Abstract
SMADs are evolutionarily conserved transducers of the differentiation and growth arrest signals from the transforming growth factor/BMP (TGF/BMP) family of ligands. Upon receptor activation, the ligand-restricted SMADs1–35 are phosphorylated in the C-terminal MH2 domain and recruit the common subunit SMAD4/DPC-4 gene to the nucleus to mediate target gene expression. Frequent inactivating mutations of SMAD4, or less common somatic mutations ofSMAD2 seen in solid tumors, suggest that these genes have a suppressor function. However, there have been no identified mutations of SMAD5, although the gene localizes to the critical region of loss in chromosome 5q31.1 (chromosome 5, long arm, region 3, band 1, subband 1) in myelodysplasia (MDS) and acute myelogenous leukemia (AML). A ubiquitously expressed novel isoform,SMAD5β, encodes a 351 amino acid protein with a truncated MH2 domain and a unique C-terminal tail of 18 amino acids, which may be the functional equivalent of inactivating mutations. The levels of SMAD5β transcripts are higher in the undifferentiated CD34+ hematopoietic stem cells than in the terminally differentiated peripheral blood leukocytes, thereby implicating the β form in stem cell homeostasis. Yeast 2-hybrid interaction assays reveal the lack of physical interactions between SMAD5β and SMAD5 or SMAD4. The expression ofSMAD5β may represent a novel mechanism to protect pluripotent stem cells and malignant cells from the growth inhibitory and differentiation signals of BMPs.
The transforming growth factor-β (TGF-β) superfamily of multifunctional cytokines regulates proliferation, differentiation, and apoptosis in hematopoietic cells and a variety of other tissues and cell types.1 The TGF-β/BMP/activin family of ligands signals by binding to specific transmembrane receptors.2 In response to ligand binding, the type II receptor, which is a serine/threonine kinase, initiates a signaling cascade by phosphorylating the serine/threonine kinase type I receptor. The type I receptor in turn phosphorylates the specific ligand-restricted SMAD, which heterodimerizes with the common mediator SMAD4/DPC4 and translocates into the nucleus to recruit transcriptional coactivators. The multiprotein complex thus assembled induces target gene expression.3-5
In the vertebrates, 8 highly conserved SMADs were identified and categorized into 3 groups: (1) ligand-restricted SMADs (SMAD1and SMAD5 respond to BMP, and SMAD2 and SMAD3respond to activin and TGF-β); (2) negative regulators (SMAD6and SMAD7); and (3) a common mediator/subunit (SMAD4/DPC4). The SMAD genes are modular because they comprise 3 major domains: (i) the highly conserved N-terminal DNA binding domain (MH1); (ii) the C-terminal regulatory domain (MH2), which is involved in SMAD-receptor interaction, SMAD homodimerization, SMAD-SMAD4 interaction, and SMAD-transcription cofactor interaction; and (iii) a variable proline/serine–enriched linker region that is the target of phosphorylation by mitogenic signals.3-5
The MH1 and MH2 domains inhibit each other in the basal state, and tumor-specific mutations enhance autoinhibition.6 In response to receptor-mediated phosphorylation, this inhibition is relieved, allowing the heterodimeric interactions through the MH2 domain. X-ray crystallographic analysis of the SMAD1 MH1 domain reveals a novel DNA-binding motif consisting of a conserved 11-residue β hairpin that can be embedded in the major groove of DNA to bind the 4–base pair (4-bp) “SMAD box” motif.7
The physiological responses to the TGF-β family of ligands, namely growth inhibition and differentiation, render this pathway a critical target for inactivation during neoplastic transformation. The common subunit SMAD4/DPC4 was initially isolated as a tumor suppressor that is homozygously deleted in patients with pancreatic cancer.8 Inactivating mutations of SMAD2 have also been identified in patients with colon cancer.9
The SMAD5 gene is localized to human chromosome 5q31 (chromosome 5, long arm, region 3, band 1) to a region of invariant allele loss (subband 1) in human myeloid leukemia. The lack of gross intragenic mutations suggests that SMAD5 is not a common target of somatic inactivation in leukemia and solid tumors.10-13Nonetheless, microinjection of mouse SMAD5 transcripts intoXenopus toad embryos induces embryonic globin synthesis analogous to the response seen with BMP-4 transcripts.14The human SMAD5 gene is transcribed into both major (8.7-kilobase [8.7-kb]) and minor (4.2-kb) transcripts in several hematolymphoid tissues including the spleen, fetal liver, appendix, thymus, lymph node, bone marrow, and peripheral blood leukocytes.10
Homozygous SMAD5 null mice undergo embryonic lethality with abnormal vasculature and blood cells.15,16 Recent studies demonstrate that SMAD1 and SMAD5, as well as the type I receptors ALK3 and ALK6, are expressed in CD34+CD38−lin− human hematopoietic stem cells, which are capable of giving rise to all the lineages in nonobese diabetic/severe combined immune deficient (NOD/SCID) mice.17 Furthermore, the CD34+CD38− lin− cells show a unique biphasic response to BMP-4, with growth inhibition at low concentrations and enhanced colony formation at higher concentrations.17
TGF-β, a potent inducer of erythroid differentiation and inhibitor of granulocytic macrophage progenitors, has a sparing effect on early progenitors.18-20 Despite the growing body of evidence, very little is known about the mechanisms underlying the differential regulation of hematopoiesis by TGF-β/BMP. Specifically, the precise regulation of hematopoietic stem cell, progenitor self-renewal, and differentiation in the presence of circulating TGF-β/BMP are poorly understood.
Here, we report an alternative splice form of SMAD5, designatedSMAD5β, which encodes the entire MH1 and linker domains, and a truncated MH2 domain. The stoichiometry between theSMAD5β and SMAD5 transcripts is higher in undifferentiated CD34+ hematopoietic bone marrow stem cells than in terminally differentiated peripheral blood cells. The SMAD5β isoform does not homodimerize or heterodimerize with SMAD5 or SMAD4. Thus, the enhanced expression of the alternative splice form may explain, in part, the refractoriness of hematopoietic stem cells to BMP-4.
Materials and methods
Genomic polymerase chain reaction
Long polymerase chain reactions (long-PCRs) were performed to obtain genomic fragments spanning adjacent exons. We used the bacterial artificial chromosome (BAC) b37i1610 as a template and the ELONGase enzyme mix (Life Technologies, Gaithersburg, MD). The following conditions of amplification were applied: initial denaturation (94°C for 2 minutes); then 30-35 cycles of denaturation (94°C for 30 seconds), annealing (55-58°C for 30 seconds), and extension (68-70°C for 2-10 minutes); and a final extension (68-70°C for 5-10 minutes). Intron 3 was amplified with primers Ex1.F and Ex2.R; intron 4, JV5.1.F2 and JV5.1.R1; intron 5, Ex3.F and Ex4.R; intron 6, Ex4.F and Ex5.R; and intron 7, Ex5.F and Ex6.R. Amplification of primers Ex1.F/Ex2.R, Ex4.F/Ex5.R, and Ex5.F/Ex6.R were reported previously.10 The remaining primers were amplified as follows: JV5.1.F2, 5′-AGC AAG TTC TGG ACC AGG AAG-3′; JV5.1.R1, 5′-ATT ATT GCT TGT ATC CAT AGG CT-3′; Ex3.F, 5′-CTA TCC TCA CTC CTA TCC TCA-3′; and Ex4.R, 5′-TAT AAA ATA CCG TAA GGC TGA-3′.
Hematopoietic cells, leukemia cells, and RNA isolation
Normal peripheral blood mononuclear cells (PBMCs) were enriched by Ficoll Hypaque (Organon Teknika, Durham, NC) gradient. Bone marrow from healthy donors was sorted for CD34 expression by fluorescence activated cell sorter (FACS). Several acute myelogenous leukemic cells with anomalies of chromosome 5q31 were used in the study. Of these, AML193 (acute myelomonocytic leukemia), TF1 (erythroleukemia), and U937 (myelomonocytic leukemia) harbor uncharacterized translocations involving the 5q31 chromosome. KG1 (acute myelogenous leukemia) cells are monosomic for chromosome 5, and ML3 (acute myelogenous leukemia) cells carry a chromosome deletion, del(5)(q13-q35), and a chromosome derivative, der(3), with insertion of (5)(q13-35) material. Additionally, HEL (erythroleukemia) and K562 (Ph+ chromosome, chronic myelogenous leukemia in erythroid blast crisis) were also included. The cells were cultured according to their growth requirements in humidified air containing 5% carbon dioxide (CO2) at 37°C. ML-3, HEL, K562, and U937 cells were cultured in Roswell Park Memorial Institute medium (RPMI 1640) with 10% fetal bovine serum (FBS), and KG1 cells in Iscove's Modified Dulbecco's medium (IMDM) with 20% FBS. The factor-dependent TF1 cell line was grown in RPMI 1640 with 10% FBS plus 5 ng/mL granulocyte macrophage–colony stimulating factor (GM-CSF), and the factor-dependent cell line AML193 was grown in IMDM with 10% FBS plus 5 μg/mL transferrin, 5 μg/mL insulin, and 5 ng/mL GM-CSF.
The total RNA was prepared from PBMCs, bone marrow hematopoietic stem cells from a normal donor flow sorted for the expression of the CD34 antigen, and leukemia cells in exponential growth by the TRIzol reagent (Gibco BRL, Gaithersburg, MD) or guanidium isothiocyanate lysis.
Radioactive reverse transcriptase–PCR
A total of 3 μg RNA from each source was reverse transcribed with oligo-dT primers, and the complimentary DNA (cDNA) pool was diluted 40-fold. The following primer pairs were used forSMAD5β: JV5.1.F4, 5′-TAA ACA ATC GTG TTG GAG AAG C-3′, and Ex4.RP, 5′-ATA CAT TCT TCA ATA TCG GCA ACT-3′. The following primer pairs were used for full-lengthSMAD5: JV5.1.F14, 5′-AAA TGT GTA CCA TTC GGA TGA G-3′, and JV5.1.R13, 5′-AAA CAG AAG ATA TGG GGT TCA G-3′, and β-2-microglobin (β-2-m) was used as an internal control. We pooled 5 pmol of each sense primer and end-labeled them with 32P ATP (adenosine 5′-triphosphate) using polynucleotide kinase in the same reaction. Unlabeled primer pairs (10 pmol) spiked with 0.1 pmol of the end-labeled reverse primer were used in each amplification reaction, and 30 cycles of amplifications were performed. The expected product sizes were 307-bp SMAD5β, 170-bpSMAD5, and 114-bp β-2-m. The linear nature of the assay conditions was first established by quantitating the products against a range of reference templates. Three independent PCRs were performed for each sample and resolved on an 8% polyacrylamide gel, and the amplifications were quantified in a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Competitor DNA constructs for quantitative PCR
To make a SMAD5β competitor construct, we designed a forward primer that will anneal to the cDNA template 54 bases downstream of the 3′ end of P1 (in the SMAD5 exon 6 at position 18 nucleotide [nt]). This was done by using a forward primer (P2, 5′-AGC CTA TGG ATA CAA GCA ATA ATT ATG AAG AGC CTA AAC ATT GGT G-3′) that contains the entire sequence of the native forward primer (P1, 5′-AGC CTA TGG ATA CAA GCA ATA AT-3′) at the 5′ end (in the SMAD5 exon 5 at position 61 nt) followed by a sequence downstream of P1. When used in PCR, this primer, together with the 3′ end reverse primer (P3′) in the SMAD5 intron 6 at position 120 nt, generated a 373-bp fragment that was cloned into pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA) as a SMAD5β competitor. The P1 and P3 primer pair yielded a 427-bp nativeSMAD5β fragment. For the SMAD5competitor construct, the primer P2 is the same as that for theSMAD5β competitor, whereas the 3′ reverse primer (P3, 5′-GAT TAA CAT TTG ACA ACA AAC CC-3′) is complementary to SMAD5 exon 6 at position 158 nt. The P2 and P3 primer pair amplified a 196-bp fragment for the SMAD5 competitor construct, and the primer P1 and P3 amplified a 250-bp native SMAD5 fragment.
Quantitation by competitive PCR
During PCR, a constant amount of synthesized cDNA (1:20 dilution, 2 μL for each reaction) was coamplified with varying concentrations of competitor plasmid DNA (TA/SMAD5 or TA/SMAD5β). PCR was performed with an initial denaturation at 94°C for 2 minutes; followed by 35 cycles of denaturation, annealing, and extension at 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 30 seconds, respectively; and a final extension of 72°C for 5 minutes. At least 2 independent PCRs were performed for each sample. PCR products were resolved by 2% agarose gel electrophoresis. The gel was stained with ethidium bromide and then photographed. The ratio of intensity between native versus competitor bands was examined for each lane.
Yeast 2-hybrid interaction assays
To analyze protein-protein interactions, the LexA-based yeast 2-hybrid system (Invitrogen) was used. The full-length cDNA sequences of SMAD5 and SMAD5β were subcloned into pHyb/LexA and pYESTrp2 vectors, respectively.SMAD5β.N345S, corresponding to a common polymorphism, as well as SMAD5.e6T, which is truncated at codon 333, were also cloned into the corresponding vectors. Sequences of all constructs were confirmed to be accurate and in-frame. The plasmids were transformed into the yeast L40 strain, and the expression was verified by immunoblots with anti-LexA antibody, which recognizes the epitope-tagged fusion proteins. The bait plasmids were initially checked for nonspecific activation (histidine prototrophy and β-galactosidase activity). Protein interactions were detected by the filter assay, which scores for β-galactosidase activity on the filter containing X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactoside).
Databases
Results
Characterization of the genomic organization of SMAD5 and identification of an alternatively spliced form,SMAD5β
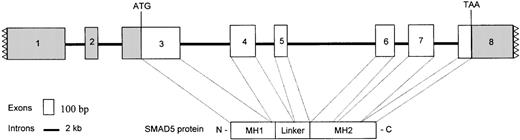
Previous studies concluded that the SMAD5 gene is made up of 8 exons, with the translation start at exon 3.13 An initial screen of the BAC b37i16 with sequence-tagged sites (STSs) derived from the 5′ and 3′ untranslated regions (UTRs) of the full-length SMAD5 cDNA were positive, indicating that the entire gene resided within the 180-kb insert. The BAC b37i16 was used as a template to determine the size of the introns. Primers designed from the known exonic and partial intronic sequences were used under multiple conditions to obtain amplifications of 1- to 10-kb genomic targets.
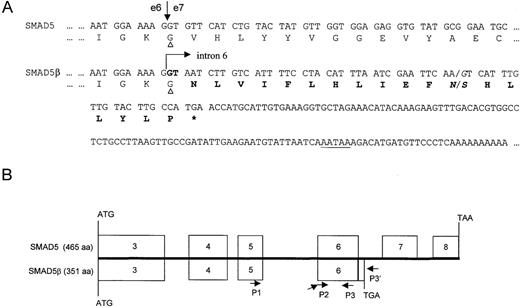
The genomic organization of SMAD5 shown in Figure1 reveals that the coding sequences are contained in a fragment of about 22.6 kb. Figure 1 also depicts the modular nature of the SMAD5 gene. The entire MH1 domain is encoded by exon 3, the unique linker region is encoded by exons 4 and 5, and the MH2 domain is encoded by exons 6-8. As we could not determine the exact size of exon 8 (3′UTR) nor identify a polyadenylation signal, the Expressed Sequence Tagged Sequences (EST) database (dbEST)22 was searched for cDNA clones with polyadenylation signal. Surprisingly, a single cDNA clone from ovarian cancer (IMAGE consortium clone ID594181) with a canonical polyadenylation (poly-A) signal and a poly-A tail was identified. Upon sequencing, the cDNA 594181 with an insert size of 770 bp was found to encode a transcript that diversified within the MH2 domain at codon 334, although the upstream linker sequences were identical toSMAD5. A comparison of the clone 594181 sequences with the partial genomic sequences from BACb37i16 revealed that the EST clone was an alternate splice form of SMAD5, in which the 5′ splice donor sequences of intron 6 were suppressed (Figure2A). We therefore designated the novel alternate splice form SMAD5β.
Organization of the human SMAD5 gene.
The boxes represent exons, and the lines denote introns. Filled boxes with jagged lines represent the partial 5′ and 3′ UTR. The SMAD5 coding region resides between the ATG start codon at exon 3 and the TAA stop codon at exon 8. The sizes of the exons are as follows: exon 3, 574 bp; exon 4, 252 bp; exon 5, 120 bp; exon 6, 222 bp; and exon 7, 257 bp. The sizes of the introns are: intron 3, 6.5 kb; intron 4, 2.1 kb; intron 5, 8.0 kb; intron 6, 1.5 kb; and intron 7, 2.5 kb. The exons corresponding to the MH1, linker, and MH2 domains are noted.
Organization of the human SMAD5 gene.
The boxes represent exons, and the lines denote introns. Filled boxes with jagged lines represent the partial 5′ and 3′ UTR. The SMAD5 coding region resides between the ATG start codon at exon 3 and the TAA stop codon at exon 8. The sizes of the exons are as follows: exon 3, 574 bp; exon 4, 252 bp; exon 5, 120 bp; exon 6, 222 bp; and exon 7, 257 bp. The sizes of the introns are: intron 3, 6.5 kb; intron 4, 2.1 kb; intron 5, 8.0 kb; intron 6, 1.5 kb; and intron 7, 2.5 kb. The exons corresponding to the MH1, linker, and MH2 domains are noted.
Alternative splice forms of SMAD5.
(A) SMAD5 and SMAD5β diverge at the junction of exon 6. Arrows denote the exon 6/exon 7 junction of SMAD5 or the exon6/intron 6 junction of SMAD5β. The 5′ splice site (GT) is given in bold. SMAD5β is transcribed through exon 6 and part of intron 6 to terminate at an alternative poly-A site, which is underlined. The novel 18 amino acids are given in bold. Note that codon 334, indicated with triangles, is a glycine (Gly) in both isoforms. The common nucleotide polymorphism and the corresponding amino acid polymorphism are italicized. (B) Comparison of the open reading frames (ORFs) of both isoforms. Schematic depicts the full-length ORFs of SMAD5 (top), which encode 465 amino acids, and the C-terminally truncated splice form SMAD5β (bottom), which encodes 351 amino acids. Both proteins are translated using the same start site and have an identical amino acid sequence through the end of exon 6 (333 amino acids).
Alternative splice forms of SMAD5.
(A) SMAD5 and SMAD5β diverge at the junction of exon 6. Arrows denote the exon 6/exon 7 junction of SMAD5 or the exon6/intron 6 junction of SMAD5β. The 5′ splice site (GT) is given in bold. SMAD5β is transcribed through exon 6 and part of intron 6 to terminate at an alternative poly-A site, which is underlined. The novel 18 amino acids are given in bold. Note that codon 334, indicated with triangles, is a glycine (Gly) in both isoforms. The common nucleotide polymorphism and the corresponding amino acid polymorphism are italicized. (B) Comparison of the open reading frames (ORFs) of both isoforms. Schematic depicts the full-length ORFs of SMAD5 (top), which encode 465 amino acids, and the C-terminally truncated splice form SMAD5β (bottom), which encodes 351 amino acids. Both proteins are translated using the same start site and have an identical amino acid sequence through the end of exon 6 (333 amino acids).
SMAD5β encodes a novel carboxyl-terminal (C-terminal) tail of 18 amino acids, which includes a glycine (G) at residue 334, a stop codon, and a short 3′ UTR (119 nt) with a canonical poly-A signal within intron 6. Furthermore, the splice donor and acceptor sites at codon 333 are also conserved in the SMA-2gene, the closest homolog of SMAD5 in C elegans, although the much smaller intron (185 bp) of C elegans does not contain a poly-A signal.23 In contrast to the full-lengthSMAD5 that encodes for a protein of 465 amino acids,SMAD5β would encode a protein of 351 amino acids with a truncated MH2 domain (Figure 2B). The novel 18 amino acid lysine-rich tail does not show any homology to sequences in the DNA and protein databases. In addition, a single nucleotide polymorphism (A/G) at 37nt, intron 6 of SMAD5, which we identified previously,10 is predicted to translate into an amino acid polymorphism of asparagine/serine (N/S) at codon 345 ofSMAD5β.
We further confirmed that the SMAD5β transcripts use the same initiation methionine (M) codon as the full-length protein, and the alternative splice form was also expressed in hematopoietic tissues. RT-PCR performed using the cDNA pool from K562 cells with primers spanning the initiation M of SMAD5 and the predicted stop codon of SMAD5β yielded the expected product of 1053 bp (Y.J. and L.N., unpublished results, February, 1999). DNA sequencing confirmed that the alternative transcript used identical 5′ sequences.
Enhanced expression of SMAD5β in normal bone marrow stem cells and in the erythroleukemia TF1 cell line
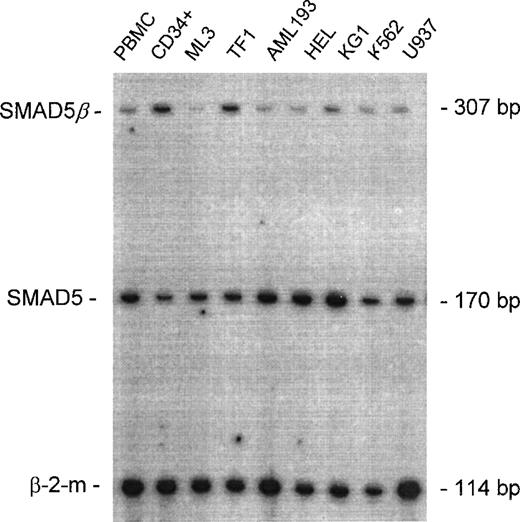
Full-length SMAD5 and theSMAD5β isoform could not be readily detected in normal and malignant cells by Northern blot analyses of total RNA. Therefore, we employed a radio-label–coupled RT-PCR (RRT-PCR) with unique primer pairs that detected either the full-length SMAD5 or the alternative splice form to screen cDNA pools from CD34+normal bone marrow stem cells and PBMCs. A panel of 7 acute myelogenous leukemia cell lines (ML3, TF1, AML193, HEL, KG1, K562, and U937), which represented different lineages and stages of myeloid maturation with and without anomalies of chromosome 5q31 (see “Materials and methods”), were examined. Products specific for both the full-length form and the shorter isoform were detected in all the cell types examined. Interestingly, the intensity of the SMAD5β product was higher than the full-length SMAD5 in CD34+ hematopoietic stem cells in contrast to the terminally differentiated PBMCs. Among the leukemia cell lines, the erythroleukemia TF1 cell line expresses the highest level of SMAD5β (Figure3).
Expression of SMAD5β in hematopoietic and leukemic cells.
A representative autoradiograph of multiplexed RT-PCR products resolved on a poly-A site. The upper band (307 bp) representsSMAD5β; the middle band (170 bp) represents the full-length SMAD5; and the lower band (114 bp) represents the internal control, β-2-m. Templates used for amplification are as indicated: peripheral blood mononuclear cells (PBMCs), normal bone marrow progenitor cells (CD34+), acute myelogenous leukemia (ML-3), erythroleukemia (TF1), acute myelogenous leukemia (AML193), erythroleukemia (HEL), acute myelogenous leukemia (KG1), chronic myelogenous leukemia in erythroid blast crisis (K562), and myelomonocytic leukemia (U937).
Expression of SMAD5β in hematopoietic and leukemic cells.
A representative autoradiograph of multiplexed RT-PCR products resolved on a poly-A site. The upper band (307 bp) representsSMAD5β; the middle band (170 bp) represents the full-length SMAD5; and the lower band (114 bp) represents the internal control, β-2-m. Templates used for amplification are as indicated: peripheral blood mononuclear cells (PBMCs), normal bone marrow progenitor cells (CD34+), acute myelogenous leukemia (ML-3), erythroleukemia (TF1), acute myelogenous leukemia (AML193), erythroleukemia (HEL), acute myelogenous leukemia (KG1), chronic myelogenous leukemia in erythroid blast crisis (K562), and myelomonocytic leukemia (U937).
We further developed a quantitative competitive PCR (QC-PCR) to quantify the absolute amount of transcripts present in the initial cDNAs from PBMCs and CD34+ and TF1 cells. HEL cells were also included as a control. The results in Figure4 suggest that the steady-state levels of SMAD5 transcripts may be similar in all these cells, as seen by equal amplification around 92.5 fmol competitor DNA. In contrast, the levels of SMAD5β vary from one cell type to another. Thus, equimolar amplification is seen around 8.75 fmol PBMCs, whereas in the CD34+ cells, equimolar products would be obtained between 43.75 and 87.5 fmol of the competitor. Similarly, the cDNA pool from TF1 cells needs a competitor template between 43.75 and 87.5 fmol to yield the same amount of products, unlike the HEL cells, which would yield equimolar products between 17.5 and 43.75 fmol.
Differential expression of SMAD5β in PBMC, CD34+, TF1, and HEL cells.
Competitor plasmids, which would yield smaller products from the same primer pair, were constructed. Serial dilutions of either the SMAD5 or SMAD5β competitor were coamplified with cDNAs from PBMCs and CD34+, TF1, and HEL cells. The positions of primers P1, P2, P3, and P3′ are indicated in Figure 2B. The 250-bp and 427-bp bands correspond to the native SMAD5 andSMAD5β, respectively, and the 196-bp and 373-bp bands correspond to the competitor templates. PCR products were resolved in 2% agarose gel and stained with ethidium bromide. The photographs were reversed to give a negative version.
Differential expression of SMAD5β in PBMC, CD34+, TF1, and HEL cells.
Competitor plasmids, which would yield smaller products from the same primer pair, were constructed. Serial dilutions of either the SMAD5 or SMAD5β competitor were coamplified with cDNAs from PBMCs and CD34+, TF1, and HEL cells. The positions of primers P1, P2, P3, and P3′ are indicated in Figure 2B. The 250-bp and 427-bp bands correspond to the native SMAD5 andSMAD5β, respectively, and the 196-bp and 373-bp bands correspond to the competitor templates. PCR products were resolved in 2% agarose gel and stained with ethidium bromide. The photographs were reversed to give a negative version.
The results depicted in Figure 4 suggest that the molar ratio of the full length to the β form is higher in PBMCs and HEL cells than in CD34+ and TF1 cells. Thus, the expression of the alternative splice form appears to be differentially regulated during normal hematopoietic maturation. The enhanced expression of the β isoform in TF1 versus HEL erythroleukemic cells suggests that the aberrant expression of the alternative splice form may be due to genetic alterations that are unique to specific cases and are not tightly correlated with the morphologic classification or stages of maturation.
SMAD5β does not homodimerize with itself and heterodimerize with SMAD5 or SMAD4
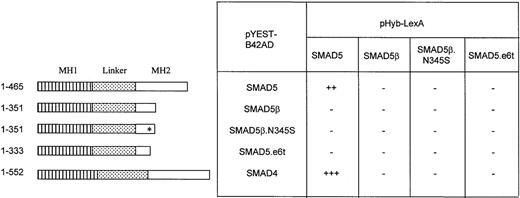
The MH2 domain truncation in SMAD5β suggests that this isoform may not readily heterodimerize with SMAD4/DPC4.6,21 However, the possibility of a homodimeric interaction due to the novel C-terminal tail or dimerization with the full-length SMAD5 could not be ruled out. Interaction studies in the yeast 2-hybrid system, which lacks endogenous SMAD, were performed to determine if this was indeed the case. Physical interactions between the proteins were scored based on the transcription activation of the β-galactosidase reporter gene. Homodimeric or heterodimeric interactions of SMAD5, SMAD5β, and SMAD4 were examined. Another SMAD5 construct in which the MH2 domain was truncated at codon 333 of exon 6 (SMAD5.e6t) and the polymorphic variant SMAD5β.N345S were also included. Results shown in Figure5 demonstrate that the full-length SMAD5 molecules can both homodimerize and heterodimerize with SMAD4, with heterodimerization being stronger than homodimerization. None of the isoforms with the truncated MH2 domain, SMAD5β, SMAD5β.N345S, or SMAD5.e6t, homodimerizes or interacts with the full-length SMAD5 or SMAD4. These results confirm that the C-terminal MH2 domain spanning amino acids 335-465 is involved in SMAD-SMAD homodimeric or heterodimeric interactions.24
SMAD5β does not heterodimerize or homodimerize.
The protein interactions of SMADs were assayed by the yeast 2-hybrid system. Various SMAD forms were expressed in frame with the LexA DNA binding domain in the pHyb-LexA plasmid (bait): the full-length forms, SMAD4, SMAD5, and SMAD5β; the polymorphic form SMAD5β.N345S; and a truncated form, SMAD5.e6t. Each SMAD was also expressed in frame with the transcription activation domain B42 in the pYEST-B42AD plasmid (prey). Transformants were tested for protein interactions using the β-galactosidase filter assay. The intensity of β-galactosidase was scored based on the intensity of the blue color, ranging from no color (−) to strongly positive (+++).
SMAD5β does not heterodimerize or homodimerize.
The protein interactions of SMADs were assayed by the yeast 2-hybrid system. Various SMAD forms were expressed in frame with the LexA DNA binding domain in the pHyb-LexA plasmid (bait): the full-length forms, SMAD4, SMAD5, and SMAD5β; the polymorphic form SMAD5β.N345S; and a truncated form, SMAD5.e6t. Each SMAD was also expressed in frame with the transcription activation domain B42 in the pYEST-B42AD plasmid (prey). Transformants were tested for protein interactions using the β-galactosidase filter assay. The intensity of β-galactosidase was scored based on the intensity of the blue color, ranging from no color (−) to strongly positive (+++).
Discussion
The genomic organization and expression of the human SMAD5gene was studied in order to understand the function of BMPs in normal hematopoiesis and leukemogenesis. Differential expression of a novel C-terminal truncated splice form, SMAD5β, provides clues on how hematopoietic stem cells may override constitutive differentiation and growth arrest cues from the BMP family of ligands.
The C-terminal truncated SMAD5β isoform
The alternative splice form characterized here is the first example of a physiological negative regulator of SMADs that may occur at the level of DNA binding or protein-protein interactions necessary for transcription. SMAD5β may act predominantly in the nucleus. This is unlike SMAD6 and SMAD7, the well-characterized negative regulators of SMAD signaling, which bind to the activated receptors and interfere with the recruitment and phosphorylation of ligand-specific SMADs.25-27 Absence of the receptor-mediated phosphorylation sites and heterodimerization domains render the β isoform a ligand-independent regulator that can confer refractoriness. This can be achieved by competing with other SMADs to bind to the SMAD response elements. The MH1 domain of the MAD gene ofDrosophila exhibits 100-fold enhanced DNA binding in vitro compared with the full-length protein.28 Alternatively, the novel C-terminal tail of the β isoform may interact with regulatory proteins to activate and/or inactivate specific target genes that are unique to stem cells. Thus, the possibility that SMAD5β is both a transcriptional coactivator and corepressor, depending upon the cellular context, cannot be excluded.
The evolutionary conservation of the splice donor and acceptor sites at codon 333 between exons 6 and 7 of the human SMAD5 gene and theSMA-2 gene of C elegans is noteworthy. Unlike the human intron, the intron in C elegans is much smaller (185 bp) and lacks a canonical poly-A signal. Thus, the SMAD5β isoform has evolved to modulate the complexity of BMP responses in mammals.
Expression of alternative splice forms in normal hematopoiesis and leukemogenesis
The high conservation of SMADs in evolution strongly suggests that the SMAD5 pathway is central to mammalian hematopoiesis. SMAD5 is implicated as a preferred transducer of signals from the BMP family of ligands. Specifically, SMAD5 mimics the BMP-4 mediated hematopoietic differentiation and globin synthesis in Xenopus laevisembryos.14 Other studies of the mesenchymal precursor cell line C2C1229,30 and the rat osteoprogenitor cells ROB-C2631 have suggested that SMAD5 is a mediator of BMP-2 or BMP-7 responses.
The restricted number of hematopoietic stem cells in the normal adult bone marrow makes this compartment a technical challenge for expression studies. Although the estimation based on the competitive PCR assay may not be absolute, Figure 4 is consistent with enhanced expression of the β isoform in undifferentiated hematopoietic stem cells. Thus, SMAD5β may manifest a dominant negative function that counteracts activation of the BMP-mediated differentiation program and may protect self-replication of the hematopoietic stem cells. Future studies on specific subpopulations of hematopoietic progenitors, as well as hematopoietic cell lines (eg, TF1) amenable to in vitro differentiation, will provide valuable clues to such a mechanism.
TGF-β has a sparing effect on primitive progenitors, in contrast to the inhibitory effect on committed GM-CSFs.18-20 TGF-β is also a potent inducer of erythroid differentiation. The role of anti-SMADs in conferring refractoriness during hematopoietic differentiation is unknown at present. Antisense oligonucleotides to SMAD5 reverse the inhibitory effects of TGF-β on the interleukin-3–mediated (IL-3–mediated) proliferative response of hematopoietic progenitors.32 Our future studies will elucidate whether the SMAD5β isoform confers refractoriness specifically to BMP-4 or to the entire TGF-β/BMP/activin family of ligands. Finally, the regulation of alternative splicing may provide clues to mechanisms governing hematopoietic stem cell homeostasis.
SMAD5β, the functional equivalent of inactivating mutations
Loss of sensitivity to the TGF-β family-mediated growth arrest represents an important step in neoplastic transformation. Refractoriness to TGF-β is achieved by inactivation of the common subunit SMAD4 or by mutations in the type II receptor, which binds the ligand. While mutations in SMAD4 appear to be associated with 50% of pancreatic and colon cancers,8 mutations in the ligand-restricted SMAD2 gene are rare (6% in colon cancer).9 Mice harboring targeted homozygous deletions of the SMAD3 gene are predisposed to colonic neoplasms,33 although intragenic mutations of the human ortholog have not been reported. Similarly, we and others have not found inactivating mutations or homozygous deletions of SMAD5in more than 200 malignant tissues and cell lines.10-13
Inactivating mutations in SMAD4 and SMAD2 render them incapable of homodimerization or heterodimerization.6 The SMAD5β isoform is analogous to the inactivating mutations in that it does not exhibit homodimeric or heterodimeric interactions (Figure 5). The aberrant expression of SMAD5β in the erythroleukemia TF1 cell line is likely to alter the erythroid differentiation response to TGF-β/BMP ligands. Furthermore, the ovarian cancer tissue from which the EST clone 594181 was isolated may have acquired refractoriness to TGF-β/BMPs by expressing the alternative splice form. Absence of homozygous deletions forSMAD5 in cancer may be in part due to the selective advantage conferred by the β isoform on the transformed phenotype. Nonetheless, the short 3′UTR of the β isoform may render the transcript unstable, and the alternative splice form may be underrepresented in the cDNA libraries.
Finally, expression of the SMAD5 protein appears to be regulated at multiple levels. Recently, we characterized low levels of DAMS, an antisense transcript to SMAD5 that is expressed in fetal tissues and a pancreatic tumor.34 Functional studies on endogenous SMAD5 isoforms will elucidate their role in normal cell growth and neoplastic transformation. Isolation and characterization of the alternative splice form is a first step toward these studies.
Acknowledgments
We thank Dr John Massague for the pCMV5/SMAD4-HA construct, Dr Shourong Zhao for RNA from CD34+ cells, and Brian Johnson and Monica Spears for manuscript preparation.
Reprints:Lalitha Nagarajan, the Department of Molecular Genetics, 1515 Holcombe Blvd, Box 45, MD Anderson Cancer Center, University of Texas, Houston 77030, TX; e-mail:lalitha@odin.mdacc.tmc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal