Abstract
Adult bone marrow is a major site for hematopoiesis, and reduction of the bone marrow cavity induces hematopoiesis in extramarrow tissues. To investigate the rudimentary intramarrow and the compensatory extramarrow hematopoiesis, particularly B lymphopoiesis, we used 3 osteopetrotic mouse strains [op/op, mi/mi, and Fos(−/−)], which are severely deficient in functional osteoclasts and therefore form inadequate bone marrow cavities. We found that bone marrow in these osteopetrotic mice supports myelopoiesis but not B lymphopoiesis, although cells that have the potential to differentiate into B lineage cells are present in the bone marrow. Although B lymphopoiesis normally occurs both in the spleen and liver of newborn mice, compensatory B lymphopoiesis in adultop/op and mi/mi mice is observed only in the liver, while myelopoiesis is enhanced in both organs. Interestingly, mice lacking the Fos proto-oncogene exhibit B lymphopoiesis in the spleen as well as liver. The amounts of expression of steel factor, Flt3/Flk-2 ligand, and interleukin-7 in the bone marrow, spleen, or liver were not significantly affected in these osteopetrotic mutants. These findings suggest that the volume of the bone marrow cavity regulates B lymphopoiesis without affecting the production of certain hematopoietic growth factors. The splenic microenvironments that support both myelopoiesis and B lymphopoiesis in the neonatal stage are lost in adults and are not reactivated even in the osteopetrotic adults unless the Fos gene is disrupted.
Hematopoiesis is initiated in the yolk sac and subsequently occurs in the paraaortic splanchnopleural mesoderm and aorta-gonad-mesonephros regions.1-3 Hematopoietic stem cells (HSCs) in the splanchnopleural mesoderm or aorta-gonad-mesonephros migrate to the fetal liver and spleen and finally localize in the bone marrow. Blood formation in extramarrow tissues gradually decreases, and adult hematopoiesis mainly occurs in the bone marrow.2-5
In adult life, extramedullary hematopoiesis is induced by low-dose X-irradiation6,7 and by osteopetrosis, fibrosis, or malignant blood diseases in which the bone marrow cavity is incapacitated for hematopoiesis.8-11 Usually, extramedullary hematopoiesis occurs in the spleen and liver, which are the sites for fetal and neonatal hematopoiesis in the normal state.12 It is speculated that adult spleen and liver maintain the potential to support hematopoiesis and that stimuli such as X-irradiation or reduction of bone cavity induce molecules essential for the support of extramedullary hematopoiesis.
Colonization from HSCs in spleen (CFU-s, or colony-forming units-spleen) depends on the presence of steel factor (hereafter abbreviated as SLF, also named stem cell factor, mast cell growth factor, and a ligand for c-Kit receptor protein tyrosine kinase and the encoding gene symbolized as Mgf). SLF-deficient steel mice and the c-Kit–deficient dominant spotting (KitW) mice lack CFU-s and have severely reduced definitive-type hematopoiesis.13-20 Another receptor protein tyrosine kinase, Flt3/Flk-2 (the encoding gene symbolized as Flt3), is also expressed in immature hematopoietic cells,21-23 and disruption of the Flt3gene results in deficiencies both in HSCs and in early B progenitor cells, while mice with combined Flt3/Flk-2 and c-Kit deficiencies exhibit more severe phenotypes.24 Thus, SLF and Flt3/Flk-2 ligand (Flt3L) are candidate critical molecules for extramedullary hematopoiesis.
For B lymphopoiesis, interleukin (IL)-7, which is produced by stromal cells that support hematopoiesis, is known to be essential.25-28 Disruption of the Il7 or its receptor (Il7r) genes results in severe lymphopenia.29-31 Overexpression of the Il7 gene induces B cell hyperplasia in the bone marrow and extramedullary organs in transgenic mice.32 33 These findings indicate that SLF, Flt3L, and IL-7 production might regulate lymphohematopoiesis.
In the current study, we used 2 different spontaneous osteopetrotic mutations, osteopetrosis (Csfmop), abbreviated as op, and microphthalmia (Mitfmi), abbreviated as mi,and the induced mutation Fos (−/−), which result in deficiencies of osteoclasts and a reduced hematopoietic “niche” in the bone marrow.34-38 Hematopoiesis in the bone marrow and in the extramedullary organs was analyzed in these osteopetrotic mice. We found that the regulation of B lymphopoiesis is different from that of hematopoiesis represented by colony-forming cells responsive to IL-3 (CFU–IL-3). Rudimentary bone marrow in the osteopetrotic mice contained CFU–IL-3 at a normal frequency, but the frequencies of B lineage cells, which are characterized by stromal cell– and IL-7–dependent B precursor cells and CFU–IL-7,39,40 were significantly diminished. However, when the bone marrow cells of these osteopetrotic mice were cultured with stromal cells in the presence of IL-7, they differentiated into B lineage cells, indicating that defective B lymphopoiesis in osteopetrotic bone marrow may result from defects in the microenvironment rather than from defective B progenitor cells. Moreover, the finding that aged op/op mice with spontaneously cured osteopetrosis had restored levels of B precursor cells in their bone marrow confirms the critical role of the hematopoietic niche on medullary B lymphopoiesis.41,42 Because numbers of peripheral blood cells, including mature B cells in fetal, newborn, and aged op/op mice are relatively normal8,43,44 and production of immunoglobulin (Ig)M and IgG antibodies is also indistinguishable from normal mice,45 46 we reasoned that the osteopetrotic mice use alternative sites for hematopoiesis. While myelopoiesis was enhanced in both the spleen and liver of adult osteopetrotic mice, extramedullary B lymphopoiesis in op/op andmi/mi mice was observed only in the liver. In contrast, Fos(−/−) spleen contained substantial numbers of CFU–IL-7.
Expression of the critical hematopoietic growth factors SLF, Flt3L, and IL-7 in the bone marrow, spleen, and liver of osteopetrotic mice was relatively indistinguishable in osteopetrotic mice and normal littermates. This suggests the possible importance of the reduction of the hematopoietic niche in the bone marrow in inducing the arrest of B lymphopoiesis and implies that compensatory extramedullary hematopoiesis is induced by as yet unknown mechanisms without an increase of production of SLF, Flt3L, or IL-7.
Materials and methods
Mice
C57BL/6 (B6) mice were purchased from Clea Japan (Yokohama, Japan); B6C3Fe-a/a- Csfmop/+ (op/+)and B6C3Fe-a/a- Mitfmi/+(mi/+) heterozygous mice were obtained from the Jackson Laboratory (Bar Harbor, ME); and 129/Sv-Fos (+/−) [Fos (+/−)] gene-targeted mice37 were obtained from Chiba University, Japan, and were originally derived from the European Molecular Biology Laboratory, Heidelberg, Germany. All mice were maintained in the Research Animal Center, Faculty of Medicine, Tottori University, Yonago, Japan. Genomic DNA from the tails of op/+ and mi/+ mice was screened to detect the mutated sequences, and that from Fos (+/−) heterozygous mice carrying the neoR gene was screened by using appropriate primers and the polymerase chain reaction (PCR).34 36 Homozygous op/op, mi/mi, andFos (−/−) mice were obtained by heterozygous sibling mating of each strain and were identified by the lack of incisors.
Cell preparation
Bone marrow cells from normal mice were collected by flushing femoral shafts using a 26G sterile needle, and those from osteopetrotic mice were obtained by crushing the femora to small pieces and using vigorous pipetting. Spleens and livers were minced and then homogenized by disruption between frosted glass slides in alpha-minimum essential medium (α-MEM; Gibco-BRL, Grand Island, NY). The cells were passed through nylon mesh and washed once. To prepare hematopoietic cells from the liver, the cells were washed 3 more times and then suspended in 40% Percoll (Gibco-BRL) and layered onto 70% Percoll. The tubes were centrifuged at 600g at 20°C for 25 minutes, and the interface between the 40% layer and the 70% layer containing hematopoietic cells was collected.47
Assays of colony-forming cells
Cells were incubated in 1 mL of α-MEM containing 1.2% methylcellulose (Muromachi Kagaku Kogyo, Tokyo, Japan), 30% fetal bovine serum (Bio-Whittaker, Walkersville, MD), 1% deionized bovine serum albumin (Sigma Chemical, St Louis, MO), 50-μM 2-mercaptoethanol, 50-U/mL streptomycin, and 50-μg/mL penicillin (Meiji Chemical, Tokyo, Japan) in the presence of 100 U/mL mouse recombinant IL-3, 100 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), or 20 U/mL IL-7. Numbers of colonies were counted on the seventh day after the inoculation39 and expressed as the mean ± SD of triplicate cultures.
Clonal expansion of B progenitor cells on stromal cell layers
The limiting dilution assay on PA648 stromal cell layers was performed as previously described.39 Briefly, stromal cell monolayers were allowed to form in 96-well culture plates (Falcon Labware, Oxnard, CA). Bone marrow cells were diluted to various concentrations, inoculated into the wells, and cultivated for 7 or 8 days in the presence of 20-U/mL mouse recombinant IL-7. Outgrowth of B lineage cells was assessed with the aid of a microscope.
Flow cytometry
Cells were incubated on ice with heat-inactivated normal rabbit serum (Gibco-BRL) and then stained with biotinylated anti-Mac-1 (M1/70, PharMingen, San Diego, CA), biotinylated goat antimouse IgM (ICN Pharmaceuticals Inc, Costa Mesa, CA), and fluorescein isothiocyanate (FITC)-conjugated anti-B220 (6B2, PharMingen). The stained cells were further incubated with phycoerythrin (PE)-labeled streptavidin (Becton Dickinson Immunocytometry Systems, San Jose, CA) and analyzed using an EPICS-XL flow cytometer (Coulter Electronics Inc, Haleah, FL).
Quantitative reverse transcription–polymerase chain reaction and Southern blotting
Total RNA was extracted from bone marrow, spleen, and liver using ISOGEN (Nippon Gene, Toyama, Japan). RNA (5 μg) was reverse-transcribed using SuperScript RNase H−reverse transcriptase (Gibco-BRL) and oligo(dT) primer according to the protocol provided by the manufacturer. PCR assays were performed in a reaction mixture containing 1 × rTaq buffer (Toyobo, Osaka, Japan), 0.16-mM dNTP, 1.5-mM MgCl2, 30-U/mL rTaqDNA polymerase (Toyobo), and each primer at 0.5 μmol/L. The forward primers and reverse primers, respectively, were as follows:Mgf, 5′-GTGGCAAATCTTCCAAATGA-3′ and 5′-CTCGGGACCTAATGTTGAAG-3′; Il7,5′-ACATCATCTGAGTGCCACA-3′ and 5′-CTCTCAGTAGTCTCTTTAG-3′; Flt3l, 5′-GGACGAGAAGCACTGCAAGG-3′ and 5′-GTGAGAGGCAGCAGCAGCAG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPD), 5′-CCATGGAGAAGGCTGGGG-3′ and 5′-CAAAGTCATGGATGACC-3′. PCR amplification of complementary DNAs (cDNAs) was performed under the following conditions: first cycle consisting of 94°C for 3 minutes, annealing for 3 minutes, and 72°C for 3 minutes, followed by 24 cycles of 94°C for 1 minute, annealing for 1 minute, and 72°C for 1.5 minutes; the temperatures for annealing were 55°C (Mgf), 57°C (Il7), 55°C (Flt3l), and 60°C (GAPD). To compare relative levels of gene expression, serially diluted cDNA (1:1, 1:8, 1:64) was amplified. The PCR products were subjected to electrophoresis on 2% agarose gels and Southern blot hybridization with appropriate [32P]-radiolabeled gene probes.
Results
B lymphopoiesis is observed in neonatal spleen but not in adult spleen of wild-type mice
The spleen is one of the main sites for perinatal hematopoiesis in normal mice.2 3 We assessed hematopoiesis in the spleens of 2-day-old neonatal and 8-week-old adult C57BL/6 mice by enumerating the cells that had the potential of colony formation in the presence of IL-3 (CFU–IL-3) and IL-7 (CFU–IL-7). Numbers of cells recovered from 2-day-old neonatal spleen and femora were approximately 20- and 10-fold lower than those from 8-week-old adult mice, respectively. Both neonatal and adult bone marrow contained CFU–IL-3 and CFU–IL-7 at approximately comparable frequencies (Table1). In the spleen, the frequency of CFU–IL-3 in adult mice was reduced to less than 1/36 of that in neonatal mice, and the total number of CFU–IL-3 in the spleens of 8-week-old mice was half of that in the spleens of 2-day-old mice. While CFU–IL-7 existed in the neonatal spleen, we did not detect CFU–IL-7 in the adult spleen (Table 1). These results indicated that adult hematopoiesis, particularly B lymphopoiesis, mainly occurs in bone marrow rather than in the spleen.
Age-related sites for hematopoiesis in normal mice
| Factor . | Cells from . | No. of colonies . | |
|---|---|---|---|
| Per 105 . | Per femur or spleen . | ||
| Bone marrow | |||
| IL-3 | 2-day-old | 281.5 ± 17.5 | 2083 ± 128 |
| IL-3 | 8-week-old | 551.5 ± 56.0 | 45 499 ± 4620 |
| IL-7 | 2-day-old | 208.5 ± 27.5 | 1543 ± 204 |
| IL-7 | 8-week-old | 158.5 ± 13.0 | 13 077 ± 1073 |
| Spleen | |||
| IL-3 | 2-day-old | 361.5 ± 47.5 | 19 087 ± 2508 |
| IL-3 | 8-week-old | 10.0 ± 5.0 | 10 300 ± 5150 |
| IL-7 | 2-day-old | 43.5 ± 17.5 | 2297 ± 924 |
| IL-7 | 8-week-old | 0 | <343 |
| Factor . | Cells from . | No. of colonies . | |
|---|---|---|---|
| Per 105 . | Per femur or spleen . | ||
| Bone marrow | |||
| IL-3 | 2-day-old | 281.5 ± 17.5 | 2083 ± 128 |
| IL-3 | 8-week-old | 551.5 ± 56.0 | 45 499 ± 4620 |
| IL-7 | 2-day-old | 208.5 ± 27.5 | 1543 ± 204 |
| IL-7 | 8-week-old | 158.5 ± 13.0 | 13 077 ± 1073 |
| Spleen | |||
| IL-3 | 2-day-old | 361.5 ± 47.5 | 19 087 ± 2508 |
| IL-3 | 8-week-old | 10.0 ± 5.0 | 10 300 ± 5150 |
| IL-7 | 2-day-old | 43.5 ± 17.5 | 2297 ± 924 |
| IL-7 | 8-week-old | 0 | <343 |
Freshly prepared bone marrow cells and spleen cells were cultured in methylcellulose semi-solid medium with IL-3 or IL-7 for 7 days. The numbers of cells recovered per femur were 0.74 × 106 and 8.25 × 106 from 2-day-old and 8-week-old mice, respectively. The numbers of cells recovered per spleen were 5.28 × 106 and 103.0 × 106 from 2-day-old and 8-week-old mice, respectively. Data are expressed as the mean ± SD of triplicate cultures.
Lack of B lymphopoiesis in the bone marrow from osteopetrotic mice
Osteopetrotic mice are known to have reduced bone marrow cavities available for hematopoiesis and to have elevated extramedullary hematopoiesis.8 We first analyzed the residual intramedullary hematopoiesis in op/op mice. Hematopoietic cells in the op/op rudimentary bone marrow cavity were obtained by crushing the femora into small pieces and using vigorous pipetting, and recovery of cells from 8-week-old op/op bone marrow was 10 to 20 times lower than that from the bone marrow of normal littermates. However, the frequencies of CFU–IL-3 and CFU-GM in op/op mice were comparable with those of the normal littermates (Table2), although in 1 experiment the frequency of CFU–IL-3 in op/op mice was only half of that in the normal littermates (Table 2, experiment no. 3). On the other hand, the frequency of CFU–IL-7 in op/op bone marrow was diminished to 1/8 of that in the bone marrow of normal littermates, and in 1 experiment (Table 2, experiment no. 3) we could not detect any colony-forming cells per 105op/op bone marrow cells.
Reduction of frequency of CFU–IL-7 in op/opbone marrow
| Factor addition . | No. of colonies per 105 cells . | |
|---|---|---|
| +/? . | op/op . | |
| Experiment no. 1 | ||
| Medium | 0 | 0 |
| IL-3 | 295.0 ± 43.3 | 363.3 ± 53.9 |
| IL-7 | 48.3 ± 11.5 | 1.7 ± 2.9* |
| Experiment no. 2 | ||
| Medium | 0 | 0 |
| IL-3 | 241.5 ± 33.5 | 230.0 ± 73.5 |
| IL-7 | 68.5 ± 12.5 | 8.3 ± 7.5* |
| Experiment no. 3 | ||
| Medium | 0 | 0 |
| IL-3 | 655.0 ± 13.2 | 335.0 ± 32.8* |
| GM-CSF | 435.0 ± 73.7 | 443.0 ± 92.5 |
| IL-7 | 76.7 ± 15.3 | 0* |
| Factor addition . | No. of colonies per 105 cells . | |
|---|---|---|
| +/? . | op/op . | |
| Experiment no. 1 | ||
| Medium | 0 | 0 |
| IL-3 | 295.0 ± 43.3 | 363.3 ± 53.9 |
| IL-7 | 48.3 ± 11.5 | 1.7 ± 2.9* |
| Experiment no. 2 | ||
| Medium | 0 | 0 |
| IL-3 | 241.5 ± 33.5 | 230.0 ± 73.5 |
| IL-7 | 68.5 ± 12.5 | 8.3 ± 7.5* |
| Experiment no. 3 | ||
| Medium | 0 | 0 |
| IL-3 | 655.0 ± 13.2 | 335.0 ± 32.8* |
| GM-CSF | 435.0 ± 73.7 | 443.0 ± 92.5 |
| IL-7 | 76.7 ± 15.3 | 0* |
Freshly prepared bone marrow cells from 8-week-old op/ophomozygotes and their normal littermates were cultured in methylcellulose semi-solid medium with IL-3, GM-CSF, or IL-7 for 7 days. Data are expressed as the mean ± SD of triplicate cultures.
Significantly different from the corresponding normal mouse cultures (P < .002).
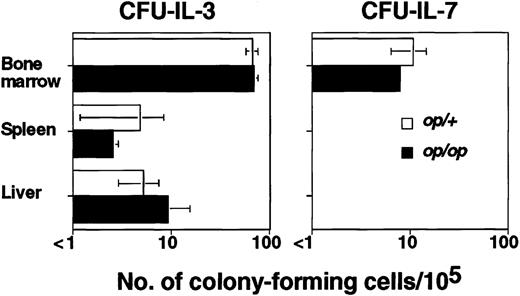
The defect of op/op mice is attributed to hematopoietic microenvironments that lack functional M-CSF produced by stromal cells, resulting in reduced osteoclast development and therefore in osteopetrosis.34,35 To determine whether the abnormality in B lymphopoiesis associated with osteopetrosis is anop/op-specific characteristic or a common phenomenon in osteopetrotic mice, we used osteopetrotic mi/mi mice mutated in the Mitf gene, which encodes a basic helix-loop-helix, leucine zipper-type transcriptional factor and affects hematopoietic cells such as osteoclasts and mast cells rather than affecting supportive microenvironments.36 The total number of CFU–IL-3 (mi/mi: 1306 ± 24 cells/femur, mi/+: 55 692 ± 7224 cells/femur) was significantly reduced inmi/mi mice, whereas the frequency of CFU–IL-3 was comparable with that of mi/+ littermates (Figure1). No CFU–IL-7 was detected (< 1/105), which is similar to the finding in op/op mice. We also studied Fos (−/−) mice, which lack osteoclasts and thus have osteopetrosis, and obtained similar results (Figure 1).
Extramedullary hematopoiesis in 8-week-old mi/miand Fos (−/−) mice.
The numbers of colony-forming cells elicited by 100 U/mL IL-3 or 20 U/mL IL-7 per 105 cells from bone marrow, spleen, liver, thymus, peripheral lymph nodes (cervical, axillary, and inguinal), mesenteric lymph nodes, Peyer patches, and peritoneal cavity were measured. Cells from tissues other than bone marrow, spleen, and liver did not form any colonies (< 1/105). Data are expressed as the mean ± SD of triplicate cultures. *Values of osteopetrotic mice that are significantly different from those of the corresponding normal littermates (P < .05).
Extramedullary hematopoiesis in 8-week-old mi/miand Fos (−/−) mice.
The numbers of colony-forming cells elicited by 100 U/mL IL-3 or 20 U/mL IL-7 per 105 cells from bone marrow, spleen, liver, thymus, peripheral lymph nodes (cervical, axillary, and inguinal), mesenteric lymph nodes, Peyer patches, and peritoneal cavity were measured. Cells from tissues other than bone marrow, spleen, and liver did not form any colonies (< 1/105). Data are expressed as the mean ± SD of triplicate cultures. *Values of osteopetrotic mice that are significantly different from those of the corresponding normal littermates (P < .05).
Next, we examined at what differentiation stage the B-cell lineage in osteopetrotic bone marrow was affected. The culture of bone marrow cells with PA6 stromal cells in the presence of IL-7 (PA6 + IL-7) allows growth and differentiation of B lineage cells more immature than CFU–IL-7.39 Using this culture system, the frequency of clonal expansion of PA6 + IL-7–dependent B lineage cells was examined. Freshly prepared bone marrow cells from op/op mice showed a significantly reduced frequency of PA6 + IL-7–dependent B lineage cells (Table3), and in some experiments we observed more than 90-fold reduction compared with normal littermates (op/op: < 1/9500, +/?: 1/103.9). These results indicate that B lymphopoiesis in op/op bone marrow is affected prior to the PA6 + IL-7–dependent stage.
Presence of B progenitor cells in op/op bone marrow
| Bone marrow cells . | Frequency of B lineage cell generation3-150 . | No. of CFU–IL-7 per 105 cells . | ||
|---|---|---|---|---|
| +/? . | op/op . | +/? . | op/op . | |
| Freshly prepared3-151 | 1/237.8 ± 144.9 | 1/454.8 ± 156.23-152 | 48.3 ± 11.5 | 1.7 ± 2.93-153 |
| Cultured with PA6 and IL-73-155 | >1/6.5 | >1/6.5 | 26.7 ± 5.8 | 103.3 ± 18.93-153 |
| Bone marrow cells . | Frequency of B lineage cell generation3-150 . | No. of CFU–IL-7 per 105 cells . | ||
|---|---|---|---|---|
| +/? . | op/op . | +/? . | op/op . | |
| Freshly prepared3-151 | 1/237.8 ± 144.9 | 1/454.8 ± 156.23-152 | 48.3 ± 11.5 | 1.7 ± 2.93-153 |
| Cultured with PA6 and IL-73-155 | >1/6.5 | >1/6.5 | 26.7 ± 5.8 | 103.3 ± 18.93-153 |
The frequency of B lineage cell generation was measured by limiting dilution analysis.
Freshly prepared bone marrow cells (62.5, 125, 250, and 500 cells/well) from 8-week-old op/op and +/? mice were cultured on PA6 in the presence of 20-U/mL IL-7 for 8 days, and the number of wells containing B lineage cells was counted.
Significantly different from the corresponding normal mouse cultures (P < .05).
Significantly different from the corresponding normal mouse cultures (P < .002).
Cells cultured with PA6 and IL-7 for 2 weeks were harvested and recultured (by seeding 25, 50, 100, or 200 cells/well [48 wells/group]) with PA6 and IL-7 for 7 days.
However, after culturing op/op bone marrow cells with PA6 and IL-7 for 2 weeks, the frequency of PA6 + IL-7–dependent B lineage cells was restored and that of CFU–IL-7 was increased (Table 3). These results suggest that the precursor cells of the B lineage were present in op/op bone marrow, but the microenvironment for B-cell differentiation was defective.
Selective reduction of B precursor cells in the bone marrow of osteopetrotic mice
Because numbers of CFU–IL-7 and PA6 + IL-7–dependent B lineage cells in osteopetrotic bone marrow were reduced, we examined whether mature B cells exist in the osteopetrotic mice by flow cytometric analysis. B220+ cells in op/op bone marrow were selectively reduced (24.3% B220+ cells in +/? bone marrow cells and 7.0% in op/op bone marrow cells), and almost all B220+ cells also expressed surface IgM (8.7% B220+ IgM+ cells in op/op bone marrow cells). In contrast, two-thirds of the B lineage cells in normal bone marrow were immature B220+ IgM− cells (8.0% B220+IgM+ cells in +/? bone marrow cells). There were few immature B lineage cells in osteopetrotic bone marrow, indicating that B lymphopoiesis may not occur in the bone marrow of osteopetrotic mice and mature B cells are generated in extramarrow tissues.
B precursor cells appear in the spontaneously cured osteopetrotic marrow of op/op mice
It has been reported that age-related progressive formation of bone marrow cavity by an unknown mechanism cures osteopetrosis inop/op mice.41 42 We have observed that some adultop/op mice (30 weeks old) had a relatively normal number of hematopoietic cells in the bone marrow (op/op: 4.23 × 106/femur, op/+ littermate: 15.6 × 106/femur). The partially curedop/op bone marrow cells showed nearly normal relative frequencies of CFU–IL-3 and CFU–IL-7 (Figure2). This result confirmed the close relationship between the amount of marrow cavity available for hematopoiesis and maintenance of B lymphopoiesis in the bone marrow, and it ruled out the possibility that M-CSF is essential to support B-cell development in the bone marrow.
Bone marrow from aged op/op mice in which osteopetrosis was spontaneously cured produced CFU–IL-7.
The numbers of CFU–IL-3 and CFU–IL-7 in 30-week-old op/ophomozygous and heterozygous littermates were examined. Data are expressed as the mean ± SD of triplicate cultures.
Bone marrow from aged op/op mice in which osteopetrosis was spontaneously cured produced CFU–IL-7.
The numbers of CFU–IL-3 and CFU–IL-7 in 30-week-old op/ophomozygous and heterozygous littermates were examined. Data are expressed as the mean ± SD of triplicate cultures.
Because op/op mice are spontaneously cured, we could not precisely determine the state of osteopetrosis of each op/opmouse; thus, the following experiments were mainly carried out usingmi/mi mice unless otherwise indicated.
Extramedullary B lymphopoiesis in osteopetrotic mice
If bone marrow is the only site for adult B lymphopoiesis, osteopetrotic mice should lack mature B lymphocytes. We analyzed whether B cells expressing both B220 and IgM were present in 8-week-oldmi/mi and op/op spleens and detected numbers of mature B cells comparable with those of normal littermates (Figure3), indicating that sites other than bone marrow for B lymphopoiesis may exist in these mice. To determine the sites for extramedullary B lymphopoiesis, we assessed the presence of the B precursor cells indicated by CFU–IL-7 in mi/mi spleens. However, we could not detect any CFU–IL-7 (< 1/105) either in mi/mi or mi/+adult spleens (Figure 1). Because our experiments could detect 1 CFU–IL-7 in 105 cells, there were fewer than 1000 CFU–IL-7 per spleen. In contrast, the frequency of CFU–IL-3 was significantly increased in mi/mi spleens (Figure 1), indicating that extramedullary hematopoiesis was induced in the spleens ofmi/mi mice whereas B lymphopoiesis was not. We also examined the numbers of CFU–IL-7 in the thymus; peripheral lymph nodes, including cervical, axillary, and inguinal nodes; mesenteric lymph nodes; Peyer patches; and peritoneal cavity; however, no colony-forming cells were detected (< 1/105 cells) from mi/mior mi/+ mice.
Presence of mature B cells in the mi/mi spleen.
Spleen cells (106) from 8-week-old mi/mihomozygotes or mi/+ normal littermates were stained with FITC-6B2 (anti-B220), biotinylated-IgM or -M1/70 (anti-Mac-1), and PE-streptavidin, and analyzed using a flow cytometer.
Presence of mature B cells in the mi/mi spleen.
Spleen cells (106) from 8-week-old mi/mihomozygotes or mi/+ normal littermates were stained with FITC-6B2 (anti-B220), biotinylated-IgM or -M1/70 (anti-Mac-1), and PE-streptavidin, and analyzed using a flow cytometer.
Further examination was done by using fractionated liver cells separated by Percoll density gradients (40%-70%). The fractionated cells, containing 64% to 84% viable hematopoietic cells, were used for further analysis. The fractionated cells from both mi/miand mi/+ livers contained CFU–IL-7, and the frequencies of both CFU–IL-3 and CFU–IL-7 in mi/mi liver cells were significantly increased (Figure 1). When op/op mice were used, we obtained results similar to those with mi/mi mice (data not shown). These results indicated that osteopetrotic mice have up-regulated hematopoiesis in both spleen and liver, but B lymphopoiesis was only up-regulated in the liver.
It was previously reported that Fos (−/−) mice have reduced numbers of mature B cells.49 This implies a lack of extramedullary B lymphopoiesis and resultant B lymphopenia in Fos(−/−) mice. However, we found that some Fos(−/−) mice have normal numbers of B cells in their spleens, although other Fos (−/−) mice have significantly reduced numbers of B cells, as reported.49 We selected theFos (−/−) mice without B lymphopenia and analyzed the presence of CFU–IL-7. Interestingly, we detected CFU–IL-7 in both spleen and liver of Fos (−/−) mice (Figure 1).
Expression of Mgf, Flt3l, and Il7 genes in the osteopetrotic mice
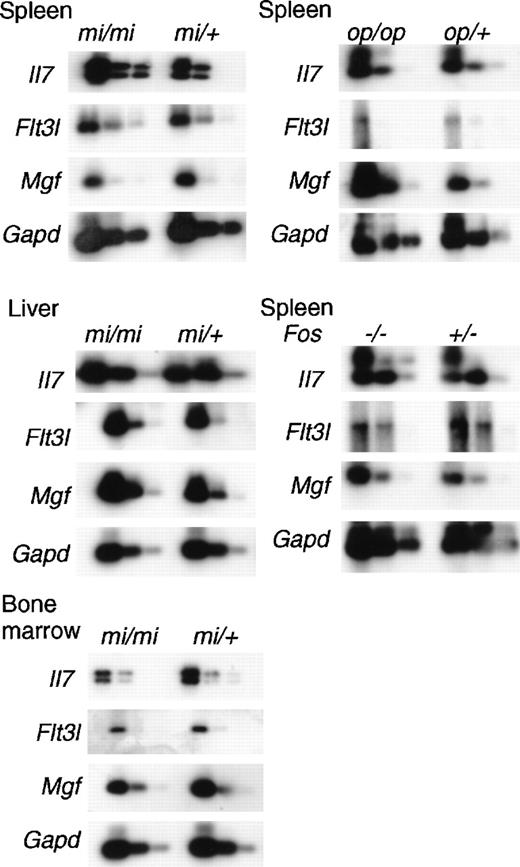
To investigate the enhanced myelopoiesis in the spleen and liver and B lymphopoiesis in the liver of osteopetrotic mice, we examined the expression of genes encoding critical hematopoietic factors, SLF, Flt3L, and IL-7, of mi/mi mice by using quantitative reverse transcription–PCR. The Mgf, Flt3l, and Il7 genes inmi/mi mice were expressed at levels relatively indistinguishable from those of normal mi/+ mice (Figure4). Myelopoiesis in spleen and liver ofmi/mi mice was enhanced, but the levels of Mgf andFlt3l gene messenger RNAs (mRNAs) did not significantly increase. In contrast, B lymphopoiesis in mi/mi bone marrow was reduced, while almost equal amounts of Il7 mRNA were detected in the bone marrow of mi/mi mice and normal littermates. Not only liver where B lymphopoiesis occurred in osteopetrotic mice, but also spleen where no B lymphopoiesis occurred, revealed a high level expression of Il7 mRNA both in mi/mi and mi/+mice. Although all osteopetrotic mouse strains showed enhanced myelopoiesis in the spleens, op/op but not mi/mispleens demonstrated increased Mgf mRNA (Figure 4). B lymphopoiesis was observed in Fos (−/−) spleen; however, significant differences between Fos (+/−) andFos (−/−) spleens in the expression of Il7gene were not detected (Figure 4). Furthermore, we did not detect any differences in Flt3l gene expression between mutants and their controls. These results suggest that although SLF, Flt3L, and IL-7 play critical roles in hematopoiesis, including B lymphopoiesis, induction or initiation of extramedullary hematopoiesis might be regulated by factors other than those we have tested here.
Expression of mRNA of Mgf, Flt3l, and Il7in the osteopetrotic mice.
Total RNAs were prepared from bone marrow, spleens, or livers of 8-week-old osteopetrotic mice. Different dilutions (1:1, 1:8, 1:64) of cDNA were subjected to PCR amplification specific for Mgf, Flt3l, Il7, and GAPD transcripts. The products were electrophoresed, blotted, and probed to detect messages for each corresponding gene.
Expression of mRNA of Mgf, Flt3l, and Il7in the osteopetrotic mice.
Total RNAs were prepared from bone marrow, spleens, or livers of 8-week-old osteopetrotic mice. Different dilutions (1:1, 1:8, 1:64) of cDNA were subjected to PCR amplification specific for Mgf, Flt3l, Il7, and GAPD transcripts. The products were electrophoresed, blotted, and probed to detect messages for each corresponding gene.
Discussion
Osteopetrotic mice have remarkably diminished total hematopoietic cell numbers in the bone marrow.8,50-52 In this study, we showed selective reduction of B lymphopoiesis in the rudimentary bone marrow of osteopetrotic mice regardless of whether the mutated gene wasCsfm, Mitf, orFos.34-38 All these mutant mice had enhanced extramedullary hematopoiesis in their spleens and livers, but B lymphopoiesis in op/op and mi/mi mice was enhanced only in the liver. Despite the fact that early stages of lympho-hematopoiesis are dependent on SLF, Flt3L, and IL-7, no significant differences of the expression of these genes were observed in the bone marrow, spleen, or liver of osteopetrotic versus normal mice.
The frequencies of stromal cell + IL-7–dependent B precursors and CFU–IL-7 in osteopetrotic bone marrow were all reduced; however, the progenitor cells that give rise to stromal cell + IL-7–dependent B precursors and CFU–IL-7 were maintained in the osteopetrotic marrow (Table 3). These results suggest that the progenitor cells of the B lineage are present in the osteopetrotic bone marrow, but the microenvironment may be defective for support of further development of B cells.
The relationship between the volume of the bone marrow cavity and medullary B lymphopoiesis is obvious in vertebrates. Intramarrow hematopoiesis first appears in amphibians,53 and the bone marrow in land Rana is an active site for hematopoiesis involving myelopoiesis and lymphopoiesis, whereas the bone marrow of aquatic amphibians such as Xenopus is rudimentary and is not a lymphopoietic tissue.54 Moreover, aquatic mammals such as Trichechiformes, with significantly diminished bone cavity,55 have been observed to carry out erythropoiesis and granulopoiesis, but not lymphopoiesis, in their vertebrae.56 Furthermore, the fact that bone marrow cells from the op/op mice spontaneously cured of osteopetrosis showed a normal frequency of CFU–IL-7 also supports the existence of such a relationship.41 42
Each lineage of hematopoietic cells in the bone marrow develops in a specialized location, indicating that local requirements necessary for each cell lineage are not identical.26 Jacobsen and Osmond5 proposed specially organized intramarrow B lymphopoiesis in the bone marrow. According to their proposal, B lymphopoiesis is centrally directed from the peripheral subendosteal area, near the bone cortex, in which the earliest B progenitor cells are located in extravascular spaces of marrow, in accordance with progression of maturation.5 Hematopoiesis in the bone marrow may be initiated first by erythropoiesis or myelopoiesis and then followed by B lymphopoiesis. Therefore, it is probable that the subendosteal microenvironment, which supports early B lymphopoiesis, is preserved, but the microenvironment required for subsequent differentiation is not retained properly in the osteopetrotic bone marrow.
We have previously reported that even in scid/scid mice, which completely lack lymphocytes and thus might have room available for hematopoiesis in all lymphoid organs, hematopoiesis is not enhanced in the spleen, indicating that the spleen cannot support adult hematopoiesis because of a microenvironmental defect, and the defect is not simply caused by a defective space or niche for hematopoiesis.7 When scid/scid mice are treated with low-dose X-irradiation (150 rad), hematopoiesis in the spleen is induced and Mgf gene expression in the spleen is up-regulated,7 indicating that SLF is one of the candidates of essential factors for the construction of hematopoietic microenvironments.57-59
It has been reported that injection of high doses of soluble SLF does not significantly increase the number of HSCs but promotes the migration of HSCs from the bone marrow to the spleen and peripheral blood.60 Recently, we prepared transgenic mice expressingMgf transgenes in the spleen, but Mgf expression was not necessarily accompanied by splenic hematopoiesis (T. Kunisada, H. Tagaya, and H. Yamazaki, unpublished observation).61 In the present study, although myelopoiesis was promoted in the spleens of osteopetrotic mice, Mgf up-regulation was not observed. All of these findings suggest that Mgf gene expression is not necessarily enhanced during extramedullary hematopoiesis.
Major histocompatibility complex class II promoter-drivenIl7-transgenic mice show extremely elevated B lymphopoiesis in the bone marrow, spleen, liver, and lymph nodes.32 33 This may suggest that insufficiency of IL-7 production in the spleen is a reason for the lack of B lymphopoiesis in the spleens of osteopetrotic mice, because hematopoiesis other than B lymphopoiesis is enhanced in osteopetrotic mice. However, Il7 mRNA is expressed in the spleen as well as liver both in mi/mi and mi/+ mice, indicating that induction of extramedullary hematopoiesis might be regulated by factors other than those tested here.
An alternative explanation for the extramedullary hematopoiesis is that not only bone marrow but also spleen and liver constitutively produce these factors sufficiently to support adult hematopoiesis, and distribution of HSCs is a crucial requirement in this process. Because HSCs in osteopetrotic mice rarely migrate into and lodge in the bone marrow, mainly due to the lack of adequate space, HSCs could only be retained in the extramedullary organs.62-68
Recently, analyses of knock-out mice targeting the genes for the chemokine SDF-1 or its receptor, CXCR4, have shown that HSCs or immature cells, especially B lineage cells, require signaling via CXCR4 for migration into and retention in the bone marrow69,70; TEL/ETV6 transcription factor null mice show virtually the same phenotypes.71 The possibility remains that SDF-1 production may be insufficient in the osteopetrotic marrow, and therefore immature B lineage cells selectively leave the bone marrow.62However, because our experiments did not detect the differences ofSdf-1 messages between mi/mi and mi/+ mice (data not shown), other explanations will have to be found for the defect of B lymphopoiesis in the osteopetrotic marrow.
Il7-transgenic mice have also been reported to display accelerated bone resorption, resulting in an increase of the bone marrow cavity.33 Because B lineage cells are known to produce osteoclast differentiation factor (also named osteoprotegerin ligand, TRANCE, and RANKL), which induces osteoclastogenesis in the presence of M-CSF,72-75 it is possible that the B lineage cells themselves control osteoclast development and regulate the volume of the bone marrow cavity.51,76 77
Lastly, it is still unclear why only Fos (−/−) spleen is able to support CFU–IL-7 generation. Unknown critical molecules for B lymphopoiesis might be activated when c-Fos transcription factor is deleted. In summary, osteopetrotic mice should provide us opportunities to elucidate molecular and structural requirements for intramedullary and extramedullary hematopoiesis.
Acknowledgments
We thank Dr Paul W. Kincade (Oklahoma Medical Research Foundation), Dr Shin-Ichi Nishikawa, Dr Minetaro Ogawa, Dr Koichi Ikuta (Kyoto University), Dr Seiji Okada (Chiba University), Dr Toru Naskano (Osaka University), and Dr Shumpei Niida (Hiroshima University) for helpful suggestions and generous gifts of reagents. We also thank Dr Toshiyuki Shibahara and Dr Takashi Iwaki for maintenance of the mice and Ms Toshie Shinohara for secretarial assistance.
Supported by grants from the Ministry of Education, Science, Sports and Culture in Japan; the Ministry of Science and Technology of Japan; the Cellular Technology Institute; Otsuka Pharmaceutical Co, Ltd; Osaka Foundation for Promotion of Clinical Immunology; and NIH grant CA20 408 (L.D.S.). T. Yamane is a research fellow of the Japan Society for the Promotion of Science.
Reprints:Shin-Ichi Hayashi, Department of Immunology, School of Life Science, Faculty of Medicine, Tottori University, 86 Nishi-machi, Yonago, Tottori 683-8503, Japan; e-mail:shayashi@grape.med.tottori-u.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal