Abstract
We examined whether activated protein C (APC) reduces ischemia/reperfusion (I/R)–induced renal injury by inhibiting leukocyte activation. In a rat model, intravenous administration of APC markedly reduced I/R-induced renal dysfunction and histological changes, whereas intravenous administration of dansyl glutamylglycylarginyl chloromethyl ketone–treated factor Xa (DEGR-FXa; active-site–blocked factor Xa), heparin or diisopropyl fluorophosphate–treated APC (DIP-APC; inactive derivative of ARC) had no effect. Furthermore, APC significantly inhibited the I/R-induced decrease in renal tissue blood flow and the increase in the vascular permeability, whereas neither DEGR-FXa, heparin, nor DIP-APC produced such effects. Renal I/R-induced increases in plasma levels of fibrin degradation products were significantly inhibited by APC, DEGR-FXa, and heparin. These observations suggest that APC reduces I/R-induced renal injury independently of its anticoagulant effects but in a manner dependent on its serine protease activity. Renal levels of tumor necrosis factor- (TNF-), rat interleukin-8, and myeloperoxidase were significantly increased after renal I/R. These increases were significantly inhibited by APC but not by DEGR-FXa, heparin, or DIP-APC. Leukocytopenia produced effects similar to those of APC. These findings strongly suggest that APC protects against I/R-induced renal injury not by inhibiting coagulation abnormalities but by inhibiting activation of leukocytes that play an important role in I/R-induced renal injury. Inhibition of leukocyte activation by APC could be explained by the inhibitory activity of TNF-.
Activated protein C (APC) is an important physiologic anticoagulant that is generated from protein C by the action of the thrombin-thrombomodulin complex on endothelial cells.1 APC regulates the coagulation system by inactivating factors Va and VIIIa.1,2 Although its precise mechanisms of action have not been elucidated, APC was shown to reduce the mortality rate of baboons challenged with lethal doses ofEscherichia coli not by inhibiting the coagulation abnormality but by reducing organ damage.3,4 Because leukocytes have been implicated in the organ damage induced by endotoxin,5it is possible that APC reduces the organ damage by inhibiting leukocyte activation. In vitro studies indicated that APC inhibits the production of tumor necrosis factor α (TNF-α) by lipopolysaccharide (LPS)-activated monocytes.6,7 In studies with findings consistent with these observations, we demonstrated that APC reduced activated neutrophil–induced pulmonary vascular injury by inhibiting TNF-α production in rats given LPS.8 9 These results strongly suggest that, in sepsis, APC may be involved in regulation of the inflammatory response, as well as the coagulation system, through inhibition of TNF-α production.
Ischemia/reperfusion (I/R) is an important pathologic mechanism leading to organ failure in shock.10 TNF-α, by activating neutrophils, plays an important role in the development of I/R-induced organ damage.11 These observations strongly suggest that APC reduces the I/R-induced organ failure by inhibiting TNF-α production. I/R is also one of the most important pathologic mechanisms in the development of acute renal failure associated with renal transplantation and septic shock.12Activated neutrophils were implicated as participating in the pathogenesis of I/R-induced renal injury by damaging endothelial cells and thereby decreasing renal blood flow.10,13 In addition, fibrin deposition in the glomerulus contributed to a decrease in glomerular filtration fraction in animals subjected to renal I/R.14 15
These findings suggest that APC may reduce I/R-induced renal injury by inhibiting leukocyte activation as well as by attenuating the coagulation abnormality. In this study, we examined whether APC reduces I/R-induced renal injury in rats. To investigate the mechanism by which APC diminishes renal injury, we analyzed the effects of APC, as well as the effects of an inactive derivative of factor Xa that inhibits thrombin generation selectively, heparin, and inactivated APC, on I/R-induced renal injury in our rat model.
Materials and methods
Protein C was purified from human plasma and activated by thrombin as described previously.16 Nitrogen mustard (NM), diisopropyl fluorophosphate (DIP), Evans blue dye, and myeloperoxidase (MPO) were purchased from Sigma (St Louis, MO); dansyl glutamylglycylarginyl chloromethyl ketone (DEGR) was from Calbiochem (San Diego, CA); and heparin was from Novo Nordisk (Gentofte, Denmark). All other reagents were of analytical grade.
Preparation of DEGR-treated factor Xa
DEGR-treated factor Xa (DEGR-FXa), a selective inhibitor of thrombin generation,17 was prepared as described previously. Briefly, factor X was purified from human plasma and activated with Russell viper venom.18,19 Activated factor X was inactivated by incubation with a 20-fold molar excess of DEGR for 30 minutes at 25°C. The mixture was then subjected to extensive dialysis against a solution containing 20 mmol/L Tris-hydrochloric acid (pH 7.4) and 100 mmol/L sodium chloride. DEGR-FXa competes with intact factor Xa for prothrombinase complex formation.20 DEGR-FXa generated as described above showed no clotting activity and a prolonged activated partial thromboplastin time in a concentration-dependent manner (0 to 300 μg/mL).
Preparation of DIP-treated APC (DIP-APC)
APC was inactivated by using DIP, according to a previously described method.6 Briefly, APC (1 mg/mL) was incubated with 10 mmol/L of DIP in phosphate-buffered saline (pH 7.4) for 2 hours and dialyzed extensively against the same buffer. The effectiveness of inactivation of APC by DIP treatment was monitored amidolytically by measuring the rate of hydrolysis of the chromogenic substrate S-2366 (Chromogenix AB, Stockholm, Sweden) at 405 nm. The activity of APC after the inactivation procedure was less than 1%.
Reduction in the number of circulating leukocytes by NM
NM was used to produce leukocytopenia in rats. NM (1 mg/kg of body weight) or saline was administered intravenously to the animals 2 days before the experiment.21 On the day of the experiment, the number of circulating leukocytes was 10 722 ± 1041 in controls (n = 6) and 3100 ± 371 in NM-treated rats (n = 6;P < .01 compared with control value).
Animal model of I/R-induced renal injury
Specific pathogen-free male Wistar rats (Nihon SLC, Hamamatsu, Japan) weighing 180 to 220 g were used throughout the experiments. Animal care and handling were performed in accordance with the guidelines of the National Institutes of Health. The rats were given water but not food for 16 hours before the experiments. The renal I/R protocol was as follows.22 The rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg; Abbott Laboratories, North Chicago, IL). In all rats, a midline incision was made, the left kidney was mobilized, the left renal vessels were ligated, and a left nephrectomy was performed. In the I/R groups, the right pedicle was clamped with a noncrushing microvascular clamp for 60 minutes. The presence of ischemia was visually confirmed by observing blanching of the kidney. During the period of renal ischemia, the rats were covered with plastic wrap to prevent evaporation. APC (100 μg/kg), DEGR-FXa (1 mg/kg), heparin (300 U/kg), DIP-APC (100 μg/kg), or saline was administered intravenously 30 minutes before reperfusion. After 60 minutes of ischemia, the clamps were removed, the wounds were closed with 3-0 silk, and the animals were returned to their cages. The rats in the sham-operation group underwent the same procedure except that clamping was not done. At specified times after reperfusion, animals were anesthetized by intraperitoneal administration of pentobarbital (50 mg/kg) and killed by exsanguination from the abdominal aorta. Blood was collected in tubes and centrifuged at 2000g for 10 minutes. Serum levels of blood urea nitrogen (BUN) and creatinine were measured with standard urease assays and picric acid reactions.11 Serum levels of fibrin degradation products [FDP(E)] were measured with a latex agglutination assay, as described previously.23
Determination of renal microvascular permeability
Renal vascular permeability was assessed by a dye method in which extravasated dye was measured as described previously.24Briefly, Evans blue dye (25 μg/kg) was injected intravenously 10 minutes before the rats were killed by exsanguination from the abdominal aorta. The kidneys were removed, weighed, and put into tubes containing 5 mL of dimethylformamide (Wako, Osaka, Japan). After centrifugation (2000g for 10 minutes), the concentration of Evans blue dye extracted in the dimethylformamide was measured with a spectrophotometer (DU-54; Beckman, Irvine, CA) at a wavelength of 610 nm by comparison with results obtained with standards of known concentration. The Evans blue dye concentration for each sample was expressed in micrograms per gram of tissue.
Measurement of blood flow in renal tissue
The rats were anesthetized with pentobarbital sodium (50 mg/kg given intraperitoneally), laparotomy was performed, and the blood flow in renal tissue was measured by using a laser Doppler flowmeter (ALF21N; Advance, Tokyo, Japan), as described previously.25 The Doppler probe was directed toward the cortex. Renal tissue blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Electrical signals from the probe were digitized and recorded in real time by using a Macintosh Performa 6310 and MacLab software.26 Results were expressed as percentages of the preischemia levels.
Histopathological studies of the kidneys
The rats' kidneys were removed 24 hours after reperfusion, fixed in 10% formalin, embedded in paraffin, sectioned (5-μm thicknesses), and stained with hematoxylin and eosin. Histological changes were evaluated by assessments of neutrophil accumulation, tubular necrosis, and vascular congestion in the outer medulla, according to the methods described by Kelly et al11 and Chiao et al,13 with some modifications. Neutrophil accumulation was evaluated by counting the neutrophils in the outer medulla, whereas vascular congestion was assessed by counting the erythrocytes in the outer medulla. The cells were counted by using an eyepiece graticule (magnification ×400). Tubular necrosis was evaluated by determining the percentage of tubules in the outer medulla in which epithelial necrosis or necrotic debris was observed. Each variable was evaluated in 20 high-power fields. Samples were analyzed by a pathologist with no knowledge of the experiment group to which the rat belonged.
Measurement of renal MPO activity
Renal MPO activity was measured as described previously.21 The rats were killed at specified times after reperfusion. The kidneys were removed, weighed, and homogenized with a homogenizer (Physcotron; Nition, Tokyo, Japan) in 10% (wt/vol) homogenization buffer (50 mmol/L phosphate buffer [pH 6.0] containing 0.5% hexadecyltrimethylammonium bromide) and sonicated for 20 seconds. After centrifugation (4500g for 20 minutes at 4°C), 0.1 mL of supernatant was added to 0.55 mL of 0.1 mol/L phosphate buffer (pH 6.0) containing 1.25 mg/mL o-dianisidine and 0.05% hydrogen peroxide. After 5 minutes, the change in absorbance at 460 nm was measured with a spectrophotometer. Purified MPO was used as a standard. Results were expressed as units of MPO activity per gram of tissue.
Measurement of renal levels of cytokines
At specified times after reperfusion, the kidneys were removed, weighed, and homogenized with a homogenizer using 0.1 mol/L phosphate buffer (pH 7.4) containing 0.05% (wt/vol) of sodium azide at 4°C. Homogenates were then sonicated for 20 seconds and centrifuged (2000g for 10 minutes at 4°C). The supernatants were stored at −80°C until measurement of cytokines. Renal levels of TNF-α and rat interleukin 8 (IL-8) were measured with use of a rat TNF-α enzyme-linked immunosorbent assay (ELISA) kit (Genzyme, Cambridge, MA) and a rat IL-8 ELISA kit (Amersham, Little Chalfont, UK), respectively. Results were expressed as picograms of the measured cytokine per gram of tissue.
Statistical analysis
Data are presented as mean ± SD values. Results were compared with an unpaired t test (single comparison) or analysis of variance followed by the Scheffe post hoc test (multiple comparisons). A level of P < .05 was considered significant.
Results
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on I/R-induced renal dysfunction
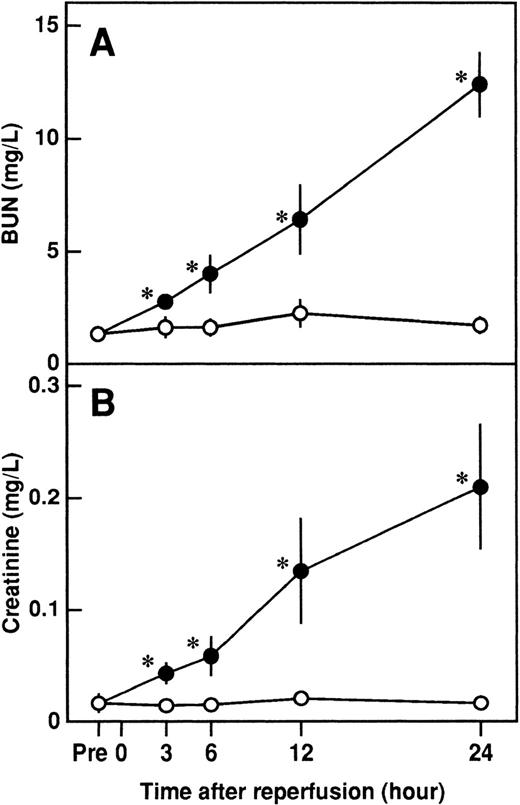
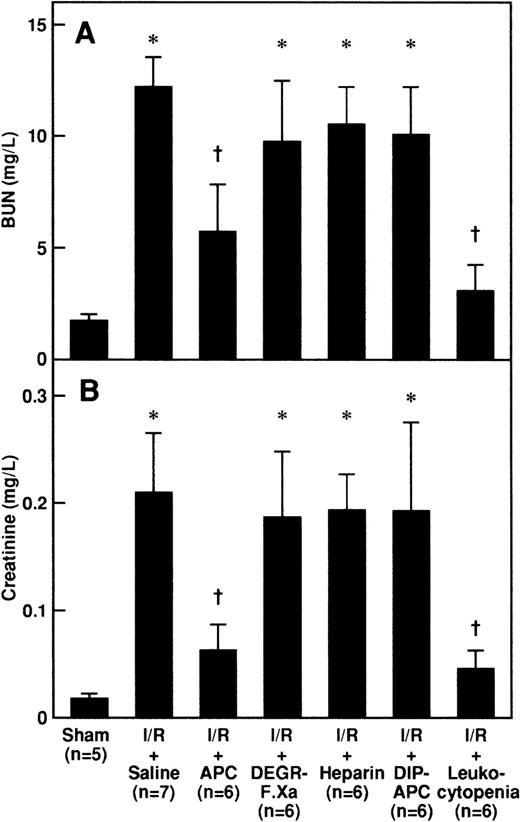
Serum levels of BUN and creatinine were significantly higher after reperfusion in rats that had I/R than in rats that had a sham operation and peaked at 24 hours after reperfusion (Figure1). Intravenous administration of APC significantly inhibited the increases in serum levels of BUN and creatinine 24 hours after reperfusion, but neither DEGR-FXa, heparin, nor DIP-APC had any effect (Figure 2). The levels of BUN and creatinine 24 hours after reperfusion were significantly decreased in rats with leukocytopenia (Figure 2).
Changes in serum levels of blood urea nitrogen (BUN) and creatinine in rats after renal ischemia and reperfusion (I/R).
Rats were subjected to uninephrectomy and 60 minutes of renal ischemia followed by reperfusion. Serum levels of BUN (A) and creatinine (B) were measured before ischemia (Pre), and 3, 6, 12, and 24 hours after reperfusion. Rats in the sham-operation group had uninephrectomy but not I/R. Data are expressed as mean ± SD values. Open circles indicate rats in the sham-operation group (n = 5); and closed circles, rats that had I/R (n = 5). *P < .01 compared with the sham-operation group.
Changes in serum levels of blood urea nitrogen (BUN) and creatinine in rats after renal ischemia and reperfusion (I/R).
Rats were subjected to uninephrectomy and 60 minutes of renal ischemia followed by reperfusion. Serum levels of BUN (A) and creatinine (B) were measured before ischemia (Pre), and 3, 6, 12, and 24 hours after reperfusion. Rats in the sham-operation group had uninephrectomy but not I/R. Data are expressed as mean ± SD values. Open circles indicate rats in the sham-operation group (n = 5); and closed circles, rats that had I/R (n = 5). *P < .01 compared with the sham-operation group.
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on I/R-induced increases in BUN and creatinine.
Serum levels of BUN (A) and creatinine (B) were measured 24 hours after reperfusion in rats subjected to 60 minutes of renal ischemia followed by reperfusion. Animals were intravenously administered saline, APC (100 μg/kg of body weight), DEGR-FXa (1 mg/kg), heparin (300 U/kg), or DIP-APC (100 μg/kg) 30 minutes before reperfusion. Leukocytopenia was induced by administration of nitrogen mustard. Data are expressed as mean ± SD values. Values in parentheses indicate the number of animals in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on I/R-induced increases in BUN and creatinine.
Serum levels of BUN (A) and creatinine (B) were measured 24 hours after reperfusion in rats subjected to 60 minutes of renal ischemia followed by reperfusion. Animals were intravenously administered saline, APC (100 μg/kg of body weight), DEGR-FXa (1 mg/kg), heparin (300 U/kg), or DIP-APC (100 μg/kg) 30 minutes before reperfusion. Leukocytopenia was induced by administration of nitrogen mustard. Data are expressed as mean ± SD values. Values in parentheses indicate the number of animals in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
Histological changes
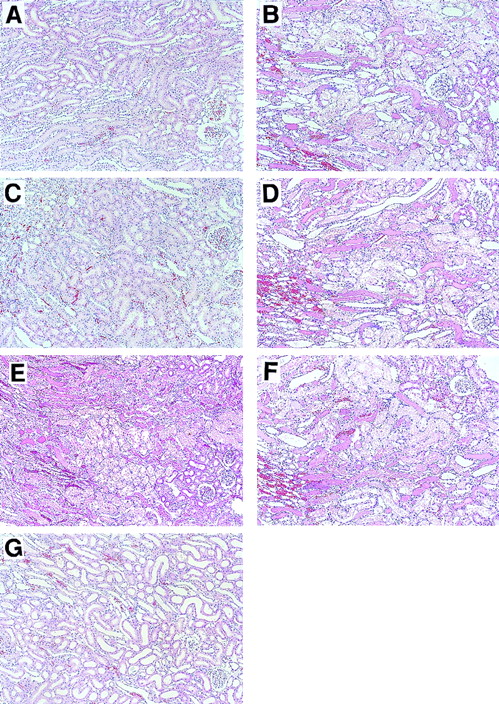
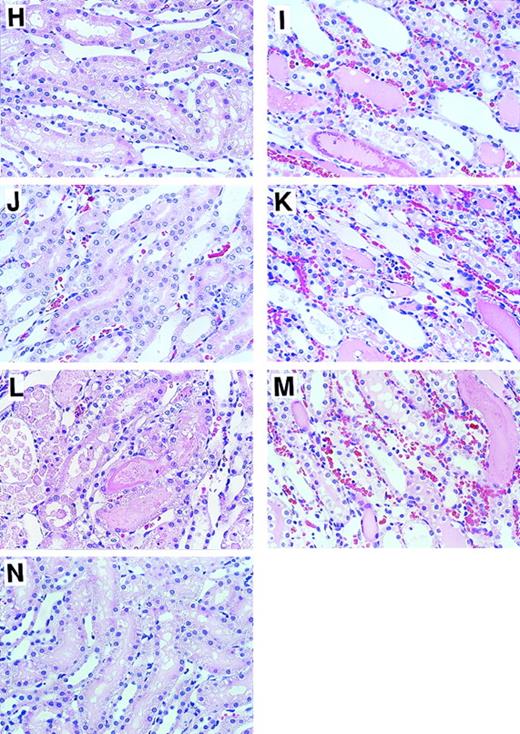
Histological examinations of kidneys were performed 24 hours after reperfusion (Figure 3). Microscopical assessment of renal tissue revealed severe tubular necrosis, loss of brush border, necrosis and sloughing of tubular cells, and obstructing cast in the outer medulla (Figures 3B and 3I); such changes were not observed in rats in the sham-operation group (Figures 3A and3H). Intravenous administration of APC markedly reduced these histological changes (Figures 3C and 3J), whereas neither DEGR-FXa (Figures 3D and 3K), heparin (Figures 3E and 3L), nor DIP-APC (Figures3F and 3M) produced such an effect. The I/R-induced histological changes were also reduced by leukocytopenia (Figures 3G and 3N). Tubular necrosis was significantly diminished by APC administration and leukocytopenia but was not reduced in rats given either DEGR-FXa or heparin (Table 1). Administration of DIP-APC also did not attenuate this change (Table 1). Neutrophil accumulation in the vasa recta of the outer medulla and vascular congestion were also found after I/R of the kidney (Figures 3B and 3I). When analyzed quantitatively, renal neutrophil accumulation and the vascular congestion induced by renal I/R were significantly reduced in rats given APC and in those with leukocytopenia (Table 1). However, neither DEGR-FXa, heparin, nor DIP-APC affected the renal changes (Table 1). Fibrin deposition was not observed in kidneys from any group.
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on renal histological feature in rats that had I/R.
Rats were subjected to 60 minutes of renal ischemia followed by reperfusion. Results are shown in rats in the sham-operation group (A and H), rats treated with I/R plus saline (B and I), rats treated with I/R plus APC (C and J); rats treated with I/R plus DEGR-FXa (D and K), rats treated with I/R plus heparin (E and L), rats treated with I/R plus DIP-APC (F and M), and rats treated with I/R and induction of leukocytopenia (G and N). For A, B, C, D, E, F, G, original magnification ×100; for H, I, J, K, L, M, and N, original magnification ×400.
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on renal histological feature in rats that had I/R.
Rats were subjected to 60 minutes of renal ischemia followed by reperfusion. Results are shown in rats in the sham-operation group (A and H), rats treated with I/R plus saline (B and I), rats treated with I/R plus APC (C and J); rats treated with I/R plus DEGR-FXa (D and K), rats treated with I/R plus heparin (E and L), rats treated with I/R plus DIP-APC (F and M), and rats treated with I/R and induction of leukocytopenia (G and N). For A, B, C, D, E, F, G, original magnification ×100; for H, I, J, K, L, M, and N, original magnification ×400.
Tubular necrosis, neutrophil accumulation, and congestion in the outer medulla in rats subjected to renal ischemia followed by reperfusion
| Treatment Group . | Tubular Necrosis (%) . | Neutrophils (/HPF) . | Erythrocytes (/HPF) . |
|---|---|---|---|
| Sham operation | 0.0 ± 0.0 | 0.1 ± 0.1 | 80.3 ± 17.1 |
| I/R + saline | 84.8 ± 9.1* | 8.3 ± 3.7* | 352.7 ± 48.1* |
| I/R + APC | 51.4 ± 11.8† | 1.9 ± 1.6† | 183.6 ± 65.9† |
| I/R + DEGR-FXa | 82.0 ± 6.8* | 8.2 ± 3.1* | 343.2 ± 67.3* |
| I/R + heparin | 81.6 ± 7.5* | 7.8 ± 2.6* | 304.4 ± 44.6* |
| I/R + DIP-APC | 80.0 ± 8.7* | 8.3 ± 3.3* | 345.6 ± 70.2* |
| I/R + leukocytopenia | 32.6 ± 12.9† | 0.8 ± 0.7† | 175.8 ± 80.4† |
| Treatment Group . | Tubular Necrosis (%) . | Neutrophils (/HPF) . | Erythrocytes (/HPF) . |
|---|---|---|---|
| Sham operation | 0.0 ± 0.0 | 0.1 ± 0.1 | 80.3 ± 17.1 |
| I/R + saline | 84.8 ± 9.1* | 8.3 ± 3.7* | 352.7 ± 48.1* |
| I/R + APC | 51.4 ± 11.8† | 1.9 ± 1.6† | 183.6 ± 65.9† |
| I/R + DEGR-FXa | 82.0 ± 6.8* | 8.2 ± 3.1* | 343.2 ± 67.3* |
| I/R + heparin | 81.6 ± 7.5* | 7.8 ± 2.6* | 304.4 ± 44.6* |
| I/R + DIP-APC | 80.0 ± 8.7* | 8.3 ± 3.3* | 345.6 ± 70.2* |
| I/R + leukocytopenia | 32.6 ± 12.9† | 0.8 ± 0.7† | 175.8 ± 80.4† |
Rats were subjected to 60 minutes of renal ischemia followed by reperfusion. Kidney samples were obtained 24 hours after reperfusion. Data are expressed as mean ± SD values from 5 experiments. HPF indicates high-power field; I/R, ischemia/reperfusion; APC, activated protein C; DEGR-FXa, dansyl glutamylglycylarginyl chloromethyl ketone–treated factor Xa; and DIP-APC, diisopropyl fluorophosphate–treated APC.
P < .01 compared with sham operation group.
P < .01 compared with I/R + saline group.
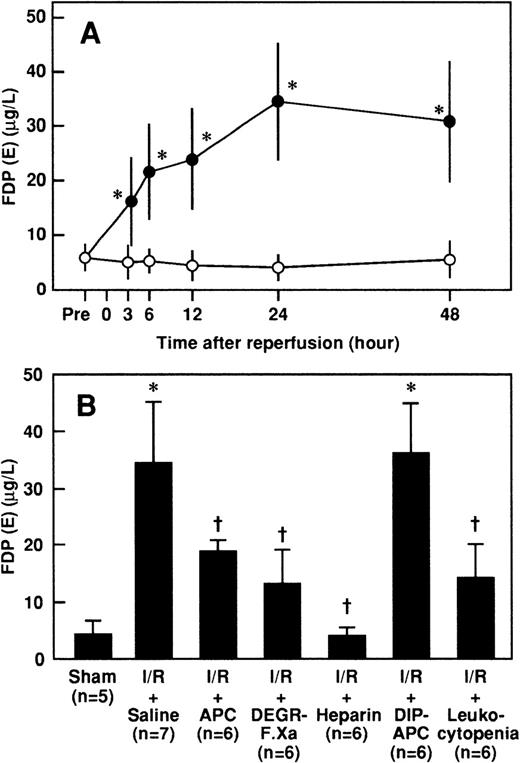
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on I/R-induced increases in serum FDP(E) levels
Serum concentrations of FDP(E) were significantly higher after reperfusion in rats that had I/R than in rats that had a sham operation and peaked at 24 hours after reperfusion (Figure4A). The increases in serum FDP(E) levels 24 hours after reperfusion were significantly inhibited by administration of APC, DEGR-FXa, or heparin but not by DIP-APC (Figure4B). Leukocytopenia significantly inhibited the increase in serum FDP(E) levels in rats subjected to renal I/R (Figure 4B).
Changes in serum levels of FDP(E) in rats that had renal I/R, and effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on I/R-induced increases in serum levels of FDP(E) 24 hours after reperfusion.
(A) Rats were subjected to uninephrectomy and 60 minutes of renal ischemia followed by reperfusion. Serum levels of FDP(E) were measured before ischemia (Pre), and 3, 6, 12, 24, and 48 hours after reperfusion. Rats in the sham-operation group had uninephrectomy only. Data are expressed as mean ± SD values. Open circles indicate rats in the sham-operation group (n = 5); and closed circles, rats that had I/R (n = 5). *P < .01 compared with the sham-operation group. (B) Rats were given intravenously administered saline, APC (100 μg/kg), DEGR-FXa (1 mg/kg), heparin (300 U/kg), or DIP-APC (100 μg/kg) 30 minutes before reperfusion. Leukocytopenia was induced by administration of nitrogen mustard. Data are expressed as mean ± SD values. Values in parentheses indicate the number of animals in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
Changes in serum levels of FDP(E) in rats that had renal I/R, and effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on I/R-induced increases in serum levels of FDP(E) 24 hours after reperfusion.
(A) Rats were subjected to uninephrectomy and 60 minutes of renal ischemia followed by reperfusion. Serum levels of FDP(E) were measured before ischemia (Pre), and 3, 6, 12, 24, and 48 hours after reperfusion. Rats in the sham-operation group had uninephrectomy only. Data are expressed as mean ± SD values. Open circles indicate rats in the sham-operation group (n = 5); and closed circles, rats that had I/R (n = 5). *P < .01 compared with the sham-operation group. (B) Rats were given intravenously administered saline, APC (100 μg/kg), DEGR-FXa (1 mg/kg), heparin (300 U/kg), or DIP-APC (100 μg/kg) 30 minutes before reperfusion. Leukocytopenia was induced by administration of nitrogen mustard. Data are expressed as mean ± SD values. Values in parentheses indicate the number of animals in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
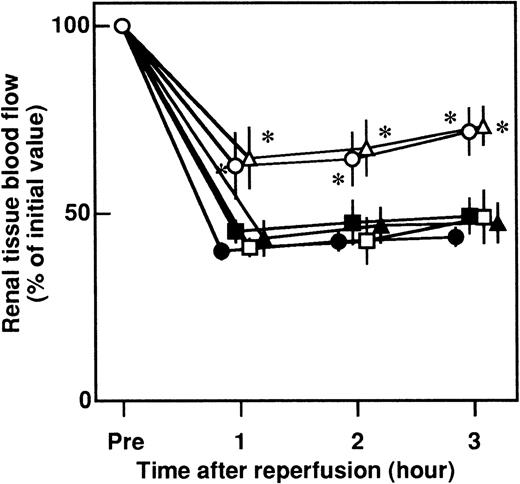
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the changes in renal tissue blood flow in rats that had renal I/R
Renal tissue blood flow decreased to approximately 40% of the preischemia level 3 hours after reperfusion (Figure5). Intravenous administration of APC significantly inhibited the I/R-induced reduction in blood flow. No significant effect was observed in rats given DEGR-FXa, heparin, or DIP-APC (Figure 5). The reduction in blood flow was significantly decreased by leukocytopenia (Figure 5).
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the renal I/R-induced decrease in renal tissue blood flow in rats.
Rats were subjected to 60 minutes of renal ischemia followed by reperfusion. Renal tissue blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Data are expressed as mean ± SD values from 5 experiments. Closed circles indicate saline-treated rats; open circles, APC-treated rats; closed squares, DEGR-FXa–treated rats; open squares, heparin-treated rats; closed triangles, DIP-APC–treated rats; and open triangles, leukocytopenic rats. *P < .01 compared with saline-treated rats that had I/R.
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the renal I/R-induced decrease in renal tissue blood flow in rats.
Rats were subjected to 60 minutes of renal ischemia followed by reperfusion. Renal tissue blood flow was measured from 30 minutes before ischemia until 3 hours after reperfusion. Data are expressed as mean ± SD values from 5 experiments. Closed circles indicate saline-treated rats; open circles, APC-treated rats; closed squares, DEGR-FXa–treated rats; open squares, heparin-treated rats; closed triangles, DIP-APC–treated rats; and open triangles, leukocytopenic rats. *P < .01 compared with saline-treated rats that had I/R.
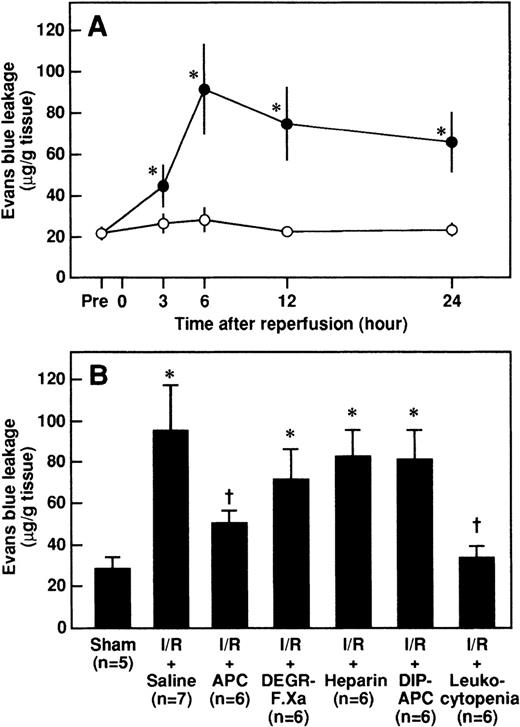
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the increase in renal vascular permeability after renal I/R
Renal vascular permeability as assessed by an increase in leakage of Evans blue dye was significantly higher after reperfusion in rats that had I/R than in rats that had a sham operation and peaked at 6 hours after reperfusion (Figure6A). Both administration of APC and leukocytopenia significantly inhibited the increases in renal vascular permeability 6 hours after reperfusion, whereas neither DEGR-FXa, heparin, nor DIP-APC had any effect (Figure 6B).
Changes in renal vascular permeability in rats after renal I/R, and effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the I/R-induced increase in vascular permeability observed 6 hours after reperfusion.
(A) Rats were subjected to uninephrectomy and 60 minutes of renal ischemia followed by reperfusion. Renal vascular permeability was evaluated by assessing leakage of Evans blue dye administered intravenously 10 minutes before the rats were killed. Vascular permeability was measured before ischemia (Pre) and 3, 6, 12, and 24 hours after reperfusion. Data are expressed as mean ± SD values. Open circles indicate rats in the sham-operation group (n = 5), and closed circles, rats that had I/R (n = 5). *P < .01 compared with the sham-operation group. (B) Rats were given intravenously administered saline, APC (100 μg/kg), DEGR-FXa (1 mg/kg), heparin (300 U/kg), or DIP-APC (100 μg/kg) 30 minutes before reperfusion. Leukocytopenia was induced by administration of nitrogen mustard. Data are expressed as mean ± SD values. Values in parentheses indicate the number of animals in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
Changes in renal vascular permeability in rats after renal I/R, and effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the I/R-induced increase in vascular permeability observed 6 hours after reperfusion.
(A) Rats were subjected to uninephrectomy and 60 minutes of renal ischemia followed by reperfusion. Renal vascular permeability was evaluated by assessing leakage of Evans blue dye administered intravenously 10 minutes before the rats were killed. Vascular permeability was measured before ischemia (Pre) and 3, 6, 12, and 24 hours after reperfusion. Data are expressed as mean ± SD values. Open circles indicate rats in the sham-operation group (n = 5), and closed circles, rats that had I/R (n = 5). *P < .01 compared with the sham-operation group. (B) Rats were given intravenously administered saline, APC (100 μg/kg), DEGR-FXa (1 mg/kg), heparin (300 U/kg), or DIP-APC (100 μg/kg) 30 minutes before reperfusion. Leukocytopenia was induced by administration of nitrogen mustard. Data are expressed as mean ± SD values. Values in parentheses indicate the number of animals in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
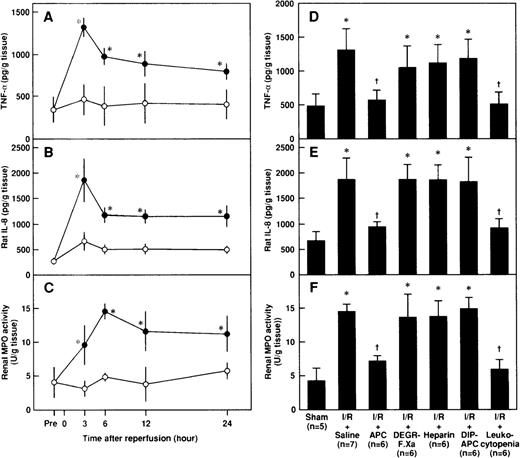
Effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on renal levels of TNF-, rat IL-8, and MPO after renal I/R in rats
Renal levels of TNF-α and rat IL-8 in rats subjected to renal I/R increased, reached their peaks at 3 hours after reperfusion, and decreased gradually thereafter (Figures 7A and 7B). Renal levels of MPO also increased after reperfusion and peaked at 6 hours after reperfusion (Figure 7C). Administration of APC significantly inhibited the I/R-induced increases in renal levels of TNF-α and rat IL-8 3 hours after reperfusion, whereas neither DEGR-FXa, heparin, nor DIP-APC produced such effects (Figures 7D and7E). Although APC inhibited the I/R-induced increases in renal levels of MPO 6 hours after reperfusion, neither DEGR-FXa, heparin, nor DIP-APC had any effect (Figure 7F). Renal tissue levels of TNF-α and rat IL-8 3 hours after reperfusion were significantly lower in rats with leukocytopenia than in those given saline (Figures 7D and 7E). The I/R-induced increases in renal levels of MPO 6 hours after reperfusion were significantly inhibited in rats with leukocytopenia (Figure7F).
Changes in renal tissue levels of cytokines and MPO after renal I/R in rats and effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia.
Shown are the renal tissue levels of tumor necrosis factor-α (TNF-α) (A), rat interleukin 8 (IL-8) (B), and MPO (C) after renal I/R and the effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the I/R-induced increases in renal tissue levels of TNF-α (D), rat IL-8 (E), and MPO (F) after reperfusion. Renal tissue levels of TNF-α, rat IL-8, and MPO were measured before ischemia (Pre) and 3, 6, 12, and 24 hours after reperfusion or sham operation. Renal tissue levels of TNF-α and rat IL-8 were measured 3 hours after reperfusion; renal tissue levels of MPO were determined 6 hours after reperfusion. Data are expressed as mean ± SD values. Values in parentheses indicate the number of rats in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
Changes in renal tissue levels of cytokines and MPO after renal I/R in rats and effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia.
Shown are the renal tissue levels of tumor necrosis factor-α (TNF-α) (A), rat interleukin 8 (IL-8) (B), and MPO (C) after renal I/R and the effects of APC, DEGR-FXa, heparin, DIP-APC, and leukocytopenia on the I/R-induced increases in renal tissue levels of TNF-α (D), rat IL-8 (E), and MPO (F) after reperfusion. Renal tissue levels of TNF-α, rat IL-8, and MPO were measured before ischemia (Pre) and 3, 6, 12, and 24 hours after reperfusion or sham operation. Renal tissue levels of TNF-α and rat IL-8 were measured 3 hours after reperfusion; renal tissue levels of MPO were determined 6 hours after reperfusion. Data are expressed as mean ± SD values. Values in parentheses indicate the number of rats in each experiment. *P < .01 compared with the sham-operation group; †P < .01 compared with the I/R plus saline group.
Discussion
In this study, we demonstrated that APC, a physiologic anticoagulant, reduced I/R-induced renal dysfunction and histological changes in rats. Although APC, DEGR-FXa (a selective inhibitor of thrombin generation), and heparin significantly inhibited the I/R-induced increases in serum levels of FDP(E), neither DEGR-FXa nor heparin diminished the I/R-induced renal dysfunction. These observations strongly suggest that the anticoagulant effect of APC may not have contributed to the reduction in I/R-induced renal injury. Because DIP-APC, an inactive derivative of APC, did not diminish the I/R renal injury, the serine protease activity of APC may be critical for its protective role.
Leukocytopenia did reduce I/R-induced renal injury, suggesting that leukocytes are critically involved in the pathologic process of such an injury. Results of a study by Kelly et al27 demonstrating that inhibition of neutrophil adhesion prevents ischemic renal injury are consistent with this hypothesis. Activated neutrophils release various inflammatory mediators that are capable of damaging endothelial cells.28 These mediators, which include neutrophil elastase and oxygen free radicals, increase vascular permeability,10,29 thereby leading to a reduction in tissue blood flow.30 In accordance with this hypothesis, leukocytopenia significantly inhibited both the increase in renal vascular permeability and the reduction in renal tissue blood flow induced by I/R in the rat model in the current study.
A preliminary experiment by our group demonstrated that ONO-5046, a selective inhibitor of neutrophil elastase,31 significantly inhibited the I/R-induced decrease in renal tissue blood flow by diminishing the increase in renal vascular permeability in the same animal model used here. Thus, it is possible that activation of neutrophils is an important cause of the development of I/R-induced renal injury through a reduction in renal tissue blood flow. Although APC significantly inhibited both the increase in I/R-induced renal vascular permeability and the reduction in renal tissue blood flow, neither DEGR-FXa, heparin, nor DIP-APC had any effect, suggesting that APC may prevent the I/R-induced decrease in renal tissue blood flow in a manner independent of its anticoagulant effects, that is, by means of some other biologic activity or activities that depend on its serine protease activity. Furthermore, it is possible that the preventive effect of APC might include the inhibition of neutrophil activation.
TNF-α activates neutrophils to release inflammatory mediators.32 Because TNF-α production is enhanced in I/R-induced renal injury,33 it is possible that TNF-α activates neutrophils to promote the release of inflammatory mediators, which may decrease renal tissue blood flow. In this study, leukocytopenia inhibited the I/R-induced increases in renal levels of both TNF-α and rat IL-8 and decreased the I/R-induced renal accumulation of neutrophils. Thus, inhibition of TNF-α production may prevent the subsequent neutrophil activation that leads to a reduction in renal tissue blood flow. Although APC does not inhibit the activation of neutrophils directly, it inhibits TNF-α production by LPS-stimulated monocytes.9 Furthermore, APC was shown to inhibit the increase in plasma levels of TNF-α in rats given LPS,9 suggesting that APC may inhibit TNF-α production and thereby inhibit neutrophil activation. In accordance with this hypothesis, we found in this study that APC inhibited the I/R-induced increases in renal levels of TNF-α and rat IL-8, as well as the renal accumulation of neutrophils. Moreover, neither DEGR-FXa, heparin, nor DIP-APC inhibited these increases, suggesting that APC may reduce I/R-induced renal injury by inhibiting neutrophil activation by means of inhibition of TNF-α production. In addition, these protective effects of APC may be mediated not by its anticoagulant effect but by its serine protease activity. Taken together, these observations suggest that inhibition of leukocyte activation could be one possible therapeutic mechanism by which APC reduces I/R-induced renal injury.
The mechanism by which APC inhibits TNF-α production by activated monocytes is not well understood. We previously showed that the serine protease activity of APC is important for the inhibition of TNF-α production by monocytes stimulated with LPS in vitro.9 Grey et al6 demonstrated that APC suppresses the production of TNF-α by LPS-stimulated monocytes by inhibiting the coupling of LPS and CD14, which stimulates production of cytokines, and this suppressive activity of APC depends on its serine protease activity. However, inhibition of TNF-α production by APC in the pathologic condition of I/R-induced tissue injury may not be related to inhibition of the coupling of LPS and CD14. Thus, alternative mechanisms may explain the inhibition of TNF-α production by APC in I/R-induced renal injury. Because oxygen free radicals play a role in I/R-induced tissue injury by stimulating monocytes to produce TNF-α,34 it is possible that APC inhibits oxygen free radical–induced TNF-α production by monocytes. We are currently investigating this possibility.
Plasma-derived APC concentrates have been shown to be effective in alleviating coagulation abnormalities in patients with disseminated intravascular coagulation (DIC).35 Because APC may be effective in reducing I/R-induced tissue injury, it can be useful not only for attenuating the coagulation abnormality but also for diminishing organ injury in patients with DIC associated with shock.
Reprints:Kenji Okajima, Department of Laboratory Medicine, Kumamoto University School of Medicine, Honjo 1-1-1, Kumamoto 860-0811, Japan; e-mail: whynot@kaiju.medic.kumamoto-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal