Abstract
The chimeric anti-CD20 MAb rituximab has recently become a treatment of choice for low-grade or follicular non-Hodgkin's lymphomas (FL) with a response rate of about 50%. In this report, we have investigated the mechanism of action of rituximab on 4 FL and 1 Burkitt's lymphoma (BL) cell lines, 3 fresh FL samples and normal B cells in vitro. Rituximab efficiently blocks the proliferation of normal B cells, but not that of the lymphoma lines. We did not detect significant apoptosis of the cell lines in response to rituximab alone. All cell lines were targets of antibody-dependent cellular cytotoxicity (ADCC). On the other hand, human complement-mediated lysis was highly variable between cell lines, ranging from 100% lysis to complete resistance. Investigation of the role of the complement inhibitors CD35, CD46, CD55, and CD59 showed that CD55, and to a lesser extent CD59, are important regulators of complement-mediated cytotoxicity (CDC) in FL cell lines as well as in fresh cases of FL: Blocking CD55 and/or CD59 function with specific antibodies significantly increased CDC in FL cells. We conclude that CDC and ADCC are major mechanisms of action of rituximab on B-cell lymphomas and that a heterogeneous susceptibility of different lymphoma cells to complement may be at least in part responsible for the heterogeneity of the response of different patients to rituximab in vivo. Furthermore, we suggest that the relative levels of CD55 and CD59 may become useful markers to predict the clinical response.
Rituximab is an anti-CD20 chimeric monoclonal antibody containing the human IgG1 and κ constant regions1 and is the first MAb approved for the treatment of low-grade and follicular non-Hogdkin's lymphoma (FL).2-7 In phase I studies, rituximab induced a rapid depletion of CD20+ normal and lymphoma cells.1-3 Phase II trials with low-grade or follicular lymphomas showed a 50% response rate,5,8whereas intermediate- to high-grade lymphomas showed a lower response rate.6 Little is known about the reason for the heterogeneity of the response of different patients. Lack of response does not seem to relate to CD20 levels because CD20 is highly expressed in most cases. A high tumor burden leading to high antibody clearance rate and low median serum antibody levels has been suggested as one possible mechanism for lack of response.5 9 Other factors have not been investigated to date.
The biologic basis for choosing the CD20 antigen as a target was its B-cell specificity, its expression at high levels during nearly all stages of B-cell differentiation and the fact that the antigen is not internalized, down-modulated, or shed by anti-CD20 antibodies.1,10 Its mechanism of action in vivo has not yet been fully clarified: It is likely to include complement-mediated cytotoxicity (CDC) and/or antibody-dependent cytotoxicity (ADCC), because an equivalent IgG4κ version of the antibody lost the capacity to deplete normal B cells in vivo in nonhuman primates.11However, different murine anti-CD20 monoclonal antibodies, in particular the 1F5 and B1 antibodies that recognize close but distinct epitopes on the CD20 molecule, have been shown to have other biologic activities on B cells (reviewed by Tedder and Engel10). 1F5 activates normal B cells from the G0 to the G1 phase of the cell cycle, but blocks their differentiation to immunoglobulin secretion.12,13 Furthermore, 1F5 can deliver activation or proliferation signals to some leukemic cells.13,14 The B1 antibody on the other hand blocks mitogen-induced proliferation and does not activate B cells.15,16 B1 inhibits B-cell proliferation induced by Staphylococcus aureus cowan I strain bacteria (SAC) or Epstein Barr virus (EBV) but has little or no activity on anti-μ or T-cell derived signals, or on phorbol myristic acetate (PMA)-induced proliferation.15-17 B1 has been suggested to act in late G1, blocking G1-S phase transition.10,17 In addition, 1F5 or rituximab may have either a proapoptotic or antiapoptotic activity on neoplastic or normal B cells, respectively.8 18-20
CD20 is a 33 to 37 kd phosphoprotein that forms tetramers and can act as a Ca++ channel21 (reviewed by Tedder and Engel10). It is phosphorylated in both normal and neoplastic B cells.22,23 It is unclear whether Ca++ fluxes or the tyrosine kinases associated with CD20 are involved in signaling B-cell activation or inhibition of proliferation.10,24 B-cell activation by 1F5 is accompanied by induction of c-myc and B-myb.25 26
In this report, we have investigated the different biologic activities of rituximab in vitro against normal B cells as well as several cell lines and fresh lymphoma samples, including possible effects on proliferation, apoptosis, CDC, and ADCC. This work was aimed at defining the likely mechanism of action of rituximab on lymphoma cells and the molecular basis for the heterogeneity of the response of different patients to this agent.
Materials and methods
Cell cultures
The BJAB Burkitt's lymphoma (BL) line was a kind gift of Dr D. Vercelli (DIBIT, San Raffaele, Milano, Italy). The DHL-4 cell line was a kind gift of Dr L. Boxer (Stanford University School of Medicine, Stanford, CA). The Karpas 422, DOHH-2 and WSU-NHL follicular lymphoma cell lines were purchased from the German Collection of Microorganisms and Cell Culture (DSM, Braunschweig, Germany). Human resting B cells were purified from freshly excised tonsils using aminoethylisothiouronium-treated sheep red blood cells and discontinuous Percoll gradients (Pharmacia, Uppsala, Sweden) as described.27 Mononuclear cells from follicular lymphoma patients were purified by Ficoll-Hypaque gradient centrifugation. All FL cell lines and fresh samples were verified to carry the t(14;18) translocation by polymerase chain reaction (PCR). The cells were cultured in RPMI 1640 medium (Seromed, Berlin, Germany) supplemented with 10% fetal calf serum (FCS) (Hyclone, Steril System, Logan, UT), glutamine (GIBCO, Paisley, Scotland), and 50 μg/mL gentamicin (GIBCO).
Antibodies and immunofluorescence
The purified 1F5 anti-CD20 antibody and G28.5 anti-CD40 were a kind gift of Dr E. A. Clark (University of Washington, Seattle, WA).12 Goat anti-μ F(ab′)2 was from Cappel (Organon Teknika Corp, West Chester, PA). The purified anti-CD19 and CD22 antibodies HD37 and HD239, respectively, were a kind gift of Dr Moldenhauer (Deutsches Krebsforschungszentrum, Heidelberg, Germany).28 Rituximab was supplied by Roche Italia (Monza, Italy). The blocking anti-CD55 antibody BRIC216 was from IGBRL Research Products, Bristol, UK. The blocking anti-CD46 antibody J4-48 was from Cymbus Biotechnology Ltd (Chandlers Ford, Hants, UK). CD59 was detected with a goat anti-CD59 antiserum. This complement inhibitor was isolated from human urine by affinity chromatography as described,29 using the rat IgG2b MAb YTH53.1 kindly provided by Prof H. Waldmann (Department of Pathology, University of Cambridge, UK.).30 The IgG fraction of the anti-CD59 antiserum was purified by chromatography on a protein G-Sepharose column (Pharmacia) and F(ab′)2 fragments prepared by digestion with pepsin (Sigma Chemical, St Louis, MO) for 4 hours at 37°C at an enzyme ratio of 1/600 (w/w). The F(ab′)2 fragment was purified by fast protein liquid chromatography on a MonoQ column (Pharmacia) and the purity of the fragments was checked by SDS-PAGE analysis. The purified F(ab′)2 fragment was used at a 1/100 dilution for blocking experiments. For phenotype analysis, indirect immunofluorescence was performed with the above antibodies and fluorescein isothiocyanate (FITC)-labeled goat antimouse Ig (Becton Dickinson, Mountain View, CA) or rabbit antigoat Ig (Sigma). Antibodies against retinoblastoma, cyclin A, cdk2, and cdk4 were from Santa Cruz (Santa Cruz, CA).
Proliferation assays
Resting tonsillar B cells were cultured at 1 × 106 cells/mL in 96-well plates. Proliferation in quadruplicate wells was assessed at different times with a 4- to 16-hour pulse of 0.0185 MBq (0.5 μCi)3H-thymidine per well. Formalin-killed SAC were from Calbiochem Behring (La Jolla, CA). Monoparametric analysis on ethanol fixed cells using propidium iodide was performed as described previously using a FACStar Plus (Becton Dickinson).27
Antibody-dependent cellular cytotoxicity assays
2 × 106 lymphoma cells were loaded with51Cr by incubation for 1 hour at 37°C with 3.7 MBq (100 μCi) 51Cr and then washed 3 times in phosphate-buffered saline (PBS). Total mononuclear cells were obtained by Ficoll-Hypaque centrifugation of buffy coats. An enrichment to 15%-30% natural killer (NK) cells was obtained by separation on a 47% Percoll gradient. 104 lymphoma cells were incubated in duplicate with or without 2 μg/mL rituximab and increasing amounts of freshly prepared enriched NK cell population for 4 hours at 37°C in complete medium. The 100 μL of cell supernatant was then collected and counted in a γ-counter.
Complement-mediated cell lysis
Cells at 106/mL were incubated with 2 μg/mL rituximab and/or blocking antibodies for 10 minutes at room temperature. Five percent to 50% normal human serum (NHS) was then added, mixed by pipetting, and incubation was carried out for an additional 1 hour at 37°C. The extent of lysis was measured by direct cell count in a Bürker chamber of trypan blue–stained samples or by fluorescence-activated cell sorter (FACS) analysis of acridine orange–stained cells on a FACStar Plus (Becton Dickinson). Briefly, lysed cells were mixed with an equal volume of 3 μg/mL acridine orange solution in PBS and analyzed immediately. Statistical analysis was performed with the Student t test.
Northern blots
Western blots
Total proteins from 1.5 × 106 cells were extracted in SDS loading buffer and boiled for 5 minutes. Samples were run in SDS-PAGE gels and elec-troblotted onto nitrocellulose (Schleicher and Schuell, Dassel, Germany) for 5 hours at 60 V, according to standard procedures. The blots were incubated with the relevant antibodies diluted in PBS containing 5% nonfat milk powder and washed in the same solution containing 0.5% Nonidet P-40. Secondary peroxidase conjugated antimouse or antirabbit antibodies were used at 1/1000 (Amersham, Buckinghamshire, UK). Detection was performed using the enhanced chemiluminescence system (ECL; Amersham).
Apoptosis assays
Apoptosis was measured with the Annexin-V-FLUOS kit (Roche Diagnostics, Milan, Italy) according to the manufacturer's instructions and analysis on the FACS.
Results
Rituximab blocks the proliferation of normal B cells induced by SAC, not by anti-μ and anti-CD40
The first step was to determine whether rituximab can block proliferation of B cells. For this purpose we purified resting human B lymphocytes from tonsils and stimulated them with either SAC bacteria or with anti-μ and anti-CD40 antibodies. As shown in Figure1A, rituximab blocked SAC-induced B-cell proliferation by about 90%, even at the lowest concentration (0.3 μg/mL). An irrelevant IgG1 antibody (My7) had no effect. On the contrary, anti-μ + anti-CD40 triggered proliferation was little affected by rituximab, with a maximal inhibition of 15%. These findings show that rituximab, like B1, is a blocking anti-CD20 antibody and that it interferes with some but not all intracellular signals.15 17
Inhibition of normal B-cell proliferation by rituximab.
(A) Resting tonsillar B cells were stimulated in vitro with SAC (open circles) or anti-μ and anti-CD40 (closed circles) in the presence of the indicated concentrations of rituximab. SAC-stimulated cells were also incubated with a control IgG1 antibody (10 μg/mL My7 IgG1) (open square). 3H-thymidine incorporation of quadruplicate wells was measured at 48 to 66 hours. The data are represented as percentage of the stimulated controls in the absence of rituximab. (B) Resting tonsillar B cells were stimulated with SAC and rituximab was added at 2 μg/mL at the indicated times. 3H-thymidine was measured at 48 to 66 hours.
Inhibition of normal B-cell proliferation by rituximab.
(A) Resting tonsillar B cells were stimulated in vitro with SAC (open circles) or anti-μ and anti-CD40 (closed circles) in the presence of the indicated concentrations of rituximab. SAC-stimulated cells were also incubated with a control IgG1 antibody (10 μg/mL My7 IgG1) (open square). 3H-thymidine incorporation of quadruplicate wells was measured at 48 to 66 hours. The data are represented as percentage of the stimulated controls in the absence of rituximab. (B) Resting tonsillar B cells were stimulated with SAC and rituximab was added at 2 μg/mL at the indicated times. 3H-thymidine was measured at 48 to 66 hours.
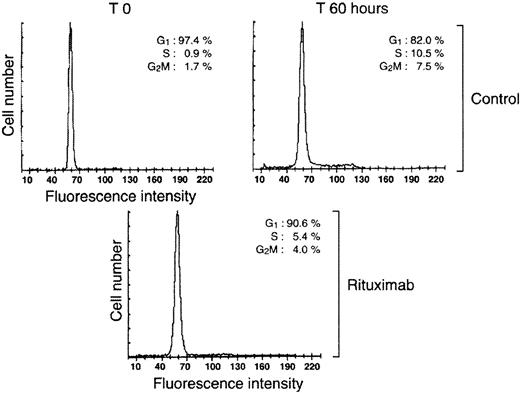
To determine at which stage of the cell cycle rituximab acts, the antibody was added at different times after stimulation of resting B cells with SAC. As shown in Figure 1B, rituximab was fully effective when added after up to 12 hours of culture, but its activity decreases thereafter, suggesting a target of action acting during mid-late G1 phase of the cell cycle. Subsequent cell cycle analysis by flow cytometry showed that B cells were blocked mostly in G1 because rituximab led to a 50% decrease in the percentage of cells in the S, G2, and M phases of the cell cycle (Figure2).
Rituximab blocks B-cell proliferation before entry into S phase.
Resting tonsillar B cells were stimulated with SAC in the presence or absence of 2 μg/mL rituximab. Cell cycle analysis was performed at the indicated times. The percentage of cells in the different phases of the cell cycle are indicated.
Rituximab blocks B-cell proliferation before entry into S phase.
Resting tonsillar B cells were stimulated with SAC in the presence or absence of 2 μg/mL rituximab. Cell cycle analysis was performed at the indicated times. The percentage of cells in the different phases of the cell cycle are indicated.
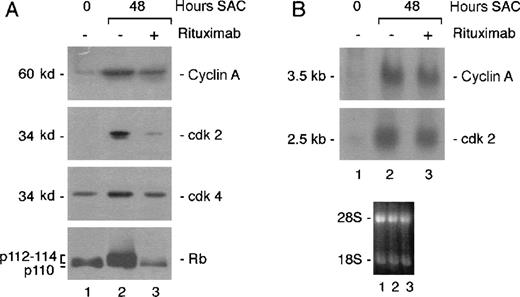
We also analyzed expression of several cell cycle proteins induced by SAC. In agreement with a block in mid-late G1, we found that rituximab inhibited SAC-induced Rb hyperphosphorylation (Figure3A). Furthermore, induction of cdk2, cdk4, and cyclin A protein expression, which is associated with G1-S phase transition, was also blocked. That equivalent amounts of proteins were loaded in each lane was verified by Ponceau red staining of the blot (data not shown). To determine whether cyclin or cdk2 expression was inhibited at the transcriptional level, expression of these molecules was analyzed at the RNA level in Northern blots. As shown in Figure 3B, rituximab did not significantly block the induction of cyclin A or cdk2 messenger RNA (mRNA) expression. These data show that the antibody affects cyclin A and cdk2 induction only at the protein level.
Rituximab blocks hyperphosphorylation of Rb and induction of cell cycle proteins.
Resting tonsillar B cells were stimulated with SAC in the presence or absence of 2 μg/mL rituximab. (A) Total protein extracts were prepared from 1.5 × 106 cells for each time point and analyzed in Western blots with the indicated antibodies. (B) Total RNA was extracted at the indicated times. RNA (20 μg/mL) was analyzed by Northern blot analysis using the indicated probes. The lower panel shows the photograph of the ethidium bromide stained blot. The data are representative of 2 independent experiments.
Rituximab blocks hyperphosphorylation of Rb and induction of cell cycle proteins.
Resting tonsillar B cells were stimulated with SAC in the presence or absence of 2 μg/mL rituximab. (A) Total protein extracts were prepared from 1.5 × 106 cells for each time point and analyzed in Western blots with the indicated antibodies. (B) Total RNA was extracted at the indicated times. RNA (20 μg/mL) was analyzed by Northern blot analysis using the indicated probes. The lower panel shows the photograph of the ethidium bromide stained blot. The data are representative of 2 independent experiments.
We next wanted to determine whether rituximab has only an inhibitory effect on B-cell proliferation, like B1, or whether it also has activating function like 1F5. To measure B-cell activation, we analyzed the expression of 2 cell cycle regulated genes, c-myc and B-myb, which are associated with cell activation and have been previously shown to be induced by the 1F5 antibody.25 26 As shown in Figure 4, rituximab did not induce c-myc or B-myb mRNA expression at 3 or 48 hours, respectively. 1F5, on the other hand, was fully effective as expected (Figure 4).
Rituximab does not activate B cells.
Resting tonsillar B cells were stimulated with 2 μg/mL rituximab or 1F5 anti-CD20 antibodies. Total RNA was extracted at 3 hours (c-myc, lanes 1-3) or 48 hours (B-myb, lanes 4-6) and analyzed for expression of the indicated mRNA by Northern blot. The photograph of the ethidium bromide stained blots is shown below to demonstrate that all lanes contained equivalent quantities of RNA.
Rituximab does not activate B cells.
Resting tonsillar B cells were stimulated with 2 μg/mL rituximab or 1F5 anti-CD20 antibodies. Total RNA was extracted at 3 hours (c-myc, lanes 1-3) or 48 hours (B-myb, lanes 4-6) and analyzed for expression of the indicated mRNA by Northern blot. The photograph of the ethidium bromide stained blots is shown below to demonstrate that all lanes contained equivalent quantities of RNA.
We conclude that rituximab, like B1, can efficiently block B-cell proliferation induced by some (SAC) but not all (anti-μ and anti-CD40) mitogenic signals,15 17 that it arrests B cells mostly in the G1 phase of the cell cycle, at least in part, through inhibition of retinoblastoma phosphorylation, and that, unlike 1F5, it has no activating function.
Effect of rituximab on proliferation and/or apoptosis of lymphoma cell lines
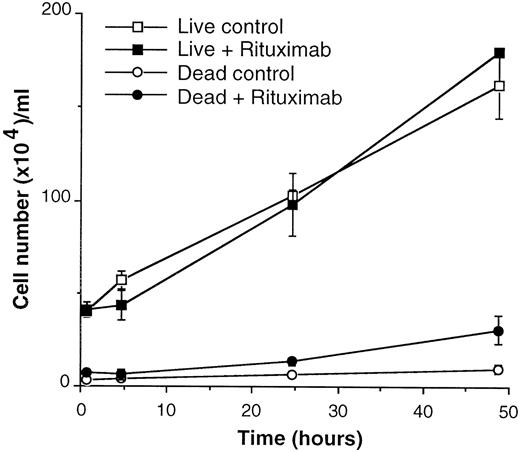
We next investigated whether rituximab also had an antiproliferative activity on 4 different follicular lymphoma lines (DOHH2, DHL-4, WSU-NHL, Karpas 422). Proliferation was measured by3H-thymidine uptake assays 24, 48, and 72 hours after the beginning of culture. We did not detect any significant effect of rituximab on proliferation of these cell lines (data not shown). These data also indicated that rituximab did not induce significant cell death in these lines, which would have been detected as a decrease in thymidine uptake. However, 2 previous reports had suggested a proapoptotic effect of rituximab on DHL-4 cells, either alone or in combination with chemotherapeutic agents.19,20 We therefore went on to verify more extensively this point. DHL-4 cells were cultured in the presence or absence of 2 to 300 μg/mL rituximab for up to 4 days and live and dead cells counted at regular intervals. As shown in Figure 5, the growth curve and number of live cells were not affected by rituximab for up to 48 hours. Only a small (8%-15%) but reproducible increase in the number of dead cells could be detected at 48 hours. The number of dead cells did not increase further at 96 hours (data not shown). We also directly measured the number of apoptotic and necrotic cells by Annexin V binding and propidium iodide staining. As shown in Table1, rituximab did not induce an increase in apoptotic or necrotic cells up to 96 hours after the beginning of culture. On the other hand, staurosporine, a known inducer of apoptosis, did induce a clear increase in early apoptotic cells at 4 hours. As expected, these cells became necrotic (Annexin V and propidium iodide positive) at later time points (Table 1). We also investigated the activation of caspase 3 and 7 using Ac-DEVD-MCA, a fluorescent substrate for these enzymes.32 We could detect a 2-fold caspase activation by staurosporine at 4 hours but not by rituximab at 4, 24, or 48 hours (data not shown). Similarly, we could detect apoptotic DNA fragmentation 6 hours after staurosporine addition but not 6 or 12 hours after rituximab (data not shown).
Rituximab alone does not induce growth inhibition or cell death of DHL-4 cells.
DHL-4 cells were plated at 4 × 105 cells/mL in the presence or absence of 10 μg/mL rituximab. Live and dead cells were counted at the indicated times by trypan blue exclusion. The results are representative of 3 independent experiments.
Rituximab alone does not induce growth inhibition or cell death of DHL-4 cells.
DHL-4 cells were plated at 4 × 105 cells/mL in the presence or absence of 10 μg/mL rituximab. Live and dead cells were counted at the indicated times by trypan blue exclusion. The results are representative of 3 independent experiments.
Rituximab does not induce apoptosis in DHL-4 cells
| Time . | Control . | +Rituximab (10 μg/mL) . | +Staurosporine (1 μmol/L) . | |||
|---|---|---|---|---|---|---|
| Apoptotic . | Necrotic . | Apoptotic . | Necrotic . | Apoptotic . | Necrotic . | |
| 0 | 0 | 1.5 | 0 | 1.5 | 1.0 | 1.5 |
| 4h | ND | ND | 1.3 | 1.6 | 17.2 | 4.2 |
| 24h | 0.3 | 1.0 | 0.3 | 1.8 | 2.4 | 32.9 |
| 48h | 0.1 | 1.8 | 0.2 | 2.1 | 0.6 | 74.6 |
| 72h | 0.1 | 1.9 | 0.2 | 3.6 | ND | ND |
| 96h | 0.1 | 1.9 | 0.2 | 2.6 | ND | ND |
| Time . | Control . | +Rituximab (10 μg/mL) . | +Staurosporine (1 μmol/L) . | |||
|---|---|---|---|---|---|---|
| Apoptotic . | Necrotic . | Apoptotic . | Necrotic . | Apoptotic . | Necrotic . | |
| 0 | 0 | 1.5 | 0 | 1.5 | 1.0 | 1.5 |
| 4h | ND | ND | 1.3 | 1.6 | 17.2 | 4.2 |
| 24h | 0.3 | 1.0 | 0.3 | 1.8 | 2.4 | 32.9 |
| 48h | 0.1 | 1.8 | 0.2 | 2.1 | 0.6 | 74.6 |
| 72h | 0.1 | 1.9 | 0.2 | 3.6 | ND | ND |
| 96h | 0.1 | 1.9 | 0.2 | 2.6 | ND | ND |
ND, Not determined.
Apoptotic cells are Annexin V positive and propidium iodide negative.
Necrotic cells are double positive for Annexin V and propidium iodide.
We conclude that rituximab alone does not inhibit proliferation or induce significant apoptosis in any of the cell lines examined.
Rituximab can induce ADCC to similar levels in different lymphoma lines
One proposed mechanism of action of rituximab in vivo is the induction of ADCC.11 We were therefore interested to determine whether the lymphoma lines could be lysed by ADCC triggered by rituximab and whether this response was variable between different cell lines. For this study, 3 FL cell lines and 1 Burkitt's lymphoma line (BJAB) were chosen. ADCC was measured in 3 independent experiments using as effector population, fresh peripheral blood mononuclear cell fractions enriched in CD16+ NK cells to 15% to 30%. All cell lines were always tested in parallel to exclude differences between the effector populations. As shown in Figure6, 2 of the cell lines were targets for natural killer activity in the absence of antibody. In particular, the WSU-NHL cell line showed a maximum of 20% killing in the absence of rituximab. All cell lines showed a 10% to 20% increase in lysis in the presence of rituximab. We conclude that rituximab can activate the ADCC of several FL and BL lines and that levels of ADCC do not vary significantly between the different cell lines.
Rituximab can trigger ADCC of lymphoma lines.
The 51Cr-labeled cell lines were cultured at 5 × 104/mL in the presence of the indicated amounts of effector cells enriched for NK cells and in the presence (closed circles) or the absence (open circles) of 2 μg/mL rituximab. The cells were incubated for 4 hours at 37°C. 51Cr released in the supernatant was measured as percentage of total 51Cr released with 1% SDS. The results are the mean and SD of 3 independent experiments.
Rituximab can trigger ADCC of lymphoma lines.
The 51Cr-labeled cell lines were cultured at 5 × 104/mL in the presence of the indicated amounts of effector cells enriched for NK cells and in the presence (closed circles) or the absence (open circles) of 2 μg/mL rituximab. The cells were incubated for 4 hours at 37°C. 51Cr released in the supernatant was measured as percentage of total 51Cr released with 1% SDS. The results are the mean and SD of 3 independent experiments.
The efficiency of lysis of different FL lines by complement is heterogeneous
CDC is known to take place in vivo and is likely to be an important mechanism for the elimination of both normal and lymphoma B cells. We have therefore tested the capacity of the different lymphoma lines to be killed by CDC in vitro. As shown in Figure7, increasing concentrations of human serum were added as a source of complement in the presence or absence of rituximab. Serum alone had little toxic effect, except at the higher concentration (50%), where about 10% to 15% lysis was usually observed (Figure 7, open squares). In the presence of rituximab, on the other hand, the different cell lines were lysed in a very heterogeneous manner: At the extremes, the DHL4 line was completely lysed with only 10% serum, whereas the Karpas 422 showed complete resistance at 25% and insignificant rituximab-dependent lysis at 50%. The BJAB and WSU-NHL cell lines showed an intermediate phenotype with an average of 25% and 50% lysis for BJAB and WSU-NHL, respectively, with 25% serum (Figure 7 and data not shown). Because 50% serum was slightly toxic on its own, a concentration of 25% was used in subsequent experiments as a standard efficacious concentration. We also tested the rituximab-dependent lysis of purified tonsillar B cells. These also showed clear heterogeneity because 80% were lysed but 20% were resistant. Similar CDC results were obtained in several experiments and by evaluating lysis either by a direct count of trypan blue negative and positive cells or by a FACS analysis of acridine orange-stained cells (which stains live cells) (see below) or propidium iodide–stained cells (which marks lysed cells). The results were found to be highly reproducible using several different batches of NHS (data not shown).
Complement-mediated lysis of lymphoma lines by rituximab.
The indicated cell lines were incubated at 1 × 106/mL in the presence (closed squares) or the absence (open squares) of 2 μg/mL rituximab and increasing concentrations of NHS. Percentage cell lysis was measured after 1 hour at 37°C by cell count with trypan blue. The data are representative of at least 3 different experiments.
Complement-mediated lysis of lymphoma lines by rituximab.
The indicated cell lines were incubated at 1 × 106/mL in the presence (closed squares) or the absence (open squares) of 2 μg/mL rituximab and increasing concentrations of NHS. Percentage cell lysis was measured after 1 hour at 37°C by cell count with trypan blue. The data are representative of at least 3 different experiments.
The degree of resistance of the lines to complement mediated lysis correlates with the levels of CD55
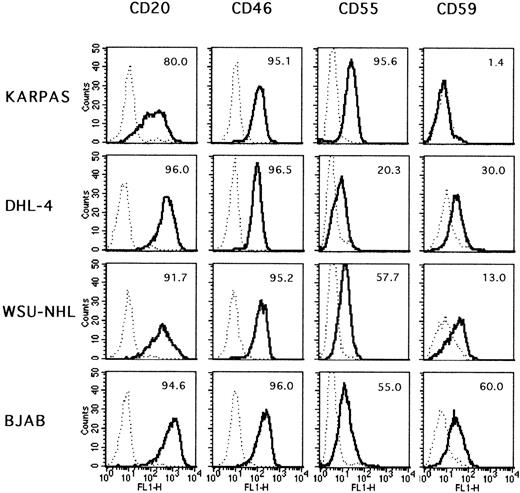
To determine the underlying mechanism accounting for the differences in susceptibility to complement of the lymphoma cell lines, we first investigated the levels of expression of CD20 itself on the cell surface. As shown in Figure 8, the 4 cell lines all expressed CD20 at high levels on more than 80% of the cells. Although a portion of Karpas 422 cells expressed CD20 at slightly lower levels than the other cell lines, this small difference could not justify a 100% difference in cell lysis between Karpas 422 and DHL-4. Furthermore, the BJAB cell line expressed very high levels of CD20 on virtually 100% of the cells but is lysed only to about 25%. We concluded that the susceptibility of the different lines to complement is not due to antigen density but to other cellular factors. Several complement inhibitors are known to be present on the surface of hematopoietic cells.33 The best characterized are CD35, CD46, CD55, and CD59. The expression pattern of these 4 molecules on the surface of the lymphoma cell lines was therefore investigated. CD35 was not detectable at significant levels on any of the cell lines (data not shown). On the contrary, CD46 was present in similar amounts on all 4 lines (Figure 8). The last 2 inhibitors, CD55 and CD59, showed a more interesting pattern in that they were variably expressed in the different cell lines. CD55 was expressed at the highest levels in the Karpas 422 cell line and at the lowest levels in the DHL-4 cell line, with the percentage of positive cells ranging from 95.6 to 20.3, respectively. The WSU-NHL and BJAB cell lines showed intermediate levels of expression (57.7 and 55.0, respectively, Figure 8). These differences were reproducible (in at least 4 separate experiments). Thus, CD55 levels correlated quite well with the resistance to complement-mediated lysis. CD59 levels were also variable among the different cell lines. Karpas 422 was essentially negative, whereas the other cell lines showed intermediate levels of expression (from 13% to 60%) (Figure 8). CD59 levels therefore did not correlate well with the degree of resistance to complement because the most resistant cell line, Karpas 422, did not express CD59, whereas the least resistant (DHL-4) was positive (30%).
Phenotype of the lymphoma cell lines.
Exponentially growing cells were stained by indirect immunofluorescence for the indicated surface markers. · · · negative control.__ positive sample. The percentages of positive cells are indicated in each plot.
Phenotype of the lymphoma cell lines.
Exponentially growing cells were stained by indirect immunofluorescence for the indicated surface markers. · · · negative control.__ positive sample. The percentages of positive cells are indicated in each plot.
CD55 and CD59 can inhibit complement-mediated cell lysis
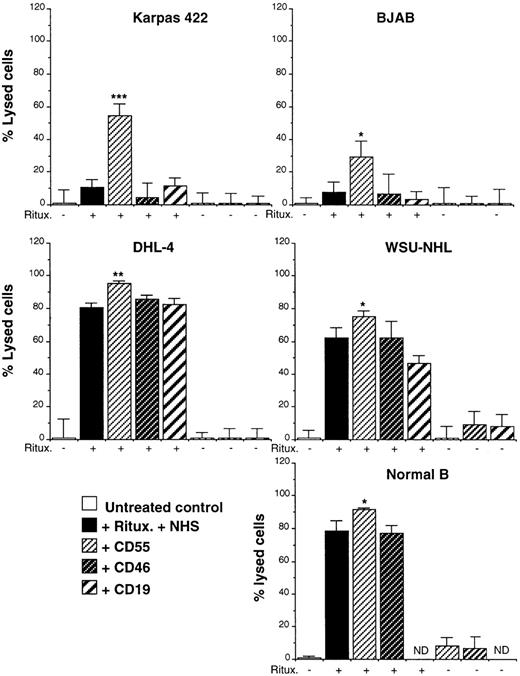
To demonstrate directly that CD55 present on the cell lines studied can indeed inhibit complement, we have carried out a complement lysis experiment in the presence or absence of antibodies that recognize and functionally block either to CD55 or to CD46.33 Both antibodies are murine IgG1 antibodies that cannot therefore activate complement by themselves. As a control an anti-CD19 IgG1 antibody (clone HD37) was used. As shown in Figure9, the anti-CD55, -CD46, or -CD19 antibodies had no effect on complement lysis in the presence of serum alone (ie, they did not activate complement by themselves). Interestingly, the anti-CD55, but not anti-CD46 or -CD19 antibodies, increased the killing of all cell lines in the presence of both rituximab and complement. This increase was statistically significant for all cell lines examined (Figure 9). Of note is that the experiment with the DHL-4 cell line was performed with only 5% serum to obtain suboptimal levels of lysis in the absence of anti-CD55. An augmented lysis was reproducibly observed in 5 separate experiments for each cell line, with a mean increase of 280% for Karpas, 140% for BJAB, 40% for WSU-NHL, and 20% for DHL-4. Thus, the cells that were most resistant to complement, Karpas 422 and BJAB, also showed the greatest response to blocking anti-CD55 antibody. We conclude that CD55 can regulate complement-mediated lysis and is a most effective inhibitor on the resistant Karpas 422 cell line.
CD55, but not CD46, inhibits complement-mediated lysis.
The indicated cells were incubated in presence (+) or absence (−) of rituximab and 25% NHS and 10 μg/mL anti-CD55, -CD46, or -CD19 (all IgG1). Cell death was measured after 1 hour at 37°C by cell count. The results are representative of at least 5 separate experiments. The statistical significance is shown by asterisks. *P < .05; ** P < .01; ***P < .001 (Student t test). ND, not determined.
CD55, but not CD46, inhibits complement-mediated lysis.
The indicated cells were incubated in presence (+) or absence (−) of rituximab and 25% NHS and 10 μg/mL anti-CD55, -CD46, or -CD19 (all IgG1). Cell death was measured after 1 hour at 37°C by cell count. The results are representative of at least 5 separate experiments. The statistical significance is shown by asterisks. *P < .05; ** P < .01; ***P < .001 (Student t test). ND, not determined.
Because CD59 has been reported to inhibit CDC in different cell types,34-37 we have also investigated the functional effect of a blocking anti-CD59 antibody (F(ab′)2 antiserum). The blocking anti-CD59 had no effect of cell viability in the presence of serum but the absence of rituximab (data not shown), indicating that it was not able to activate complement by itself. The same antibody, however, induced a 6% to 20% increase in complement-mediated lysis triggered by rituximab in all cell lines, except Karpas 422, which does not express CD59 (Table 2). As shown previously (Figure 9), the blocking anti-CD46 antibody had no effect. Interestingly, anti-CD55 synergized with anti-CD59 on the WSU-NHL and BJAB cell lines, which were lysed completely in the presence of both blocking antibodies. No such synergy could be observed in DHL-4 or Karpas 422 (Table 2). The data are representative of at least 3 independent experiments.
Effects on complement-mediated cytotoxicity of blocking CD46, CD55, and CD59
| Conditions . | DHL-4 . | Karpas . | WSU-NHL . | BJAB . |
|---|---|---|---|---|
| Medium | 0* | 0 | 0 | 0 |
| NHS† | 0.8 | 2 | 2.3 | 1.3 |
| R‡ + NHS | 62.4 | 7.8 | 48.2 | 55.4 |
| R + NHS + anti-CD55 | 75 | 39.9 | 56.8 | 73.8 |
| R + NHS + anti-CD46 | 54.8 | 0.7 | 46.8 | 56.7 |
| R + NHS + anti-CD59 | 83.7 | 5.6 | 67.5 | 61.4 |
| R + NHS + anti-CD55/CD59 | 83.9 | 26.8 | 90.6 | 95.9 |
| Conditions . | DHL-4 . | Karpas . | WSU-NHL . | BJAB . |
|---|---|---|---|---|
| Medium | 0* | 0 | 0 | 0 |
| NHS† | 0.8 | 2 | 2.3 | 1.3 |
| R‡ + NHS | 62.4 | 7.8 | 48.2 | 55.4 |
| R + NHS + anti-CD55 | 75 | 39.9 | 56.8 | 73.8 |
| R + NHS + anti-CD46 | 54.8 | 0.7 | 46.8 | 56.7 |
| R + NHS + anti-CD59 | 83.7 | 5.6 | 67.5 | 61.4 |
| R + NHS + anti-CD55/CD59 | 83.9 | 26.8 | 90.6 | 95.9 |
The data are the % lysed cells above that of the untreated control. They are representative of 3 independent experiments.
Normal human serum (NHS) was used at 25% for all cell lines, except for DHL-4 in which 5% was added.
R: 2 μg/mL rituximab.
We conclude that both CD55 and CD59 are functional on several of the cell lines examined and that higher expression of CD55 can explain at least in part the resistance to complement of the Karpas 422 cell line.
CD55 and CD59 also affect lysis of fresh FL cells
We next collected blood samples from 3 cases of t(14;18) FL patients. The phenotype of the purified peripheral blood mononuclear cells (PBMCs) from the patients is shown in Table3. Patients 1 and 3 contained 96% to 98% CD20-positive neoplastic B cells and 1% to 3% T lymphocytes. Patient 2 contained only about 50% CD20-positive neoplastic B cells and 37% T lymphocytes. The cells from all patients expressed the complement inhibitors CD46, CD55, and CD59 with somewhat variable intensity. We next measured complement-mediated lysis of the same mononuclear cell preparations. As shown in Table 4, patient 1 showed about 60% lysis in the presence of both serum and rituximab. Killing is increased to about 90% in the presence of blocking anti-CD55 and to 83% in the presence of anti-CD59. Virtually complete lysis (93%) was obtained with both anti-CD55 and anti-CD59. These effects were statistically significant. Anti-CD46 had no effect. Patients 2 and 3 showed a different pattern of lysis: CD20-positive cells were essentially completely lysed with rituximab and complement alone. Indeed, blocking the function of CD55 or CD59 or of both together only marginally increased CDC of these populations, confirming that most CD20-positive cells were killed by rituximab and complement alone. To verify the specificity of CDC on blood mononuclear cells, CDC was also performed on PBMCs from a normal donor. Rituximab and complement lysed only 5% to 10% of normal PBMCs even in the presence of blocking CD55 and CD59. This percentage corresponds to the number of CD20-positive cells in the sample (data not shown).
Phenotype of fresh follicular non-Hodgkin's lymphoma samples
| Antigen . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| CD20 | 98 (1035) | 50 (497) | 96 (317) |
| CD3 | 3 (401) | 37 (392) | 1 (68) |
| CD46 | 98 (73) | 66 (20) | 80 (52) |
| CD55 | 98 (62) | 61 (24) | 96 (25) |
| CD59 | 81 (98.6) | 90 (161) | 34 (32) |
| Antigen . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| CD20 | 98 (1035) | 50 (497) | 96 (317) |
| CD3 | 3 (401) | 37 (392) | 1 (68) |
| CD46 | 98 (73) | 66 (20) | 80 (52) |
| CD55 | 98 (62) | 61 (24) | 96 (25) |
| CD59 | 81 (98.6) | 90 (161) | 34 (32) |
The data are expressed as % positive cells and (mean fluorescence intensity).
Complement lysis of fresh follicular non-Hodgkin's lymphoma samples
| Condition . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Medium | 0 | 0 | 0 |
| NHS4-150 | 0 | 2.3 (2.3) | 0 |
| R4-151 | 6.1 (6.1) | 3.2 (3.2) | 4.9 (2.5) |
| NHS + R | 59.4 (0.4) | 51.2 (1.3) | 85 (5.1) |
| NHS + R + anti-CD46 | 54.9 (2.9) | 50.6 (2.1) | 71.8 (4.8) |
| NHS + R + anti-CD55 | 89.7 (2.2)‡ | 58.0 (3.9) | 95 (3.2) |
| NHS + R + anti-CD59 | 83.6 (6.5)4-153 | 54.8 (9.4) | 82.7 (2.6) |
| NHS + R + anti-CD55/CD59 | 93 (3)‡ | 55.9 (5.0) | 93.1 (2.5) |
| Condition . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Medium | 0 | 0 | 0 |
| NHS4-150 | 0 | 2.3 (2.3) | 0 |
| R4-151 | 6.1 (6.1) | 3.2 (3.2) | 4.9 (2.5) |
| NHS + R | 59.4 (0.4) | 51.2 (1.3) | 85 (5.1) |
| NHS + R + anti-CD46 | 54.9 (2.9) | 50.6 (2.1) | 71.8 (4.8) |
| NHS + R + anti-CD55 | 89.7 (2.2)‡ | 58.0 (3.9) | 95 (3.2) |
| NHS + R + anti-CD59 | 83.6 (6.5)4-153 | 54.8 (9.4) | 82.7 (2.6) |
| NHS + R + anti-CD55/CD59 | 93 (3)‡ | 55.9 (5.0) | 93.1 (2.5) |
The % lysed cells was determined by acridine orange and trypan blue exclusion, in triplicates, after 3 hours incubation in the indicated conditions. The results are the means and range of 2 separate experiments. The statistical significance was calculated from the blocking antibody treated samples relative to the those incubated with NHS + R only.
NHS = normal human serum; R = Rituximab.
NHS was used at 25%.
5 μg/ml of Rituximab was used.
P < .01 (Student t test).
P < .05.
We conclude that the different FL patients studied showed a different susceptibility to lysis with rituximab and complement. Interestingly, lysis of the most resistant FL cells could be increased significantly with blocking anti-CD55 and/or CD59 antibodies, demonstrating a role for these inhibitors also on fresh FL cells.
Discussion
In this work, we have investigated the mechanism of action of rituximab on 5 human lymphoma cell lines, 4 of the follicular type, and 1 Burkitt, on normal B lymphocytes as well as on 3 fresh cases of follicular lymphoma. We have examined the potential effect of rituximab on B-cell proliferation, activation, apoptosis, antibody-dependent cell mediated cytotoxicity (ADCC) and complement-mediated cytotoxicity (CDC). These studies suggest that complement and complement inhibitors are likely to play a role in the heterogeneity of the response of different FL patients to rituximab in vivo.
We show here that rituximab inhibits efficiently SAC but not anti-μ and anti-CD40–induced proliferation of normal B cells. Unlike 1F5, rituximab did not activate B cells to express c-myc or B-myb. This is the first demonstration that rituximab is a blocking antibody. Similarly to B1, it shows specificity for some mitogenic signals and not others.15,17 The mechanism for this specificity is not clear at present because the intracellular signals with which B1 or rituximab interfere have not yet been defined. These may include Ca++ or kinase-mediated events or both.10,21,24We have shown here with time course assays and cell cycle analysis that rituximab blocks cells in mid-late G1 but before entry into S phase. In agreement with these data, we have found that hyperphosphorylation of retinoblastoma protein, as well as cdk2, cdk4, and cyclin A induction, is inhibited by rituximab. Interestingly, inhibition of cdk2 and cyclin A takes place at the protein but not the RNA level, suggesting that signals involved in the regulation of cdk2 and cyclin A protein stability or expression are blocked by CD20. The molecular mechanism by which rituximab inhibits cdk and cyclin A induction and Rb phosphorylation is beyond the scope of this article but is likely to be a major cause of the proliferation block induced by the antibody.38
In contrast to normal B cells, rituximab did not block the proliferation of 4 FL cell lines. This finding suggests that the signals induced by CD20 either are not active in the cell lines or are not sufficient to block proliferation. This effect could be related to the lack of inhibition of growth factor–induced proliferation of normal B cell by B1 antibody.16 The lack of inhibition of the cell lines could be a specific property of long-term culture because the proliferation and/or differentiation of some fresh leukemic B cells have been shown as being blocked by B1 antibody.13Thus, the blocking activity of rituximab could also be a relevant property for neoplastic B cells in vivo.
1F5 and rituximab has been reported previously to induce apoptosis of Ramos or DHL4 cells, respectively.18-20 The data presented here do not support the conclusion that rituximab on its own has a significant proapoptotic activity for FL cells, including DHL-4. We detected a less than 15% increase in cell death after 48 to 96 hours incubation. Furthermore, Annexin V binding, an early marker of apoptosis, or caspase 3/7 activity, were not up-regulated 4 to 96 hours after the addition of rituximab in vitro. The reasons for the discrepancies with the previous report are not clear but may be due to culture conditions.20 Altogether our data demonstrate that rituximab on its own is not proapoptotic, at least in vitro. We cannot exclude, however, that rituximab has proapoptotic activity against FL cells in vivo or that, as suggested by others,19 it can cooperate with other proapoptotic signals, such as those delivered by chemotherapeutic drugs.
We have next investigated whether the lymphoma lines were targets for either ADCC or CDC. The results presented show that, whereas the 4 cell lines studied are all targets of ADCC and show marginal differences in the extent of lysis, the sensitivity to complement varies dramatically between the different targets. These differences were highly reproducible, were not dependent on the batches of serum, and were therefore a property of the cell lines. The enormous difference of susceptibility to complement between monoclonal leukemic cell lines is of particular interest because such differences may be at least in part the basis for differences in the responses of different patients to rituximab in vivo. The data presented here on only 3 fresh cases of FL clearly do not allow us to draw firm conclusions on this point but do support such a hypothesis: Also, the fresh lymphoma cells showed heterogeneity with regard to complement lysis. In this context, it is worth recording that ADCC and CDC are likely to be responsible for the in vivo normal B-cell depletion because the equivalent IgG4 anti-CD20 antibody, which lacks both functions, does not deplete B cells in monkeys.11
Study of the 4 major cell surface complement inhibitors, namely, CD35, CD46, CD55, and CD59, pointed to a role for CD55 in determining resistance to CDC: (i) Levels of expression of CD20 were roughly equivalent in all lines. (ii) CD55 was the only inhibitor whose expression correlated with resistance to complement. (iii) Blocking of CD55 function led to a net increase in CDC, particularly in the complement-resistant cell lines Karpas 422 and BJAB. Indeed, Karpas 422 could be lysed to 50% in the presence of blocking anti-CD55. (iv) Anti-CD55 antibody was also effective on fresh FL cells: Patient 1 showed only partial lysis (55%-60%) in the absence of anti-CD55 and was completely lysed (90%-95%) in its presence. Anti-CD46 had no such effect. CD55 has been shown previously to play a role in inhibiting CDC of BL-cell lines by the alternative pathway,34 of HIV-1 infected lymphocytes or different tumor cells by the classical pathway.33,35,39,40 Our data, together with the report that patients lacking CD55 do not show clinical signs of hemolytic anemia,18 41 suggest that the inhibition of CD55 during rituximab treatment could be clinically feasible to increase the response of resistant patients to the drug.
CD59 blocks the last steps of complement activation and has been shown to inhibit CDC of some BL-cell lines, erythrocytes,34,40,42T cells,35 renal carcinoma cell lines,37 and melanoma lines.36 In agreement with previous reports, blocking anti-CD59 antibodies increased CDC in all lymphoma cell lines that express detectable levels of the protein. In addition, in 2 cell lines (BJAB and WSU-NHL), anti-CD59 antibodies were synergistic with anti-CD55. Indeed, near complete lysis of both cell lines could be obtained in the presence of the 2 antibodies. Blocking CD59 also increased lysis of fresh FL cells, again suggesting the relevance of this antigen in vivo. A study of a larger group of patients will be required to determine the role of the different surface antigens and is beyond the scope of this article. However, we cannot exclude that factors other than CD55 and CD59 contribute to the differences in complement susceptibility.
To conclude, we suggest that study of the expression of both CD55 and CD59 on the surface of neoplastic B cells, in addition to that of CD20, will be important to predict their possible response to rituximab in vivo. This knowledge may also be important to predict infusion-related side effects in patients with circulating tumor cells. In addition, novel and still more efficacious immunotherapeutic strategies could include either the combined administration of rituximab, together with blocking anti-CD55/59 antibodies, or the production of bispecific anti-CD20/CD55 or CD20/CD59 reagents.39
Supported in part by grants from Roche Italia, the Associazione Italiana Ricerca sul Cancro (AIRC), The Istituto Superiore di Sanità (ISS, Rome, AIDS project 30a.0.29), the Consiglio Nazionale della Ricerche (CNR): target project on biotechnology (no. 97.01278.PF49 and 99.00496.PF4), the Associazione Paolo Belli, Lotta alla Leucemia, and by Biomed 2 concerted action (no. BMH4-CT96-1005).
Reprints:Martino Introna, Laboratory of Molecular Immunohematology, Department of Immunology and Cell Biology, Istituto Ricerche Farmacologiche Mario Negri, via Eritrea 62, 20157 Milano, Italy; e-mail: martino@irfmn.mnegri.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal