We read with great interest the report by Marquardt et al1 regarding the beneficial effect of fucose supplementation in a patient with leukocyte adhesion deficiency type II (LAD II). Not only was improvement noted in neutrophil adhesion function, but also the patient's developmental delay was reduced. It should be noted that fucose treatment was started when the child was already more than 1 year of age.
From the time of our initial report of LAD II,2 we have discovered another 2 infants, a male and a female, affected by LAD II. They were both of Arabic origin, the parents were close relatives, and they live in the same area as the previous ones.
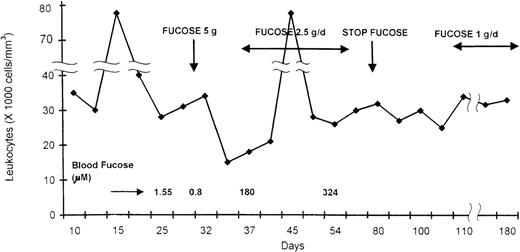
No evidence of consanguinity between the families is present. Their birth length and weight was normal for gestational age. Both children presented with febrile illnesses, and a complete blood count showed a very high leukocyte count (above 40 000 cells/mm3). CD15a was not expressed on leukocyte surfaces, and the children had the Bombay blood group phenotype. CD18, CD11a, and CD11b were expressed normally, and the diagnosis of LAD II was made. Because no anti-H antibodies were detected, we decided to start fucose supplementation at the age of 4 weeks, after obtaining informed consent from the parents. As no data regarding fucose administration in humans is currently available, we estimated that around 200 mg/kg per day may achieve the desired normal blood fucose level.3 A loading dose of 5-gram fucose (Pfanctiehl, IL) was given orally to patient 1 (in 4 divided doses). A day later a marked decrease in the leukocyte count was noted (Figure).
Leukocyte count during fucose administration in an infant with LAD II.
Leukocyte count during fucose administration in an infant with LAD II.
Hypoglycemia developed, and the dose was decreased to 2.5 g, followed by an increase in leukocyte count to 80 000/mm3. Although the baseline of blood-free fucose was less than 50% of normal (Figure), repeated fucose measurements showed very high levels while the child was on therapy, which were comparable to those obtained by Marquardt et al1 using a high dose of fucose. Fucose concentration in samples was determined using both gas chromatographs coupled with mass spectrometry and the enzymatic assay with fucose dehydrogenase.3,4
Urine fucose levels in the patient while on therapy were 10 times higher (1.2 μmol/L) than in control (25-110 μmol/L). Still, no decrease in leukocyte count was observed, and CD15a expression was not detected on leukocyte surfaces.
After a month, the dose of fucose was decreased to 1 g/d and was continued for up to 12 months. Unfortunately no change in leukocytes counts or CD15a expression was noted, and her psychomotor retardation continue to deteriorate. Her weight, length, and head circumference were all below the third percentile for age. The same unfavorable results were seen in the male infant with LAD II, with whom therapy also started at age 1 month.
How can one explain the different results of fucose supplementation in our 2 patients and Marquardt et al's patient? The widespread lack of L-fucose on several different glycoconjugates seems to exclude any impairment of fucosyl-transferase activities and to favor a general defect in L-fucose metabolism. Lymphocytes from 1 of our patients display a significant reduction of GDP-D-mannose-4,6 dehydratase (GMD) activity, even though no qualitative or quantitative defects are observed for this enzyme, thus suggesting the presence of inhibitory mechanisms.5 Recently, Lubke et al6found that the activity of this enzyme was normal in their LAD II patient, but a defect in the import of GDP fucose into Golgi-enriched vesicles was found. A decreased import of GDP-L-fucose into the Golgi-enriched vesicles was also observed in our patient (Tonetti, unpublished results), and the consequent increase in the cytosolic concentration of GDP-L-fucose, which is a very good noncompetitive feedback inhibitor for GMD, can explain the defect in the enzymatic activity observed. Our in vitro studies on cells from the LAD II patient indicated that administration of L-fucose is able to restore the expression of fucosylated antigens on the cell membrane.7 But the concentrations used in vitro were many times higher than those achievable in vivo. In Marquardt et al's patient, fucose administration was able to correct not only the expression of various glycoproteins on the cell surface but even some of the neurological defects,1 while in our 2 patients no effect was observed.
We believe, therefore, that although the phenotype of LAD II in our 4 Arabic patients is very similar to the Turkish patient reported by Marquardt et al, the biochemical defect is somehow different. It is clear that the increased fucose delivery to the cell through the scavenger pathway is enough to overcome the Golgi-uptake defect in Marquardt et al's patient. Still, in our patients the specific defect in fucose import by the Golgi apparatus seems more profound and could not be overcome by increasing fucose delivery to the cell, at least at the concentrations that can be obtained in vivo. In order to clarify this very interesting issue, complementation studies using cells from the different patients should be performed in order to find the primary molecular genetic defect.
Fucose supplementation in leukocyte adhesion deficiency type II
Rambam Medical Center
B Rappaport School of Medicine
Technion
Haifa, Israel
Universität Münster
Münster, Germany
Universität Münster
Münster, Germany
Section of Biochemistry
University of Genova
Genova, Italy
Universität Münster
Münster, Germany
Universität Münster
Münster, Germany
Drs Etzioni and Tonetti report on 2 newly discovered leukocyte adhesion deficiency type II (LAD II) patients and their attempts to treat these 2 patients with oral fucose. The treatment did not lead to detectable improvements and did not cause re-expression of the carbohydrate epitope sLex (CD15s). These results need to be compared with our recently published results on the successful treatment of LAD II by oral fucose.1-1
Taking a closer look at the quantitative details reveals that the fucose doses used in the described therapy were considerably lower than those used in our therapy. Those doses would not have been sufficient to rescue expression of sLex (CD15s) in our patient. Although the presented description of the therapy is very brief, we will try to compare the outcome with our recently published results.
Comparing the fucose doses used by Dr Etzioni with ours clearly shows that they are not sufficient to cause significant expression of the carbohydrate epitope sLex (CD15s). Expression of P-selectin ligands (monitored with P-selectin-IgG) is much earlier observed during fucose therapy and would have been the better epitope to monitor the outcome of the therapy.
The doses reported in the letter are not given as an amount of fucose administered per kilogram of body weight (b.w.) but as a total amount given per day per patient. The body weight of the patient is not reported. Since the starting dose was aimed at 4 daily doses of 200 mg/kg, and a total loading dose of 5 g was given for 1 day, the weight of the baby can be calculated as 6.25 kg. Since we usually give 5 doses per day, this would amount to a single dose of 160 mg/kg in our therapy. This dose was only given for 1 day during therapy of the Arabic patient. We observed significant expression of sLex (15% to 20% of normal expression) only above 5 single daily doses of 250 mg/kg, a dose level that was slowly reached after more than 60 days of treatment during which doses were steadily increased. We continuously increased our doses up to 492 mg/kg, a dose that we reached at day 277 of therapy. We observed the first signs of the expression of P-selectin ligands at a dose above 100 mg/kg.
The starting dose of 5 g/d was given to the Arabic patient only for a single day, followed by 1 week without fucose (according to the graph). The therapy was restarted with half the dose (comparable to single daily doses of 80 mg/kg if 5 doses per day are given, as in our therapy). After 1 month of treatment, the dose was reduced to 1 g/d (32 mg/kg of 5 single doses per day). If we take into account the rapid gain of body weight of a baby over time, these doses per kilogram would in fact be even lower. Clearly, these doses would not have been sufficient to rescue expression of significant amounts of sLex in our patient. No details were reported about the attempted therapy of the second patient (doses, duration of treatment).
In contrast to our paper,1-1 the letter only reports on total peripheral leukocyte counts, not on neutrophil counts. Our patient still has a mild lymphocytosis, leading to mild elevation of total leukocyte counts (12 000 leukocytes/μL). Soon after the onset of therapy, we observed a dramatic reduction of peripheral neutrophil counts.1-1 We agree with Drs Etzioni and Tonetti that treatment of their patient with 2.5 grams fucose per day (corresponding to 5 daily doses of 80 mg/kg) for 1 month might possibly have had a chance to reduce peripheral leukocyte counts in our patient, although treatment with this dose was not long in duration and the dose was at the lower limit. If this were the case, it would argue for a different sensitivity of the LAD II defect to the rescue by externally added fucose. Although unknown, it is still conceivable that the same genes would be mutated in the different patients, since different parts of the molecules could be affected (see below).
The total serum fucose concentrations determined during therapy are surprisingly high, given the low doses of fucose that were administered. Unfortunately, only 2 measurements are documented.
The letter reports that a loading dose of 5 grams of fucose was followed by hypoglycemia. We controlled for this over the whole period of our therapy and never found a sign of hypoglycemia, although for most of our therapy we gave much higher doses of fucose. Considering the biochemical pathways of glucose and fucose and the lack of interaction between them, it is unlikely that application of fucose could cause hypoglycemia.
The letter compares the unpublished therapy data of the 2 new patients with published in vitro data obtained with cells from 1 of the 2 first patients. It is important to keep in mind that these data were obtained with different patients. Determination of reduced GDP-D-mannose-4,6 dehydratase activity in lysates of lymphoblasts were probably obtained with the cells of 1 of the older LAD II patients.1-2 The rescue of α1,6-core fucosylation in fibroblasts and lymphoblastoid cells was also described for cells of 1 of the 2 older patients.1-3 It is unclear whether similar results were obtained for cells of the 2 new patients. It is also unclear from which patient the cells that allowed us to determine that GDP-L-fucose import into Golgi vesicles was defective were isolated (referred to as Tonetti, unpublished results).
We stated in our paper that the psychomotor development of the patient improved during fucose treatment. Indeed, before therapy the patient showed a severe psychomotor retardation, as evidenced by a total score of 28.5 EQ (Griffiths test; 100 EQ is equivalent to the fiftieth percentile).1-4 Reassessment after 3 months of fucose therapy showed a significant increase in psychomotor functioning, with a total score of 45 EQ. But naturally we do not know how the psychomotoric abilities of the patient would have developed in the absence of fucose treatment. Of course, the developmental defects of the patient have not been normalized. We have clearly stated in our paper that “at two years of age the boy is still severely retarded. He does not speak yet, but is able to actively turn around when lying on his back, takes toys that are presented in his hands, and starts to sit briefly without support.”
It is interesting and important that Drs Tonetti and Etzioni have detected a defect in GDP-fucose import into Golgi vesicles of 1 of the 4 Arabic patients (unpublished). Indeed, this would hint at a genetic defect similar to that published in our case.1-5 In agreement with Drs Tonetti and Etzioni, we can well imagine that different mutated alleles of a protein that mediates GDP-fucose import could lead to functional defects of this protein of various severity. Thus, an increase in the cytosolic GDP-fucose level that might be sufficient to overcome a low-efficiency GDP-fucose import in cells that express one mutated allele might not be sufficient to rescue the import in cells expressing another, more severely defective allele. It would be interesting to know whether expression of fucosylated glycoconjugates in cells of the 2 new patients could be rescued by culturing these cells in the presence of externally added fucose and, if so, what fucose concentrations would be necessary. Cloning of the gene(s) coding for a GDP-fucose transporter will allow testing of whether the defect in LAD II is indeed due to mutations in such gene(s). This will also allow researchers to finally determine if and to what extent the molecular defects in different LAD II patients vary.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal