Abnormal interaction of sickle red blood cells (SS RBC) with the vascular endothelium has been implicated as a factor in the initiation of vasoocclusion in sickle cell anemia. Both von Willebrand factor (vWf) and thrombospondin (TSP) play important roles in mediating SS RBC–endothelium interaction and can bind to the endothelium via Vβ3 receptors. We have used monoclonal antibodies (MoAb) directed against Vβ3 and IIbβ3 (GPIIb/IIIa) integrins to dissect the role of these integrins in SS RBC adhesion. The murine MoAb 7E3 inhibits both Vβ3 and IIbβ3 (GPIIb/IIIa), whereas MoAb LM609 selectively inhibits Vβ3, and MoAb 10E5 binds only to IIbβ3. In this study, we have tested the capacity of these MoAbs to block platelet-activating factor (PAF)–induced SS RBC adhesion in the ex vivo mesocecum vasculature of the rat. Infusion of washed SS RBC in preparations treated with PAF (200 pg/mL), with or without a control antibody, resulted in extensive adhesion of these cells in venules, accompanied by frequent postcapillary blockage and increased peripheral resistance units (PRU). PAF also caused increased endothelial surface and interendothelial expression of endothelial vWf. Importantly, pretreatment ofthe vasculature with either MoAb 7E3 F(ab′)2 or LM609, but not 10E5 F(ab′)2, after PAF almost completely inhibited SS RBC adhesion in postcapillary venules, the sites of maximal adhesion and frequent blockage. The inhibition of adhesion with 7E3 or LM609 was accompanied by smaller increases in PRU and shorter pressure-flow recovery times. Thus, blockade of Vβ3 may constitute a potential therapeutic approach to prevent SS RBC–endothelium interactions under flow conditions.
Sickle (SS) cell anemia is characterized by recurring episodes of painful vasoocclusive crisis and multiple organ damage. Although hemoglobin S (HbS) polymerization under deoxygenated conditions is central to the pathophysiology of this disease, multiple factors (both primary and secondary) may participate in the initiation of a vasoocclusive episode.1 Among these factors, abnormal adhesion of SS red blood cells (RBC) to the vascular endothelium may play an important role in vasoocclusion.2-7 SS RBC adhesion to the endothelium occurs at venular wall shear rates,8,9 and both ex vivo and in vivo studies have revealed that venules are the exclusive sites of SS RBC adherence in animal models.6,9 SS RBC adhesion could potentially reduce the effective luminal diameters of postcapillary venules, resulting in selective trapping of dense SS RBC and increased red blood cell transit times, thus providing a greater opportunity for HbS polymerization.6 7
Multiple adhesion molecules may participate in SS RBC–endothelial interactions. Potential red blood cell receptors include the integrin receptor α4β1 and CD36 (glycoprotein IV [GPIV]), both of which have been found on SS stress reticulocytes,10-14 the type of reticulocytes present only in patients undergoing erythropoietic stress. Other red blood cell surface molecules may also be involved because nonstress reticulocytes and other SS RBC are also capable of adhering to the vascular endothelium.15,16 For example, repeated sickling in vivo and oxidative stress17 may result in red blood cell membrane damage,18 leading to abnormal surface exposure of membrane components (eg, band-3 and sulfatides)19 20 that might mediate SS RBC adhesion.
Several adhesive proteins are implicated in SS RBC adhesion. Unusually large molecular weight forms of von Willebrand factor (vWf) and thrombospondin (TSP) are known to enhance adhesion of SS RBC to endothelial cells.13,14,21-25 TSP and vWf are both present in platelets and endothelial cells and can be released into the local environment under appropriate stimulation. In fact, elevated levels of both adhesive proteins have been identified in the plasma of patients with sickle cell anemia.26-28 SS RBCs also show an increased adhesion to immobilized laminin.20,29 In addition, α4β1 integrin and CD36 could mediate binding of SS stress reticulocytes to vascular endothelium via vascular cell adhesion molecule-1 (VCAM-1), expressed on cytokine-stimulated endothelium, and TSP, respectively.10-14
Endothelial receptors involved in the adhesion of SS RBC could include the integrin receptor αVβ3, which is expressed on endothelial cells as well as other cells.30 Both vWf and TSP can bind to αVβ3 receptors,31-34 and laminin is also reported to bind to αVβ3 in addition to β1 receptors.35 These adhesive proteins can also bind to sulfatides.36-38 Thus, tripartite adhesive complexes (RBC receptor-adhesion protein-endothelial receptor) may contribute to SS RBC adhesion. Based on known interactions between these components, a number of such complexes may plausibly exist, including CD36-TSP-αVβ3 integrin, sulfatide-TSP-αVβ3, sulfatide-vWf-αVβ3, and sulfatide-laminin-αVβ3 (or several potential β1 integrins). Thus, αVβ3 may play an important role in SS RBC adhesion to the endothelium.
In flow systems, peptides and antibodies that inhibit αVβ3 function have been shown to inhibit SS RBC adhesion to endothelium, although the peptides were not absolutely specific for αVβ3,39,40and in one study both E-selectin and VCAM-1 also seemed to play important roles.40 Moreover, cultured endothelial cells were used for these studies, and thus, may differ from the endothelium of intact vessels.
In the present studies, we have addressed the role of αVβ3 integrin in SS RBC adhesion by testing the effects of monoclonal antibodies (MoAbs) specific for αVβ3 and the related receptor αIIbβ3 on SS RBC–endothelium interaction in the artificially perfused, ex vivo mesocecum vasculature of the rat. We have previously used the ex vivo vasculature for investigating the role of both vWf and TSP in adhesion of SS RBC in the microcirculation.22,25 To enhance SS RBC adhesion, we pretreated the mesocecum with platelet-activating factor (PAF), a potent inflammatory agent that exerts a wide variety of effects such as release of vWf from the endothelium, vasoconstriction, increased venular permeability, neutrophil activation, and platelet aggregation.41,42 Sickle cell patients have almost twice the plasma levels of PAF compared with normal subjects,43raising the possibility that it, or cytokines that produce similar effects, may be important in the pathophysiology of SS cell vasoocclusion. The strong clinical association between infections that may initiate cytokine production and SS painful crises provides an additional plausible link between increases in agents that can affect the endothelium and SS RBC–mediated vasoocclusion.
Materials and methods
Monoclonal antibodies
F(ab′)2 fragments of MoAbs 7E3 (anti-αIIbβ3 + αVβ3),44 10E5 (anti-αIIbβ3),45 and the control irrelevant antibody OC125 were generously provided by Centocor, Inc (Malvern, PA). 7E3 F(ab′)2 reacts with rat αIIbβ3 and αVβ3 (KD 7 and 9 μg/mL).46 MoAb LM609 (anti-αVβ3) was kindly provided by Dr David Cheresh (Scripps Institute, La Jolla, CA). It is not known whether 7E3 and LM609 bind to the ligand-bound forms of the αVβ3 receptors.
Preparation of cells
Heparinized blood was obtained with informed consent from normal (AA) adults (n = 3) and from sickle cell anemia patients (n = 11) who were not in crisis and had not received a blood transfusion in the preceding 4 months.
After removal of the buffy coat, blood was washed three times in normal saline, once in bicarbonate Ringer-albumin solution (118 mmol/L NaCl, 5 mmol/L KCl, 2.5 mmol/L CaCl2, 0.64 mmol/L MgCl2, 27 mmol/L NaHCO3, 0.5% bovine albumin, equilibrated with 95% O2 and 5% CO2; pH 7.4; osmolarity, 295 mosm/kg), and resuspended in Ringer-albumin solution. In each case, hematocrit (Hct) was adjusted to 30% for perfusion studies.
Preparation and perfusion of rat mesocecum vasculature
Perfusion studies were performed in the isolated, acutely denervated, and artificially perfused rat mesocecum vasculature (n = 46) according to the method of Baez et al47 as modified by Kaul et al6,48 for the infusion of erythrocytes. Details of the procedure have been described elsewhere.48 Arterial perfusion pressure in the mesocecum was maintained at 60 mm Hg, and venous outflow pressure was kept at 3.8 mm Hg. During perfusion with Ringer-albumin solution containing 3% bovine albumin, a 0.2-mL bolus of a given red blood cell sample (Hct 30%) was injected over ∼5 seconds. Peripheral resistance units (PRU) were determined as described49 and expressed in mm Hg/mL/min/g. PRU = ΔP/Q, where ΔP is the arteriovenous pressure difference and Q is the rate of venous outflow (mL/min) per gram of tissue weight. Pressure flow recovery time (Tpf), defined as the time (seconds) required for the arterial pressure and the venous outflow to return to their baseline levels, was determined after the red blood cell injection.
Intravital microscopic observations and adhesion quantification
Direct intravital microscopic observations and simultaneous video-recording of the microcirculatory events were performed with an Olympus microscope (model BH-2; Olympus Corp, Lake Success, NY) equipped with a television camera (Cohu, 5000 Series; Cohu, San Diego, CA) and a Sony U-matic video recorder (model VO5800; Sony, Teaneck, NJ). The number of adherent SS cells per 100 μm2 was calculated from the counts of individual adherent cells and the surface area (μm2) of the inner wall of the vessel segment as described previously.6 Adhesion data for each experimental group were pooled for statistical comparisons.
Perfusion experiments with PAF and monoclonal antibodies
PAF supplied in chloroform solution (Sigma, St Louis, MO) was first diluted in dimethyl sulfoxide (DMSO; Sigma), followed by serial dilutions in Ringer-albumin. Rat mesocecum was isolated and perfused with 40 mL of Ringer-albumin containing PAF (200 pg/mL) for 10 minutes. After a 5-minute incubation period, the preparation was perfused as above, and a bolus of AA or SS RBC was injected.
In experiments designed to evaluate the effects of 7E3 F(ab′)2 or 10E5 F(ab′)2, the preparation was first infused with the respective antibody (50 μg/mL in 5 mL Ringer-albumin). Control experiments were performed using a control MoAb (OC125) F(ab′)2 of the same subtype (IgG1) as 7E3. After a 30-minute incubation at room temperature, the preparation was perfused with PAF solution (200 pg/mL in 40 mL) containing the MoAb (50 μg/mL). Thereafter, a bolus of RBC was injected. In experiments using the MoAb LM609, the preparation was first infused with the MoAb (50 μg/mL in 3 mL) and incubated for 30 minutes as above. This was followed by infusion with PAF (200 pg/mL in 40 mL). A bolus of RBCs was then injected. The control for these experiments was an intact mouse IgG MoAb at the same concentration.
Immunohistochemistry
Cryostat sections (6 μm) of fresh-frozen tissue were postfixed in acetone. Sections were treated with 3% hydrogen peroxide, and then immunoperoxidase staining was performed using primary rabbit antihuman vWf polyclonal antibody (Dakopatts, Glostrup, Denmark), diluted 1:400 in phosphate-buffered saline (PBS) containing 5% bovine serum albumin (BSA; Sigma). Control sections were incubated with normal rabbit Ig (Dakopatts) in BSA-PBS. After a 30-minute incubation, the sections were treated with goat antirabbit IgG conjugated with horseradish peroxidase (Dakopatts) at 1:250 (30 minutes). After three washes in PBS, sections were treated for 2 to 3 minutes with freshly prepared 3′3′-diaminobenzidine (DAB [Sigma]). The slides were counterstained with 0.05% toluidine blue. Sections were mounted with Cytoseal mounting medium (Stephens Scientific, Riverdale, NJ).
Statistical analysis
Paired t-test or unpaired Student's t-test was applied to analyze PRU data as indicated in the tables and Results. Linear regression line analysis of the number of adhered cells/100 μm2 (Y) versus the venular diameter (X) was performed. Comparisons of the regression lines between experimental groups were performed using multiple linear regression analysis50 as indicated in Results. The various statistical tests or tests for hypotheses were performed using a Type I error of .05 and were two-tailed. The statistical analysis was performed using the Statgraphic Plus (version 3.1) program for Windows (Manugistics, Inc, Rockville, MD).
Results
Effects of PAF on vascular endothelial von Willebrand factor
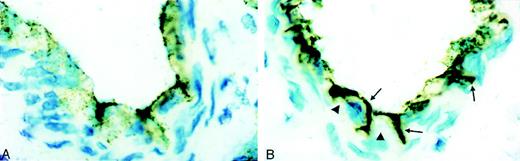
In untreated preparations, immunoperoxidase staining for vWf in postfixed frozen sections showed a positive granular pattern in the cytoplasm and often a linear pattern on the luminal surface of endothelial cells (Figure 1A). There was little or no reactivity at interendothelial cell junctions. In contrast, in PAF-treated vasculature, distinct endothelial contraction and separation of endothelial cell junctions were observed. Heavy deposition of vWf could be seen on the luminal aspect of many endothelial cells as well as in the interendothelial cell gaps (Figure1B,arrows); this was accompanied by almost complete depletion of vWf inside some cells (Figure 1B, arrowheads).
Immunoperoxidase staining for vWf in control and PAF-treated vessels.
(A) Control (untreated) vessels show granular reaction for vWf in the cytoplasm. In addition, vWf is found associated with the endothelial surface, and little reactivity is noticed at the interendothelial cell junctions. (B) In PAF-treated preparations, there is distinct endothelial cell contraction that is indicative of increased venular permeability. Heavy deposits of vWf are seen on the endothelial cell surface and in the interendothelial cell gaps (arrows). Many cells show almost complete depletion of vWf in the cytoplasm (arrowheads).
Immunoperoxidase staining for vWf in control and PAF-treated vessels.
(A) Control (untreated) vessels show granular reaction for vWf in the cytoplasm. In addition, vWf is found associated with the endothelial surface, and little reactivity is noticed at the interendothelial cell junctions. (B) In PAF-treated preparations, there is distinct endothelial cell contraction that is indicative of increased venular permeability. Heavy deposits of vWf are seen on the endothelial cell surface and in the interendothelial cell gaps (arrows). Many cells show almost complete depletion of vWf in the cytoplasm (arrowheads).
The effect of PAF on the hemodynamic behavior of AA and SS RBC
Table 1 depicts hemodynamic parameters in the absence or presence of PAF in the ex vivo mesocecum vasculature. In infusion groups 1 and 3, which comprised the untreated preparations, the PRU values were 4.0 and 4.2 mm Hg/mL/min/g, respectively, when Ringer-albumin solution was perfused through the vasculature. In infusion groups 2 and 4, which comprised the preparations treated with PAF, the baseline PRU values were 5.1 and 6.4 mm Hg/mL/min/g, respectively, 40% higher on average than the PRU values in the untreated preparations. Because the dose of PAF employed does not alter arteriolar diameter (as determined in separate preparations), the increase in PRU with PAF treatment reflects the effect on venous outflow of tissue edema secondary to PAF-induced increases in venular permeability.
The effect of PAF on hemodynamic behavior of SS RBC in the mesocecum vasculature
| Infusion . | No. . | PRU (mm Hg/mL/min/g) . | ΔPRU (%) . | Tpf (s) . | |
|---|---|---|---|---|---|
| Ringer . | RBC . | ||||
| 1. AA RBC | 4 | 4.0 ± 0.1 | 4.6 ± 0.1 | 15.0 ± 2.3 | 31.5 ± 1.9 |
| 2. PAF/AA RBC | 4 | 5.1 ± 0.7 | 6.0 ± 0.9 | 17.6 ± 1.7 | 37.0 ± 6.3 |
| 3. SS RBC | 4 | 4.2 ± 1.0 | 5.2 ± 1.3 | 25.2 ± 3.0* | 49.9 ± 2.3† |
| 4. PAF/SS RBC | 4 | 6.4 ± 1.9 | 9.0 ± 2.5 | 40.5 ± 6.7‡ | 90.8 ± 13.91-153 |
| Infusion . | No. . | PRU (mm Hg/mL/min/g) . | ΔPRU (%) . | Tpf (s) . | |
|---|---|---|---|---|---|
| Ringer . | RBC . | ||||
| 1. AA RBC | 4 | 4.0 ± 0.1 | 4.6 ± 0.1 | 15.0 ± 2.3 | 31.5 ± 1.9 |
| 2. PAF/AA RBC | 4 | 5.1 ± 0.7 | 6.0 ± 0.9 | 17.6 ± 1.7 | 37.0 ± 6.3 |
| 3. SS RBC | 4 | 4.2 ± 1.0 | 5.2 ± 1.3 | 25.2 ± 3.0* | 49.9 ± 2.3† |
| 4. PAF/SS RBC | 4 | 6.4 ± 1.9 | 9.0 ± 2.5 | 40.5 ± 6.7‡ | 90.8 ± 13.91-153 |
Values are mean ± SD. In experiments using PAF, the vasculature was first pretreated with PAF (200 pg/mL) followed by a bolus infusion of SS RBC (Hct 30%, 0.2 mL).
Abbreviations: ΔPRU, PRU RBC/PRU Ringer; Tpf, pressure-flow recovery time to the baseline level.
P < .002 and
P < .0001, compared with respective values for AA RBC.
P < .024 and
P < .013, compared with respective values for SS RBC.
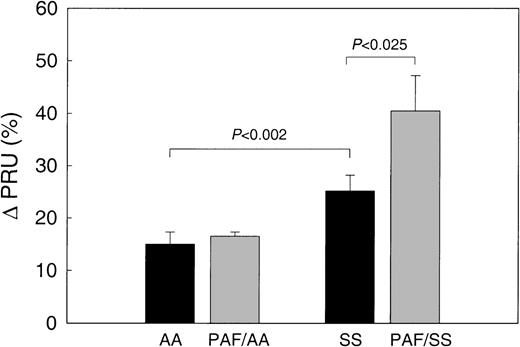
When AA RBCs were infused into untreated preparations, the PRU values increased by 15.0% ± 2.3% compared with the baseline (Table 1, infusion group 1; Figure 2), and when AA RBCs were infused into the PAF-treated preparations (infusion group 2), the PRU values increased by 17.6% ± 1.7% (Figure 2). In contrast, when SS RBCs were infused into the untreated preparations (infusion group 3), the PRU values increased 25.2% ± 3.0%, which is a 68% greater increase than the increase with AA RBCs (P< .002). Similarly, when SS RBCs were infused into the PAF-treated preparations (infusion group 4), the PRU values increased by 40.5% ± 6.7%, which is a 130% greater increase than when AA RBCs were infused into PAF-treated preparations (infusion group 2;P < .002), and a 61% greater increase than when SS RBCs were infused into untreated preparations (infusion group 3; P = .024).
The effect of PAF on the hemodynamic behavior of AA and SS RBC in the ex vivo mesocecum vasculature.
In untreated (control) preparations, SS RBC (n = 4) resulted in a 1.6-fold increase in the PRU compared with AA RBC (P < .002). In PAF (200 pg/mL)-treated preparations, SS RBCs caused a 1.6-fold increase in PRU compared with SS RBC infusions from the same patients in untreated preparations (P < .025, pairedt-test). In contrast, PAF had no effect on PRU for AA RBC.
The effect of PAF on the hemodynamic behavior of AA and SS RBC in the ex vivo mesocecum vasculature.
In untreated (control) preparations, SS RBC (n = 4) resulted in a 1.6-fold increase in the PRU compared with AA RBC (P < .002). In PAF (200 pg/mL)-treated preparations, SS RBCs caused a 1.6-fold increase in PRU compared with SS RBC infusions from the same patients in untreated preparations (P < .025, pairedt-test). In contrast, PAF had no effect on PRU for AA RBC.
The Tpf values correlated with the PRU responses. Thus, with AA RBCs, PAF treatment only modestly increased the Tpf (from 31.5 ± 1.9 seconds to 37.0 ± 6.3 seconds, a nonsignificant difference; Table1, infusion groups 1 and 2). The Tpf of untreated preparations infused with SS RBCs was 49.9 ± 2.3 seconds, which was significantly greater than the Tpf of untreated preparations infused with AA RBCs (infusion group 1; P < .0001). The longest Tpf was observed with the infusion of SS RBCs into PAF-treated preparations (90.8 ± 13.9 seconds), which was significantly longer than the values with either AA RBC infusion into PAF-treated preparations (infusion group 2;P < .001) or SS RBC infusion into untreated preparations (infusion group 3; P = .013).
Intravital microscopy
Direct microscopic observations and analysis of videotapes revealed that AA RBCs showed little or no adhesion to postcapillary venules in untreated or PAF-treated preparations. In sharp contrast, infusion of SS RBCs into untreated preparations resulted in prominent adhesion of these cells, which occurred exclusively to the venular endothelium. In the affected venules, adhesion was inversely related to the vessel diameter, which is in accord with our previous observations.6,15 In preparations pretreated with PAF, the increase in adhesion of SS RBC was most prominent and frequently led to obstruction of small-diameter venules (Figure 3). As we previously showed,6 7 the postcapillary blockage may involve contribution from SS RBCs adherent to the endothelium and subsequent trapping of dense SS RBCs. The number of adherent SS RBC/100 μm2 in individual venules was plotted as a function of venular diameter. As shown in Figure 4, the number of adherent SS RBCs/100 μm2 increased as the venular diameter decreased. Linear regression analysis of the data, using the equation Y = a + bX, confirmed a strong inverse correlation between the number of adherent RBC/100 μm2and the vessel diameter in both control and PAF-treated preparations (control/SS, r = −.80, P < .00001; PAF/SS,r = −.86, P < .00001). Furthermore, a comparison of the two regression lines confirmed significantly increased adhesion of SS RBC in the PAF-treated group as revealed by differences in Y intercepts (P < .0001; Figure 4).
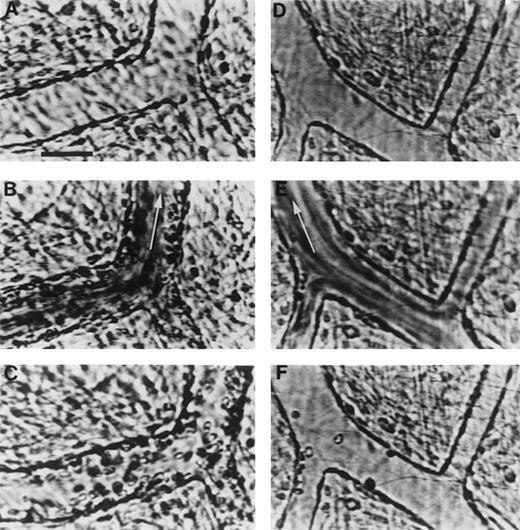
Videomicrographs showing inhibition of PAF-induced SS RBC adhesion in the ex vivo mesocecum vasculature in the presence of MoAb 7E3 F(ab′)2.
(A-C) Ex vivo preparation treated with OC125 F(ab′)2(control antibody) and PAF: (A) clear venular lumen during artificial perfusion with Ringer-albumin; (B) the passage of SS RBCs after a bolus infusion is accompanied by adhesion of these cells in venules; (C) after the passage of the bolus, a large number of SS RBC are seen adhering to the vessel wall during perfusion with Ringer-albumin. (D-F) Ex vivo preparation treated with MoAb 7E3 and PAF: (D) venules during the artificial perfusion; (E) rapid flow of SS RBCs in the vessels after a bolus infusion; (F) after the passage of the bolus, only few SS RBC adhere to the vessel wall. Visit the article on the website (www.bloodjournal.org) to download the video.
Videomicrographs showing inhibition of PAF-induced SS RBC adhesion in the ex vivo mesocecum vasculature in the presence of MoAb 7E3 F(ab′)2.
(A-C) Ex vivo preparation treated with OC125 F(ab′)2(control antibody) and PAF: (A) clear venular lumen during artificial perfusion with Ringer-albumin; (B) the passage of SS RBCs after a bolus infusion is accompanied by adhesion of these cells in venules; (C) after the passage of the bolus, a large number of SS RBC are seen adhering to the vessel wall during perfusion with Ringer-albumin. (D-F) Ex vivo preparation treated with MoAb 7E3 and PAF: (D) venules during the artificial perfusion; (E) rapid flow of SS RBCs in the vessels after a bolus infusion; (F) after the passage of the bolus, only few SS RBC adhere to the vessel wall. Visit the article on the website (www.bloodjournal.org) to download the video.
Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in control and PAF-treated preparations.
The adhesion of SS RBC shows strong inverse correlation with the venular diameter (control/SS: r = −.80, P< .00001; PAF/SS: r = −.86, P < .00001). Both intercepts and slopes of the regression lines show significant differences and show that PAF causes a significantly greater adhesion of SS RBC in small-diameter venules, the sites of frequent blockage (SS control intercept 0.41 ± 0.04 [mean ± SE], PAF/SS intercept 1.05 ± 0.08, P < .0001).
Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in control and PAF-treated preparations.
The adhesion of SS RBC shows strong inverse correlation with the venular diameter (control/SS: r = −.80, P< .00001; PAF/SS: r = −.86, P < .00001). Both intercepts and slopes of the regression lines show significant differences and show that PAF causes a significantly greater adhesion of SS RBC in small-diameter venules, the sites of frequent blockage (SS control intercept 0.41 ± 0.04 [mean ± SE], PAF/SS intercept 1.05 ± 0.08, P < .0001).
The effect of monoclonal antibodies on SS RBC adhesion
Control antibody OC125 F(ab′)2 and 7E3 F(ab′)2 (antiαIIbβ3 + αVβ3) were tested in five different experiments in combination with PAF; in each experiment, the blood from a single SS patient was tested in both a preparation pretreated with control F(ab′)2 and a preparation pretreated with 7E3 F(ab′)2 (total, 5 patients and 10 preparations). In four additional experiments, 10E5 F(ab′)2 (anti-αIIbβ3) was tested with the SS RBCs from two SS patients. Briefly, the preparation was first treated with a given antibody, and then perfused for 10 minutes with a combination of the antibody and PAF (see “Materials and Methods”).
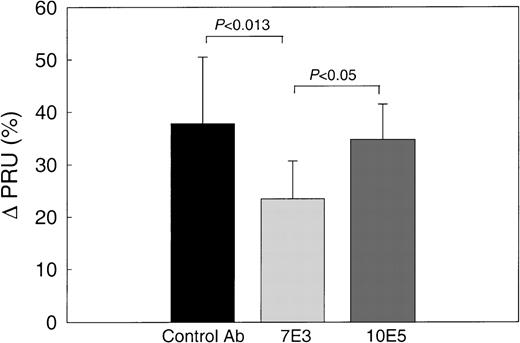
The baseline PRU values in these experiments (Table 2A) (7.3 ± 1.6, 6.2 ± 1.6, and 8.8 ± 4.3 mm Hg/mL/min/g for preparations pretreated with control antibody, 7E3, or 10E5, respectively) were somewhat higher than those for the untreated preparations in the previous experiments (Table 1), reflecting interpreparation variations. When SS RBCs were infused into the control antibody–pretreated and 10E5 F(ab′)2–pretreated preparations, the PRU increased by 37.8% ± 12.7% and 34.7% ± 6.8% (Figure 5), values similar to the 40.5% ± 6.7% increase in PRU in experiments using PAF and SS RBCs without antibody reported in Table 1 and Figure 2. In contrast, the PRU values increased by only 23.5% ± 7.2% in the preparations pretreated with 7E3 F(ab′)2, which is significantly less than the increases with either the control antibody (P < .0013) or 10E5 F(ab′)2 (P < .05). The Tpf results were in accord with the PRU data (Table 2) because the Tpf values of both the control antibody–pretreated preparation (97.0 ± 9.7 seconds) and the 10E5-pretreated preparation (105.0 ± 31.0 seconds) were significantly greater than the Tpf values of the 7E3 F(ab′)2–pretreated preparation (62.0 ± 5.7 seconds; P < .003 and P < .02, respectively).
The effect of 7E3 F(ab′)2 and LM609 on hemodynamic behavior of SS RBC in PAF-treated mesocecum vasculature
| A: 7E3 . | |||||
|---|---|---|---|---|---|
| Experiment . | No. . | PRU (mm Hg/mL/min/g) . | ΔPRU (%) . | Tpf (s) . | |
| Ringer . | RBC . | ||||
| 1. Control Ab/PAF + Control Ab/SS RBC | 5 | 7.3 ± 1.6 | 10.1 ± 2.8 | 37.8 ± 12.7 | 97.0 ± 9.7 |
| 2. 7E3/PAF + 7E3/SS RBC | 5 | 6.2 ± 1.6 | 7.7 ± 2.3 | 23.5 ± 7.2* | 62.0 ± 5.7† |
| 3. 10E5/PAF + 10E5/SS RBC | 4 | 8.8 ± 4.3 | 12.1 ± 6.7 | 34.7 ± 6.8 | 105.0 ± 31.0 |
| A: 7E3 . | |||||
|---|---|---|---|---|---|
| Experiment . | No. . | PRU (mm Hg/mL/min/g) . | ΔPRU (%) . | Tpf (s) . | |
| Ringer . | RBC . | ||||
| 1. Control Ab/PAF + Control Ab/SS RBC | 5 | 7.3 ± 1.6 | 10.1 ± 2.8 | 37.8 ± 12.7 | 97.0 ± 9.7 |
| 2. 7E3/PAF + 7E3/SS RBC | 5 | 6.2 ± 1.6 | 7.7 ± 2.3 | 23.5 ± 7.2* | 62.0 ± 5.7† |
| 3. 10E5/PAF + 10E5/SS RBC | 4 | 8.8 ± 4.3 | 12.1 ± 6.7 | 34.7 ± 6.8 | 105.0 ± 31.0 |
| B: LM609 . | |||||
|---|---|---|---|---|---|
| Experiment . | No. . | PRU (mm Hg/mL/min/g) . | ΔPRU (%) . | Tpf (s) . | |
| Ringer . | RBC . | ||||
| 1. Control Ab/PAF/SS RBC | 4 | 9.3 ± 2.4 | 14.0 ± 3.5 | 50.3 ± 8.7 | 117.5 ± 9.6 |
| 2. LM609/PAF/SS RBC | 4 | 7.6 ± 2.4 | 9.6 ± 3.5 | 25.1 ± 8.0‡ | 82.0 ± 9.32-153 |
| B: LM609 . | |||||
|---|---|---|---|---|---|
| Experiment . | No. . | PRU (mm Hg/mL/min/g) . | ΔPRU (%) . | Tpf (s) . | |
| Ringer . | RBC . | ||||
| 1. Control Ab/PAF/SS RBC | 4 | 9.3 ± 2.4 | 14.0 ± 3.5 | 50.3 ± 8.7 | 117.5 ± 9.6 |
| 2. LM609/PAF/SS RBC | 4 | 7.6 ± 2.4 | 9.6 ± 3.5 | 25.1 ± 8.0‡ | 82.0 ± 9.32-153 |
Values are mean ± SD. In these experiments, the vasculature was first incubated with either control (OC125) or 7E3 F(ab′)2antibodies (50 μg/mL, 5 mL) for 30 minutes followed by infusion of PAF (200 pg/mL, 40 mL) containing a given antibody. In one control experiment, the preparation was preinfused with PAF only.
Abbreviations: ΔPRU, PRU RBC/PRU Ringer; Tpf, pressure-flow recovery time to the baseline level.
P < .013 and P < .05 compared with ΔPRU for control Ab and 10E5 groups, respectively.
P < .0003 and P < .02 compared with Tpf for control Ab and 10E5 groups, respectively.
P < .006 compared with respective values for the control antibody group.
P < .02 compared with respective values for the control antibody group.
The effect of MoAb 7E3 F(ab′)2 on hemodynamic behavior of SS RBC in PAF-treated ex vivo mesocecum vasculature.
In preparations treated with OC125 F(ab′)2 (control antibody) or 10E5 F(ab′)2, SS RBC resulted in a comparable increase in the PRU as observed in the PAF-treated preparations (see Figure 1). In contrast, when the preparation was treated with MoAb 7E3 and PAF, SS RBC resulted in a significantly lower PRU compared with either control or 10E5.
The effect of MoAb 7E3 F(ab′)2 on hemodynamic behavior of SS RBC in PAF-treated ex vivo mesocecum vasculature.
In preparations treated with OC125 F(ab′)2 (control antibody) or 10E5 F(ab′)2, SS RBC resulted in a comparable increase in the PRU as observed in the PAF-treated preparations (see Figure 1). In contrast, when the preparation was treated with MoAb 7E3 and PAF, SS RBC resulted in a significantly lower PRU compared with either control or 10E5.
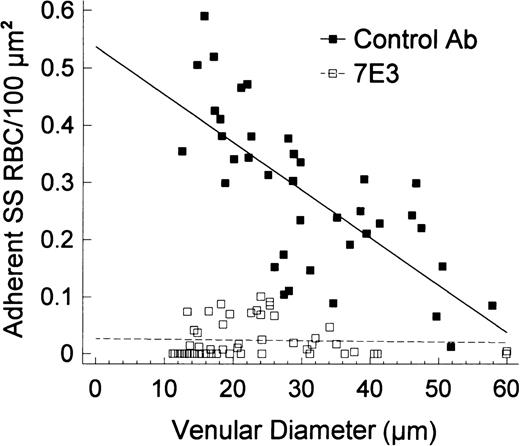
In preparations pretreated with the control antibody and PAF, there was pronounced adhesion of SS RBC to the venular endothelium (Figure 3A and C). In marked contrast, in preparations pretreated with 7E3 F(ab′)2, there was minimal SS RBC adhesion in four experiments and moderate SS RBC adhesion in one experiment (Figure 3D and F). Quantitative analysis of the number of adherent SS RBCs per 100 μm2 as a function of venular diameter revealed marked inhibition of SS RBC adhesion by 7E3 F(ab′)2 even in the smaller-diameter venules, the sites of maximal adhesion. As a result, the Y-intercept of the data plotted in Figure 6 was markedly different for the preparations treated with the control F(ab′)2 (0.55 SS RBC/100 μm2) and the 7E3 F(ab′)2(0.02 SS RBC/100 μm2; P < .0001). The slopes were also significantly different (P < .00001) because the 7E3 F(ab′)2–treated preparations did not demonstrate an inverse relationship between SS RBC adhesion and venular diameter. Furthermore, no obstruction of small-diameter postcapillary venules was evident in the presence of 7E3.
Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in preparations treated with 7E3 F(ab′)2 (or a control antibody) and PAF.
In the presence of OC125 F(ab′)2 (control antibody), PAF caused a pronounced adhesion of SS RBC that was inversely correlated with the vessel diameter (r = −.72, P < .00001). In contrast, 7E3 resulted in marked inhibition of SS RBC adhesion in venules, particularly in small-diameter venules (the sites of maximal adhesion and frequent blockage) that resulted in a lack of correlation between adhered cells and the vessel diameter (r = −.04, P > .78). A comparison of the two regression lines revealed pronounced differences in intercepts (OC125/PAF/SS 0.54 ± 0.04, 7E3/PAF/SS 0.03 ± 0.01; P < .0001).
Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in preparations treated with 7E3 F(ab′)2 (or a control antibody) and PAF.
In the presence of OC125 F(ab′)2 (control antibody), PAF caused a pronounced adhesion of SS RBC that was inversely correlated with the vessel diameter (r = −.72, P < .00001). In contrast, 7E3 resulted in marked inhibition of SS RBC adhesion in venules, particularly in small-diameter venules (the sites of maximal adhesion and frequent blockage) that resulted in a lack of correlation between adhered cells and the vessel diameter (r = −.04, P > .78). A comparison of the two regression lines revealed pronounced differences in intercepts (OC125/PAF/SS 0.54 ± 0.04, 7E3/PAF/SS 0.03 ± 0.01; P < .0001).
Microscopic analysis of the preparations treated with 10E5 F(ab′)2 revealed results similar to those seen with the control antibody, with pronounced adhesion of SS RBC in venules and frequent postcapillary blockage.
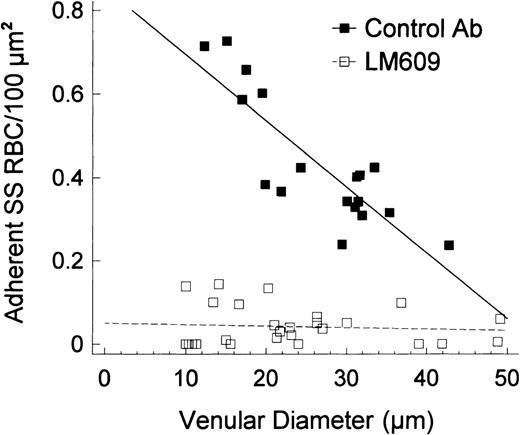
To further test the role of αVβ3 in SS RBC adhesion, we pretreated four preparations with control IgG and four preparations with LM609 IgG (each 50 μg/mL); all of the preparations were then treated with PAF (200 pg/mL). With the control antibody, the infusion of a bolus of SS RBCs resulted in a 50.3% ± 8.7% increase in PRU, whereas with LM609, the increase was only 25.1% ± 8.0% (P < .006; Table 2B). Similarly, the Tpf was signficantly greater with the control antibody (117.5 ± 9.6 seconds) than with LM609 IgG (82.0 ± 9.3 seconds, P < .02). Microscopic examination revealed that LM609, like 7E3 F(ab′)2, dramatically inhibited SS RBC adhesion (Figure 7; P < .0001 for the Y-intercept values and slopes).
Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in preparations treated with LM609 (or IgG) and PAF.
In the presence of IgG, PAF caused a pronounced adhesion of SS RBC that was inversely correlated with the vessel diameter (r = −.85,P < .00001). In contrast, LM609 caused a significant inhibition of SS RBC adhesion, particularly in small-diameter venules (the sites of maximal adhesion and frequent blockage), resulting in a lack of correlation between the diameter and the number of adherent cells (r = −.08, P > .68). A comparison of the two linear regression lines confirmed marked differences between intercepts (control Ab/PAF/SS 0.85 ± 0.07, LM609/PAF/SS 0.05 ± 0.02;P < .0001).
Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in preparations treated with LM609 (or IgG) and PAF.
In the presence of IgG, PAF caused a pronounced adhesion of SS RBC that was inversely correlated with the vessel diameter (r = −.85,P < .00001). In contrast, LM609 caused a significant inhibition of SS RBC adhesion, particularly in small-diameter venules (the sites of maximal adhesion and frequent blockage), resulting in a lack of correlation between the diameter and the number of adherent cells (r = −.08, P > .68). A comparison of the two linear regression lines confirmed marked differences between intercepts (control Ab/PAF/SS 0.85 ± 0.07, LM609/PAF/SS 0.05 ± 0.02;P < .0001).
Discussion
Our data show that: (1) in the ex vivo mesocecum vasculature, PAF promotes SS RBC–endothelium interactions, and this is associated with increased endothelial surface expression of vWf; and (2) antibodies directed against αVβ3 inhibit adhesion of SS RBC to the PAF-treated vasculature.
We chose PAF to stimulate endothelial cells in our studies because it has been shown to be elevated in the plasma of patients with sickle cell anemia43 and because it has been shown to cause release of endothelial vWf,41 an agent that we and others have reported increases SS RBC adhesion to endothelium.21,22 The concentration of PAF used in this ex vivo, plasma-free study is ∼50% of normal plasma PAF levels (393 ± 65 pg/mL), and ∼25% of the levels found in SS patients (797 ± 62 pg/mL).43 We previously showed that desmopressin, another agent that causes release of vWf from endothelial cells, also results in enhanced SS RBC–endothelial interaction.22
Because αVβ3 can bind vWf and because endothelial cells have αVβ3 on their luminal surface, we tested whether antibodies to αVβ3 would inhibit adhesion to the postcapillary venules. Importantly, both anti-αVβ3 antibodies we tested (7E3 and LM609) dramatically inhibited SS RBC adhesion under shear flow conditions. Because 7E3 also reacts with αIIbβ3,44 we also tested an antibody (10E5) that inhibits αIIbβ3,45 but not αVβ3. This antibody did not inhibit SS RBC adhesion, supporting the hypothesis that it is 7E3's anti-αVβ3 reactivity that results in the decrease in SS RBC adhesion. Likewise, TSP-enhanced adhesion of SS RBCs to cultured endothelium is abolished by anti-αVβ3 antibodies.34 Taken together, these results indicate that interfering with TSP and vWF binding to the endothelium can effectively inhibit SS RBC adhesion.
Consistent with our previous findings,6 9 the small-diameter venules, probably because of the diameter constraint, low red blood cell velocities, and perhaps endothelial differences compared with arterioles, were the sites of maximal adhesion and frequent blockage in PAF-treated preparations. The inhibition of SS RBC adhesion by anti-αVβ3 antibodies almost completely abolished postcapillary blockage in the present studies. The inhibition of adhesion also resulted in improved hemodynamic behavior of SS RBCs as shown by significantly smaller increases in the peripheral resistance and shortened pressure-flow recovery times. Thus, effective inhibition of SS RBC adhesion can alleviate adhesion-induced vasooclusion.
Although we used washed SS RBC and perfused the vasculature with plasma-free buffer, we cannot rigorously exclude a contribution from a small number of platelets that may have remained or the products they may have released, including TSP, vWf, and fibrinogen. Because TSP, vWf, and fibrinogen can bind to αVβ3, it is possible that some of the effects of the anti-αVβ3 antibodies were caused by inhibition of binding of these adhesive glycoproteins. Similarly, we cannot rigorously exclude the possibility that very small amounts of thrombin may have been generated in our near plasma-free system. Studies by Manodori et al51 found that thrombin can enhance SS RBC adhesion to endothelium with a peak effect at ∼0.1 U/mL, via a mechanism that involves interendothelial cell gap formation.
In conclusion, we have shown that PAF causes a significant increase in SS RBC adhesion, accompanied by an increase in vWf expression on the endothelial surface. This indicates that PAF could be a potential in vivo participant in the pathophysiology of sickle cell vasoocclusion. Pretreatment of the ex vivo vasculature with either of two different monoclonal antibodies to αVβ3 results in a significant inhibition of adhesion and a smaller increase in peripheral resistance. Thus, blockade of αVβ3 receptors may constitute a potential therapeutic approach to prevent SS RBC–endothelium interactions and related vasoocclusion in sickle cell anemia.
Note added in proof. After this manuscript was submitted for publication, Solovey et al52 reported that circulating endothelial cells from patients with sickle cell anemia exhibit increased expression of αVβ3, lending support to a role for αVβ3 in human SS red blood cell adhesion to the vasculature.
Supported by HL45931 (D.K.K.), the Bronx Comprehensive Sickle Cell Center (R.L.N., D.K.K.), and HL19278 (B.S.C.).
B.S.C. is an inventor of abciximab, and in accordance with federal law and the patent policy of the Research Foundation of the State University of New York, shares in royalties paid to the Foundation for the sale of abciximab.
Reprints:D. K. Kaul, Department of Medicine, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY 10461; e-mail:kaul@aecom.yu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 4. Linear regression plots for the number of adherent SS RBC/100 μm2 according to the venular diameter in control and PAF-treated preparations. / The adhesion of SS RBC shows strong inverse correlation with the venular diameter (control/SS: r = −.80, P< .00001; PAF/SS: r = −.86, P < .00001). Both intercepts and slopes of the regression lines show significant differences and show that PAF causes a significantly greater adhesion of SS RBC in small-diameter venules, the sites of frequent blockage (SS control intercept 0.41 ± 0.04 [mean ± SE], PAF/SS intercept 1.05 ± 0.08, P < .0001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/2/10.1182_blood.v95.2.368/6/m_bloo00253004x.jpeg?Expires=1763528211&Signature=D8eUDQdml~FZbzKF9rEEduqTA9pl32cZXXCZKusgFnVYFXfTOW0QQrRpVmlMIp0xtfkvodw8TCusT3VVdAuxPHPN6~PexIt2MS3-ZB42JjCwm6bVTab-bf4ogQDEZnEG4yvcu4ONxjCHqthy8G8iW~jesKMbSrAxyeajsXtceVj6MbyV9CCkOH2Q2oLnZQ5lm1QjBVFlhmasRC0zVOxEND1gdzp1ePLZXsHj-hJmr3WAAlkSwF0kv6rMBRFtkv6a0o~w917bLppK3m2C8NW0YyJFV6JsFoY8KJ1J5j02wyptb9q6scQhvYYzUl-bHFMa5JuhSCwCC1adGQTOiDifdA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal