions and suggest a mechanism for the homing of HSPC into the extravascular spaces of the BM, including the improved ability of more primitive HSPC to engraft in the BM after transplantation.
Materials and methods
E-, P-, and L-selectin substrates
The E-, P-, and L-selectin-IgG chimeras used in this study were a gift from Ray Camphausen (Genetics Institute, Cambridge, MA). The chimeras consisted of the lectin, epidermal growth factor, and multiple short consensus repeat domains for human E-, P-, or L- selectin linked to the Fc region of human IgG1. Controls or selectin chimeras were incubated on silanated glass microscope slides (Sigma, St. Louis, MO) in modified flexiPERM wells (Sigma) at 2.0 μg/mL in phosphate-buffered saline (PBS) overnight at 4°C. Slides were washed with PBS, then incubated with blocking buffer for 1 hour at 22°C to block nonspecific adhesion. Blocking buffer is PBS containing 2% bovine serum albumin (BSA) (Sigma) that is heated at 56°C for 30 minutes before blocking to denature the BSA. Control slides were made by incubating only with blocking buffer or by incubating with human IgG1 (Calbiochem, San Diego, CA).
Antibodies and reagents
All antibodies used were murine monoclonal antibodies (mAbs) directed against the appropriate human molecule. Chimera-coated slides were incubated for 1 hour in 20 μg/mL anti-E-selectin (68-5H11, IgG1, Pharmingen, San Diego, CA), anti-P-selectin (G1, IgG1, Ancell, Bayport, MN), or anti-L-selectin (DREG-56, IgG1, Caltag, Burlingame, CA) in PBS and then inserted into the flow chamber. Cell suspensions at 1 × 106/mL were incubated for 30 minutes at 4°C with 10 μg/mL anti-PSGL-1 (PL1, IgG1, Ancell) or 2 μg/mL anti-CD34 (QBEND/10, IgG1, Biosource International, Camarillo, CA) in RPMI 1640 medium.
Isolation of cell populations
A single cell suspension of FL tissue (18 weeks gestation) was minced, pipetted in complete RPMI medium several times, and passed through a cell strainer. The cells were pelleted (5 minutes, 1100 rpm) and the red blood cells lysed with NH4Cl lysis buffer. After lysis, the cell suspension was diluted 5-fold with complete RPMI and pelleted. This cell pellet was resuspended in complete RPMI in preparation for isolation of CD34+ cells as described later. Post-Ficoll ABM mononuclear cells were obtained from Poietic Technologies (Gaithersburg, MD). The mononuclear cells from ABM or FL were then enriched for CD34 by immunomagnetic positive selection (CD34 progenitor cell isolation kit; Miltenyi Biotech, Inc, Auburn, CA). Briefly, CD34+ HSPC were indirectly magnetically labeled using a monoclonal hapten-conjugated CD34 antibody (QBEND/10, IgG1, 0.5 μg/106 cells) and colloidal superparamagnetic MACS microbeads conjugated to an anti-hapten antibody. The magnetically labeled cells were then enriched on positive selection columns in a magnetic field. CD34 content was assessed by FACS and purity was routinely > 90%.15 For experiments involving CD34+CD38+/− fractions, the CD34+fraction from the column was stained with murine mAbs against human CD34 (581, FITC, Pharmingen, San Diego, CA) and CD38 (HIT2, R-PE, Caltag, Burlingame, CA) for FACS sorting and analysis. Cells collected from FACS sorting were always strongly positive for CD34+. The percentage of CD34+ cells that were sorted as CD38− ranged from 11% to 21% of the live cell gate. The proportion of the live cell gate that was sorted as CD38+ varied from 39% to 50%. The purity of FACS purified cells was routinely higher than 95%.
Flow chamber
All experiments were conducted in a parallel plate flow chamber with a tapered channel design that allows for a linear variation of shear stress down the length of the flow channel at a single flow rate and channel height. This design is ideal for these experiments as it allows us to measure adhesion at many different shear stresses in a single experiment, thus minimizing the necessary supply of ABM or FL cells, which are expensive. The design is based on Hele-Shaw flow theory between parallel plates and has been previously described.16 17 The plates were separated by 250 μm Duralastic sheeting (Allied Biomedical, Paso Robles, CA), which compressed to 180 μm when the flow chamber was fully assembled and tightened. During experiments, the chamber was secured on the stage of a Nikon Diaphot inverted phase contrast microscope (Melville, NY) connected to a monochrome CCD video camera (Cohu, Inc., San Diego, CA) and an S-VHS videocassette recorder (Model SVO-9500MD; Sony Electronics, Park Ridge, NJ). Buffer and cell suspensions were drawn through the chamber by an infusion/withdrawal syringe pump (Harvard Apparatus, South Natick, MA).
Adhesion experiments
E-, P-, or L-selectin coated slides were placed in the well of the flow chamber that was assembled in PBS to prevent air bubbles and then secured on the microscope stage. The perfusion buffer or cell suspension flow rate was set to 0.004 21 mL/s (0.253 mL/min), which for a channel height of 180 μm, gives an observable range in wall shear rates from 77 s−1 to 487 s−1down the length of the channel. The chamber was perfused with RPMI 1640 medium (Gibco BRL, Life Technologies, Rockville, MD) supplemented with 10 mmol/L HEPES for 15 minutes; then the cell suspension (1 × 105/mL) was introduced. Data were collected by stepping down the chamber from inlet to outlet in 5.0-mm steps, allowing at least 1 minute between steps. Cell interaction with the surface was recorded for future analysis at a total magnification of 300× (using a 10× objective). All experiments were performed at room temperature (22°C). Perfusion buffer with 5 mmol/L EDTA (Sigma) or 10 μg/mL fucoidan (Sigma) was used for inhibition experiments with these reagents. Fucoidan is a sialylated, fucosylated plant polysaccharide that has been shown to block the carbohydrate binding domains of L- and P- selectin, but not E-selectin, in a specific, saturable fashion.18
Data analysis
To determine the rolling flux (cells rolling/min/mm2), the number of rolling interactions in each field of view, consisting of a 0.438 mm2 area, were manually counted for 1 minute at each shear stress. Cells were counted if they rolled for > 10 cell diameters while remaining in the field of view. Firmly attached cells were not included in rolling calculations. Cells were considered firmly attached if they remained stationary for > 10 seconds. Velocity measurements were obtained from recorded data using National Instruments' image acquisition (IMAQ) PCI-1408 frame grabber board (Austin, TX), IMAQ software, and LabVIEW 4.0. Virtual instruments (VIs) used in LabVIEW were developed to determine rolling velocities. Briefly, VIs automatically advanced the VCR a specified number of frames, grabbed a designated number of frames spaced equally apart, converted each captured frame into a binary image according to user supplied criteria, detected cells on each image according to user inputted criteria, and recorded these cell positions as coordinates on a 2-dimensional array. Another VI was then used to plot cell trajectories by using the coordinate arrays from the images and representing each detected cell from each grabbed frame as a point on a background plot. Trajectories were then manually selected for and instantaneous and average velocities in both the x and y directions, along with the standard deviation of the average velocities, were automatically calculated and sent to a tab delimited text file that could be imported into spreadsheet and graphing programs. Instantaneous velocity was calculated by dividing the displacement of a rolling cell by the time between incremental captured frames. Average velocity was calculated by averaging the instantaneous velocities for a given trajectory. The time between incremental captured frames was set to 3 seconds, or 90 frames, for E- and P-selectin and 0.5 seconds, or 15 frames, for L-selectin. The total time for each analyzed trajectory was 30 seconds, or 10 iterations, for E- and P-selectin, and 10 seconds, or 20 iterations, for L-selectin. Rolling concentration, an index of overall rolling efficiency, is calculated as rolling flux/mean velocity, giving units of concentration (cells per microliter). For shear rates in which there were < 2 rolling cells, rolling concentration was set equal to zero because mean rolling velocity cannot be determined.
Free stream velocity calculations
Free stream velocities were calculated using the theory of Goldman, Cox, and Brenner,19 20 for a 10-μm-diameter sphere at particle to surface separation of 50 nm and range from 195 μm/s at a shear rate of 77 s−1 to 1232 μm/s at a shear rate of 487 s−1. At this particle to surface separation, it is assumed that a cell would be able to interact with the selectin surface. Free stream velocity gives an estimate of the velocity of a cell very close to, but not rolling on, the selectin surface.
Statistics
Experiments were performed in triplicate when possible. Comparisons of mean values over specified ranges of shear rates were examined with 2-tailed paired Student t tests, with significance taken atP < .05. Data are presented as mean ± SEM, except where indicated.
Results
In order to characterize the interaction of human HSPC with selectins, we immobilized E-, P-, or L-selectin chimeras on the surface of a parallel plate flow chamber. Selectin chimeras consisting of the extracellular domains of the selectins fused to the Fc domain of human IgG1 were adsorbed to silanated glass microscope slides. Interactions of HSPC purified from ABM and FL with the selectin surfaces were observed at wall shear rates ranging from 77 s−1 to 487 s−1 that are similar to the wall shear rates ranging from 23.7 s−1 to 167.9 s−1 that have been observed in murine BM microvessels.5 Rolling velocity, rolling flux, and rolling concentration at each shear rate observed were determined and used to compare populations of cells. Rolling flux (rolling cells counted/min/mm2) gives an indication of the relative number of cells rolling, whereas rolling concentration (=rolling flux/rolling velocity) is a measure of the overall rolling efficiency of cells (cells that roll to a greater extent and slower than other cells roll more efficiently).
We used the CD34 and CD38 cell surface markers to define and isolate subpopulations of ABM and FL cells. Immunomagnetic separation and FACS sorting and analysis were used. CD34+ ABM and FL cells were compared with more differentiated CD34− cells. Comparisons were also made between CD34+CD38− ABM cells and committed progenitor cells represented by CD34+CD38+cells.
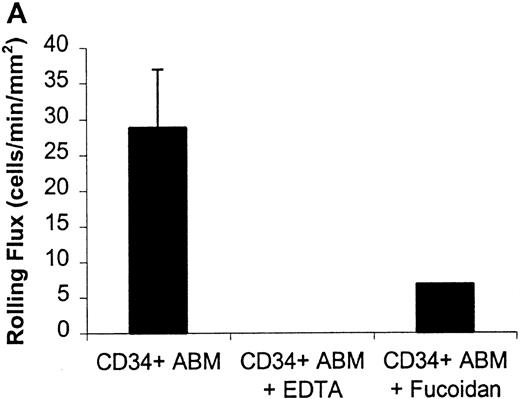
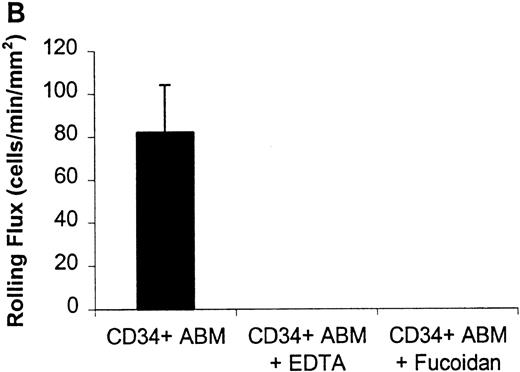
CD34+ ABM and FL cells rolled on surfaces to which E-, P-, or L-selectin chimeras had been immobilized, under physiologic flow conditions (Figures 1 to 5). Control substrates composed of adsorbed human IgG1 or adsorbed BSA alone did not support any cell rolling or firm adhesion (data not shown). Monoclonal antibodies specific for E-, P-, and L-selectin greatly reduced rolling interactions of CD34+ FL on corresponding E-, P-, and L-selectin surfaces (Figure 1 and data not shown). CD34+ ABM cell rolling on P- and L-selectin was calcium dependent as shown by inhibition with EDTA (Figure 2). Rolling on P- and L-selectin was blocked by fucoidan (Figure 2). Also, previously bound and rolling cells were released immediately on infusion of 5 mmol/L EDTA or 10 μg/mL fucoidan. These observations indicate that CD34+ABM and FL cells express functional E-, P-, and L-selectin ligands and that their interactions with the selectins are specific.
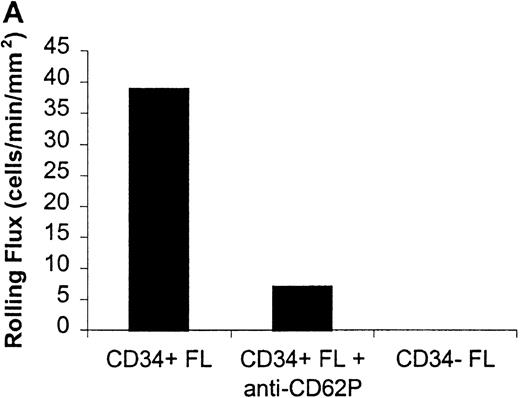
Rolling flux of CD34+ and CD34− FL cells on P- and L-selectin at a shear rate of 210 s−1.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on P- or L-selectin chimeras adsorbed at 2 μg/mL on silanated glass. Rolling flux, rolling velocity, and rolling concentration was determined at 10 shear rates on P- and L-selectin, but only the rolling flux at a representative shear rate of 210 s−1 is shown. In control experiments, chimera-coated slides were incubated with mAb. (A) Rolling flux on P-selectin. Control experiment used the anti-P selectin (CD62P) mAb, G1. (B) Rolling flux on L-selectin. Control experiment used the anti-L selectin (CD62L) mAb, DREG-56. Data presented are from single experiments.
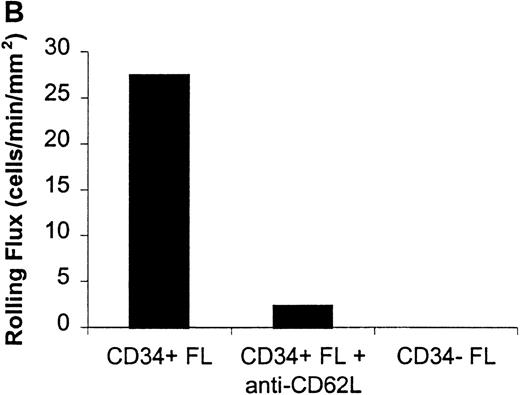
Rolling flux of CD34+ and CD34− FL cells on P- and L-selectin at a shear rate of 210 s−1.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on P- or L-selectin chimeras adsorbed at 2 μg/mL on silanated glass. Rolling flux, rolling velocity, and rolling concentration was determined at 10 shear rates on P- and L-selectin, but only the rolling flux at a representative shear rate of 210 s−1 is shown. In control experiments, chimera-coated slides were incubated with mAb. (A) Rolling flux on P-selectin. Control experiment used the anti-P selectin (CD62P) mAb, G1. (B) Rolling flux on L-selectin. Control experiment used the anti-L selectin (CD62L) mAb, DREG-56. Data presented are from single experiments.
Rolling flux of CD34+ ABM cells on P- and L-selectin at a shear rate of 210 s−1.
Rolling flux, rolling velocity, and rolling concentration were determined at 10 shear rates for P- and L-selectin, but only the rolling flux at a representative shear rate of 210 s−1 is shown. In control experiments, cells were perfused with 5 mmol/L EDTA or 10 μg/mL fucoidan. (A) Rolling flux on P-selectin chimera. (B) Rolling flux on L-selectin chimera. Data for control experiments represents single experiments and is compared with the mean rolling flux for 3 independent experiments ± SEM.
Rolling flux of CD34+ ABM cells on P- and L-selectin at a shear rate of 210 s−1.
Rolling flux, rolling velocity, and rolling concentration were determined at 10 shear rates for P- and L-selectin, but only the rolling flux at a representative shear rate of 210 s−1 is shown. In control experiments, cells were perfused with 5 mmol/L EDTA or 10 μg/mL fucoidan. (A) Rolling flux on P-selectin chimera. (B) Rolling flux on L-selectin chimera. Data for control experiments represents single experiments and is compared with the mean rolling flux for 3 independent experiments ± SEM.
CD34+ ABM cells rolled on E-, P-, and L-selectin at wall shear rates ranging from 77 s−1 to 487 s−1 (Figures3-5). Free stream velocities for cells very close to, but not interacting with, the selectin surfaces were calculated to range from 195 μm/s at a shear rate of 77 s−1 to 1232 μm/s at a shear rate of 487 s−1 (see “Materials and Methods”). Thus, for all the shear rates observed, velocities of cells rolling along the selectin surfaces (Figures 3B, 4B, and 5B) are much slower than free stream velocities. Both CD34+ and CD34−cells rolled much faster on L-selectin than on E- or P-selectin. Rolling velocities were 1- to 2-fold higher on P-selectin than on E-selectin. Rolling velocities of CD34+ ABM cells ranged from 0.7 to 1.0 μm/s on E-selectin, 0.8-1.9 μm/s on P-selectin, and 24-118 μm/s on L-selectin. Increases in shear rate resulted in increases in rolling velocities. However, a 6-fold increase in shear rate produced only a 1.4-fold increase in rolling velocities on E-selectin, a 2.4-fold increase on P-selectin, and a 4.8-fold increase on L-selectin. Rolling flux (Figures 3A, 4A, and 5A) and rolling concentration (Figures 3C, 4C, and 5C) generally decreased with increases in shear rate on E-, P-, and L-selectin. An apparent shear threshold effect21 22 was observed on L-selectin in which rolling flux and concentration increased with increases in shear rate up to 150 s−1 and then decreased (Figure 5A, C) and also on P-selectin in which rolling flux and concentration peaked at approximately 200 s−1 (Figure 4A and C).
CD34+ and CD34− ABM cells rolling on E-selectin.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on E-selectin chimera adsorbed at 2 μg/mL on silanated glass. (A) Rolling flux. Rolling flux was determined at 10 shear rates for each experiment. (B) Rolling velocity. Rolling velocities were determined for every rolling cell at each shear rate for which there were 2 or more rolling cells and then averaged at each shear rate for each experiment. At higher shear rates for which there were < 2 rolling cells, mean rolling velocity is not presented. (C) Rolling concentration. Rolling concentration ( = rolling flux/mean rolling velocity) was determined at 10 shear rates for each experiment. Each data point represents results from 2 independent experiments. Data are presented as mean ± SEM.
CD34+ and CD34− ABM cells rolling on E-selectin.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on E-selectin chimera adsorbed at 2 μg/mL on silanated glass. (A) Rolling flux. Rolling flux was determined at 10 shear rates for each experiment. (B) Rolling velocity. Rolling velocities were determined for every rolling cell at each shear rate for which there were 2 or more rolling cells and then averaged at each shear rate for each experiment. At higher shear rates for which there were < 2 rolling cells, mean rolling velocity is not presented. (C) Rolling concentration. Rolling concentration ( = rolling flux/mean rolling velocity) was determined at 10 shear rates for each experiment. Each data point represents results from 2 independent experiments. Data are presented as mean ± SEM.
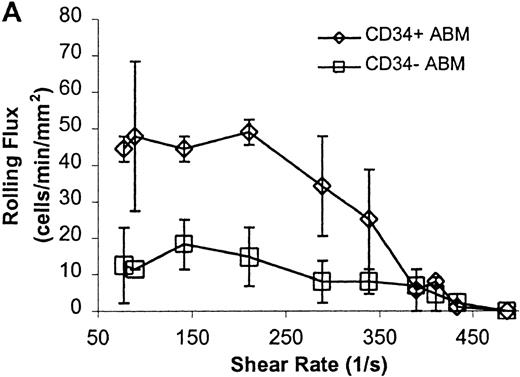
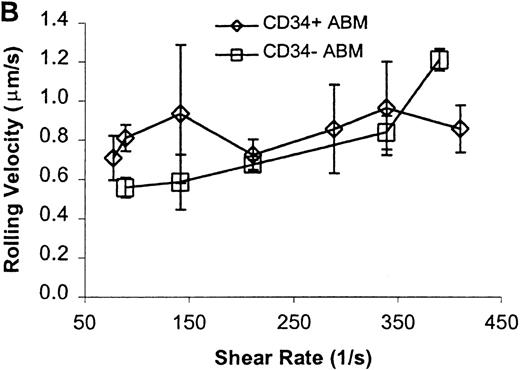
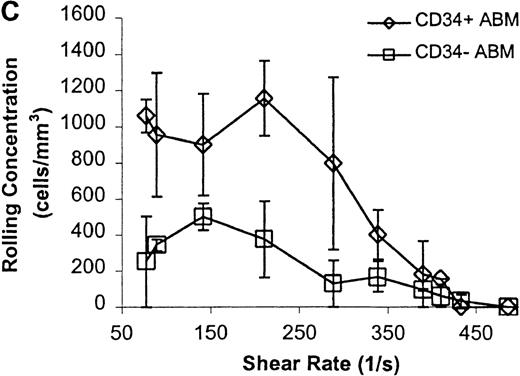
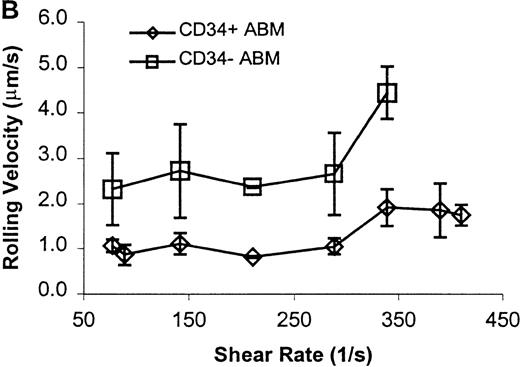
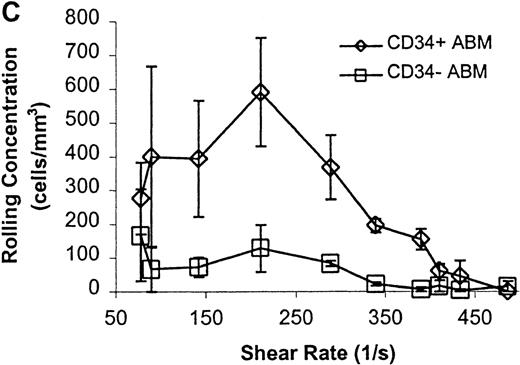
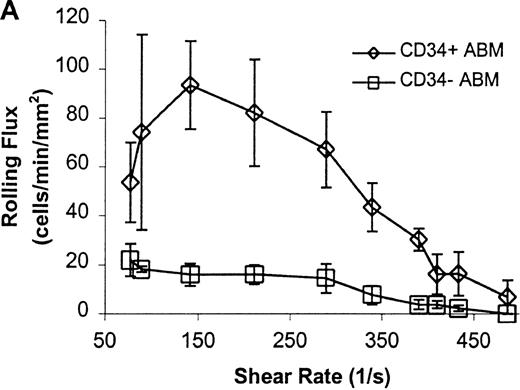
CD34+ and CD34− ABM cells rolling on P-selectin.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on P-selectin chimera adsorbed at 2 μg/mL on silanated glass. (A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
CD34+ and CD34− ABM cells rolling on P-selectin.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on P-selectin chimera adsorbed at 2 μg/mL on silanated glass. (A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
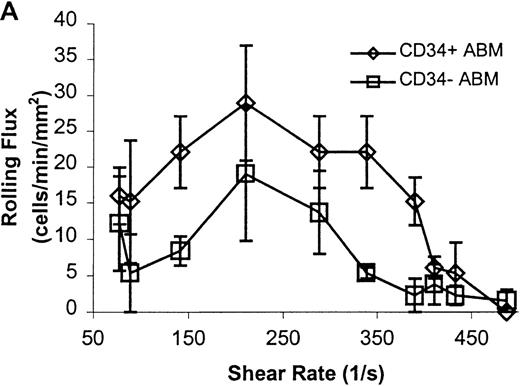
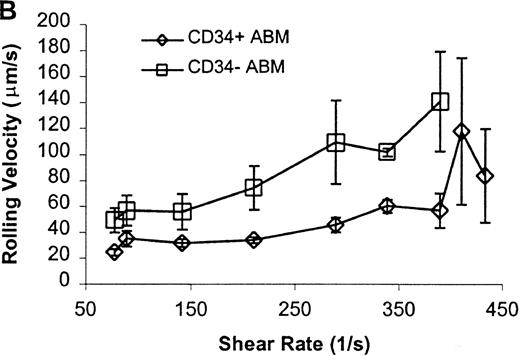
CD34+ and CD34− ABM cells rolling on L-selectin.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on L-selectin chimera adsorbed at 2 μg/mL on silanated glass. (A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
CD34+ and CD34− ABM cells rolling on L-selectin.
Cells were subjected to wall shear rates from 487 s−1to 77 s−1 on L-selectin chimera adsorbed at 2 μg/mL on silanated glass. (A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
Significant differences in rolling properties were observed for CD34+ cells compared with CD34−cells. CD34− cells are those cells that were not selected by CD34+ magnetic separation. CD34+ ABM and FL cells had a higher rolling flux than CD34− cells on E-, P-, and L-selectin (Figures 1, 3A, 4A, and 5A). For ABM cells, this effect was the most pronounced on L-selectin, but was significant across a range of shear rates from 77 s−1 to 339 s−1 on E-selectin (P = .002), 77 s−1 to 390 s−1 on P-selectin (P = .0005), and 77 s−1 to 433 s−1 on L-selectin (P = .0006). Differences were greater when comparing CD34+ and CD34− FL cells. CD34− FL cells showed greatly reduced E-, P-, and L-selectin ligand activity compared with CD34+ FL cells with little rolling of CD34− FL cells at any shear rate observed (Figure 1and data not shown). CD34+ ABM cells rolled more slowly than CD34− ABM cells on P- and L-selectin (Figures 4B and 5B), but not on E-selectin (Figure 3B). The reduction in rolling velocities on P- and L-selectin was significant across a range of shear rates from 77 s−1 to 410 s−1(P = .001 and P = .003, respectively). Rolling concentration was much lower for CD34− ABM cells than CD34+ ABM cells on E-, P-, and L-selectin (Figures 3C, 4C, and 5C). This effect was most pronounced on L-selectin, but was significant at shear rates from 77 s−1 to 339 s−1 on E-selectin (P = .001) and 77 s−1 to 390 s−1 on P- and L-selectin (P = .001 and P = .002, respectively).
Few differences between ABM and FL cells were observed on E-, P-, or L-selectin. Comparison of CD34+ versus CD34− FL cells generally revealed the same trends in rolling flux (Figure 1), rolling velocity (data not shown), and rolling concentration (data not shown) as CD34+ versus CD34− ABM cells.
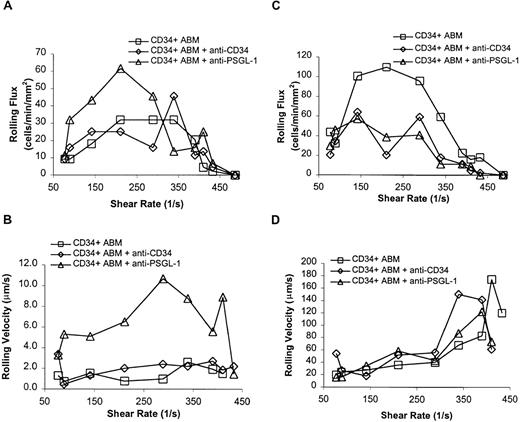
Neither the CD34-specific mAb QBEND/10 nor PSGL-1- specific mAb PL1 significantly reduced the rolling flux of CD34+ ABM cells on P-selectin (Figure 6A). However, PSGL-1 mAb PL1 greatly increased rolling velocities on P-selectin from 77 s−1 to 410 s−1(P = .0005, Figure 6B), indicating at least partial blocking of the P-selectin ligands on CD34+ ABM cells with this mAb. The CD34 mAb QBEND/10 had no effect on rolling velocities on P-selectin. The addition of either CD34 or PSGL-1 mAbs reduced rolling flux on L-selectin from 77 s−1 to 410 s−1 (P = .02 for each, Figure 6C) but had no effect on rolling velocities (Figure 6D). These results indicate that PSGL-1 acts as a ligand for P-selectin on the surface of CD34+ ABM cells as shown by increased rolling velocities. The epitope recognized by the QBEND/10 mAb on CD34 does not act as a ligand for P-selectin. Less conclusive results were obtained on L-selectin, but it appears that both PSGL-1 and CD34 on the surface of CD34+ ABM cells may both play a modest role in mediating interactions with L-selectin as shown by the effect of the mAbs on rolling fluxes.
Antibody blocking experiments for CD34+ ABM cells on P- and L- selectin.
Cells were preincubated with mAb indicated before being perfused over P- or L-selectin chimera surface. The CD34 mAb used was QBEND/10.32,36 The PSGL-1 mAb used was PL-1.31 32 (A) Rolling flux on P-selectin. (B) Rolling velocity on P-selectin. (C) Rolling flux on L-selectin. (D) Rolling velocity on L-selectin. Each data point represents results from a single experiment.
Antibody blocking experiments for CD34+ ABM cells on P- and L- selectin.
Cells were preincubated with mAb indicated before being perfused over P- or L-selectin chimera surface. The CD34 mAb used was QBEND/10.32,36 The PSGL-1 mAb used was PL-1.31 32 (A) Rolling flux on P-selectin. (B) Rolling velocity on P-selectin. (C) Rolling flux on L-selectin. (D) Rolling velocity on L-selectin. Each data point represents results from a single experiment.
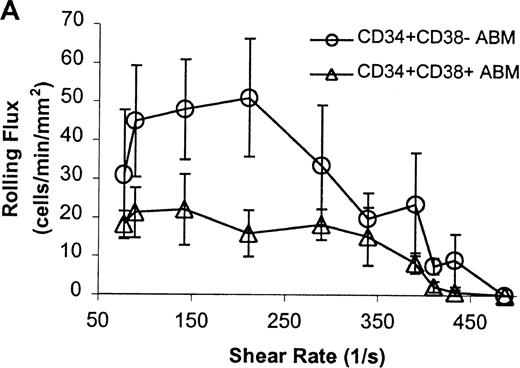
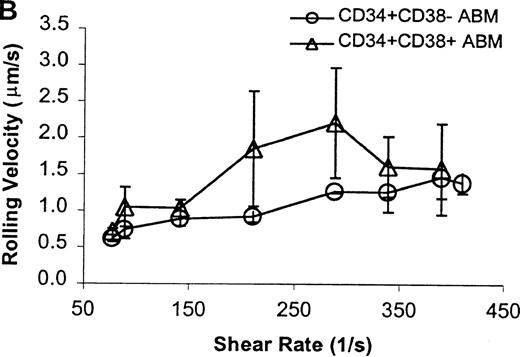
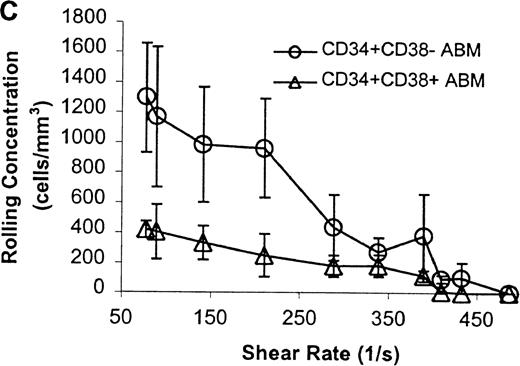
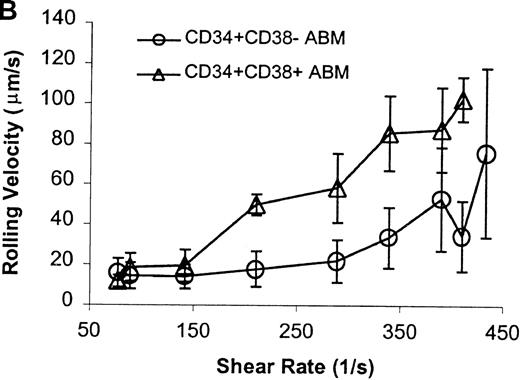
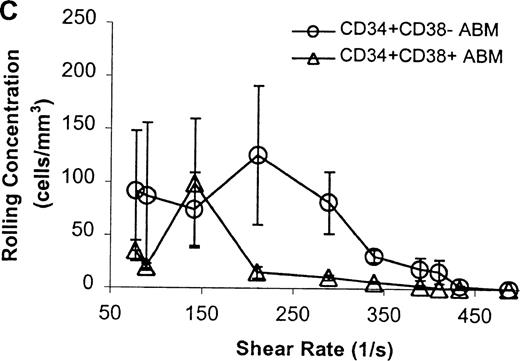
The CD34+ ABM population was further fractionated into the CD38+ and CD38− subpopulations by FACS sorting and analysis. The rolling efficiency of CD34+CD38− ABM cells was greater than for CD34+CD38+ ABM cells. Rolling flux was greater for CD34+CD38− ABM cells than CD34+CD38+ ABM cells on E-selectin from 77 s−1 to 211 s−1 (P = .01, data not shown), on P-selectin from 77 s−1 to 289 s−1 (P = .005, Figure7A), and on L-selectin from 211 s−1 to 339 s−1 (P = .02, Figure 8A). This difference was most evident on P-selectin. CD34+CD38− ABM cells rolled slower than CD34+CD38+ ABM cells on P-selectin from 211 s−1 to 289 s−1 (P = .007, Figure 7B) and on L-selectin from 211 s−1 to 410 s−1(P = .003, Figure 8B). The reduction in rolling velocity was most pronounced on L-selectin. Thus, the fast rolling of CD34+ ABM cells on L-selectin could be resolved into faster (CD34+CD38+) and slower (CD34+CD38−) rolling populations (Figure8B). Rolling concentration was greater for CD34+CD38− cells than CD34+CD38+ cells on E-selectin at shear rates from 77 s−1 to 211 s−1(P = .01, data not shown), on P-selectin from 77 s−1 to 289 s−1 (P = .003, Figure 7C), and on L-selectin from 77 s−1 to 339 s−1 (P = .04, Figure 8C). This difference was the most evident on P-selectin. Interestingly, a maximum in rolling flux and rolling concentration on L-selectin (Figure 8A, C) was seen with CD34+CD38− ABM cells, suggesting that the shear threshold effect on L-selectin21 is preserved for this subpopulation.
CD34+CD38− ABM and CD34+CD38+ ABM cells rolling on P-selectin.
(A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
CD34+CD38− ABM and CD34+CD38+ ABM cells rolling on P-selectin.
(A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
CD34+CD38− ABM and CD34+CD38+ ABM cells rolling on L-selectin.
(A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
CD34+CD38− ABM and CD34+CD38+ ABM cells rolling on L-selectin.
(A) Rolling flux. (B) Rolling velocity. (C) Rolling concentration. Each data point represents results from 3 independent experiments. Data are presented as mean ± SEM.
Discussion
During BM and purified HSPC transplantation, as well as in normal physiologic processes, HSPC must home to the BM. The similarities between HSPC and leukocyte trafficking invite comparison and suggest that an examination of selectin-mediated HSPC adhesion is warranted. In vitro flow chambers have been an effective tool to elucidate leukocyte rolling mechanisms23-25 and thus should be useful for elucidating the molecular mechanisms of HSPC rolling. The increased ability of more primitive HSPC populations to roll on selectins may be an important feature that contributes to their ability to home to the marrow spaces and subsequently engraft.8 9
Human HSPC isolated from ABM and FL rolled on E-, P-, and L-selectin under physiologic flow conditions. Results indicated the presence of ligands on HSPC that supported rolling and are calcium dependent, as shown by inhibition of rolling with EDTA. Rolling was inhibited by antibodies to each of the selectins and by fucoidan on P- and L-selectin. These results give direct evidence of ligands for E-, P-, and L-selectin on human HSPC.
Our results also show that rolling, characterized by rolling velocity, rolling flux, and rolling concentration, differs during the progression from primitive stem/progenitor hematopoietic cells to committed progenitor cells to lineage committed cells. Our results indicate that primitive ABM and FL stem/progenitor cells (CD34+ and CD34+CD38−) roll more efficiently than more differentiated cells (CD34− and CD34+CD38+, respectively) on E-, P-, and L-selectin. This is the first study to show that HSPC demonstrate different capacities for rolling on selectins, based on their progression in hematopoietic development.
Both in vivo and in vitro studies, including this study, are limited because of the rarity of the HSPC and the associated difficulty in isolating a large enough number of cells for multiple experiments. As such, there remains much to be done in studying the interactions of HSPC with selectins. Cell lines, such as KG1a, a CD34+human hematopoietic progenitor cell line, or animal HSPC were not used because of the difficulty in extrapolating results to nontransformed human HSPC. Animal or transformed human HSPC may express completely different or slightly modified ligands and receptors than primary HSPC. Fine differences in carbohydrate chemistry can greatly affect interactions between the selectins and their ligands on the cell surface.17 26 Analysis of primary HSPC isolated from human ABM and FL cells provides the most direct test of the mechanism of human HSPC transplantation.
There is considerable evidence for the expression of selectins and their ligands by BM endothelial cells and HSPC. BM endothelial cells express E- and P-selectin,4,27 as well as CD34.4 HSPC express L-selectin,5,28-30PSGL-1,5,27,31,32 and CD34.10-12 It should also be noted that L-selectin has been reported to express sialyl LewisX (sLeX) and to function as a counter-receptor for E- and P-selectin.33 Because the endothelium of BM microvessels represents a continuous barrier5 that homing HSPC must cross to nest in the BM extravascular space, it is certainly plausible that a critical step in this process is the adhesion of HSPC to E- and P-selectin expressed by the endothelium of BM microvessels and/or L-selectin expressed by HSPC adherent to BM microvessels, which could be presented to other HSPC in flow.
The HSPC cell surface molecules responsible for the homing of HSPC to the BM are currently not known. However, our initial results suggest roles for PSGL-1 and possibly CD34 as evidenced by antibody blocking experiments on substrates of P- and L-selectin. The PSGL-1 mAb PL1 greatly increased rolling velocities of CD34+ ABM cells on P-selectin, indicating that PSGL-1 acts as a ligand for P-selectin on CD34+ ABM cells. Both the PSGL-1 mAb PL1 and the CD34 mAb QBEND/10 modestly reduced the rolling flux of CD34+ABM cells on L-selectin. One recognized difficulty with studying CD34 recognition with antibodies is that CD34 is a heterogeneous ligand that is differentially glycosylated in different phenotypes (reviewed in Krause et al34), and study of its activity would be greatly aided by a more diverse repertoire of antibodies against CD34. Further experiments are necessary to completely elucidate the selectin ligands responsible for mediating the rolling on selectin surfaces.
Differential expression of selectin ligands may be a way of determining which HSPC can home to the BM most effectively. The increased rolling ability on E-, P-, and L-selectin for CD34+ cells compared with CD34− cells suggests that differential expression of selectin ligands on ABM and FL cells exists. These results, in conjunction with a similar increase in rolling efficiency on E-, P-, and L-selectin for CD34+CD38−ABM cells compared with CD34+CD38+ ABM cells, indicate a correlation between selectin ligand expression on the surface of HSPC and their progression in hematopoietic development. More immature HSPC roll at a higher concentration on E-, P-, and L-selectin than more mature HSPC. As mentioned earlier, immature HSPC, as defined by the presence of CD34 and lack of CD38 cell surface antigens, are enriched for stem cell activity, as measured by long-term multilineage hematopoietic engraftment ability, relative to other more differentiated hematopoietic precursor populations (CD34+CD38− or CD34−).8 9 This suggests a link between selectin ligand expression and stem cell activity, including propensity for engraftment. We propose that the increased rolling efficiency of more primitive HSPC allows them to be retained in greater numbers by the endothelium of BM microvessels, allowing more of these cells to extravasate into the BM stromal matrix. Thus, primitive HSPC are able to home more effectively than their more differentiated progeny.
These results suggest a role for the selectins in the homing of HSPC to the BM. We hypothesize that HSPC may home to the BM as a result of binding to E-, P-, or L-selectin or combinations of these. This process might mimic the known multistep process for extravasation of mature leukocytes. In the initial steps, HSPC would roll on E- and P-selectins expressed by BM microvessel endothelial cells or on L-selectin expressed by HSPC already adherent to the BM endothelium. Subsequent steps would involve stronger binding of integrin molecules on HSPC to their ligands on the BM endothelium resulting in firm adhesion of the HSPC to the vessel walls. HSPC may then transverse through the endothelial layer, adhere to the BM extracellular stromal matrix, and undergo proliferation and maturation in the BM extravascular space.
Our results generally agree well with and may provide advantages over in vivo studies that show a role for selectins in the rolling of HSPC in murine BM microvessels5 and also in the homing of HSPC to the BM of lethally irradiated knockout mice.7 In studies with knockout mice deficient in E- and P-selectin,5-7 there may exist compensation mechanisms, such as elevated cytokine levels,6 that can affect both results and conclusions about molecular mechanisms. Similarly, in normal animals, many molecular and cellular interactions may play a role in homing, making it difficult to test hypotheses. Such complications do not exist in a reconstituted system such as ours. Although a previous study utilizing intravital microscopy in mice found that rolling of an injected cell line in normal adult BM microvessels did not involve L-selectin,5we do not rule out a role for L-selectin in the homing of HSPC to the BM. As mentioned earlier, cell lines may possess different or modified adhesion molecules than primary cells. Also, interactions involving L-selectin may play a more important role in BM affected by chemotherapy or radiation or in developing fetal BM. In vitro experiments have shown that neutrophils use L-selectin to accumulate near already adherent neutrophils.21,35 36We hypothesize that a similar process may occur for HSPC that would allow accumulation of HSPC at sites of extravasation in BM microvessels.
Our results do not exclude a role for other adhesion molecules or chemoattractants that may be critical for firm arrest of rolling cells or subsequent extravasation. Also, because the selectins are expressed on many tissues, additional mechanisms to ensure specific homing of HSPC to the BM must be required. Perhaps, specificity is provided by other adhesion molecules or unique chemoattractants secreted within the BM microenvironment. Also, the selectins could be inducibly upregulated or constitutively expressed on BM endothelial cells. Evidence of constitutive expression of E- and P-selectin on murine BM venules and sinusoids, in the absence of inflammation, has been found.5 This same study also found that bone venules, in immediate vicinity to BM venules and sinusoids, did not support rolling of HSPC, suggesting that BM microvessels express the selectins selectively as well.
Because the success of BM and HSPC transplantation depends on the efficient seeding of grafted cells in the recipient's BM, the further characterization of HSPC trafficking is of great importance. The evidence that more primitive subpopulations of HSPC that engraft better in BM during transplantation and that bind preferentially to selectin substrates invites examination of additional subpopulations of HSPC. Results have shown that the subfraction of CD34+CD38− ABM cells expressing c-mpl, the thrombopoietin receptor, engrafts significantly better in a severe combined immunodeficient-human bone model than the subfraction lacking c-mpl expression,15 inviting investigation of whether this subpopulation is further endowed for preferential homing to the BM. These preferential homing abilities of the most primitive HSPC suggest that evolution has developed a natural method for retention of stem cells in the rich BM environment, a mechanism that can be further exploited in BM transplantation through additional fractionation of donor BM before transplantation.
Acknowledgments
We thank Heather McIntosh and John Ninos for the purification of the human stem/progenitor cell populations used in this study.
Drs Kerr and Hammer contributed equally to this article.
Submitted May 19, 1999; accepted September 17, 1999.
Supported by a Whitaker Foundation fellowship to A.W.G. and National Institute of Health (NIH) grants RO1 DK54767-01 to W.G.K. and HL18208 to D.A.H.
Reprints:Daniel A. Hammer, Department of Chemical Engineering, 311A Towne Building, 220 S 33rd St, Philadelphia, PA 19104.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal