Abstract
The Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1), present on the surfaces of parasitized red blood cells (pRBC), mediates rosetting, a virulent phenotype. Here, we show that pRBC specifically bind heparan sulfate (HS) and heparin onto their surfaces and that the rosetting ligand PfEMP1 specifically adheres to heparin–Sepharose when extracted from the surfaces of radioiodinated infected RBC. An analysis of the binding properties of the different regions of PfEMP1 provides evidence that the Duffy-binding–like domain-1 (DBL-1) is the predominant ligand involved in HS and heparin binding. Soluble DBL-1 requires a minimal heparin fragment size of a 12-mer (≈4 kd) for binding and is critically dependent on N-sulfation. A 12-mer is also the minimal heparin fragment that disrupts naturally formed rosettes. DBL-1 binds specifically to erythrocytes and also to HS from endothelial cells and human aorta but not to chondroitin sulfate A, suggesting that different PfEMP1s mediate adhesion to distinct glycosaminoglycans in individual malaria parasites. Present data suggest that HS on endothelial cells may also be involved in the sequestration of pRBC. Elucidation of these binding mechanisms opens up new possibilities for therapeutic strategies targeting adhesive interactions of pRBC.
The mature intraerythrocytic stages of the malaria parasite Plasmodium falciparum mediate multiple adhesive interactions with host cell surfaces, and, as a consequence, mature parasites are absent from the peripheral circulation. This phenomenon is known as sequestration, and it protects parasitized red blood cells (pRBC) from splenic clearance and attack from the immune system.1 One important adhesive interaction of pRBC is the binding to endothelial cells lining the vasculature (cytoadherence), a phenomenon that predominantly occurs in postcapillary venules. PRBC also adhere to uninfected RBC (rosetting). The rosetting phenotype has been associated with the occurrence of severe malaria (ie, cerebral malaria) and anemia.2-4 Rosetting is mediated by the parasite-derived antigen P falciparum erythrocyte membrane protein 1 (PfEMP1),5,6 a high-molecular–weight polypeptide encoded by the large and diverse family of var genes. Several studies have now established that PfEMP1 is the protein that mediates cytoadhesion and that a significant portion of the antigenicity variation of the pRBC-surface is caused by PfEMP1.7-9Complement receptor 1 (CR1) has been identified as an important receptor for PfEMP1-mediated rosetting.5 Recently, we have suggested that heparan sulfate (HS) or HS-like glycan structures on the surfaces of uninfected RBC may act as receptors for rosetting and that glycosaminoglycan (GAG)-binding motifs of PfEMP1 mediate this binding.6
GAGs are long carbohydrate chains modifying protein cores of proteoglycans, which are ubiquitously found in plasma membranes and extracellular matrices. These linear anionic carbohydrate chains are composed of alternating hexuronic acid and hexosamine units. HS and heparin are composed of the repeating disaccharide unit (-4GlcAβ1-4GlcNAcα1-) variably modified by epimerization of the glucuronic to the iduronic acid and N- andO-sulfation at different positions, resulting in a heterogeneous pattern of sequences. Heparin is the most extensively modified form of the glucosaminoglycans composed predominantly of the disaccharide (-4IdoA(2-OSO3)α1-4GlcNSO3(6-OSO3)α1-), and it can be considered a highly sulfated form of HS. Chondroitin sulfate (CS) chains, on the other hand, are composed of the repeating disaccharide (-4GlcAβ1-3GalNAcβ1-). CS do not containN-sulfated galactosamines, yet these GAGs are alsoO-sulfated at various positions.
The purpose of the current study was to characterize the interaction of the rosetting ligand PfEMP1 with the putative adhesion receptors of glycan nature, the heparin-related polysaccharides.
Materials and methods
Parasites and rosetting
Parasites were cultivated in malaria culture medium (RPMI-1640–HEPES, 25 mmol/L sodium bicarbonate, 10 μg/mL gentamicin) containing 10% human serum (blood group AB Rh-positive) according to standard procedures.10 The FCR3S1 strain and 2 FCR3S1-derived lines, cloned by micromanipulation—FCR3S1.2 (R+, high rosetting phenotype) and FCR3S1.6 (R− , low rosetting phenotype)11—were used in the assays.
Expression of PfEMP1 domains
The pGEX-4T-1 vector was used as described.6 Briefly, DBL-1, CIDR, and DBL-4 fragments were amplified with specific primers. The amplified fragments were inserted into the EcoR1 cloning site for pGEX-4T-1 downstream of the glutathione S-transferase (GST) sequence and expressed in Escherichia coli (BL21). Expression of fusion proteins was induced with 0.1 mmol/L isopropyl-β-D-thiogalactoside at 25°C for 3 to 4 hours, and the fusion proteins were purified on glutathione–Sepharose as indicated by the manufacturer (GST Gene Fusion System; Pharmacia-Upjohn, Uppsala, Sweden).
Polysaccharides
Heparin and HS from porcine intestine used for cell-binding assays were obtained from Løvens Kemiske Fabrik (Ballerup, Denmark). Bovine lung heparin (a gift from The Upjohn Company, Kalamazoo, MI) was purified as described.12 Heparin sulfite (HS) from bovine kidney, lung, and aorta were a gift from Seikagaku (Tokyo, Japan).13 Human aorta HS was a generous gift from E. Feyzi (University of Uppsala, Uppsala, Sweden).14 HS was isolated from3H-glucosamine–labeled bovine aorta endothelial cells GM 7373 (generous gift from M. Presta, University of Brescia, Brescia, Italy) as described.15 Heparin–fluorescein isothiocyanate (heparin–FITC; average MWt 18 000 and 1.29 mol dye/mol heparin) was obtained from Molecular Probes (Leiden, Holland). Heparin–Sepharose (Hi-trap) was purchased from Pharmacia Upjohn (Hi-trap; Uppsala, Sweden). Heparin–albumin gold and albumin gold were bought from Sigma (St Louis, MO). Chondroitin sulfate A (CSA) from bovine nasal cartilage, chondroitin sulfate C (CSC) from bovine tracheal cartilage, and dermatan sulfate from porcine skin were generous gifts from A. Malmström (University of Lund, Lund, Sweden).
Bovine heparin and HS were radiolabeled byN-3H-acetylating free amino groups to a specific activity of 20 000 dpm/μg, 23 000 dpm/μg, 20 000 dpm/μg, and 10 000 dpm/μg for heparin, kidney, lung, and aorta HS, respectively, as described.16 Chondroitin sulfate (CS) were N-3H-acetylated as described17 to specific activities of 8 × 105 dpm/μg for CSA, 2 × 105 dpm/μg for dermatan sulfate, and 6 × 105 dpm/μg for CSC, respectively. Selective chemical modification of bovine lung heparin and preparation of size-defined fragments were as described previously15 16(see also Table 1). The specific activity of the 3H-labeled heparin fragments was 106dpm/nmol oligosaccharide.
Heparin preparations used for rosette disruption assays on FCR3S1.2 parasites
| Preparation* . | Sulfate groups/ disaccharide unit† . | IC50 of rosette disruption‡ . |
|---|---|---|
| Native | 2.7 | 8 μg/mL |
| N-desulfated/N-acetylated | 1.6 | >1000 μg/mL |
| 2-O-desulfated | 1.9 | 80 μg/mL |
| 6-O-desulfated | 1.7 | 90 μg/mL |
| N-/2-O-desulfated | 1.1 | ne1-153 |
| N-/6-O-desulfated | 0.7 | ne |
| 2-O-/6-O-desulfated | 1.3 | 105 μg/mL |
| N-/2-O-/6-O-desulfated | 0.3 | ne |
| Preparation* . | Sulfate groups/ disaccharide unit† . | IC50 of rosette disruption‡ . |
|---|---|---|
| Native | 2.7 | 8 μg/mL |
| N-desulfated/N-acetylated | 1.6 | >1000 μg/mL |
| 2-O-desulfated | 1.9 | 80 μg/mL |
| 6-O-desulfated | 1.7 | 90 μg/mL |
| N-/2-O-desulfated | 1.1 | ne1-153 |
| N-/6-O-desulfated | 0.7 | ne |
| 2-O-/6-O-desulfated | 1.3 | 105 μg/mL |
| N-/2-O-/6-O-desulfated | 0.3 | ne |
Bovine lung heparin was selectively modified as described.15
Average number of sulfated groups/single disaccharide unit as calculated from compositional analysis.
Concentration of component needed to achieve 50% disruption of rosettes.
ne, no effect.
Rosette disruption assay
Rosette disruption by various GAGs was performed as described.18 Briefly, glycoconjugates diluted in RPMI medium were added to the cultures in 96-well microtiter plates at variable concentrations, as indicated, and were followed by 30 minutes of incubation at room temperature. The suspension was mixed with a small amount of acridine orange solution and examined using epifluorescence microscopy (Nikon). Inhibition was estimated by comparing the rosetting rate of each sample (300 cells counted) with that of a sample without additives.
Treatment of erythrocytes and infected erythrocytes
Enzymatic treatments of cultures were performed essentially as described.19 Briefly, cells were subjected to treatment with trypsin (100 IU/mL, 37°C, pH 7.5; Sigma) for 5 minutes, and the reaction was stopped with an excess of soybean protease inhibitor (Sigma). Alternatively, cells were treated with Clostridium perfringens neuraminidase (0.1 IU/mL, 37°C, pH 6; Sigma) for 30 minutes. The samples were washed twice in phosphate-buffered saline (PBS) and divided in 2 portions after the enzymatic digestions. One portion was resuspended in malaria culture medium containing 10% human serum, and the rosetting rate was assessed after 30 minutes as indicated above. The second portion was subjected to labeling with heparin–FITC.
Binding of heparin to the surfaces of infected erythrocytes
Parasite cultures were washed 3 times in PBS and incubated in the presence of heparin–FITC at a concentration of 100 μg/mL for 30 minutes at room temperature. Cells were then washed 3 times in PBS. An aliquot was mounted on a glass slide and mixed with an ethidium bromide solution to counterstain. Three hundred infected RBC were counted using epifluorescence microscopy. The fluorescence rate was expressed as the number of fluorescent late-stage–infected RBC relative to the total number of late-stage–infected RBC.
Competition of FITC-conjugate binding by heparin, HS, and CSA was assayed by simultaneously adding a fixed concentration of FITC-conjugate (200 μg/mL) and a variable concentration of competitor to the parasite culture. After a 30-minute incubation at room temperature, the culture was washed and treated as described above.
Electron microscopy
Parasite cultures grown to trophozoite stage were washed 3 times in PBS and incubated for 30 minutes at room temperature with heparin–albumin gold or albumin gold at a concentration of 1:100. After 2 washes in PBS, the cell suspension pellet was fixed in buffered 1% glutaraldehyde/1% paraformaldehyde solution, postfixed in osmium tetroxide, dehydrated in a graded ethanol series, and embedded in Durcopan resin (Fluka AG, Buchs, Switzerland). Ultrathin sections were contrasted with uranyl acetate and lead citrate and examined in a JEOL 100CX transmission electron microscope at 80 kV (JEOL, Tokyo, Japan).
Binding of parasitized erythrocyte extracts to heparin–Sepharose
Erythrocytes infected with the FCR3S1.2 parasites were surface iodinated with sodium iodide 125I by the lactoperoxidase method.11 The intact radioiodinated pRBC were enriched to more than 95% in Percoll–sorbitol gradients and sequentially extracted with 1% Triton X-100 followed by 2% sodium dodecyl sulfate (SDS). Fifty μL of a 1:1 suspension of heparin–Sepharose in PBS was incubated for 16 hours at 4°C with 50 μL SDS extract in 1 mL binding buffer (25 mmol/L HEPES–RPMI 1640 pH 7.4, 0.5% Triton X-100, 1% IgG-free bovine serum albumin, and protease inhibitors). Inactivated, uncoupled Sepharose was used as a control. For competition, 2.5 or 25 mg/mL heparin, HS, or CSA was added to the incubation mixture. After incubation, the Sepharose matrix was washed 5 times with 1 mL cold PBS. The bound polypeptides were solubilized in 5% SDS sample buffer, separated by SDS–PAGE in a 5% to 8.5% gradient acrylamide gel, and detected by PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
GAG binding assay with recombinant PfEMP1 domains
For direct in-solution binding studies, recombinant DBL-1-GST, CIDR-GST, and DBL-4-GST proteins were incubated at the indicated concentrations (see figure legends) together with radiolabeled GAGs or GAG fragments in 200 μL Tris-buffered saline (50 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 0.1% bovine serum albumin) for 1 hour at room temperature. After incubation, the protein was trapped along with bound radiolabeled GAG chains on a nitrocellulose filter (pore size 0.45 μm), as described.20 The bound GAG chains were dissociated from the protein by 2 mol/L NaCl and quantified by scintillation counting. In a series of competition experiments, the proteins were incubated with radiolabeled full-length heparin together with a variable concentration of nonlabeled native heparin or desulfated heparin preparations as competitors.
GAG affinity chromatography on DBL-1 column
One milligram purified DBL-1-GST was immobilized to 1 mL NHS-Sepharose (Hi-trap; Pharmacia) according to the manufacturer's instructions, and the column was equilibrated with PBS. Radiolabeled GAGs were applied, and the unbound GAGs were eluted with 5 mL PBS followed by elution of bound GAGs with a stepwise gradient of NaCl as indicated. Then 1-mL fractions were collected and analyzed for radioactivity by liquid scintillation counting. No binding of heparin or other GAGs was observed to a control column with GST–protein used under the same conditions.
Results
Heparin binds to the surface of pRBC
To identify the cells and the cellular ligands involved in heparin-mediated rosette disruption, a heparin–FITC conjugate was incubated with cultures of asexual stage parasites. PRBC readily bound heparin–FITC, whereas no detectable binding was registered on uninfected RBC in the same culture (Figure1A), confining the heparin ligand to pRBC. The interaction was also examined using electron microscopy. Heparin–albumin gold particles readily bound to the surface of pRBC (7.3 gold particles/pRBC section, SD 4.5; Figure 1B), whereas albumin gold exhibited no significant binding (0.4 gold particles/pRBC section, SD 0.6). Uninfected RBC did not bind any of the conjugates (data not shown).
Binding of heparin-conjugates to surfaces of infected erythrocytes.
(A) Heparin–FITC was allowed to bind to a living culture. The pRBC were counterstained with ethidium bromide and visualized by UV microscopy. (B) For electron microscopy, heparin-albumin-gold particles were applied to the specimen as described in “Materials and methods.” Photograph shows cell surface of uninfected RBC (upper part) and pRBC (lower part). The scale bar represents 0.2 μm.
Binding of heparin-conjugates to surfaces of infected erythrocytes.
(A) Heparin–FITC was allowed to bind to a living culture. The pRBC were counterstained with ethidium bromide and visualized by UV microscopy. (B) For electron microscopy, heparin-albumin-gold particles were applied to the specimen as described in “Materials and methods.” Photograph shows cell surface of uninfected RBC (upper part) and pRBC (lower part). The scale bar represents 0.2 μm.
Correlation between heparin binding to pRBC and rosetting rates of the culture
The rosetting clone FCR3S1 and 2 clonal populations, derived from this parasite, of different rosetting phenotypes (FCR3S1.2, R+; FCR3S1.6, R−) were assayed for heparin binding. High rosetting rates were paralleled by a high heparin-binding capacity in strain FCR3S1.2, whereas a low rosetting rate correlated with a correspondingly low level of heparin binding in strain FCR3S1.6 (Figure2A). The binding of heparin–FITC could be abolished by competition with heparin or HS but not with CSA, confirming the selectivity for heparin-related polysaccharides (Figure2B). The rosetting ligands have previously been found to be highly sensitive to trypsin treatment.11 19 Indeed, the treatment by trypsin abolished heparin binding at the same rate as rosetting, supporting the notion that heparin binding is mediated by a trypsin-sensitive molecule such as PfEMP1 (Figure2C). Neuraminidase, on the other hand, had an effect neither on the rosetting nor on the heparin-binding properties (data not shown).
Correlation between rosetting and heparin binding of 3 different Plasmodium populations.
(A) Rosetting rates (▪) and heparin–FITC binding rates (□) for FCR3S1 and 2 clonal populations, the high rosetting clone FCR3S1.2 and the low rosetting clone FCR3S1.6. Rates were determined as described in “Materials and methods.” (B) Competition of heparin–FITC binding to living cultures of FCR3S1.2 by heparin (—•—), HS (—○—), and CSA (—◊—). (C) Sensitivity of rosetting (—▪—) and FITC-heparin-binding (—◊—) of FCR3S1.2 cultures to trypsin treatment as indicated in “Materials and methods.” All results are shown as mean and standard variation from 3 separate experiments.
Correlation between rosetting and heparin binding of 3 different Plasmodium populations.
(A) Rosetting rates (▪) and heparin–FITC binding rates (□) for FCR3S1 and 2 clonal populations, the high rosetting clone FCR3S1.2 and the low rosetting clone FCR3S1.6. Rates were determined as described in “Materials and methods.” (B) Competition of heparin–FITC binding to living cultures of FCR3S1.2 by heparin (—•—), HS (—○—), and CSA (—◊—). (C) Sensitivity of rosetting (—▪—) and FITC-heparin-binding (—◊—) of FCR3S1.2 cultures to trypsin treatment as indicated in “Materials and methods.” All results are shown as mean and standard variation from 3 separate experiments.
Importance of polysaccharide fragment length and sulfation for rosette disruption
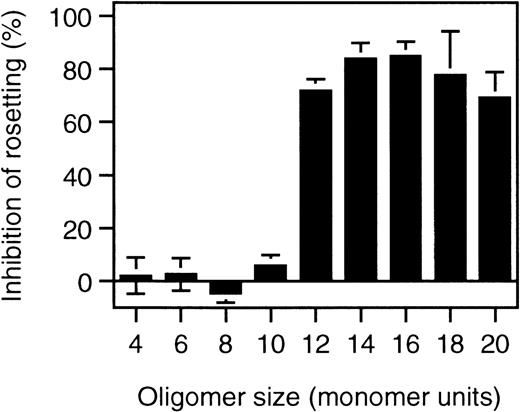
To identify the minimal requirements for rosette disruption, desulfated heparin molecules and heparin fragments were tested. Competition is most critically dependent on N-sulfation, whereas O-sulfation plays a minor role in the competition behavior of heparin (Table 1). When different lengths of heparin fragments were tested, molecules shorter than 10 sugar units (10 mer) had no effect, whereas 12-mer fragments or longer effectively disrupted rosettes (Figure 3).
Rosette disruption by heparin fragments.
Rosetting parasite cultures (FCR3S1.2) were subjected to treatment with heparin-fragments of defined size at 1 mg/mL as indicated in “Materials and methods” and the effect of rosette disruption quantitated as percent of control cultures subjected to parallel treatment without polysaccharide added.
Rosette disruption by heparin fragments.
Rosetting parasite cultures (FCR3S1.2) were subjected to treatment with heparin-fragments of defined size at 1 mg/mL as indicated in “Materials and methods” and the effect of rosette disruption quantitated as percent of control cultures subjected to parallel treatment without polysaccharide added.
Heparin binds native PfEMP1 from the FCR3S1.2 strain
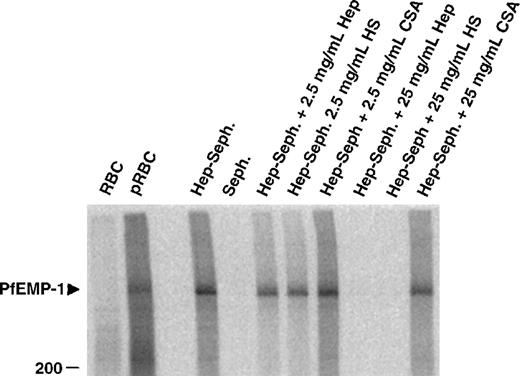
To identify the parasite-derived molecules involved in the heparin binding of pRBC, extracts from cell surface iodinated infected erythrocytes were mixed with heparin–Sepharose beads and were incubated in the presence or absence of competing polysaccharides. One band, identical with the formerly suggested rosetting ligand PfEMP1, was precipitated from FCR3S1.2-infected erythrocytes (Figure4). Again, this binding could be competed by heparin or HS but not by CSA, confirming the nature of this PfEMP1 as a ligand for heparin-related polysaccharides.
Affinity purification of infected erythrocyte membrane proteins by heparin–Sepharose.
Cell extracts of surface 125I-labeled FCR3S1.2 pRBC were incubated with Sepharose-linked heparin and the bound fraction analyzed by SDS-PAGE as described in “Materials and methods.” The specificity of binding was assessed by competition with the indicated amounts of soluble heparin, HS and CSA.
Affinity purification of infected erythrocyte membrane proteins by heparin–Sepharose.
Cell extracts of surface 125I-labeled FCR3S1.2 pRBC were incubated with Sepharose-linked heparin and the bound fraction analyzed by SDS-PAGE as described in “Materials and methods.” The specificity of binding was assessed by competition with the indicated amounts of soluble heparin, HS and CSA.
The rosetting domain DBL-1 of PfEMP1 binds heparin in a size- and sulfation-dependent manner
PfEMP1 of FCR3S1.2 is a multidomain protein with 3 extracellular domains containing potential GAG-binding sites.6 Individual domains were expressed as GST-fusion proteins, and their heparin-binding capacity was assessed by in-solution binding to [3H] heparin. A Kd approximately equal to 3 μmol/L could be estimated from these experiments for DBL-1, whereas both CIDR and DBL-4 showed only marginal binding, and no estimation of the affinity was possible though identical concentrations of protein were used for all 3 domains in the binding assays. When size-defined [3H] heparin fragments were tested in the same assay, DBL-1 showed prominent binding with a preference for 12-mer and larger fragments, whereas neither CIDR nor DBL-4 bound extensively (Figure 5B). The control protein GST alone did not bind heparin in any of the assays (data not shown). These results confined the major heparin binding to the DBL-1 domain of PfEMP1 and paralleled the results on the cellular level. By affinity chromatography with the immobilized DBL-1 domain, similar fragment size dependence could be observed. Unbound and slightly retarded fragments smaller than an 8 mer were washed out at salt concentrations of 0.2 mol/L NaCl, whereas bound fragments required salt concentrations larger than 0.4 mol/L NaCl. In contrast to the in-solution assay, the smallest heparin fragment bound by the immobilized protein was a 10 mer. The affinity of the protein for the 10 mer is most likely the result of the different exposure of the protein in the column compared with the in-solution assay (data not shown). When chemically desulfated heparin fragments of defined size (12 mer) were tested by affinity chromatography, these preparations bound more weakly to the column than fully sulfated heparin 12 mer, especially in the absence of N-sulfation (data not shown).
Binding of PfEMP1 domains to heparin and heparin fragments.
(A) [3H] Heparin binding to 4 μg of DBL-1 (—•—), CIDR (—♦—), and DBL-4 (—○—) in an in-solution assay was performed with increasing concentrations of [3H] heparin and protein together with bound polysaccharide was recovered by membrane filtration as described in “Materials and methods.” (B) Size defined [3H] heparin fragments were allowed to bind to the proteins under the same conditions as in A: DBL-1 (—•—), CIDR (—♦—), and DBL-4 (—○—).
Binding of PfEMP1 domains to heparin and heparin fragments.
(A) [3H] Heparin binding to 4 μg of DBL-1 (—•—), CIDR (—♦—), and DBL-4 (—○—) in an in-solution assay was performed with increasing concentrations of [3H] heparin and protein together with bound polysaccharide was recovered by membrane filtration as described in “Materials and methods.” (B) Size defined [3H] heparin fragments were allowed to bind to the proteins under the same conditions as in A: DBL-1 (—•—), CIDR (—♦—), and DBL-4 (—○—).
DBL-1 binds to HS from different tissues but not to CS
A cellular receptor for pRBC and PfEMP-1 is most likely not heparin, confined to connective tissue-type mast cells, but rather HS found ubiquitously in mammalian cells. We therefore tested a series of different HS, including preparations from human, bovine and porcine tissues (aorta, lung, liver, kidney) and endothelial cells, as well as CSA, dermatan sulfate, and CSC from bovine and porcine tissues (bovine nasal cartilage, porcine skin, and bovine nucleus pulposus cartilage, respectively) on the DBL-1 affinity column. Indeed, all the HS preparations bound to DBL-1 and were eluted by 0.4 to 0.6 mol/L NaCl (ie, they showed similar binding strength as heparin eluted by 0.8 mol/L NaCl). The tested CS preparations from the different sources containing mainly CSA, dermatan sulfate, and CSC, respectively, did not bind to the column, confirming the identity of the PfEMP1 and DBL-1 as heparin- and HS-specific ligands and excluding CS as a receptor for this rosetting ligand (Figure 6).
DBL-1 affinity chromatography of HS and CS.
Affinity chromatography of GAGs was performed under identical conditions as described in Figure 5C with different HS and CS preparations. (A) human aorta HS, (B) bovine endothelial HS, (C) bovine cartilage CSA.
DBL-1 affinity chromatography of HS and CS.
Affinity chromatography of GAGs was performed under identical conditions as described in Figure 5C with different HS and CS preparations. (A) human aorta HS, (B) bovine endothelial HS, (C) bovine cartilage CSA.
Discussion
Rosetting and cytoadherence are considered to be the prime virulence factors involved in the cause of severe malaria. Reverting the sequestration of pRBC could become an important tool in the treatment of the acute phases of severe disease, but there is still a lack of knowledge of the precise molecular interactions between the host and the parasite. The fact that a high proportion of rosettes from fresh clinical isolates is sensitive to HS and heparin19motivated a search for the molecular features of this interaction. Here, we have scrutinized the role of heparin–HS as a potential receptor and potent inhibitor of rosette formation. We have characterized the interaction between different domains of rosetting PfEMP1 and consolidated the function of the DBl-1 domain as the rosetting domain. We have also identified important molecular features in heparin–HS, ie, oligosaccharide chain length and sulfation, required for optimal ligand–receptor interaction. Importantly, the rosetting domain DBl-1 was found to have affinity for heparan sulfate from human aorta and from bovine endothelial cells, suggesting that this binding affinity may also be implicated in cytoadherence to the vascular endothelial cell lining.
The capacity of heparin and heparin-like molecules to disrupt rosettes could be caused by binding to the ligand on the infected erythrocyte or, hypothetically, to the receptor molecules on the noninfected erythrocyte. Yet the binding of the heparin conjugates to infected, but not to uninfected, erythrocytes and the strict correlation between heparin binding and rosetting of differentPlasmodium clones clearly supports the view that a ligand on the infected erythrocytes, PfEMP1, is responsible for the interaction with heparin. The precipitation of PfEMP1, the rosetting ligand of (R+) FCR3S1.2, by heparin–Sepharose confirms this hypothesis. The inability of CS to abolish the binding of heparin conjugates or the rosetting in FCR3S1.2 cultures and the dependence onN-sulfated polysaccharides defines PfEMP1 as a heparin- and HS-specific ligand in this parasite clone. Importantly, PfEMP1 is not detectable in surface radioiodinated pRBC of the (R−) clone FCR3S1.6, and pRBC from that clone are not agglutinated by hyperimmune sera.11 Consequently, the binding of heparin–FITC was low.
The PfEMP1 of FCR3S1.2 is a multidomain protein, and several consensus amino acid sequences, implicated in GAG binding,21 have been identified in all 3 extracellular domains.6 We, therefore, tested their GAG-binding capacity with the result that DBL-1 exhibits by far the strongest binding to all the tested GAGs compared with the 2 other domains. The binding specificity of DBL-1 for the glucosaminoglycans HS and heparin, its dependence onN-sulfation, and its size dependence for a 12-mer heparin fragment, all clearly mirror the situation of the intact rosetting cells, further confirming the role of this domain in the rosetting process. This suggests the N-terminal DBL-1 domain as a predominant ligand for cellular receptors, though it cannot exclude the participation of the other domains in the context of the intact molecule, which is, however, unfortunately too large for it to be amenable to expression and testing with currently available expression systems.
Many proteins exert their physiological roles through binding to HS.22,23 Molecular mimicry and adaptation of parasites to use GAGs or other polysaccharides as receptors are also well-described phenomena.24 In terms of the parasite survival strategy, it is therefore not surprising that essentially all types of HS tested can serve as receptors with marginal differences in affinities, depending on the overall charge level of these chains, suggesting common sequences within the chains as potential binding sites. On the other hand, CS, and specifically CSA, identified as a receptor for endothelial and placental binding of pRBC25-27 are clearly not receptors for the PfEMP1 of the FCR3S1.2 strain. A unique feature of the heparin-binding properties of both the native PfEMP1 and of recombinant DBL-1 is the strong dependence on N-sulfation. This contrasts with previous findings because several cellular factors known to bind to heparin/HS show strict dependence on O-sulfation and occasionally, as in the case of antithrombin-3 or basic fibroblast growth factor, a specific type of O-sulfation. TheN-sulfation–specific feature of rosetting and PfEMP1 ligand binding opens an interesting possibility to a therapeutic strategy for a glycomimetic. The criteria of Plasmodium binding (eg,N-sulfation) could be conserved, whereas potential sequence motifs necessary for binding physiologically relevant factors of the host could be avoided (eg, 3-O-sulfation of antithrombin-3 binding site) to reduce the unfavorable side effects of antimalarial treatment.
We have suggested that rosetting binding is mediated by HS or HS-like structures on the surfaces of the uninfected RBC, via GAG-binding motifs, on the DBL-1 domain of PfEMP1, but the identity of these molecules has to await further characterization.6 Field data have shown that a substantial portion of clinical isolates is sensitive to HS and heparin19 and that approximately 75% of fresh clinical isolates show some binding to FITC-labeled heparin. This phenotype was commonly found among patients with severe malaria (A. Heddini et al, manuscript submitted for publication). Interestingly, we have found that the DBL-1 domain of FCRS1.2 (but not CIDR or DBL-4) binds to HS/HS-like GAGs on normal CHO cells (Q. Chen et al, unpublished data), suggesting that DBL-1/HS may support binding not only for rosetting but, for example, for endothelial binding. Other studies have established an important role for CSA as a receptor in endothelial cytoadherence.25-27Members from the PfEMP1 family were recently suggested to be the corresponding parasite-derived ligands.28 29
Rosetting is a heterogeneous phenomenon, and current data suggest that glycans play a common but not an exclusive role. Additional receptors, such as CR1,5 the ABO blood group antigens,30 serum factors,31 and CD 36,32 participate in rosette formation in a strain-dependent manner. More complex, multimolecular interactions involving PfEMP1 and more than one receptor moiety are therefore also possible. We have recently shown that the A and B blood group antigens function as coreceptors to other receptors, such as HS-like GAGs, in rosetting (A. Barragan et al, manuscript submitted for publication) and that the rosetting domain of PfEMP1 may have affinity for more than 1 rosetting receptor (Q. Chen et al, unpublished data). CR1 is not likely to be involved in rosetting in the parasite lines used in this study because the binding is not inhibited by soluble CR15 or CR1 antibodies (A. Barragan unpublished data). The same reasoning holds true for CD36.33 However, although CR1-dependent rosetting of the strain R29 is unaffected by heparitinase treatment,6heparin has, to some extent, an antirosetting effect on this strain.34 Thus, heparin–HS may interfere with the CR1/PfEMP1 interaction in that strain. This broadens the potential application of heparin-related polysaccharides as anti-adhesive agents.
This paper provides the molecular characterization of the heparin-binding rosetting phenotype recently associated with disease severity (A. Heddini et al, manuscript submitted for publication). A method to detect this phenotype easily in clinical and field settings using soluble receptor (heparin–FITC-conjugate) is also provided. This is the first study that scrutinizes binding and evaluates the affinity of GAGs to all domains of a PfEMP1 variant that mediates rosetting. We have also identified molecular features of heparin–HS, such as molecular size (12-mer oligosaccharide chain) and specific sulfation (N-sulfation) that are determinant to the binding. Taken together, the current data suggest an important role for DBL-1 and HS or HS-like structures in rosette formation, but they also suggest that HS on endothelial cells may be involved in the sequestration of pRBC. Polysaccharides could be potential candidates to develop antiadhesive molecules targeting carbohydrate-mediated binding. Our results provide evidence that PfEMP1 molecules with affinity for heparin-related polysaccharides can be targeted and blocked using appropriate polysaccharides. Elucidation of these lectin-like interactions will enable new therapeutic strategies directed to virulence factors in severe P falciparum malaria.
Acknowledgments
We thank Dr Anders Malmström for providing CSA, dermatan sulfate, and CSC. We also thank Dr E. Feyzi for supplying us with human aortic HS, Dr M. Presta for the GM 7373 cells, and Dr G. Guzman and Dr E. Linder for help with the photographs.
Supported by Karolinska Institutet, INCO-DC contract IC-18-CT98-0362, the Swedish Medical Research Council, and Polysackaridforskning AB.
Reprints:Mats Wahlgren, Microbiology and Tumor Biology Center, Karolinska Institutet and Swedish Institute for Infectious Disease Control, Box 280, S-171 77 Stockholm, Sweden.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 5. Binding of PfEMP1 domains to heparin and heparin fragments. / (A) [3H] Heparin binding to 4 μg of DBL-1 (—•—), CIDR (—♦—), and DBL-4 (—○—) in an in-solution assay was performed with increasing concentrations of [3H] heparin and protein together with bound polysaccharide was recovered by membrane filtration as described in “Materials and methods.” (B) Size defined [3H] heparin fragments were allowed to bind to the proteins under the same conditions as in A: DBL-1 (—•—), CIDR (—♦—), and DBL-4 (—○—).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/11/10.1182_blood.v95.11.3594/5/m_bloo01121005x.jpeg?Expires=1769088392&Signature=SqplnpZTMKPS5dn860o1TKGWjM1LPZIRNyE0Fx7Q3WggwbtseXvMq6SrM8xiHKFnPUh1HeoVSMHnoszOEoCBO48qr7uPahoBdhJqMbGDyC7crV3e97H2gjwF6Z6uORIa5rZcWn6rsX0c-Z81QzLyphkoN0gQL8Z8SJdV6l7LIs0iVi4EpEydAqaGaK4JxkvVqkZyVkWTXmZI8DmgyRQr~M7Y-VNGmWojH9dBI40GcrcoGBu6LB9FJUknOy1w01WhPYkMk1ncvOpWAGHSjIkJRgBBuNk6~zBPZqy9FBEWMK2ka~C2x2JLQ3LBbF1kTt0LcHn3vY31r2C1ST8ZV4vzdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal