Allogeneic stem cell transplantation is the only treatment that can restore a normal hematopoiesis in Fanconi anemia (FA). In this retrospective multicenter study, we analyzed the results of this approach using HLA-matched unrelated bone marrow donors, and tried to identify covariates predicting the outcome of the transplant. From January 1985 to June 1998, 69 FA patients were transplanted with unrelated HLA-matched donors. Patients' characteristics before and after transplant were provided by the European group blood and marrow transplant registry and were analyzed in collaboration with the European Fanconi Anemia Registry. The 3-year probability of survival was 33%. Extensive malformations, a positive recipient cytomegalovirus serology, the use of androgens before transplant, and female donors were associated with a worse outcome. Primary graft failures were observed more frequently when female donors were used, mainly because the grafts contained lower nucleated cell doses per kilogram of recipient body weight compared with grafts coming from male donors. The probability of grade III-IV acute graft-versus-host disease (GVHD) was 34%. Elevated serum alanine/aspartate transaminases before transplantation; limb, urogenital tract, or nephrologic malformations; and non–T-cell-depleted grafts were predictors of severe acute GVHD. This study shows the dramatic impact of preexisting congenital malformations on the outcome of FA patients transplanted with HLA-matched unrelated donors. If the use of T-cell depletion has led to a dramatic reduction of acute GVHD incidence, no significant outcome improvement was observed with this approach, mainly because of an increased risk of graft failure.
Fanconi anemia (FA) is an autosomal recessive disorder belonging to the group of chromosomal instability syndromes.1 Skeletal and urogenital malformations, skin hyperpigmentation, and pancytopenia are the classical hallmarks of the disease.2 However, atypical presentations are frequently reported with patients more than 20 years of age and no morphological abnormalities.3 The diagnosis is usually confirmed by increased chromosomal breakage in FA cells following exposure to DNA cross-linking agents such as mitomycin C and diepoxybutane, or less frequently by a cell cycle study displaying an arrest of the cells in G2/M phase.4-7 The natural history of FA is to evolve toward progressive bone marrow failure, which is most often lethal before the end of the second decade of life without treatment. The disease also predisposes to myelodysplasia and acute myeloid leukemia with a greater than 50% actuarial risk of developing these complications by 40 years of age.8 A transient improvement in hematopoietic functions can be achieved with androgens, corticosteroids, or hematopoietic growth factors.9-12However, allogeneic stem cell transplantation is currently the only treatment option that can restore normal hematopoiesis in FA patients. A greater than 70% probability of 5-year survival can be achieved with this approach if an HLA-matched sibling donor is available.13-18 For patients lacking such a donor, stem cell transplantation using HLA-matched unrelated donors can be proposed, but previous results have been disappointing, mostly because transplants were performed in patients with advanced disease.19 Moreover, until now, it was not possible to define predictors of better outcome since the results were confined to small series of patients.20 21 This retrospective multicenter study on behalf of the European Group for Blood and Marrow Transplantation, in collaboration with the European Fanconi Anemia Registry, analyzes the results of 69 allogeneic stem cell transplantation in FA patients using HLA-matched unrelated donors and tries to identify factors predicting the outcome.
Materials and methods
Patients
From January 1985 to June 1998, data from 69 consecutive patients with FA undergoing allogeneic stem cell transplantation with HLA-matched unrelated donors were sent by 16 centers referring to the European Group for Blood and Marrow Transplantation–Aplastic Anemia Working Party or to the European Fanconi Anemia Registry. Of these transplants, 90% were performed after 1990, and two thirds during the last 5 years of the study. Patients were included if lymphocyte chromosome breaks increased after exposure to DNA cross-linking agents was observed. Patients who received cord blood transplants were not included in the study. Characteristics of the patients are summarized in Table 1. For the overall group, median age at diagnosis was 6.3 years (range, birth to 27.4); median age at the time of transplant was 10.8 years (range, 4.0 to 37.4); and median time from diagnosis to transplant was 42 months (range, 3 to 384). The extent of the malformative syndrome was assessed according to the number of anatomic sites involved.2 Skin abnormalities were not considered to be contributing to the extent of the malformative syndrome as they were observed in more than 90% of the patients. Each of the following areas was considered as one anatomic site: (1) head, including eyes (small eyes, strabismus, epicanthal folds, hypertelorism), ears (deafness, abnormal shape, atresia, dysplasia, low set, canal stenosis, abnormal middle ear), face (microcephaly, micrognathia, triangular face), and neck (short, sprengel); (2) limbs, including thumbs and radii abnormalities (absent, hypoplastic, short, supernumerary, bifid, or low set for thumbs, and absent or hypoplastic for radii), hypoplastic thenar eminence, clinodactyly, 6 fingers, absent first metacarpal, abnormal or short fingers, dysplastic ulnae; (3) kidneys; (4) gastrointestinal tract; (5) urogenital tract; and (6) cardiovascular system. Forty-six patients had at least 1 malformation of the head; 27 patients had limb malformations; 9 patients had malformations of the cardiovascular system; and 8 had malformations of the gastrointestinal tract. Among the 23 patients with kidney malformations, 26% (n = 6) also had urogenital tract malformations, whereas in the group of patients with no kidney malformation, 11% had urogenital tract malformations (n = 5), a difference that did not reach a significant level (P = .17). Even if more than 1 malformation was observed in 1 defined anatomic site (eg, ears, eyes, and neck, for the head), this site was considered only once when assessing the extent of the malformative syndrome. According to these definitions, the malformative syndrome was considered as extensive if at least 3 sites were involved, which meant involving at least 1 deep organ (kidneys, gastrointestinal, or urogenital tract, cardiovascular system). For example, the malformative syndrome was considered to be extensive if the patient had absent thumb, microcephaly, and pelvic kidney, whereas it was considered to be limited if the patient had clinodactyly, absent thumb, hypoplastic radii, microcephaly, and strabismus. The severity of the bone marrow failure syndrome at the time of transplant was defined according to the number of severe cytopenia observed. Criteria used to define severe cytopenia were an absolute neutrophil count ≤ 0.5 × 109/L, a platelet count ≤ 20 × 109/L or 20 or more pretransplant platelet transfusions, and a hemoglobin level ≤ 80 g/L or 20 or more pretransplant red blood cell transfusions. Seven patients had no severe cytopenia and had not received any transfusion at the time of transplant. In these cases, the transplant was considered either because of rapidly decreasing blood counts or because a cytogenetic clonal abnormality on bone marrow cells was observed. Eleven patients (16%) had pretransplant features of myelodysplasia (n = 8) or acute myeloid leukemia (n = 3).
Characteristics of the patients
| Covariates . | No. . |
|---|---|
| Recipient gender | |
| Male | 36 |
| Female | 33 |
| Recipient cytomegalovirus serology | |
| Negative | 41 |
| Positive | 24 |
| Malformative syndrome | |
| Limited (<3 sites) | 44 |
| Extensive (≥3 sites) | 23 |
| Androgen therapy before transplant | |
| No | 27 |
| Yes | 41 |
| Elevated transaminases before conditioning | |
| No | 51 |
| Yes | 15 |
| Number of pretransplant transfusions | |
| <20 | 23 |
| ≥20 | 43 |
| Hemogram at transplant | |
| Hemoglobin level | |
| <80 g/L | 20 |
| ≥80 g/L | 45 |
| Absolute neutrophil count | |
| <0.5 109/L | 19 |
| ≥0.5 109/L | 47 |
| Platelet count | |
| <20 109/L | 14 |
| ≥20 109/L | 50 |
| Covariates . | No. . |
|---|---|
| Recipient gender | |
| Male | 36 |
| Female | 33 |
| Recipient cytomegalovirus serology | |
| Negative | 41 |
| Positive | 24 |
| Malformative syndrome | |
| Limited (<3 sites) | 44 |
| Extensive (≥3 sites) | 23 |
| Androgen therapy before transplant | |
| No | 27 |
| Yes | 41 |
| Elevated transaminases before conditioning | |
| No | 51 |
| Yes | 15 |
| Number of pretransplant transfusions | |
| <20 | 23 |
| ≥20 | 43 |
| Hemogram at transplant | |
| Hemoglobin level | |
| <80 g/L | 20 |
| ≥80 g/L | 45 |
| Absolute neutrophil count | |
| <0.5 109/L | 19 |
| ≥0.5 109/L | 47 |
| Platelet count | |
| <20 109/L | 14 |
| ≥20 109/L | 50 |
Transplant procedure
Characteristics of the transplant procedures are listed in Table2. All but 2 patients received a conditioning regimen including irradiation: either total body irradiation (n = 39, 56%) or thoracoabdominal irradiation (n = 28, 41%). The 2 patients who were not irradiated received higher doses of cyclophosphamide. A combination of cyclophosphamide 40 to 45 mg/kg total dose, anti-T serotherapy (anti-thymocyte globulin or monoclonal antibody), and total body irradiation was the most frequently used conditioning regimen (39%). Total body irradiation (P < .001) and anti-thymocyte globulin (P < .02) were more often used during the conditioning of patients who received T-cell–depleted grafts. Bone marrow was the hematopoietic stem cell source used in all but 1 case where peripheral blood progenitor cells were used. Concerning the non–T-cell–depleted grafts (n = 43), the median nucleated cell dose infused per kilogram of recipient body weight was 3.17 × 108 (range, .23-13.5, n = 41). The median dose of CD34+ cells infused was 2 × 106 per kilogram of recipient body weight (range, .27-4.30) for the patients who received a T-cell–depleted graft using CD34+ selection (n = 16). Growth factors, mainly granulocyte-colony stimulating factor, were introduced during the first week after graft infusion in 9 cases. Recipient/donor HLA matching was based on reagents available when the typing was performed. Patients and donors were typed for HLA-A and HLA-B with the use of serological techniques. High-resolution molecular typing for HLA-DRB1 was used in 46 pairs (67%). Although various graft-versus-host disease (GVHD) prophylaxes were reported by individual centers, 3 associations were used predominantly: cyclosporine A (CsA) plus corticosteroids (n = 16, 23%), cyclosporine plus methotrexate (n = 13, 19%), and CsA plus anti-T serotherapy (n = 20, 29%). Twenty-six grafts were T-cell depleted (38%) with the use of either CD34+ selection (n = 16) or negative selection based on ex vivo monoclonal antibodies strategies (n = 10). Acute and chronic GVHD was graded according to the Seattle criteria.22 23 Analysis involving chronic GVHD was based upon patients who had neutrophil recovery and survived longer than 100 days from transplant.
Characteristics of the transplant procedures
| Covariates . | No. . |
|---|---|
| Conditioning regimens | |
| Cyclophosphamide (Cy) total dose | |
| Cy 20 mg/kg | 14 |
| Cy 40 to 45 mg/kg | 53 |
| High-dose Cy (≥60 mg/kg) | 2 |
| Irradiation | |
| Total body irradiation (TBI) | 39 |
| Thoracoabdominal irradiation (TAI) | 28 |
| No irradiation | 2 |
| Details | |
| Cy 20 mg/kg + TAI ± Anti-T serotherapy | 5 |
| Cy 20 mg/kg + TBI ± Anti-T serotherapy | 7 |
| Cy 40 mg/kg + TAI ± Anti-T serotherapy | 14 |
| Cy 40-45 mg/kg + TBI ± Anti-T serotherapy | 30 |
| Cy 40 mg/kg + Arac + TAI + Anti-T serotherapy | 6 |
| Others with irradiation | 5 |
| Others without irradiation | 2 |
| Graft-versus-host disease prophylaxis | |
| Without cyclosporine A (CsA) | 4 |
| Cyclosporine A alone | 9 |
| CsA + corticosteroids | 16 |
| CsA + methotrexate ± corticosteroids | 15 |
| CsA + anti-T serotherapy + methotrexate | 5 |
| CsA + anti-T serotherapy ± corticosteroids | 20 |
| Ex vivo T-cell depletion | |
| No | 43 |
| Yes | 26 |
| Covariates . | No. . |
|---|---|
| Conditioning regimens | |
| Cyclophosphamide (Cy) total dose | |
| Cy 20 mg/kg | 14 |
| Cy 40 to 45 mg/kg | 53 |
| High-dose Cy (≥60 mg/kg) | 2 |
| Irradiation | |
| Total body irradiation (TBI) | 39 |
| Thoracoabdominal irradiation (TAI) | 28 |
| No irradiation | 2 |
| Details | |
| Cy 20 mg/kg + TAI ± Anti-T serotherapy | 5 |
| Cy 20 mg/kg + TBI ± Anti-T serotherapy | 7 |
| Cy 40 mg/kg + TAI ± Anti-T serotherapy | 14 |
| Cy 40-45 mg/kg + TBI ± Anti-T serotherapy | 30 |
| Cy 40 mg/kg + Arac + TAI + Anti-T serotherapy | 6 |
| Others with irradiation | 5 |
| Others without irradiation | 2 |
| Graft-versus-host disease prophylaxis | |
| Without cyclosporine A (CsA) | 4 |
| Cyclosporine A alone | 9 |
| CsA + corticosteroids | 16 |
| CsA + methotrexate ± corticosteroids | 15 |
| CsA + anti-T serotherapy + methotrexate | 5 |
| CsA + anti-T serotherapy ± corticosteroids | 20 |
| Ex vivo T-cell depletion | |
| No | 43 |
| Yes | 26 |
The day of graft infusion was defined as day 0. The day of neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count ≥ 0.5 × 109/L. Primary graft failure was diagnosed if neutrophil recovery was not reached by day 30. Since the earliest neutrophil recovery occurred at day 9, patients who died before day 9 were not considered in the analysis of predictors of neutrophil recovery. Secondary graft failure was defined as a recurrent pancytopenia with an absolute neutrophil count < 0.5 × 109/L, in the absence of severe acute GVHD. Patients who did not achieve neutrophil recovery by day 30 were not considered for the analysis of risk factors associated with secondary graft failure. The day of platelet recovery was defined as the first of 7 days with a minimal platelet count of 20 × 109/L without transfusion.
Statistical analysis
Survival was assessed on the date of last patient contact and analyzed on September 1, 1998. The probabilities of hematopoietic recovery, secondary graft failure, acute GVHD, overall survival, and day-100 transplant-related mortality were estimated from the time of transplant, according to the Kaplan-Meier product-limit method.24 Groups were compared with the use of the 2-tailed log-rank test. Covariates found significant at a P value of less than .20 were subsequently introduced in a Cox proportional hazards model and selected with the use of a stepwise procedure.25 Identification of influential observations was achieved with the use of 1-step delta beta diagnoses for each coefficient together with the generalized Cook's distance.26 Collinearity between significant covariates was assessed according to Weissfeld's criteria.27 Potential interactions between significant covariates were tested by adding cross-product terms to the final models. Departure from the proportional hazards assumption was assessed by the use of the method based on partial residuals and by a graphical approach.28 29 Covariates not satisfying the proportional hazards assumption were reintroduced as time-dependent covariates, rather than via stratification, in order to obtain estimates of the relative risk associated with them. When groups were compared according to continuous covariates, the Mann-Whitney U test was used for difference in medians. According to the group sizes, the chi-square or Fisher exact test was used to compare categorical covariates. SPIDA 6.0 software for DOS was used for all statistical analyses.
Results
Hematopoietic recovery
Fifty-five of the 69 FA patients had a neutrophil recovery, which occurred at a median time of 14 days from graft infusion (range, 9 to 33). The overall day-30 probability of achieving an absolute neutrophil count ≥ 0.5 × 109/L for at least 3 days was 83% (SE, 5%). The probability of achieving a platelet count ≥ 20 × 109/L for at least 7 days without transfusions during the first 2 months after transplant was 69% (SE, 7%), with a median of 17 days (range, 9 to 62) to reach this end point. The following covariates significantly affecting neutrophil recovery in univariate analysis were subsequently introduced in the Cox proportional hazards model: donor gender (female 77% versus male 92%,P = .01), elevated serum transaminases (no 80% versus yes 100%, P < .05), recipient age at transplant (< 10 years 89% versus ≥ 10 years 79%, P = .05), T-cell depletion (no 88% versus yes 76%, P = .12), and number of pretransplant cytopenia (tri-cytopenia 69% versus no or 1 or 2 cytopenia 87%, P = .12). For the patients receiving non–T-cell–depleted transplants, a nucleated cell dose infused ≥ 3 × 108/kg of recipient body weight was associated with a higher probability of neutrophil recovery (96% versus 82%, P = .03). When T-cell depletion via CD34+ selection was used, a dose of CD34+ cells infused ≥ 2 × 106 per kilogram of recipient body weight was also associated with an improved neutrophil recovery (100% versus 57%, P < .02). However, since the nucleated cell doses infused were only informative for 41 patients, and the number of CD34+ cells infused in 16 patients, these covariates were not initially introduced in the Cox proportional hazards model. Therefore, in the multivariate analysis, a recipient age at transplant of less than 10 years (relative risk [RR], 2.90; 95% confidence interval, 1.56-5.38; P = .001), a pretransplant elevation of the serum alanine/aspartate transaminases greater than twice the upper normal value (RR, 3.60; 95% CI, 1.75-7.41; P < .001), the use of a male donor (RR, 1.89; 95% CI, 1.04-3.42; P < .04) (no significant directionality of the sex effect), and absence of a severe 3-lineage cytopenia before starting the conditioning regimen (RR, 2.49; 95% CI; .97-6.37; P < .06) were covariates associated with a higher probability of neutrophil recovery. As patients who received non–T-cell–depleted grafts from male donors had received higher nucleated cell doses than patients who received grafts from female donors (cell dose ≥ 3 × 108/kg: 39% versus 70%, P = .05), a second Cox proportional hazards model was built to delineate the effect of these 2 covariates on the neutrophil recovery of the 41 patients transplanted with non–T-cell–depleted grafts and for whom the cell dose was available (information lacking in 2 cases). In this final model, a recipient age at transplant of less than 10 years (RR, 2.43; 95% CI, 1.17-5.08;P < .02), a pretransplant elevation of the serum alanine/aspartate transaminases greater than twice the upper normal value (RR, 2.60; 95% CI, 1.13-6.00; P = .025), and a nucleated cell dose infused ≥ 3 × 108 per kilogram of recipient body weight (RR, 2.35; 95% CI, 1.12-4.95;P = .025) were significantly associated with a higher neutrophil recovery rate.
The probability of secondary graft failure at 1 year was 19% (SE, 7%). The median time from transplant to secondary graft failure was 69 days (range, 25 to 270). Since this complication occurred in 7 cases among the 55 patients at risk, discrete covariates dichotomizing the population in groups of < 10 patients were not analyzed. Neither the cell dose infused (P = .45), nor the use of T-cell depletion (P = .70) was associated with secondary graft failure. The following covariates having a potential effect on this complication in univariate analysis were considered in the Cox proportional hazards model: hemoglobin level at transplant (69% if < 80 g/L versus 3% if ≥ 80 g/L, P < .001), limb abnormalities (28% if none versus 0% if present, P = .05) number of pretransplant transfusions (0% if < 20 versus 30% if ≥ 20, P = .03), donor gender (36% if female donor versus 8% if male donor,P = .10), conditioning regimen including serotherapy (0% without serotherapy versus 25% with serotherapy, P = .11), and recipient cytomegalovirus serology (10% if negative versus 51% if positive, P = .14). In multivariate analysis, the only strong predictor of secondary graft failure was a hemoglobin level < 80 g/L at the time of transplant (RR, 22.90; 95% CI, 2.74-191.25; P < .01). None of the patients who developed secondary graft failure and did not receive a second transplant were alive at last follow-up time. Ten patients received a second transplant for primary graft failure (n = 8) or secondary graft failure (n = 2). First transplant was T-cell–depleted in 6 cases. The median time between first and second transplant was 53 days (range, 34 to 302). At the last follow-up, 2 patients were alive. Causes of death were poor engraftment or graft rejection with fungal (n = 3), viral (n = 2), or bacterial (n = 2) infections, and severe acute GVHD (n = 1).
Graft-versus-host disease
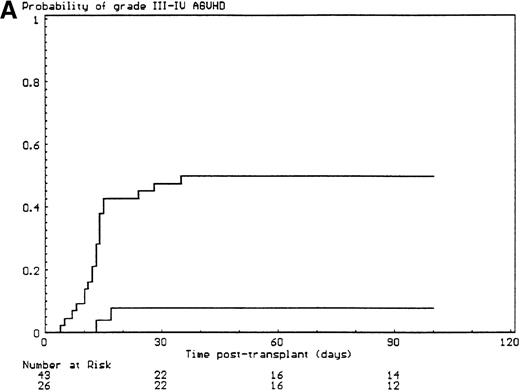
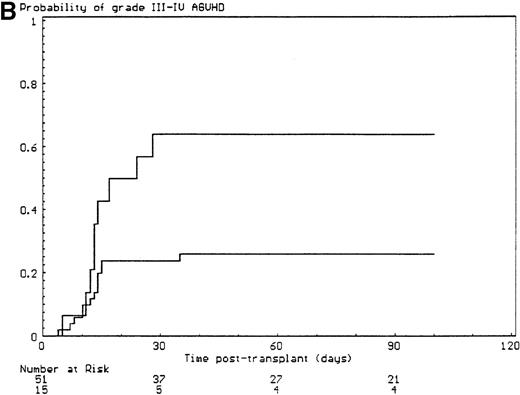
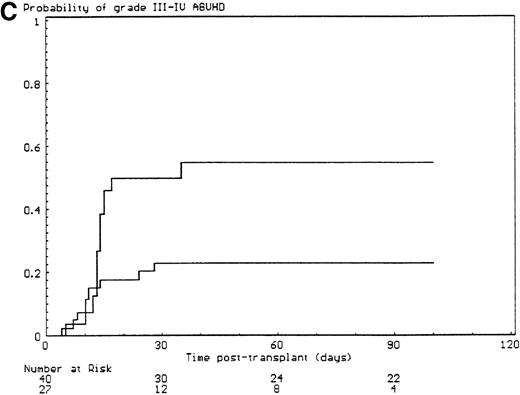
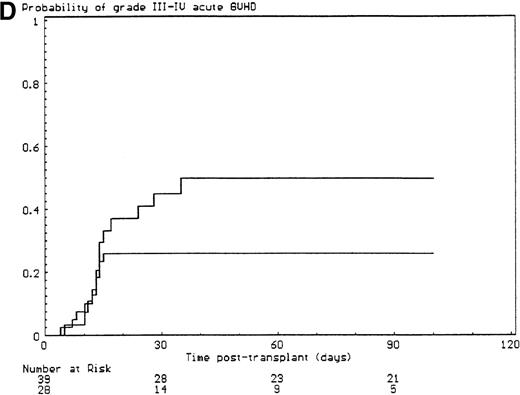
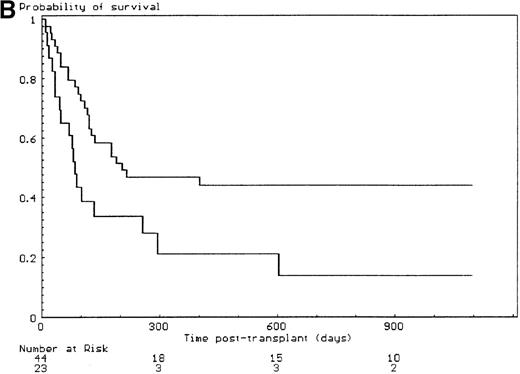
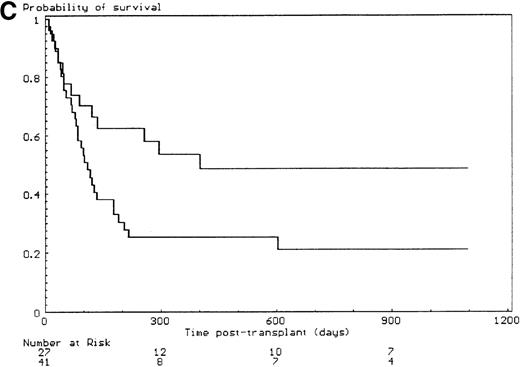
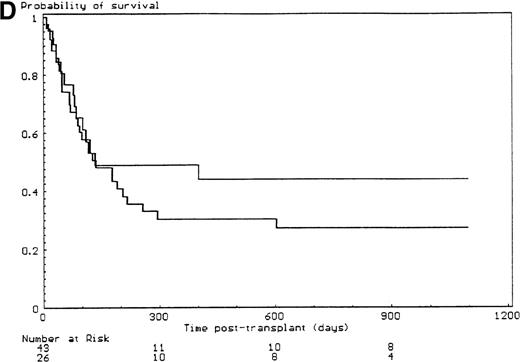
The probabilities of developing grade II-IV and grade III-IV acute GVHD for the overall group of patients were 43% (SE, 6%) and 34% (SE, 6%), respectively. All but 6 of the 29 patients who developed grade II-IV acute GVHD had, in fact, grade III-IV acute GVHD (P < .001). Since the incidence of grade 2I-IV acute GVHD was higher in the group of patients who had extensive malformations (30% if limited versus 47% if extensive) but did not reach significance in univariate analysis (P = .28), each anatomic site that was used to define the extent of malformation was analyzed separately. Malformations of the limbs (55% if present versus 23% if not, P < .02) and of the urogenital tract and/or the kidneys (50% if present versus 26% if not, P = .04) had a significant effect on the incidence of grade III-IV acute GVHD, the latter covariate only after day 14 after transplant (time-dependent covariate). There was no collinearity between these 2 covariates. Covariates introduced in the Cox proportional hazards model, according to their significant level in univariate analysis, were T-cell depletion, the serum alanine/aspartate transaminases values before starting the conditioning regimen, anti–T-cell serotherapy administration and irradiation during the conditioning regimen, and malformations of the limbs and of the urogenital tract and/or the kidneys. Absence of T-cell depletion (RR, 20.00; 95% CI, 2.54-142.86;P = .004) (Figure 1A), malformations of the urogenital tract and/or the kidneys (RR after day 14, 6.60; 95% CI, 1.38-31.64;P < .02) (Figure 1D), elevated serum alanine/aspartate transaminases value before starting the conditioning regimen (RR, 2.52; 95% CI, 1.06-6.01; P < .04) (Figure 1B), and limb malformations (RR, 2.55; 95% CI, 1.05-6.17; P < .04) (Figure 1C) remained significantly associated with a high risk of severe acute GVHD in multivariate analysis.
Predictors of grade III-IV acute GVHD.
(A) Effect of T-cell depletion. Patients who received a T-cell–depleted transplant had an estimated day-100 probability of grade III-IV acute GVHD of 8% versus 50% if no T-cell depletion was performed (P < .001, log-rank test). (B) Effect of the pretransplant hepatic transaminase values before starting the conditioning regimen. Patients with elevated serum transaminase greater than twice the upper normal values had an estimated day-100 probability of grade III-IV acute GVHD of 64% versus 26% if it was below that level (P = .01, log-rank test). (C) Effect of the limb malformations. Patients with malformations of the limbs had an estimated day-100 probability of grade III-IV acute GVHD of 55% versus 23% if no limb malformation was observed (P < .02, log-rank test). (D) Effect of the urogenital and kidney malformations. Patients with urogenital and/or kidney malformations had an estimated day-100 probability of grade III-IV acute GVHD of 50% versus 26% if such malformations were not present (P = .04, time-dependent Cox proportional hazards model).
Predictors of grade III-IV acute GVHD.
(A) Effect of T-cell depletion. Patients who received a T-cell–depleted transplant had an estimated day-100 probability of grade III-IV acute GVHD of 8% versus 50% if no T-cell depletion was performed (P < .001, log-rank test). (B) Effect of the pretransplant hepatic transaminase values before starting the conditioning regimen. Patients with elevated serum transaminase greater than twice the upper normal values had an estimated day-100 probability of grade III-IV acute GVHD of 64% versus 26% if it was below that level (P = .01, log-rank test). (C) Effect of the limb malformations. Patients with malformations of the limbs had an estimated day-100 probability of grade III-IV acute GVHD of 55% versus 23% if no limb malformation was observed (P < .02, log-rank test). (D) Effect of the urogenital and kidney malformations. Patients with urogenital and/or kidney malformations had an estimated day-100 probability of grade III-IV acute GVHD of 50% versus 26% if such malformations were not present (P = .04, time-dependent Cox proportional hazards model).
Forty patients were alive 100 days after the first transplant and assessable for chronic GVHD. Extensive chronic GVHD occurred in 8 cases, and limited chronic GVHD in 9 cases. Among the 4 patients assessable after a second transplant, 1 developed extensive chronic GVHD.
Survival
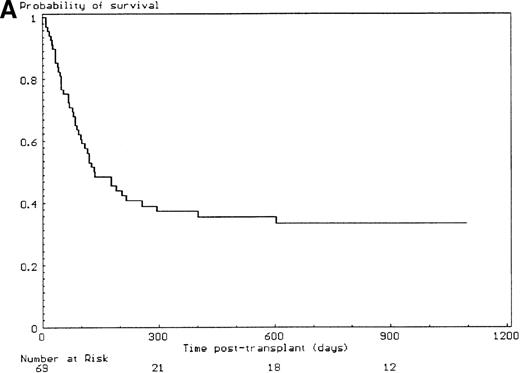
The median follow-up time for the 25 patients alive at last contact was 27 months (range, 4 months to 14 years); 11 patients were alive and well more than 3 years from transplant. The 3-year probability of survival was 33% (SE, 6%) (Figure 2A), and the day-100 transplant-related mortality was 39% (SE, 6%). From 1985 to 1998, with time considered as a continuous or as a discrete covariate, no significant improvement of outcome was observed over the years of transplant. The primary causes of death were acute GVHD (n = 18); primary or secondary graft failure (n = 13); chronic GVHD (n = 4); bacterial (n = 3), viral (n = 2), and fungal (n = 2) infections; and veno-occlusive disease of the liver (n = 1). Covariates assessed in univariate analysis for a potential effect on the day-100 transplant-related mortality and the 3-year probability of survival are listed in Table 3. Among the 14 patients older than 15 years of age at the time of transplant, 2 were alive at last follow-up, giving a 3-year estimated survival of 14% (SE, 9%). The outcome of the patients who had developed a malignancy before transplant was similar to the patients who remained in aplastic phase from diagnosis to transplant (35% without versus 27% with a malignant evolution, P = .38). The nucleated cell dose infused per kilogram of recipient body weight in the non–T-cell–depleted group had no significant effect on the survival (17% if < 3 × 108/kg versus 35% if ≥ 3 × 108/kg, P = .14). Survival was not affected by the type of irradiation (total body irradiation 35% versus thoracoabdominal irradiation 33%, P = .77), the cyclophosphamide total dose (34% at 20 mg/kg versus 31% at 40 mg/kg,P = .82), the use of anti-thymocyte globulin in the conditioning regimen (21% without versus 44% with anti-thymocyte globulin, P = .19), and the type of immunosuppressive drugs used for prevention of GVHD. In multivariate analysis, extensive malformations, elevation of the serum alanine/aspartate transaminases before starting the conditioning regimen, and recipient positive cytomegalovirus serology were covariates associated with a high day-100 transplant-related mortality (Table4). Extensive malformations, positive recipient cytomegalovirus serology, use of androgens before transplant, and use of a female donor (no significant directionality of the sex effect) were significantly associated with a worse 3-year overall survival (Table 5, Figure 2B, C, D). The 3-year probability of survival for the patients who developed grade 2I-IV acute GVHD was 0% compared with 47% in whom this complication was not observed (P < .01).
Outcome and influence of the malformative syndrome extent, the use of androgens before transplantation, and of T-cell depletion on the 3-year overall survival.
(A) 3-year outcome of the 69 FA patients transplanted with HLA-matched unrelated donors. (B) Effect of the malformative syndrome extent. Patients with a limited malformative syndrome (< 3 anatomic sites) had a 3-year survival of 44% versus 14% if the syndrome was extensive (≥ 3 anatomic sites) (P = .01, log-rank test). (C) Effect of pretransplant androgen therapy. Patients who did not receive androgen therapy had a 3-year survival rate of 49% versus 21% if they had received androgen therapy (P < .03, log-rank test). (D) Effect of T-cell depletion. Patients who received a T-cell–depleted transplant had a 3-year survival of 44% versus 27% if the graft was not T-cell–depleted (P = .33, log-rank test).
Outcome and influence of the malformative syndrome extent, the use of androgens before transplantation, and of T-cell depletion on the 3-year overall survival.
(A) 3-year outcome of the 69 FA patients transplanted with HLA-matched unrelated donors. (B) Effect of the malformative syndrome extent. Patients with a limited malformative syndrome (< 3 anatomic sites) had a 3-year survival of 44% versus 14% if the syndrome was extensive (≥ 3 anatomic sites) (P = .01, log-rank test). (C) Effect of pretransplant androgen therapy. Patients who did not receive androgen therapy had a 3-year survival rate of 49% versus 21% if they had received androgen therapy (P < .03, log-rank test). (D) Effect of T-cell depletion. Patients who received a T-cell–depleted transplant had a 3-year survival of 44% versus 27% if the graft was not T-cell–depleted (P = .33, log-rank test).
Predictors of day-100 transplant-related mortality (TRM) and 3-year survival (univariate analyses)
| Covariates . | No. . | Day-100 TRM . | Log Rank PValue . | 3-Year Survival . | Log-Rank P Value . |
|---|---|---|---|---|---|
| Recipient age at transplant | |||||
| <10 years | 29 | 31% | .17 | 39% | .18 |
| ≥10 years | 44 | 45% | 28% | ||
| Elevated serum transaminases | |||||
| No | 51 | 32% | .03 | 36% | .11 |
| Yes | 15 | 60% | 24% | ||
| Androgens before transplant | |||||
| No | 27 | 30% | .22 | 49% | <.03 |
| Yes | 41 | 47% | 21% | ||
| Transfusions before transplant | |||||
| <20 | 23 | 30% | .40 | 37% | .66 |
| ≥20 | 43 | 42% | 32% | ||
| Severe cytopenia before transplant | |||||
| None, 1 or 2 | 55 | 37% | .23 | 35% | .32 |
| 3 | 12 | 50% | 25% | ||
| Malignancy before transplant | |||||
| No | 58 | 38% | .49 | 35% | .41 |
| Yes | 11 | 46% | 27% | ||
| Pretransplant CMV recipient serology | |||||
| Negative | 41 | 32% | .14 | 39% | .09 |
| Positive | 24 | 51% | 18% | ||
| Donor gender | |||||
| Female | 37 | 46% | .14 | 22% | <.01 |
| Male | 32 | 32% | 48% | ||
| Cyclophosphamide total dose | |||||
| 20 mg/kg | 14 | 36% | .79 | 34% | .82 |
| 40-45 mg/kg | 53 | 42% | 31% | ||
| Irradiation for conditioning | |||||
| TAI | 28 | 39% | .89 | 33% | .77 |
| TBI | 39 | 36% | 35% | ||
| Anti-T serotherapy for conditioning | |||||
| No | 19 | 47% | .51 | 21% | .19 |
| Yes | 50 | 36% | 44% | ||
| T-cell depletion | |||||
| No | 43 | 42% | .61 | 27% | .33 |
| Yes | 26 | 35% | 44% | ||
| Methotrexate for GVHD prophylaxis | |||||
| No | 47 | 45% | .22 | 29% | .08 |
| Yes | 22 | 27% | 44% |
| Covariates . | No. . | Day-100 TRM . | Log Rank PValue . | 3-Year Survival . | Log-Rank P Value . |
|---|---|---|---|---|---|
| Recipient age at transplant | |||||
| <10 years | 29 | 31% | .17 | 39% | .18 |
| ≥10 years | 44 | 45% | 28% | ||
| Elevated serum transaminases | |||||
| No | 51 | 32% | .03 | 36% | .11 |
| Yes | 15 | 60% | 24% | ||
| Androgens before transplant | |||||
| No | 27 | 30% | .22 | 49% | <.03 |
| Yes | 41 | 47% | 21% | ||
| Transfusions before transplant | |||||
| <20 | 23 | 30% | .40 | 37% | .66 |
| ≥20 | 43 | 42% | 32% | ||
| Severe cytopenia before transplant | |||||
| None, 1 or 2 | 55 | 37% | .23 | 35% | .32 |
| 3 | 12 | 50% | 25% | ||
| Malignancy before transplant | |||||
| No | 58 | 38% | .49 | 35% | .41 |
| Yes | 11 | 46% | 27% | ||
| Pretransplant CMV recipient serology | |||||
| Negative | 41 | 32% | .14 | 39% | .09 |
| Positive | 24 | 51% | 18% | ||
| Donor gender | |||||
| Female | 37 | 46% | .14 | 22% | <.01 |
| Male | 32 | 32% | 48% | ||
| Cyclophosphamide total dose | |||||
| 20 mg/kg | 14 | 36% | .79 | 34% | .82 |
| 40-45 mg/kg | 53 | 42% | 31% | ||
| Irradiation for conditioning | |||||
| TAI | 28 | 39% | .89 | 33% | .77 |
| TBI | 39 | 36% | 35% | ||
| Anti-T serotherapy for conditioning | |||||
| No | 19 | 47% | .51 | 21% | .19 |
| Yes | 50 | 36% | 44% | ||
| T-cell depletion | |||||
| No | 43 | 42% | .61 | 27% | .33 |
| Yes | 26 | 35% | 44% | ||
| Methotrexate for GVHD prophylaxis | |||||
| No | 47 | 45% | .22 | 29% | .08 |
| Yes | 22 | 27% | 44% |
CMV indicates cytomegalovirus; TAI, thoracoabdominal irradiation; TBI, total body irradiation; GVHD, graft-versus-host disease.
Predictors of day-100 transplant-related mortality (TRM)
| Covariates . | RR . | 95% CI . | P Value . |
|---|---|---|---|
| Malformative syndrome | |||
| Limited (<3 sites) | 1.00 | ||
| Extensive (≥3 sites) | 3.24 | 1.42-7.40 | .005 |
| Serum transaminases before transplant procedure | |||
| <2 × normal upper value | 1.00 | ||
| ≥2 × normal upper value | 3.50 | 1.47-8.35 | .005 |
| Recipient cytomegalovirus serology before transplant | |||
| Negative | 1.00 | ||
| Positive | 2.75 | 1.17-6.45 | .02 |
| Covariates . | RR . | 95% CI . | P Value . |
|---|---|---|---|
| Malformative syndrome | |||
| Limited (<3 sites) | 1.00 | ||
| Extensive (≥3 sites) | 3.24 | 1.42-7.40 | .005 |
| Serum transaminases before transplant procedure | |||
| <2 × normal upper value | 1.00 | ||
| ≥2 × normal upper value | 3.50 | 1.47-8.35 | .005 |
| Recipient cytomegalovirus serology before transplant | |||
| Negative | 1.00 | ||
| Positive | 2.75 | 1.17-6.45 | .02 |
RR indicates relative risk; CI, confidence interval.
Predictors of 3-year overall survival
| Covariates . | RR . | 95% CI . | P Value . |
|---|---|---|---|
| Malformative syndrome | |||
| Limited (<3 sites) | 1.00 | ||
| Extensive (≥3 sites) | 2.96 | 1.54-5.71 | .001 |
| Donor gender | |||
| Male | 1.00 | ||
| Female | 2.33 | 1.19-4.54 | .01 |
| Androgens before transplant | |||
| No | 1.00 | ||
| Yes | 2.11 | 1.07-4.15 | .03 |
| Recipient cytomegalovirus serology before transplant | |||
| Negative | 1.00 | ||
| Positive | 1.90 | 1.00-3.60 | <.05 |
| Covariates . | RR . | 95% CI . | P Value . |
|---|---|---|---|
| Malformative syndrome | |||
| Limited (<3 sites) | 1.00 | ||
| Extensive (≥3 sites) | 2.96 | 1.54-5.71 | .001 |
| Donor gender | |||
| Male | 1.00 | ||
| Female | 2.33 | 1.19-4.54 | .01 |
| Androgens before transplant | |||
| No | 1.00 | ||
| Yes | 2.11 | 1.07-4.15 | .03 |
| Recipient cytomegalovirus serology before transplant | |||
| Negative | 1.00 | ||
| Positive | 1.90 | 1.00-3.60 | <.05 |
RR indicates relative risk; CI, confidence interval.
Discussion
This retrospective multicenter study is the first to address on a large scale the results of allogeneic stem cell transplantation using HLA-matched unrelated donors in FA, and to identify, in multivariate analysis, factors associated with the main endpoints, that is, survival, engraftment and acute GVHD. In this study, the 3-year overall survival rate was 33%, with a day-100 transplant-related mortality of 39%, mainly because one third of the patients developed grade III-IV acute GVHD (acute GVHD was responsible for 13 of the 26 deaths occurring before day 100 after transplant).
For the first time, a correlation between the phenotype and outcome has been observed. Patients with extensive congenital malformations had a 3-year survival of 14% compared with 44% for those whose malformations were limited. This strong and independent predictor of survival reflected a high mortality rate during the initial phase of the transplant procedure (day-100 transplant-related mortality of 57% versus 27%, P < .02). This finding was not related to a difference in terms of neutrophil recovery (85% versus 86%,P = .91), but rather to a higher incidence of grade III-IV acute GVHD (47% versus 30%, P = .28) and to significantly more early secondary graft failures (before day 100; 22% versus 3%,P = .05), when malformations were extensive. If the extent of the malformations was a major predictor of severe acute GVHD, it also appeared that malformations in some anatomic sites, for example, of the urogenital tract or/and kidneys and limbs, were more frequently associated with severe acute GVHD. The association between this complication and urogenital and/or kidney malformations probably reflected an impaired renal function and some difficulties to maintain CsA at efficient and nontoxic levels throughout the initial period after transplant. The reason for the association of limb abnormalities with severe acute GVHD remained unknown, but this might reflect genetic differences. For FANC Cmutations, it has already been demonstrated that a genomic mutation (IVS4 + 4 A > T) was associated with a more severe phenotype.30-32 In the present study, because complementation group analyses and genotyping were performed in only a few patients, it was impossible to search for potential associations between outcome, phenotype, and genotype.
Unexpectedly, an elevation of the serum alanine/aspartate transaminase values before starting the conditioning regimen was associated with a higher neutrophil recovery rate. There was no clear explanation of this finding, but it probably reflected an abnormal hepatic metabolism of the drugs used for the conditioning regimen and/or the prevention of acute GVHD. A high day-100 transplant-related mortality rate was also observed in this group of patients having abnormal liver function tests. This finding was not due to an increased incidence of lethal veno-occlusive disease of the liver33since the only patient who died from this complication had no elevated serum alanine/aspartate transaminases before transplant. The association found between this covariate and the occurrence of grade III-IV acute GVHD was the most probable explanation, as 9 of the 11 patients with abnormal pretransplant transaminases values died of acute GVHD. Notably, elevated pretransplant serum alanine/aspartate transaminase values were associated with pretransplant androgen therapy (P < .08), one of the significant predictors of 3-year overall survival in multivariate analysis. Therefore, whereas giving androgens to FA patients with cytopenia may keep patients without HLA-matched sibling donors alive for many years, the appearance of hepatic abnormalities should probably lead physicians to rapidly consider a transplant with an HLA-matched unrelated donor, if such a donor is available.
Donor gender was also a major prognostic factor in this study, mainly because of a higher incidence of primary graft failures when female donors were used. There was no directionality of the sex effect of donor/recipient mismatch. These findings were probably explained by the fact that unmanipulated bone marrow grafts coming from female donors contained less nucleated cells per kilogram of recipient body weight (medians: 2.67 × 108/kg versus 4.30 × 108/kg in male, P < .05). This difference of cell dose infused according to donor gender was probably not related to a difference in the recipients' body weight, as the median age of the recipients according to this covariate were not significantly different (11 years if female donors were used versus 10 years if male donors were used, P = .22) and led us to suspect a less efficient harvesting of the bone marrow stem cells when female donors were used.
A significant association between a positive recipient cytomegalovirus serology and a worse outcome after allogeneic stem cell transplantation has already been reported in previous studies,34 with the deleterious effect of this covariate being generally related to the association of GVHD, immunodeficiency, and infections— especially non-Candida fungal infections35—rather than to the occurrence of cytomegalovirus disease.36 In this study, patients with a positive cytomegalovirus serology had a higher day-100 transplant-related mortality (51% versus 32%, P = .14) without an increased incidence of severe acute or chronic GVHD. Secondary graft failures were more frequently observed in patients with a positive cytomegalovirus serology (10% if negative versus 51% if positive, P = .14), a finding that could partially explain the worse outcome of these patients.
As expected, a dramatic reduction of grade III-IV acute GVHD was obtained with bone marrow T-cell depletion, whatever the type of graft manipulation. However, this impressive result was not associated with a significant improvement of the outcome, even if the 3-year overall survival was 44% with T-cell–depleted grafts compared with 27% without (P = .33). This was due to the fact that if acute GVHD stopped being the predominant lethal complication in the T-cell–depleted group, primary and secondary graft failures became the predominant cause of death when such a graft manipulation was performed. When patients who received T-cell–depleted grafts via CD34+ selection were considered, the number of CD34+ hematopoietic progenitor cells infused appeared to be significantly associated with neutrophil recovery. More patients need to be studied to statistically validate the potential cut-off value of 2 × 106 CD34+ cells/kg that was found significant in this study, but our finding argues in favor of trying to obtain as many CD34+ hematopoietic stem cells as possible before the graft is processed and fosters the use of peripheral blood progenitor cells rather than bone marrow cells.
It was not possible to assess, in this multicenter retrospective study, the effect of high-resolution molecular HLA typing on the outcome of patients transplanted with unrelated HLA-matched donors, especially for HLA class-I alleles. However, according to the recent results reported by the Japanese teams37 and the Seattle group,38 a better selection of unrelated donors via “complete” molecular HLA typing should contribute to improving the outcome of FA patients transplanted with HLA-matched unrelated donors.
Reprints:E. Gluckman, Service de Greffe de Moelle “Trèfle 3,” Hôpital Saint-Louis, 1 Avenue Claude Vellefaux, F-75 475 Paris Cedex 10, France; e-mail:eliane.gluckman@chu-stlouis.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal