Abstract
To gain insight into the mechanisms controlling apoptosis of dendritic cells (DC), human monocyte-derived DC were analyzed for their expression of CD95 (Fas/Apo-1) and their response to CD95 ligation. Although DC expressed the CD95 molecule on their membrane, they did not undergo apoptosis on CD95 ligation unless sensitized by cycloheximide. In parallel, DC synthesized c-FLIPL, an inhibitor of the CD95-mediated death-signaling cascade. We also demonstrated that bisindolylmaleimide down-regulates c-FLIPL expression in DC and, in parallel, allows CD95-mediated apoptosis in these cells. In contrast, Bcl-2, Bcl-xl, and Bax levels were not affected by bisindolylmaleimide. We conclude that DC resist CD95- mediated apoptosis in association with c-FLIPLexpression and that the immunosuppressive potential of bisindolylmaleimide previously observed at the T-cell level also involves facilitation of CD95-mediated DC apoptosis.
Interactions between CD95 (Fas/Apo-1) and its ligand were shown to be essential for the development of lymphocyte apoptosis during activation-induced cell death.1 Because homeostasis of the immune system also depends on dendritic cells (DC), which play critical roles in the induction of immune responses,2 it is important to define the factors controlling DC survival. Therefore, we first analyzed the expression of CD95 on monocyte-derived human DC and the sensitivity of these cells to CD95-mediated apoptosis. Because we found that DC resisted CD95 ligation and synthesized c-FLIPl, an inhibitor of death receptors,3 we then determined the effects on DC of bisindolylmaleimide derivatives, agents that have been shown to sensitize tumor cells and T lymphocytes to CD95 death signaling.4
Materials and methods
Culture medium and reagents
The culture medium was RPMI 1640 (Bio-Whittaker, Verviers, Belgium) supplemented with 2 mmol/L L-glutamine, 20 μg/mL gentamicin, 50 μmol/L 2-mercaptoethanol, 1% nonessential amino acids, and 10% fetal-calf serum (PanSystems, Aidenbach, Germany). Recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) was obtained from Novartis (Basel, Switzerland). Recombinant interleukin 4 (IL-4) was provided by Schering-Plough (Kenilworth, NJ). Agonistic antihuman CD95 monoclonal antibody Ab (mAb; clone CH11) was purchased from Immunotech (Marseille, France), recombinant human tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) from R&D Systems Europe Ltd (Abingdon, United Kingdom), lipopolysaccharide (LPS) from Escherichia coli (0128:B12) from Sigma Chemicals (Bornem, Belgium), bisindolylmaleimide I, II, III, IV, and VIII from Alexis Biochemicals (San Diego, CA) and cycloheximide from Sigma Chemicals.
Generation of monocyte-derived DC
DC were generated from peripheral blood mononuclear cells as described by Romani et al.5 Briefly, peripheral blood mononuclear cells from healthy volunteers were isolated by density centrifugation of heparin-treated blood on Lymphoprep (Nycomed, Oslo, Norway), resuspended in culture medium, and allowed to adhere to 75-cm2 tissue-culture flasks. After 2 hours at 37°C, nonadherent cells were removed and adherent cells were cultured in 20 mL of medium containing GM-CSF (800 U/mL) and IL-4 (500 U/mL). Every 2 days, 1 mL of fresh medium containing IL-4 and GM-CSF was added. After 7 days of culture, nonadherent cells corresponding to the DC-enriched fraction were harvested, washed, and used for subsequent experiments. As previously reported,6 the DC-enriched fraction obtained with this protocol routinely contains more than 95% of DC as assessed by morphologic and fluorescence-activated cell-sorter (FACS) analysis.
Flow cytometry
For detection of CD95 membrane expression, cells were washed in phosphate-buffered saline (PBS) supplemented with 0.5% bovine serum albumin and 10 mmol/L sodium azide and incubated for 30 minutes at 4°C with phycoerythrin (PE)-conjugated anti-CD95 mouse IgG1 mAb (Becton Dickinson, Mountain View, CA) or a corresponding isotype-matched control. Analysis was done with a FACS Calibur flow cytometer (Becton Dickinson). For detection of apoptosis, double staining with fluorescein isothiocyanate (FITC)–conjugated annexin V and propidium iodide (PI) was performed on 105 DC washed with PBS and resuspended in 200 μL of annexin V–binding buffer (Becton Dickinson) containing 5 μL FITC annexin V (Pharmingen, San Diego, CA). After 10 minutes of incubation in the dark at room temperature, cells were washed, 1 μg/mL PI (Sigma Chemicals) was added, and flow cytometric analysis was performed. In some experiments, hypodiploid nuclei (subG1 peak) were detected by PI staining as described previously.7 Briefly, 105 DC were fixed in 70% cold ethanol for 45 minutes, washed before incubation with 100 μL RNase A (1 mg/mL) (Sigma Chemicals) and 200 μL PI (100 μg/mL) (Sigma Chemicals), and subjected to flow cytometry analysis.
Western blot analysis
DC (107) were washed with cold PBS and lysed at 4°C for 30 minutes in borate-buffered saline containing 1% Brij 97, 10 μg/mL pepstatin, 5 μg/mL leupeptin, 10 μg/mL aprotinin, 1 mmol/L sodium orthovanadate, 5 mmol/L sodium fluoride, 5 mmol/L EDTA, and 2 mmol/L phenylmethyl sulfonyl fluoride (Sigma Chemicals). After 5 minutes of sonication, lysates were spun at 13 000 rpm for 15 minutes, supernatants were collected, and protein concentrations were determined with use of the Micro BCA Protein Assay reagent kit (Pierce, Rockford, IL). For detection of c-FLIPL, Bcl-xL, and Bax, 10 μg of each protein lysate was electrophoresed; 30 μg was used for the Bcl-2 analysis. Lysates were separated on 10% sodium dodecyl sulfate–polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes (Amersham Pharmacia, Roosendaal, Netherlands). Membranes were blocked with 10% dry milk in PBS and Tween.
After washing with PBS and Tween, the blots were incubated for 1 hour with the following antibodies: NF6 mouse mAb antihuman c-FLIPL,8 mouse mAb antihuman Bcl-2 (Dako, Glostrup, Denmark), rabbit polyclonal IgG antihuman Bcl-xL(Santa Cruz, Santa Cruz, CA), and rabbit polyclonal IgG antihuman Bax (Santa Cruz). Blots were washed again with PBS and Tween and incubated with the following second antibodies: horseradish peroxidase (HRP)–labeled sheep antimouse antibodies (Amersham Pharmacia) for c-FLIPL and Bcl-2 detection and HRP-labeled donkey antirabbit IgG antibodies (Amersham Pharmacia) for Bcl-xL and Bax detection. Blots were developed by using an enhanced chemiluminescence detection system (ECL; Amersham Pharmacia). The expression levels of c-FLIPL in the presence and absence of bisindolylmaleimide were compared with densitometry using Gel-Doc 1000 (Bio-Rad, Nazareth, Belgium).
Results
Monocyte-derived DC express CD95 but resist CD95-mediated apoptosis
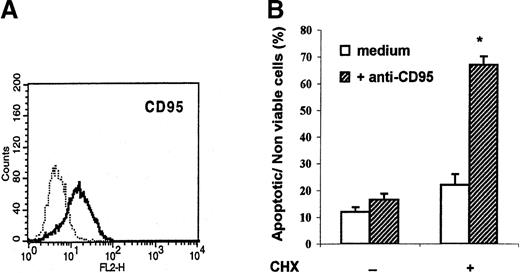
As expected from previous studies,6 plastic-adherent peripheral blood mononuclear cells cultured for 6 days in GM-CSF and IL-4 had the typical phenotype of immature DC: they expressed HLA-DR, CD80, CD86, CD40, and CD1a and were negative for CD14 and CD83 (data not shown). These cells also expressed CD95 (Figure1, panel A). However, culture of DC for 18 hours in the presence of the CH-11 agonistic anti-CD95 mAb did not increase their apoptosis rate (Figure 1, panel B). A similar observation was made when soluble recombinant CD95 ligand was used as the agonist (data not shown). Because cycloheximide is known to sensitize several types of cells to apoptosis by preventing synthesis of antiapoptotic proteins,9 10 these experiments were repeated in the presence of cycloheximide (10 μg/mL), which by itself only marginally enhanced DC apoptosis. As shown in Figure 1(panel B), the CH-11 mAb readily induced apoptosis in cycloheximide-sensitized DC, thereby demonstrating that the CD95 death pathway can function in DC when de novo protein synthesis is inhibited.
Monocyte-derived dendritic cells (DC) express CD95 but resist CD95-mediated apoptosis.
(A) Flow cytometry analysis of CD95 membrane expression on DC generated from plastic-adherent peripheral blood mononuclear cells cultured for 6 days in granulocyte-macrophage colony-stimulating factor and interleukin 4. (B) DC were incubated in medium alone (open columns) or in the presence of agonistic anti-CD95 monoclonal antibody (mAb; 1 μg/mL) (solid columns) with or without cycloheximide (CHX; 10 μg/mL). After 18 hours, the percentages of apoptotic or nonviable cells were assessed with use of flow cytometry after staining with fluorescein isothiocyanate–conjugated annexin V and propidium iodide (PI). Data are shown as the mean (± SEM) of the sums of annexin V–single-positive cells, annexin V– and PI–double-positive cells, and PI–single-positive cells in 9 experiments on samples from 9 different donors. * P = .0005 compared with DC incubated with CHX in medium alone (Wilcoxon 2-tailed test).
Monocyte-derived dendritic cells (DC) express CD95 but resist CD95-mediated apoptosis.
(A) Flow cytometry analysis of CD95 membrane expression on DC generated from plastic-adherent peripheral blood mononuclear cells cultured for 6 days in granulocyte-macrophage colony-stimulating factor and interleukin 4. (B) DC were incubated in medium alone (open columns) or in the presence of agonistic anti-CD95 monoclonal antibody (mAb; 1 μg/mL) (solid columns) with or without cycloheximide (CHX; 10 μg/mL). After 18 hours, the percentages of apoptotic or nonviable cells were assessed with use of flow cytometry after staining with fluorescein isothiocyanate–conjugated annexin V and propidium iodide (PI). Data are shown as the mean (± SEM) of the sums of annexin V–single-positive cells, annexin V– and PI–double-positive cells, and PI–single-positive cells in 9 experiments on samples from 9 different donors. * P = .0005 compared with DC incubated with CHX in medium alone (Wilcoxon 2-tailed test).
Monocyte-derived DC constitutively synthesize c-FLIPL
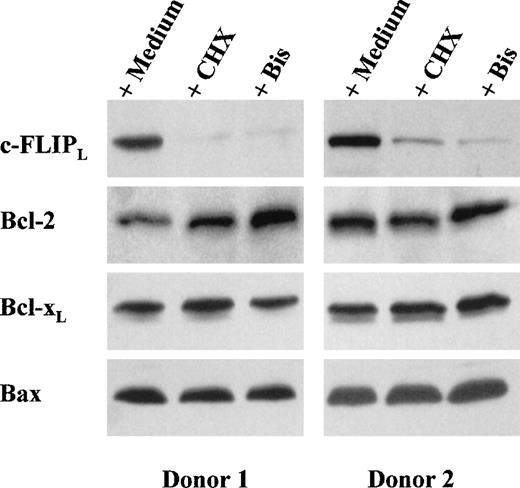
To gain insight into the nature of the proteins that might prevent CD95-mediated apoptosis in DC, we used Western blotting to analyze the expression by DC of Bcl-2, Bcl-xl, Bax, and c-FLIPL, an inhibitory protein of caspase-8 implicated in the control of CD95-mediated apoptosis in T lymphocytes.11As shown in Figure 2, DC expressed the 3 members of the Bcl-2 family as well as c-FLIPL. Incubation of DC for 12 hours with cycloheximide strongly decreased their expression of c-FLIPL, whereas their expression of Bcl-2, Bcl-xl, and Bax was not significantly modified (Figure 2). This observation indicates that DC synthesize c-FLIPL and that the turnover of this protein is faster than that of the Bcl-2 family members. In fact, c-FLIPLdown-regulation and sensitivity to CD95-mediated apoptosis were already apparent after 4 hours of incubation with cycloheximide (Figure3).
CHX and bisindolylmaleimide (Bis) down-regulate c-FLIPL expression.
DC were incubated for 12 hours in medium alone or in the presence of either CHX (10 μg/mL) or Bis III (20 μmol/L). Whole-cell extracts were electrophoresed and probed with anti-FLIPL mAb (NF6), anti-Bcl-2 mAb, anti-Bcl-xL rabbit polyclonal antibody (Ab), or anti-Bax rabbit polyclonal Ab, followed by an appropriate second Ab. Two representative experiments of 10 performed on samples from different blood donors are shown.
CHX and bisindolylmaleimide (Bis) down-regulate c-FLIPL expression.
DC were incubated for 12 hours in medium alone or in the presence of either CHX (10 μg/mL) or Bis III (20 μmol/L). Whole-cell extracts were electrophoresed and probed with anti-FLIPL mAb (NF6), anti-Bcl-2 mAb, anti-Bcl-xL rabbit polyclonal antibody (Ab), or anti-Bax rabbit polyclonal Ab, followed by an appropriate second Ab. Two representative experiments of 10 performed on samples from different blood donors are shown.
Treatment of DC with CHX or Bis results in decreased levels of c-FLIPL and sensitivity to CD95-mediated apoptosis.
(A) DC were incubated in medium alone or in the presence of either CHX (10 μg/mL) or Bis III (20 μmol/L). At the indicated times, whole-cell extracts were electrophoresed and probed with anti-FLIPL mAb (NF6) followed by an appropriate second Ab. Data are representative results from 3 experiments done on samples from different blood donors. (B) DC were incubated with agonistic anti-CD95 mAb (0.5 μg/mL) in the presence of CHX (10 μg/mL) or Bis III (20 μmol/L). After 4, 8, or 24 hours, cells were fixed, DNA was stained with PI, and the percentage of hypodiploid nuclei (subG1 peak) was analyzed with use of flow cytometry. Data are representative results from 2 experiments done on samples from different blood donors.
Treatment of DC with CHX or Bis results in decreased levels of c-FLIPL and sensitivity to CD95-mediated apoptosis.
(A) DC were incubated in medium alone or in the presence of either CHX (10 μg/mL) or Bis III (20 μmol/L). At the indicated times, whole-cell extracts were electrophoresed and probed with anti-FLIPL mAb (NF6) followed by an appropriate second Ab. Data are representative results from 3 experiments done on samples from different blood donors. (B) DC were incubated with agonistic anti-CD95 mAb (0.5 μg/mL) in the presence of CHX (10 μg/mL) or Bis III (20 μmol/L). After 4, 8, or 24 hours, cells were fixed, DNA was stained with PI, and the percentage of hypodiploid nuclei (subG1 peak) was analyzed with use of flow cytometry. Data are representative results from 2 experiments done on samples from different blood donors.
Bisindolylmaleimide down-regulates c-FLIPL expression and facilitates CD95-mediated apoptosis in monocyte-derived DC
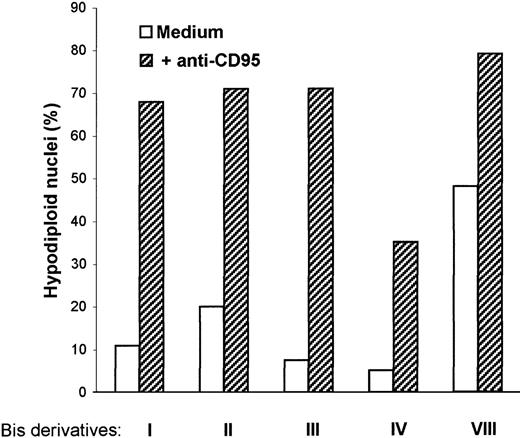
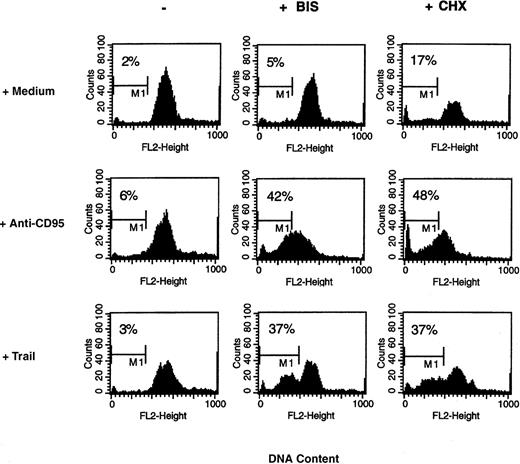
Bisindolylmaleimide derivatives were shown to sensitize tumor cells and T lymphocytes to CD95-mediated apoptosis in vitro and in vivo.4 We were interested in determining whether these agents could also modify DC responsiveness to CD95 ligation. First, we observed that combined treatment with CH-11 mAb and each bisindolylmaleimide derivative (I, II, III, IV, and VIII) at a concentration of 20 μmol/L resulted in DC apoptosis (Figure4). Because incubation of DC with bisindolylmaleimide VIII alone was by itself cytotoxic, whereas incubation with the other derivatives did not affect DC survival, we decided to use bisindolylmaleimide III for further analysis. We then observed that in the presence of bisindolylmaleimide, the CH-11 mAb induced apoptosis in DC in a reproducible manner, as assessed by both staining with annexin V and PI and enumeration of cells with hypodiploid nuclei (Table 1 and Figure5). Moreover, bisindolylmaleimide, like cycloheximide, sensitized DC to apoptosis triggered by TRAIL, another ligand of the tumor necrosis factor (TNF) family (Figure 5).
Bis derivatives facilitate apoptosis induced by anti-CD95 mAb.
DC were incubated in medium in the presence of anti-CD95 mAb (0.5 μg/mL) with or without Bis I, II, III, IV, or VIII (20 μmol/L). After 18 hours, cells were fixed, DNA was stained with PI, and the percentage of hypodiploid nuclei (subG1 peak) was analyzed with use of flow cytometry. Data are representative results from 3 experiments done on samples from different blood donors.
Bis derivatives facilitate apoptosis induced by anti-CD95 mAb.
DC were incubated in medium in the presence of anti-CD95 mAb (0.5 μg/mL) with or without Bis I, II, III, IV, or VIII (20 μmol/L). After 18 hours, cells were fixed, DNA was stained with PI, and the percentage of hypodiploid nuclei (subG1 peak) was analyzed with use of flow cytometry. Data are representative results from 3 experiments done on samples from different blood donors.
Cell-labeling results indicating facilitation by bisindolylmaleimide (Bis) of CD95-mediated apoptosis in human dendritic cells (DC)
| Treatment of DC . | Cell-labeling results (% DC)* . | PI+ . | Hypodiploid nuclei (%)† . | ||
|---|---|---|---|---|---|
| Annexin V− PI− . | Annexin V+ PI− . | Annexin V+ PI+ . | |||
| Medium | 87.2 ± 2.5 | 3.3 ± 0.7 | 7.8 ± 1.6 | 1.6 ± 0.2 | 3.8 ± 0.8 |
| Anti-CD95 | 85.8 ± 2.8 | 3.7 ± 0.8 | 8.6 ± 1.8 | 1.9 ± 0.4 | 8.8 ± 2.4 |
| Bis | 84.7 ± 4.3 | 6.3 ± 1.3 | 7.4 ± 2.6 | 1.6 ± 0.5 | 9.6 ± 1.9 |
| Bis + Anti-CD95 | 52.6 ± 1.4 | 12.4 ± 2.6‡ | 22.4 ± 1.3‡ | 12.4 ± 3.0‡ | 46.1 ± 6.71-153 |
| Treatment of DC . | Cell-labeling results (% DC)* . | PI+ . | Hypodiploid nuclei (%)† . | ||
|---|---|---|---|---|---|
| Annexin V− PI− . | Annexin V+ PI− . | Annexin V+ PI+ . | |||
| Medium | 87.2 ± 2.5 | 3.3 ± 0.7 | 7.8 ± 1.6 | 1.6 ± 0.2 | 3.8 ± 0.8 |
| Anti-CD95 | 85.8 ± 2.8 | 3.7 ± 0.8 | 8.6 ± 1.8 | 1.9 ± 0.4 | 8.8 ± 2.4 |
| Bis | 84.7 ± 4.3 | 6.3 ± 1.3 | 7.4 ± 2.6 | 1.6 ± 0.5 | 9.6 ± 1.9 |
| Bis + Anti-CD95 | 52.6 ± 1.4 | 12.4 ± 2.6‡ | 22.4 ± 1.3‡ | 12.4 ± 3.0‡ | 46.1 ± 6.71-153 |
DC were incubated for 18 hours in medium alone or in the presence of either CH-11 anti-CD95 monoclonal antibody (1 μg/mL) or Bis III (20 μmol/L).
DC were stained with fluorescein isothiocyanate-conjugated (FITC) annexin V followed by propidium iodide (PI) and analyzed with use of flow cytometry. Data represent mean (±SEM) percentage of cells in each quadrant of dot-plot analysis from 6 independent experiments on samples from 6 different donors.
P < .05 compared with DC incubated in medium alone (Wilcoxon two-tailed test).
DC were fixed before incubation with PI and RNase. Data represent mean (±SEM) percentage of hypodiploid nuclei measured by flow cytometry in 9 independent experiments on samples from 9 different donors.
P < .005 compared with DC incubated in medium alone (Wilcoxon two-tailed test).
Bis facilitates apoptosis triggered by anti-CD95 mAb and human tumor necrosis factor–related apoptosis-inducing ligand (TRAIL).
DC were incubated in medium alone or in the presence of anti-CD95 mAb (1 μg/mL) or TRAIL (250 ng/mL) with or without Bis III (20 μmol/L) or CHX (10 μg/mL). After 18 hours, cells were fixed, and DNA was stained with PI and analyzed with use of flow cytometry. The percentage of hypodiploid nuclei (subG1 peak) is indicated on each plot. Data are from 1 experiment representative of 5 done.
Bis facilitates apoptosis triggered by anti-CD95 mAb and human tumor necrosis factor–related apoptosis-inducing ligand (TRAIL).
DC were incubated in medium alone or in the presence of anti-CD95 mAb (1 μg/mL) or TRAIL (250 ng/mL) with or without Bis III (20 μmol/L) or CHX (10 μg/mL). After 18 hours, cells were fixed, and DNA was stained with PI and analyzed with use of flow cytometry. The percentage of hypodiploid nuclei (subG1 peak) is indicated on each plot. Data are from 1 experiment representative of 5 done.
Because DC incubated with bisindolylmaleimide became sensitive to CD95-mediated apoptosis, we analyzed the effect of bisindolylmaleimide on c-FLIPL expression. As shown in Figure 2, bisindolylmaleimide dramatically inhibited c-FLIPLaccumulation in DC. In 6 different experiments performed on samples from 6 different blood donors, the mean (± SEM) optical-density ratio corresponding to c-FLIPL expression in bisindolylmaleimide-treated DC relative to that in untreated DC was 7% ± 2% (P < .005). In contrast, bisindolylmaleimide did not modify Bcl-2, Bcl-xl,or Bax expression (Figure 2). As was the case with cycloheximide, c-FLIPL down-regulation and sensitivity to CD95-mediated apoptosis had already occurred after 4 hours of incubation with bisindolylmaleimide (Figure 3).
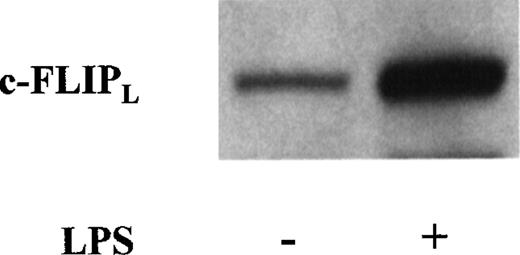
c-FLIPL up-regulation on LPS-induced DC maturation
To determine whether DC maturation could modify c-FLIPLexpression, we compared the levels of c-FLIPL in immature and LPS-activated mature DC. As shown in Figure6, which depicts results representative of 5 different experiments, incubation of DC for 24 hours with 1 μg/mL LPS clearly increased the cells' expression of c-FLIPL. Moreover, mature DC, like immature cells, resisted CD95 triggering (Table 2).
c-FLIPL expression is up-regulated on lipopolysaccharide (LPS)-induced DC maturation.
DC were incubated in medium with or without LPS (1 μg/mL). After 24 hours, whole-cell extracts were electrophoresed and probed with anti-FLIPL mAb (NF6) followed by an appropriate second Ab. Data are representative results from 5 experiments done on samples from different blood donors.
c-FLIPL expression is up-regulated on lipopolysaccharide (LPS)-induced DC maturation.
DC were incubated in medium with or without LPS (1 μg/mL). After 24 hours, whole-cell extracts were electrophoresed and probed with anti-FLIPL mAb (NF6) followed by an appropriate second Ab. Data are representative results from 5 experiments done on samples from different blood donors.
Results indicating resistance of mature DC to CD95-mediated apoptosis
| Treatment of DC . | Nonviable cells (%) . | |
|---|---|---|
| Immature DC . | Mature DC . | |
| Medium | 19.7 ± 2.2 | 11.7 ± 2.4 |
| Anti-CD95 | 24.7 ± 2.8 | 16.5 ± 2.8 |
| Treatment of DC . | Nonviable cells (%) . | |
|---|---|---|
| Immature DC . | Mature DC . | |
| Medium | 19.7 ± 2.2 | 11.7 ± 2.4 |
| Anti-CD95 | 24.7 ± 2.8 | 16.5 ± 2.8 |
DC were incubated with or without lipopolysaccharide (1 μg/mL) for 24 hours, washed, and cultured in medium in the absence or presence of anti-CD95 (CH11; 1 μg/mL) for 24 hours. The percentage of nonviable cells was determined with use of annexin V-FITC and PI staining and fluorescence-activated cell sorting. Data represent mean (±SEM) values from 5 independent experiments on samples from 5 different donors. There was no significant difference between values obtained with medium and those obtained with anti-CD95 or between values obtained with mature DC and those obtained with immature DC.
Discussion
The interactions of DC with activated T cells involve several membrane-bound molecules and soluble factors that control cell survival and death. Thus, CD40 ligation enhances DC survival,12 and interactions between TNF-related activation-induced cytokine (TRANCE) and TRANCE receptor regulate DC and T-cell apoptosis during clustering of DC and T cells.13,14 With respect to interactions between CD95 and CD95L, ligation of CD95 on murine DC by CD95L expressed on antigen-specific CD4+ Th1 cells was shown to be one of the mechanisms leading to DC apoptosis, which could in turn contribute to down-regulation of T-cell activation.15 In contrast, another study found that murine DC derived from bone marrow or spleen tissue were resistant to apoptosis through the CD95 pathway.16 In this study, we observed that human monocyte-derived DC are resistant to CD95 ligation. This finding is consistent with previous observations in DC derived from human cord blood, in which CD95 ligation induced apoptosis in only a minor fraction of the DC.12 On the other hand, Koppi et al17 reported significant responses of monocyte-derived DC to the CH-11 anti-CD95 mAb. We therefore assume that the conditions of DC generation might influence their sensitivity to CD95. The relevance of our findings to the fate of DC during natural immune responses in humans is unknown, but because monocyte-derived DC are currently used in clinical trials as cancer vaccines, it is important to determine the factors promoting their survival.
The resistance of monocyte-derived DC to CD95-mediated apoptosis was associated with the expression of c-FLIPL. This protein, which is homologous to caspase-8, blocks the early events of the CD95 signal-transduction pathway by inhibiting recruitment of caspase-8 into the death-inducing signaling complex.3 A regulatory role for c-FLIPL was initially demonstrated in human T-cell lines.11 However, involvement of c-FLIPL in the resistance of peripheral blood T cells to CD95-mediated death is controversial because it was observed in some studies11,18but not others.8 In tumor cells, the expression of c-FLIPL was found to be associated with resistance to apoptosis induced by CD95 or TRAIL-receptor ligation.10,11Interestingly, inhibition of protein synthesis in certain tumor cells resulted in both c-FLIPL down-regulation and loss of resistance to TRAIL-induced death.10 We observed a similar correlation in monocyte-derived DC, in which the decrease in c-FLIPL expression when cells were cultured with cycloheximide was paralleled by acquisition of CD95 sensitivity. Together with the observations on the effects of bisindolylmaleimide, these data strongly suggest an important role for c-FLIPLin the control of DC susceptibility to death-receptor ligation, although a contribution by other factors remains possible.
Our findings are consistent with the data of Ashanasy et al,16 who found that high levels of c-FLIP expression in murine DC were correlated with resistance to CD95-mediated cell death. Moreover, c-FLIP was demonstrated to be the main negative regulator of the CD95 signaling pathway in macrophages. Monocyte differentiation into macrophages was shown to be associated with up-regulation of c-FLIP and decreased sensitivity to CD95-mediated apoptosis. Overexpression of c-FLIP protected monocytes from CD95-mediated apoptosis, whereas c-FLIP inhibition in macrophages induced apoptosis.19 We can therefore assume that c-FLIP is up-regulated during the differentiation of monocytes into DC. We also found that c-FLIPL expression was strongly enhanced on LPS-induced DC maturation and that mature DC, like immature cells, were not susceptible to CD95-mediated killing.
Bisindolylmaleimide was found to potentiate CD95-induced apoptosis strongly in murine activated T cells both in vitro and in vivo and to thereby block the development of experimental T-cell–mediated autoimmune diseases.4 The mechanisms by which bisindolylmaleimide acts on T cells and DC seem to be different, since T cells were not affected by bisindolylmaleimide I, II, and IV but these derivatives were active on DC. The hypothesis that bisindolylmaleimide sensitizes DC to CD95 signaling through c-FLIPL down-regulation is consistent with our observation that bisindolylmaleimide also facilitated DC apoptosis triggered by the TRAIL receptor, another death receptor inhibited by c-FLIPL. Because bisindolylmaleimide derivatives are potent inhibitors of protein kinase C, which was shown to control the CD95 death pathway,20-22 we are currently investigating the role of protein kinase C in the control of c-FLIPL expression.
We conclude that aside from its action at the T-cell level, bisindolylmaleimide may down-regulate immune responses by facilitating apoptosis of DC on interaction with cells expressing CD95L or related death-receptor ligands.
Supported by the Centre de Recherche Interuniversitaire en Vaccinologie sponsored by the Région Wallonne and SmithKline Beecham Biologicals (Belgium) and by a Pôle d'Attraction Interuniversitaire (Belgium).
Reprints:Michel Goldman, Hôpital Erasme, Department of Immunology, 808, Route de Lennik, B-1070 Brussels, Belgium; e-mail:mgoldman@ulb.ac.be.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal