Abstract
A key feature of maturation of dendritic cells is the down-regulation of antigen-processing and up-regulation of immunostimulatory capacities. To study the differential expression of transcription factors in this process, we investigated the nuclear translocation and DNA binding of Rel/NF-κB and octamer factors during in vitro generation and maturation of dendritic cells compared with macrophage development. RelB was the only factor strongly up-regulated during the generation of both immature dendritic cells and macrophages. Cytokine-induced maturation of dendritic cells resulted in an increase in nuclear RelB, p50, p52, and especially c-Rel, whereas cytokine-treated macrophages responded poorly. This up-regulation of NF-κB factors did not correlate with lower levels of cytosolic NF-κB inhibitors, the IκBs. One IκB, Bcl-3, was strongly expressed only in mature dendritic cells. Furthermore, generation and maturation of dendritic cells led to a continuous down-regulation of the octamer factor Oct-2, whereas monocytes and macrophages displayed high Oct-2 levels. A similar pattern of maturation-induced changes in transcription factor levels was found in cultured murine epidermal Langerhans cells, suggesting a general physiological significance of these findings. Finally, this pattern of differential activation of Rel and octamer factors appears to be suitable in determining the maturation stage of dendritic cells generated by treatment with different cytokine combinations in vitro. (Blood. 2000;95:277-285)
Dendritic cells constitute a system of antigen-presenting cells that control immunity by interacting with lymphocytes.1 Classical myeloid-derived dendritic cells are specialized in several ways to specifically activate primary T cell responses.2 Immature dendritic cells, such as epidermal Langerhans cells, develop from hematopoietic precursors and reside as sentinels at body surfaces and interstitial spaces. They are equipped to capture antigens and to produce immunogenic major histocompatibility complex [MHC]–peptide complexes. In the presence of maturation-inducing stimuli, such as inflammatory cytokines, dendritic cells develop into potent T cell stimulators by up-regulating adhesion and costimulatory molecules. Furthermore, they migrate into secondary lymphoid organs to select and stimulate rare antigen-specific T cells. This scenario of cascadelike changes in functional capacities was first elucidated with the use of Langerhans cells as a model system,3 but was later recognized as a principle feature of dendritic cells derived from different organs.4
Distinct alterations in gene expression of dendritic cells are a prerequisite for the maturation process. However, little is known about dendritic cell–specific gene regulation and signal transduction. The family of Rel/NF-κB transcription factors plays a pivotal role in the regulation of immunological processes, as demonstrated by the phenotype of several Rel/IκB-deficient mice.5-8 RelB-deficient mice show a multifocal and mixed infiltration of inflammatory cells in several tissues and impaired cellular immunity. These animals also exhibit a dramatically reduced number of dendritic cells in the thymus,5,6 recently found to be due to a selective loss of myeloid-related, but not of lymphoid-related, dendritic cells.9 In a few studies, high expression of Rel proteins was detected mainly in mature dendritic cells.10-13 In the developing mouse, the highest RelB levels are found in interdigitating dendritic cells of thymic medulla and the deep cortex of lymph nodes.14 In addition, RelB expression correlates with the activation of their antigen-presenting capacity, a property of mature dendritic cells.11
NF-κB is a dimer composed of virtually any of the 5 mammalian Rel proteins (p65/RelA, c-Rel, RelB, p50/NF-κB1, and p52/NF-κB2), which are structurally characterized by the Rel homology domain.15-17 The Rel homology domain mediates important functions, such as specific DNA binding, nuclear translocation, and protein-protein interactions. The nuclear translocation and activity of NF-κB factors is controlled by a family of cytoplasmic inhibitory proteins, the IκBs.18,19 The IκBs interact with NF-κB dimers, thereby blocking their nuclear translocation. The individual IκBs differ in their affinities for various NF-κB dimers and in their inducible degradation.20 Many external stimuli lead to a site-specific phosphorylation of IκBs followed by their proteolytic degradation and release of active NF-κB, which is then translocated into the nucleus.21 22
The genes controlled by NF-κB factors encode proteins of principal importance for the immune system, such as MHC I and II molecules, cytokines and their receptors (eg, interleukin[IL]-2 and the α-chain of the IL-2R), or cell adhesion molecules, such as the intercellular cell adhesion molecule 1.15,19 Most of these proteins are also expressed in dendritic cells and markedly up-regulated when dendritic cells switch from an antigen-capturing to an antigen-presenting mode.23
Octamer factors are another class of transcription factors that play a central role in the immune system. The octamer motif was originally identified as a regulatory promoter element necessary for B cell–specific expression of immunoglobulin (Ig) genes.24,25 Oct-1 and Oct-2 are the 2 most prominent octamer factors. Whereas Oct-1 is expressed ubiquitously, Oct-2 expression is restricted largely to B lymphocytes.24-26Oct-2 appears to be involved in the late phases of B cell development and the proliferation of mature B cells.27 28 However, nothing is known about its role in the development of macrophages or maturation of dendritic cells.
To elucidate the expression of Rel/NF-κB and octamer factors during the generation and maturation of dendritic cells, we studied the nuclear appearance and DNA binding of these factors in human and murine dendritic cells and macrophages. We demonstrate that in both monocyte-derived dendritic cells and macrophages, the expression of Rel/NF-κB and octamer transcription factors is differentially regulated and that expression patterns are found specific for the developmental states of dendritic cells and macrophages.
Materials and methods
Cell culture and preparation of human dendritic cells, macrophages, and murine Langerhans cells
Human dendritic cells were prepared from peripheral blood monocytes according to Romani et al.29 For maturation of dendritic cells, cells were cultured either in monocyte-conditioned medium29 or in a cytokine cocktail of IL-1α (500U/ml), IL-6, tumor necrosis factor[TNF]–α, IL-1β (all 1000U/ml; Strathmann Biotech, Hannover, Germany) and PGE2(10-8M; Sigma, Deisenhofen, Germany)30 for 3 days. The yield of dendritic cells was normally between 5% and 10% of the initially processed PBMC, with an average purity higher than 80%. For preparation of macrophages, CD14[+]cells were isolated by adherence of monocytes (5 × 107/10cm2) to cell culture dishes in R0 medium (RPMI 1640/ 2mM L-glutamine/ 50 μg/ml gentamicin) containing 1% autologous plasma. The nonadherent cells were removed by 2 × gentle washing with warm PBS. Adherent monocytes were cultured for 7 days in X-VIVO-15 medium (BioWhittaker, Walkersville, MD) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 50 μg/ml gentamicin, 100 U/ml granulocyte-macrophage-stimulating factor (GM-CSF) and 10 U/ml M-CSF. The adherent cells were collected and analyzed by flow cytometry for the expression of Mph-specific markers (CD14, C36, CD64, CD68) and the absence of dendritic cell markers (CD1a, CD83). Epidermal cells were prepared from the ear skin of mice according to Schuler and Koch.31 Fresh Langerhans cells (day 0) were prepared from epidermal cells by mismatched panning31 with α-class II antibodies (Abs) (α—I-E: 14-4-4S and α—I-A: MK-D6, both from American Type Culture Collection). Alternatively, trypsinized epidermal cells were first cultured in R0 medium with 10% FCS and with 1000U/ml muGM-CSF for 1 day prior to mismatched panning of Langerhans cells or cultured for 3 days and then enriched by density centrifugation with the use of a dense bovine serum albumin–gradient. The purity of each Langerhans cell preparation was between 80% and 95% as assessed by fluorescence-activated cell sorter staining.
Antibodies for immunodetection and electrophoretic mobility shift assays (EMSAs)
The following polyclonal antibodies were used for immunodetection: α–p65-NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA, sc-109), α–c-Rel (sc-070), α–RelB (sc-226), α–p50-NF-κB (Dr. N. Rice, NCI, Frederick, MD, Ab 1141), α–p52-NF-κB (Upstate Biotechnology, Lake Placid, NY, Ab 06-413), α–κBα (sc-371), α–IκBβ (sc-945), α–IκBε (Dr. N. Rice; Ab 891 & 1775), α–Bcl-3 (sc-185), α–Oct-1 (Dr. T. Wirth, Würzburg, Germany) and α–Oct-2 (sc-233). For supershift EMSAs, 2 different antibodies were used: α–p50-NF-κB (sc-114) and α–Oct-1 (sc-232).
Flow cytometry
Flow cytometry was used to assess the differentiation of monocyte precursors into dendritic cells or macrophages. Usually, 105 cells in 100 μl PBS/1% bovine serum albumin were incubated with 1 μg primary antibody for 30 minutes on ice, washed once, and then incubated with the fluorescein isothiocyanate (FITC)– or phosphatidylethanolamine (PE)–coupled secondary antibody under the same conditions. The antibodies used were CD1a (OKT6), α–HLA class II (L243 and 9.3F10, all American Type Culture Collection, Rockville, MD); CD14, CD25, CD64, CD86 (IT2.2) (all Pharmingen, Hamburg, Germany); CD68 (KP1, Dako, Glostrup, Denmark); CD83 (Immunotech, Hamburg, Germany); CD115 (3-4A4-E4, Calbiochem, Bad Soden, Germany); CD36 (mAb 89, Serotec/Camon, Wiesbaden, Germany); and FITC- or PE-conjugated α–mouse or α– rat Ig (Dianova, Hamburg, Germany). The stained cells were analyzed on a florescence-activated cell scan (Becton Dickinson, Heidelberg, Germany).
Preparation of protein extracts and immunoblotting
Nuclear and cytoplasmic protein extracts from all cells were prepared according to Schreiber et al33 with slight modifications as described.34 Protein concentrations were determined by a Bradford assay.35 For immunoblot assays, 6 to 10 μg of protein extract were separated on 8% or 10% sodium dodecyl sulfate–polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes. Equal loading was confirmed by Ponceau S staining (Sigma, St. Louis, MO). The purity of the nuclear protein extracts was controlled by immunoblotting with the use of the p50-NF-κB-specific antibody also detecting the p105 precursor exclusively located in the cytoplasm. The cytoplasmic extracts were checked for contaminating nuclear proteins by immunoblotting with the use of the Oct-2-specific antibody. Oct-2 is found only in the nucleus.
EMSAs and supershift EMSAs
For each EMSA, 2 to 4 μg nuclear protein extracts were incubated with 6000 cpm (equivalent to approximately 0.25 ng) of a32P-labeled oligonucleotide probe. The reaction mixtures contained 1 μg poly (dIdC) per 4 μg nuclear protein as nonspecific competitor. As a control for specificity of DNA binding, a 50 mol/L excess of an unlabeled κB-specific probe was added to nuclear proteins. The samples were incubated for 30 minutes on ice and fractionated on a nondenaturing 6% polyacrylamide gel at 200 V/15 cm. One μl of each of the Rel- and octamer-specific antibodies was added in supershift EMSA. The following oligonucleotide probes were used for EMSAs: Consensus NF-κB binding site derived from κB enhancer element of the IL-2 promoter ('TCEdA > C')36: 5′-GACCAAGAGGGATTTCACCCCTAAATC-3′. Consensus octamer site from the immunoglobulin heavy chain enhancer37: 5′-AGCAGAAATGCAAATTATACCCG-3′
Results
Differentiation of monocytes into dendritic cells and macrophages
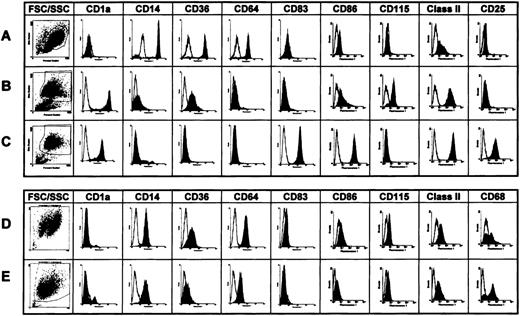
To identify molecular events involved in dendritic cell differentiation, we used the established GM-CSF/IL-4 protocol for generation of immature dendritic cells from monocytes (Figure 1A). This resulted in a population of nonadherent, highly veiled, strongly CD1a and class II positive and CD14, CD64 negative cells after a 7-day culture (Figure 1B). Treatment of these cells for 3 days with monocyte-conditioned medium induced the typical phenotype of mature dendritic cells characterized by up-regulated CD83, CD25 (IL-2Rα-chain), and the costimulatory molecule CD86 (Figure 4C). In addition, monocyte-conditioned medium decreased, by a factor of 5 to 10, the number of dendritic cells required to stimulate maximal T cell proliferation in the mixed leukocyte reaction (data not shown). Alternatively, culture of monocytes in X-VIVO-15 medium with low doses of GM-CSF and M-CSF resulted in the generation of adherent CD1a−, CD14+, and CD64+ macrophages (Figure 1D) with low T cell stimulatory capacity (data not shown). Treatment of these cells with monocyte-conditioned medium led to enhanced expression of the Mph marker CD68 (Figure 1E), but not of costimulatory molecules or CD83.
Phenotypes of human monocytes and of human dendritic cells and macrophages generated in vitro.
The expression of cell surface proteins characteristic for dendritic cells (CD1a, MHC class II, CD83, CD115) and macrophages (CD14, CD36, CD64, CD68), and dendritic cell maturation (CD86, CD25) were investigated by (A) fluorescence-activated cell sorter analysis of monocytes, (B) immature dendritic cells, (C) mature dendritic cells, (D) macrophages (on day 7), and (E) monocyte-conditioned medium–treated macrophages. Cells were generated in vitro and florescence-activated cell sorter analyses performed as described in Materials and Methods. Dot plots: Forward scatter on the x-axis and side scatter on the y-axis. Histograms: Fluorescence intensity in a logarithmic scale (x-axis) was blotted against cell numbers (y-axis).
Phenotypes of human monocytes and of human dendritic cells and macrophages generated in vitro.
The expression of cell surface proteins characteristic for dendritic cells (CD1a, MHC class II, CD83, CD115) and macrophages (CD14, CD36, CD64, CD68), and dendritic cell maturation (CD86, CD25) were investigated by (A) fluorescence-activated cell sorter analysis of monocytes, (B) immature dendritic cells, (C) mature dendritic cells, (D) macrophages (on day 7), and (E) monocyte-conditioned medium–treated macrophages. Cells were generated in vitro and florescence-activated cell sorter analyses performed as described in Materials and Methods. Dot plots: Forward scatter on the x-axis and side scatter on the y-axis. Histograms: Fluorescence intensity in a logarithmic scale (x-axis) was blotted against cell numbers (y-axis).
Generation of immature dendritic cells and macrophages leads to the specific accumulation of nuclear RelB
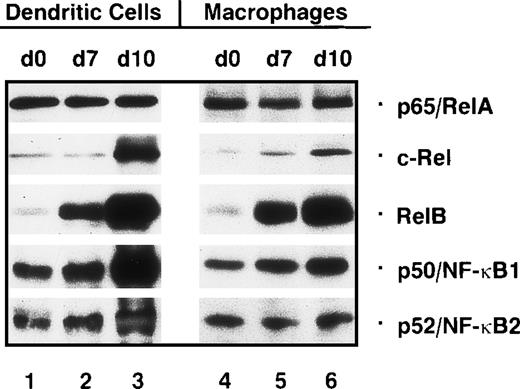
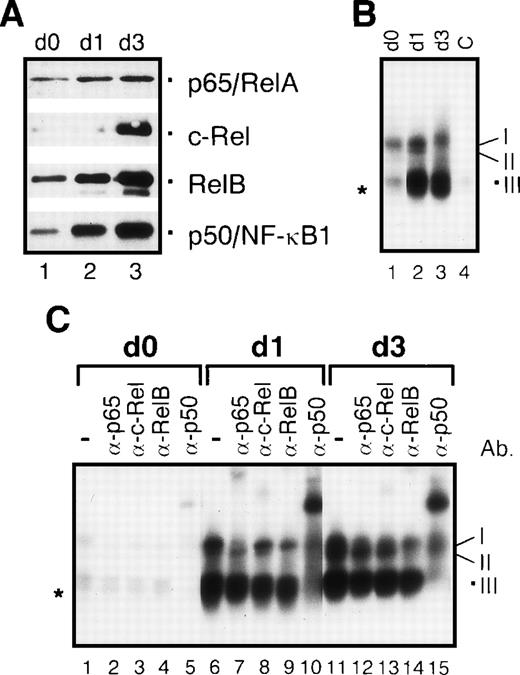
We first wanted to know whether the concentrations of nuclear Rel proteins change during the generation of immature dendritic cells from monocytes. In immunoblot assays, using nuclear protein extracts from monocyte precursors (day 0; Figure 2, lane 1) and immature dendritic cells (day 7; Figure 2, lane 2) the expression of p65/RelA, c-Rel, p50/NF-κB1 and p52/NF-κB2 was found to be similar in monocytes and immature dendritic cells. In contrast, a striking increase in the expression of nuclear RelB was observed in immature dendritic cells. A similar increase in the nuclear concentrations of RelB was also detected in macrophages. The pattern of other nuclear Rel/NF-κB proteins was also very similar, if not identical to that found in immature dendritic cells (Figure 2, lanes 4-6).
Nuclear Rel proteins are differentially expressed during generation and maturation of human dendritic cells and development of macrophages in vitro.
Immunoblots were performed with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), mature dendritic cells (day 10, lane 3), differentiated macrophages (day 7, lane 5), and macrophages treated with monocyte-conditioned medium for another 3 days (day 10, lane 6). Proteins were separated on an 8% sodium dodecyl sulfate–polyacrylamide gel and electrophoretically transferred to a nitrocellulose membrane.
Nuclear Rel proteins are differentially expressed during generation and maturation of human dendritic cells and development of macrophages in vitro.
Immunoblots were performed with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), mature dendritic cells (day 10, lane 3), differentiated macrophages (day 7, lane 5), and macrophages treated with monocyte-conditioned medium for another 3 days (day 10, lane 6). Proteins were separated on an 8% sodium dodecyl sulfate–polyacrylamide gel and electrophoretically transferred to a nitrocellulose membrane.
Maturation of dendritic cells by cytokines results in a specific nuclear accumulation of c-Rel
Monocyte-conditioned medium–induced maturation of immature dendritic cells resulted in nuclear accumulation of p50, p52, and RelB detected by immunoblot analysis. Again, p65 remained unchanged, whereas the strongest up-regulation was observed for c-Rel (Figure 2, lanes 2 and 3). In contrast, treatment of macrophages with monocyte-conditioned medium resulted in only a moderate increase in nuclear p50, p52, RelB, and c-Rel levels (Figure 2, lanes 5 and 6).
Increased nuclear translocation of Rel proteins during the generation of dendritic cells and macrophages also results in an increase in DNA binding
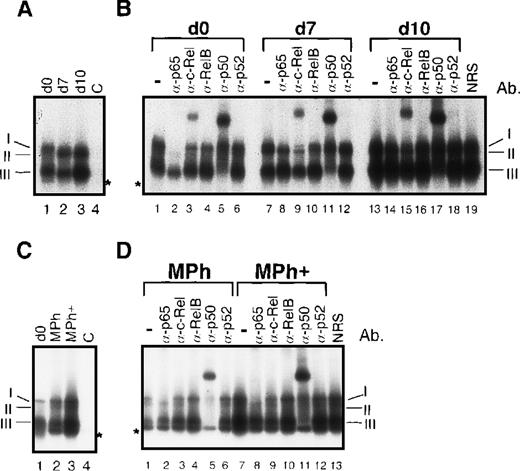
To determine whether the enhanced nuclear concentration of Rel proteins also results in an enhanced DNA binding, we studied the DNA binding of nuclear Rel proteins from dendritic cells and macrophages by EMSAs and supershift EMSAs. A strong increase in DNA binding activity was observed after induction of dendritic cell maturation (Figure3A, lane 3), resulting in 2 major DNA/protein bands. Both consisted of 2 complexes. The slower migrating band consisted of p65/RelA-containing heterodimers (complex I) and RelB-containing heterodimers (complex II) as detected by supershift EMSAs (Figure 3B). Complex I is composed of p65/p50 and p65/c-Rel heterodimers (Figure 3B; eg, see lanes 2, 3, and 5). The ‘classical’ NF-κB complex, p65/p50, is best seen after supershift with the α-c-Rel-specific antibody (Figure 3B, lane 9). The second, slightly faster, migrating complex visible after c-Rel supershifting represents the RelB/p50 heterodimer (complex II) (Figure3B, lane 9). Although no supershift signal was detected with the use of a RelB-specific antibody (Figure 3B, lanes 10 and 16), complex II disappeared after addition of α-RelB, clearly indicating the presence of RelB. This is best recognizable by a comparison of lane 9 with lane 10 of figure 3B. The RelB/p50 dimer of lane 9 was no longer visible as a distinct band in lane 10. Here only the p65 heterodimers with a slightly lower electrophoretic mobility were detectable. RelB forms heterodimers not only with p50 (Figure 3B, lanes 11 and 17) but also with c-Rel (Figure 3B, lanes 9 and 15). It should be noted that complex II is visible only in immature (day 7) and mature dendritic cells (day 10) but not in peripheral blood monocytes (day 0) (Figure 3A; compare lane 1 with lanes 2 and 3). A specific supershift signal was observed in dendritic cells with the use of the c-Rel-specific antibody (Figure3B, lanes 3, 9 and 15). This signal distinctly increased during dendritic cell maturation (Figure 3B; compare lanes 9 and 15). The band with the highest electrophoretic mobility consisted of p50/p50 homodimers (complex III) and of a complex of unknown composition (marked by an asterisk in Figure 3).
Increased nuclear translocation of Rel proteins during in vitro generation of human dendritic cells and macrophages results in an increase in κB-specific DNA binding activity.
(A) EMSAs performed with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), and mature dendritic cells (day 10, lane 3). As a control for specificity of DNA binding, an excess of an unlabeled κB-specific probe (C, lane 4) was added to nuclear proteins from monocytes. The 3 detectable κB-specific DNA binding complexes are designated I through III (see below). The asterisk indicates a complex of unknown composition. The signals of free probes were cut off. (B) Supershift EMSAs were performed with nuclear proteins from monocytes (day 0, lanes 1-6), immature dendritic cells (day 7, lanes 7-12), and mature dendritic cells (day 10, lanes 13-19). For supershift EMSAs, 1 μl of each of the Rel protein–specific antibodies was added to the extracts as indicated (lanes 2-6, 8-12, and 14-18). As a control for the specificity of supershifts, 1 μl of normal rabbit serum (NRS) was added to nuclear extracts from mature dendritic cell (lane 19). The 3 indicated κB-specific DNA complexes are composed of p50 homodimers (III) and RelB/p50 and RelB/c-Rel heterodimers (II). Complex I consists of 2 NF-κB complexes: p65/p50 and p65/c-Rel heterodimers. The asterisk indicates a complex of unknown composition. (C) EMSAs using nuclear proteins from monocytes (day 0, lane 1), differentiated macrophages (lane 2), and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lane 3). The specificity of DNA binding was controlled by addition of an excess of unlabeled κB-specific probe to nuclear extracts from monocytes (C, lane 4). (D) Supershift EMSAs using nuclear proteins from macrophages (lanes 1-6) and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lanes 7-13).
Increased nuclear translocation of Rel proteins during in vitro generation of human dendritic cells and macrophages results in an increase in κB-specific DNA binding activity.
(A) EMSAs performed with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), and mature dendritic cells (day 10, lane 3). As a control for specificity of DNA binding, an excess of an unlabeled κB-specific probe (C, lane 4) was added to nuclear proteins from monocytes. The 3 detectable κB-specific DNA binding complexes are designated I through III (see below). The asterisk indicates a complex of unknown composition. The signals of free probes were cut off. (B) Supershift EMSAs were performed with nuclear proteins from monocytes (day 0, lanes 1-6), immature dendritic cells (day 7, lanes 7-12), and mature dendritic cells (day 10, lanes 13-19). For supershift EMSAs, 1 μl of each of the Rel protein–specific antibodies was added to the extracts as indicated (lanes 2-6, 8-12, and 14-18). As a control for the specificity of supershifts, 1 μl of normal rabbit serum (NRS) was added to nuclear extracts from mature dendritic cell (lane 19). The 3 indicated κB-specific DNA complexes are composed of p50 homodimers (III) and RelB/p50 and RelB/c-Rel heterodimers (II). Complex I consists of 2 NF-κB complexes: p65/p50 and p65/c-Rel heterodimers. The asterisk indicates a complex of unknown composition. (C) EMSAs using nuclear proteins from monocytes (day 0, lane 1), differentiated macrophages (lane 2), and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lane 3). The specificity of DNA binding was controlled by addition of an excess of unlabeled κB-specific probe to nuclear extracts from monocytes (C, lane 4). (D) Supershift EMSAs using nuclear proteins from macrophages (lanes 1-6) and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lanes 7-13).
The differentiation of macrophages was also accompanied by a moderate up-regulation of κB-specific DNA binding activity (Figure 3C, lanes 1 and 2), and a further increase was observed after monocyte-conditioned medium treatment (Figure 3C, lanes 2 and 3). As in mature dendritic cells, a RelB-containing binding complex (II) was detected in fully differentiated macrophages (Figure 3C, lanes 2 and 3; Figure 3D lanes 4 and 10). The patterns of supershifts performed with Mph extracts revealed a composition of 4 complexes, very similar to those in mature dendritic cells (compare Figures 3B and 3D). However, a c-Rel-specific supershift signal was observed only after longer exposures of the films, indicating a reduced binding activity of c-Rel in macrophages compared with dendritic cells.
Maturation of dendritic cells correlates with an increase in IκBα, IκBε, and Bcl-3
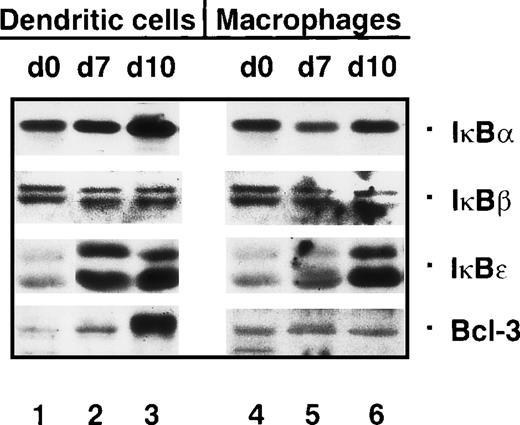
The inducible degradation of IκBs is crucial for NF-κB activation. Therefore, we investigated whether the strong increase in the nuclear concentrations of Rel proteins during dendritic cell maturation might be mediated by a sustained degradation of one or several IκBs. However, immunoblot analysis of cytoplasmic proteins showed that this is not the case. The concentrations of IκBs either remained constant (IκBβ) or were increased (IκBα Ικßε, and Bcl-3) during the maturation of dendritic cells (Figure 4, lanes 1-3). In particular, this was observed for Bcl-3, whose expression was strongly up-regulated in mature dendritic cells. IκBε, detected as 2 isoforms,38 was found to be poorly expressed in monocytes (Figure 4, lane 1), but more strongly expressed in immature and mature dendritic cells (Figure 4, lanes 2 and 3).
The concentration of Bcl-3 is strongly increased in mature dendritic cells.
Immunoblots with cytoplasmic proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), mature dendritic cells (day 10, lane 3), macrophages (day 7, lane 5), and macrophages treated with monocyte-conditioned medium for another 3 days (day 10, lane 6).
The concentration of Bcl-3 is strongly increased in mature dendritic cells.
Immunoblots with cytoplasmic proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), mature dendritic cells (day 10, lane 3), macrophages (day 7, lane 5), and macrophages treated with monocyte-conditioned medium for another 3 days (day 10, lane 6).
In vitro–generated macrophages showed a small decrease in IκBα and IκBβ compared with monocytes (Figure 4, lanes 4 and 5), while IκBα levels were slightly up-regulated following monocyte-conditioned medium treatment (Figure 4, lane 6). IκBε was relatively weakly expressed in macrophages and was up-regulated upon monocyte-conditioned medium treatment. In contrast to the strong induction of Bcl-3 in mature dendritic cells, monocyte-conditioned medium treatment did not induce Bcl-3 in macrophages (Figure 4, lane 6).
Interestingly, immunoblot analysis of cytoplasmic protein extracts performed in parallel showed that the total cellular concentration of Rel proteins was up-regulated (data not shown) during generation of dendritic cells and macrophages. Thus the increase of active, nuclear Rel protein is due principally to an enhanced expression of these transcription factors and not to a sustained degradation of IκBs.
Differential expression of Rel proteins during maturation of epidermal mouse Langerhans cells corresponds to the expression pattern in human dendritic cells
To confirm the data obtained for the human dendritic cells generated and maturated in vitro, we analyzed mouse epidermal Langerhans cells representing the “gold standard” of a dendritic cell maturation system. Freshly prepared murine Langerhans cells correspond in their differentiation stage to immature human dendritic cells. Immunoblot analyses using highly enriched, freshly explanted Langerhans cells showed a strong expression of RelB, p65, and p50, but not c-Rel (Figure5A, lane 1). This pattern corresponds to that of immature human dendritic cells. Maturation of Langerhans cells by GM-CSF induced strong c-Rel expression, a gradual increase in the concentrations of RelB and p50, and a slight increase in p65, very similar to the increase in Rel proteins during human dendritic cell maturation (Figure 5A, lane 3). In contrast to human dendritic cell, we were unable to detect p52 at any stage of murine Langerhans cell maturation. However, the pattern of κB-specific DNA binding complexes in Langerhans cells at defined stages of maturation was comparable to those obtained for human dendritic cells (Figures 5B and C; compare with Figures 3A and B). Separate control experiments using normal rabbit serum were also performed (data not shown). No unspecific supershift signals were observed in these experiments.
Changes of nuclear Rel proteins during maturation of epidermal mouse Langerhans cells correspond to those in human dendritic cells.
(A) Immunoblots of nuclear proteins from freshly explanted Langerhans cells (immature phenotype, day 0, lane 1), Langerhans cells cultured for 24 hours (intermediate phenotype, day 1, lane 2), and maturated Langerhans cells (mature phenotype, day 3, lane 3). (B) EMSAs performed with nuclear proteins. The protein extracts were prepared from freshly explanted Langerhans cells (day 0, lane 1), Langerhans cells cultured for 24 hours (day 1, lane 2), and mature Langerhans cells cultured for 3 days (day 3, lane 3). As a control for DNA binding specificity, an excess of unlabeled κB-specific probe was added to nuclear extracts from mature Langerhans cells (C, lane 4). The 3 detectable κB-specific DNA binding complexes are designated I-III (see below). The asterisk indicates an additional complex of unknown composition. (C) Supershift EMSAs with nuclear proteins from freshly explanted Langerhans cells (day 0, lanes 1-5), Langerhans cells cultured for 24 hours (day 1, lanes 6-10), and mature Langerhans cells after 3 days in culture (day 3, lanes 11-15). For supershift EMSAs, 1 μl of each of the Rel protein–specific antibodies was added to the extracts as indicated. The 3 detectable κB-specific DNA complexes are composed of p50 homodimers (III), RelB/p50 heterodimers (II), and p65/p50 and, probably, p65/c-Rel heterodimers (I).
Changes of nuclear Rel proteins during maturation of epidermal mouse Langerhans cells correspond to those in human dendritic cells.
(A) Immunoblots of nuclear proteins from freshly explanted Langerhans cells (immature phenotype, day 0, lane 1), Langerhans cells cultured for 24 hours (intermediate phenotype, day 1, lane 2), and maturated Langerhans cells (mature phenotype, day 3, lane 3). (B) EMSAs performed with nuclear proteins. The protein extracts were prepared from freshly explanted Langerhans cells (day 0, lane 1), Langerhans cells cultured for 24 hours (day 1, lane 2), and mature Langerhans cells cultured for 3 days (day 3, lane 3). As a control for DNA binding specificity, an excess of unlabeled κB-specific probe was added to nuclear extracts from mature Langerhans cells (C, lane 4). The 3 detectable κB-specific DNA binding complexes are designated I-III (see below). The asterisk indicates an additional complex of unknown composition. (C) Supershift EMSAs with nuclear proteins from freshly explanted Langerhans cells (day 0, lanes 1-5), Langerhans cells cultured for 24 hours (day 1, lanes 6-10), and mature Langerhans cells after 3 days in culture (day 3, lanes 11-15). For supershift EMSAs, 1 μl of each of the Rel protein–specific antibodies was added to the extracts as indicated. The 3 detectable κB-specific DNA complexes are composed of p50 homodimers (III), RelB/p50 heterodimers (II), and p65/p50 and, probably, p65/c-Rel heterodimers (I).
Octamer factors are also differentially expressed during generation and maturation of human dendritic cells
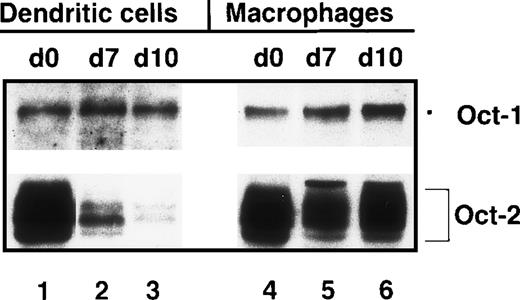
In addition to Rel/NF-κB factors, we investigated several other transcription factors, such as AP-1, NF-AT, and octamer factors, for their expression during the generation and maturation of dendritic cells and differentiation of macrophages. In contrast to AP-1 and NF-AT factors, whose concentrations remained unchanged during these processes (data not shown), the octamer factors displayed a differential expression. While the concentration of Oct-1 remained almost constant, a strong, gradual down-regulation of Oct-2, which is highly expressed in monocytes, was observed in dendritic cells (Figure6, lanes 1-3). This down-regulation started as early as day 3 after GM-CSF/IL-4 treatment of monocytes (data not shown). All different isoforms of Oct-239 were found to be down-regulated (Figure 6, lanes 2 and 3). The maturation of dendritic cells resulted in an almost complete loss of Oct-2 (Figure 6, lane 3). This loss of Oct-2 expression is accompanied by a down-regulation of the cell surface molecule CD36 (see Figure 1). The CD36 gene has been shown to be mainly controlled by Oct-2 in murine B cells and some Mph cell lines.40
Down-regulation of Oct-2 expression during in vitro differentiation of human dendritic cells but not macrophages.
Immunoblots with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), mature dendritic cells (day 10, lane 3), macrophages (day 7, lane 5), and macrophages treated with monocyte-conditioned medium for another 3 days (day 10, lane 6).
Down-regulation of Oct-2 expression during in vitro differentiation of human dendritic cells but not macrophages.
Immunoblots with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), mature dendritic cells (day 10, lane 3), macrophages (day 7, lane 5), and macrophages treated with monocyte-conditioned medium for another 3 days (day 10, lane 6).
In contrast to the profound down-regulation of Oct-2 during dendritic cell differentiation, Oct-2 was only slightly down-regulated during Mph development (Figure 6, lane 5), and following monocyte-conditioned medium treatment, Oct-2 concentrations even appeared to be up-regulated again (Figure 6, lane 6). Thus, the expression pattern of Oct-2 completely differs during the differentation to dendritic cell and macrophages. Furthermore, contrary to in vitro–generated human dendritic cells, no correlation between Oct-2 and CD36 expression was found during the development of human macrophages.
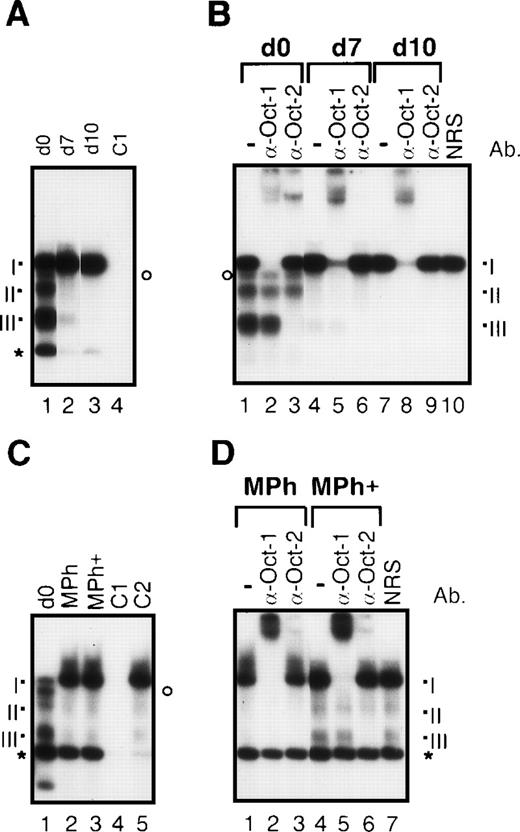
The gradual loss of Oct-2 expression during dendritic cell differentation was also detected at the level of DNA binding (Figure7). Four prominent DNA/protein complexes were detected in EMSAs with the use of an octamer binding site. As shown in supershift EMSAs, the slowest migrating complex I contains Oct-1 (Figure 7B, lanes 2, 5 and 8). The formation of this complex increases slightly during dendritic cell maturation (Figures 7A and 7B) as well as differentiation of macrophages (Figures 7C and 7D). Although only complex III reacted with the Oct-2–specific antibody used in these supershift assays, the generation of complexes II and III (and of a further, less prominent complex that can be seen best after supershifting Oct-1 as in lane 2 of Figure 7B, marked by a circle) is probably due to the binding of different Oct-2 isoforms. The synthesis of multiple Oct-2 isoforms is known to be due to alternative splicing.39 The formation of both Oct-2 complexes disappeared during the generation and maturation of dendritic cells. A fourth prominent complex migrating faster than complex III (indicated by an asterisk in Figure 7) is probably due to an unspecific binding since its formation is suppressed by both an excess of unlabeled octamer- and κB-specific binding probes (see Figures 7A and C, lane 4; and Figure 7C, lane 5). In contradiction to the sustained expression of Oct-2, the intensity Oct-2–specific binding complexes (Figure 7C, lane 1-3) decreases during Mph differentiation, indicating a less efficient DNA binding capacity of Oct-2 in these cells. However, contrary to mature dendritic cells, Oct-2 complexes can clearly be recognized in supershift EMSAs using nuclear proteins from macrophages cultured for 10 days in vitro (Figure 7D, lanes 5-7).
Different octamer-specific DNA binding complexes in human dendritic cells and macrophages.
(A) EMSAs with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), and mature dendritic cells (day 10, lane 3). As a control for specificity of DNA binding, an excess of an unlabeled octamer binding probe was added to nuclear proteins from monocytes (C1, lane 4). The 3 detectable octamer-specific complexes are designated I to III (see below). The asterisk indicates a prominent unspecific band, and the circle marks another unshiftable complex, probably containing Oct-2. As shown by supershift EMSAs (see B), the 3 detectable octamer-specific DNA complexes are composed of Oct-1 (complex I) and different isoforms of Oct-2 (complexes II and III). The signals of the free probe are cut off. (B) Supershift EMSAs with nuclear proteins from monocytes (day 0, lanes 1-3), immature dendritic cells (day 7, lanes 4-6), and mature dendritic cells (day 10, lanes 7-10). For supershift assays, 1 μl of each of the octamer-factor–specific antibodies was added as indicated (lanes 2 + 3, 5 + 6, and 8 + 9). As a control for specificity of the supershifts, 1 μl of NRS was added to nuclear protein from mature dendritic cells (lane 10). (C) EMSAs with nuclear proteins from monocytes (day 0, lane 1), macrophages (lane 2), and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lane 3). The specificity of DNA binding was controlled by addition of an excess of an octamer-specific probe (C1, lane 4) and of a κB-specific probe (C2, lane 5) to nuclear extracts from Mph+ cells. (D) Supershift EMSAs with nuclear proteins from macrophages (lanes 1-3) and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lanes 4-7).
Different octamer-specific DNA binding complexes in human dendritic cells and macrophages.
(A) EMSAs with nuclear proteins from monocytes (day 0, lanes 1 and 4), immature dendritic cells (day 7, lane 2), and mature dendritic cells (day 10, lane 3). As a control for specificity of DNA binding, an excess of an unlabeled octamer binding probe was added to nuclear proteins from monocytes (C1, lane 4). The 3 detectable octamer-specific complexes are designated I to III (see below). The asterisk indicates a prominent unspecific band, and the circle marks another unshiftable complex, probably containing Oct-2. As shown by supershift EMSAs (see B), the 3 detectable octamer-specific DNA complexes are composed of Oct-1 (complex I) and different isoforms of Oct-2 (complexes II and III). The signals of the free probe are cut off. (B) Supershift EMSAs with nuclear proteins from monocytes (day 0, lanes 1-3), immature dendritic cells (day 7, lanes 4-6), and mature dendritic cells (day 10, lanes 7-10). For supershift assays, 1 μl of each of the octamer-factor–specific antibodies was added as indicated (lanes 2 + 3, 5 + 6, and 8 + 9). As a control for specificity of the supershifts, 1 μl of NRS was added to nuclear protein from mature dendritic cells (lane 10). (C) EMSAs with nuclear proteins from monocytes (day 0, lane 1), macrophages (lane 2), and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lane 3). The specificity of DNA binding was controlled by addition of an excess of an octamer-specific probe (C1, lane 4) and of a κB-specific probe (C2, lane 5) to nuclear extracts from Mph+ cells. (D) Supershift EMSAs with nuclear proteins from macrophages (lanes 1-3) and macrophages treated with monocyte-conditioned medium for another 3 days (Mph+, lanes 4-7).
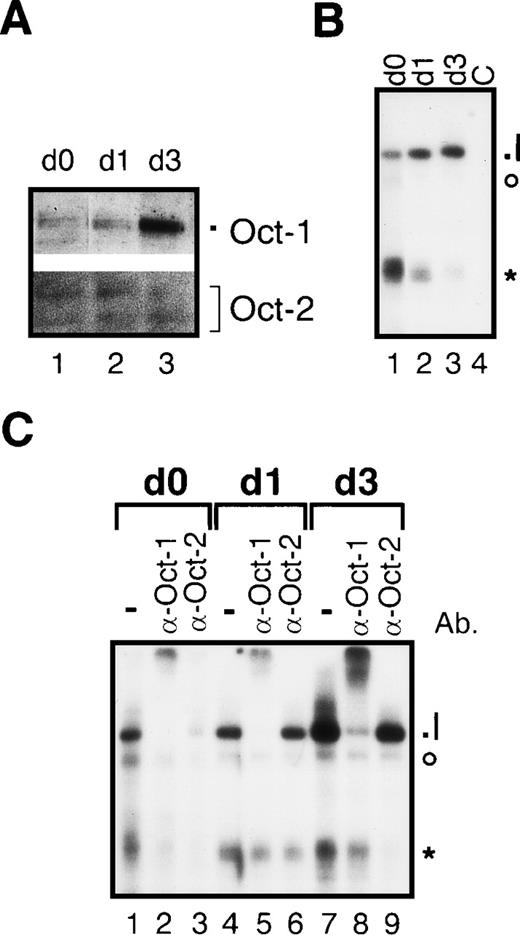
In nuclear protein extracts from freshly prepared mouse Langerhans cells only small amounts of Oct-1 and Oct-2 could be detected by immunoblotting (Figure 8 A, lane 1). Maturation of Langerhans cells resulted in an increase in concentrations of Oct-1 but not Oct-2 (Figure 8A, lane 3). According to the down-regulation of Oct-2 expression in immature dendritic cells, no Oct-2–specific DNA binding complex could be found in Langerhans cells (Figures 8B and 8C). The only detectable specific complex consisted of Oct-1 (complex I), which increased during Langerhans cell maturation (Figures 8B, lanes 1-3, and 8C lanes 2, 5, and 8). Thus, the pattern of Oct-1 and Oct-2 expression found for human dendritic cells is also typical for murine Langerhans cells.
Octamer factor expression in mouse epidermal Langerhans cells.
(A) Immunoblots with nuclear proteins from freshly explanted Langerhans cells (immature phenotype, day 0, lane 1), Langerhans cells cultured for 24 hours (intermediate phenotype, day 1, lane 2), and maturated Langerhans cells (mature phenotype, day 3, lane 3). (B) EMSAs with nuclear proteins from freshly explanted Langerhans cells (day 0, lane 1), Langerhans cells cultured for 24 hours (day 1, lane 2), and mature Langerhans cells cultured for 3 days (day 3, lane 3). As a control for specificity of DNA binding, an excess of unlabeled octamer probe was added to nuclear extract from mature Langerhans cells (C, lane 4). The octamer-specific complex is designated I (see below). The asterisk and the circle indicate prominent unspecific complexes. The signals of the free probe are cut off. (C) Supershift EMSAs performed with nuclear extracts from freshly explanted Langerhans cells (day 0, lanes 1-3), Langerhans cell cultured for 24 hours (day 1, lanes 4-6), and maturated Langerhans cells after 3 days in culture (day 3, lanes 7-9). For supershift EMSA, 1 μl of each of the octamer-factor–specific antibodies was added as indicated (lanes 2 + 3, 5 + 6, and 8 + 9). The detectable octamer-specific DNA complex is composed of Oct-1 (I).
Octamer factor expression in mouse epidermal Langerhans cells.
(A) Immunoblots with nuclear proteins from freshly explanted Langerhans cells (immature phenotype, day 0, lane 1), Langerhans cells cultured for 24 hours (intermediate phenotype, day 1, lane 2), and maturated Langerhans cells (mature phenotype, day 3, lane 3). (B) EMSAs with nuclear proteins from freshly explanted Langerhans cells (day 0, lane 1), Langerhans cells cultured for 24 hours (day 1, lane 2), and mature Langerhans cells cultured for 3 days (day 3, lane 3). As a control for specificity of DNA binding, an excess of unlabeled octamer probe was added to nuclear extract from mature Langerhans cells (C, lane 4). The octamer-specific complex is designated I (see below). The asterisk and the circle indicate prominent unspecific complexes. The signals of the free probe are cut off. (C) Supershift EMSAs performed with nuclear extracts from freshly explanted Langerhans cells (day 0, lanes 1-3), Langerhans cell cultured for 24 hours (day 1, lanes 4-6), and maturated Langerhans cells after 3 days in culture (day 3, lanes 7-9). For supershift EMSA, 1 μl of each of the octamer-factor–specific antibodies was added as indicated (lanes 2 + 3, 5 + 6, and 8 + 9). The detectable octamer-specific DNA complex is composed of Oct-1 (I).
Differential expression of Rel and octamer factors reflects the maturation stage of dendritic cells generated after treatment with various stimuli
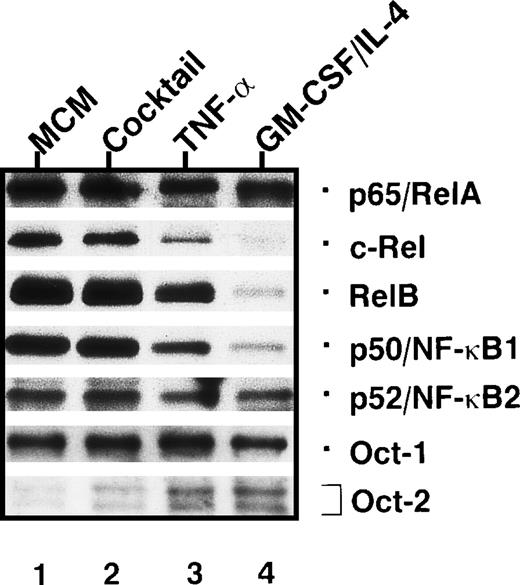
For maturation of dendritic cells in vitro, several protocols have been applied, including different cytokines and/or maturation-promoting media. To determine the maturation status of human dendritic cells generated by different stimuli, we used the expression of Rel and octamer factors as a marker. In these experiments, the dendritic cells were grown in media containing human plasma instead of fetal calf serum because fetal calf serum often contains components, such as lipopolysaccharide, that induce dendritic cell maturation. The expresssion of Rel and octamer proteins in mature dendritic cells generated by monocyte-conditioned medium treatment (Figure 9, lane 1) was compared with that in dendritic cells whose maturation was induced by a defined cytokine cocktail consisting of IL-1α and β, IL-6, TNF-α and PGE2 (Figure 9, lane 2). Further, these monocyte-conditioned medium-treated dendric cells were compared to dendritic cells treated only with TNF-α (Figure 9, lane 3), and to dendritic cells arrested at the immature state by culture in GM-CSF/IL-4–containing medium (Figure 9, lane 4). The patterns of transcription factors in mature dendritic cells generated with the cytokine cocktail were almost identical to those from dendritic cells generated by monocyte-conditioned medium (Figure 9, lanes 1 and 2). In contrast, TNF-α alone was unable to induce full dendritic cell maturation. Compared with mature dendritic cells generated by monocyte-conditioned medium, TNF-α induced only a minor up-regulation of RelB, p50, and c-Rel typical for mature dendritic cells (Figure 9, lane 3). In addition, significant levels of Oct-2, typical for immature dendritic cells, were detected in dendritic cells treated with TNF-α. Dendritic cells arrested at the immature stage showed a continuous expression of Oct-2 and a poor expression of Rel proteins (Figure 9, lane 4), which was below the Rel protein levels found in immature dendritic cells (Figure 2, lane 2). The low levels of nuclear Rel proteins in maturation-arrested dendritic cells might be due both to a decreased expression and to an enhanced degradation of Rel proteins induced by cell death. This conclusion is supported by an increased apoptosis observed in these cells (data not shown).
Patterns of nuclear Rel/NF-κB and octamer factors detected in dendritic cells after treatment with various stimuli.
Immunoblots of nuclear proteins from immature dendritic cells treated with various stimuli for 3 days. These stimuli either preserve the immature state of dendritic cells (GM-CSF/IL-4) or induce their maturation (all other stimuli). The stimuli used were monocyte-conditioned medium (lane 1); a cytokine cocktail consisting of IL-1α, IL-1β, IL-6, TNF-α, and PGE2 (lane 2); TNF-α (lane 3); and GM-CSF/IL-4 (lane 4).
Patterns of nuclear Rel/NF-κB and octamer factors detected in dendritic cells after treatment with various stimuli.
Immunoblots of nuclear proteins from immature dendritic cells treated with various stimuli for 3 days. These stimuli either preserve the immature state of dendritic cells (GM-CSF/IL-4) or induce their maturation (all other stimuli). The stimuli used were monocyte-conditioned medium (lane 1); a cytokine cocktail consisting of IL-1α, IL-1β, IL-6, TNF-α, and PGE2 (lane 2); TNF-α (lane 3); and GM-CSF/IL-4 (lane 4).
Discussion
Dendritic cells have the unique capacity to switch from antigen-processing to antigen-presenting cells capable of stimulating naive T cells. This process involves specific alterations in gene expression reflecting the differential activation of specific transcription factors. Mice deficient for RelB, a member of the Rel/NF-κB factor family, showed a severe impairment of dendritic cell function.5 6 This prompted us to study the expression of Rel/NF-κB factors during (1) the generation of immature dendritic cells derived from human monocytes and (2) the cytokine-induced maturation of human and mouse dendritic cells.
During the generation of dendritic cells from monocytes, RelB was the only nuclear protein found to be strongly up-regulated, emphasizing a role for RelB during early development of dendritic cells.12 However, we also found a comparable up-regulation of RelB in monocyte-derived macrophages, indicating that up-regulation of RelB is not a property specific to immature dendritic cells. Furthermore, since RelB-deficient mice still possess immature dendritic cells, such as epidermal Langerhans cells, RelB might be replaceable by other transcription factors in the early stages of dendritic cell development. Interestingly, RelB-deficient mice miss mature dendritic cells in lymphoid organs.5,6 This finding indicates a predominant role of Rel/NF-κB factors in the maturation but not the generation of immature dendritic cells. Such a role has been also suggested by another study using a dendritic cell–like murine cell line, demonstrating that NF-κB activation is an important signaling pathway involved in dendritic cell maturation.13
We have shown that all Rel proteins, except p65, were strongly expressed during cytokine-induced human dendritic cell maturation, indicating that the up-regulation of κB-dependent gene expression is pivotal for this process. Surprisingly, c-Rel, which has not been implicated in dendritic cell maturation so far, was found to be drastically up-regulated. This suggests an important role for c-Rel in dendritic cell function, although no obvious defect in cellular immune responses has been reported in c-Rel–deficient mice.41 A similar pattern of increased Rel protein concentrations was also found in mature mouse epidermal Langerhans cells, whereas cytokine-treated macrophages showed only a moderate, or no, increase in Rel factor concentrations. Therefore, the up-regulation of c-Rel, RelB, and p50 expression reflects a specific response of immature dendritic cells toward maturation-inducing cytokines and marks a distinct biological difference to macrophages, a further cell type of the myelo-monocytic lineage.
The increased expression of nuclear NF-κB factors observed in mature dendritic cells seems to be due to enhanced transcription of Rel genes. Instead of distinctly lower steady-state levels of IκB proteins, we reproducibly detected increased levels of IκBα and ε in immature and mature dendritic cells as well as in macrophages, especially after monocyte-conditioned medium treatment. This can be explained by the NF-κB–mediated control of IκB gene expression.15
A strong increase in Bcl-3 concentrations was detected in mature dendritic cells, but not in macrophages. Bcl-3 not only is able to function as an NF-κB-inhibitor, but also exerts (after formation of ternary complexes with p50 and p52) transactivating properties.8,15 Interestingly, Bcl-3–deficient mice display severe defects in T cell–mediated immune responses. The cellular basis of this phenotype is unknown.8 Our observation of selective Bcl-3 up-regulation in mature dendritic cells suggests this factor to be specifically involved in dendritic cell maturation, and a disturbance of this process, as in Bcl-3–deficient mice, might contribute to the observed defects in T cell responses.
In addition to the Rel/NF-κB factors, the activity of octamer factors seems also to be involved in the differentiation of dendritic cells. A strong down-regulation of Oct-2 expression starts rapidly after the onset of monocyte differentiation to immature dendritic cells. Cells cultured for 3 days in the presence of GM-CSF/IL-4 exhibit already much lower levels of Oct-2 than monocytes (data not shown). In contrast, monocyte-derived macrophages show only a moderate down-regulation of Oct-2, indicating a molecular hallmark distinguishing dendritic cells from macrophages.
The decrease in Oct-2 concentration during dendritic cell differentiation correlates with a loss of CD36 expression. CD36 was recently identified as an important receptor for the uptake of apoptotic vesicles and virally infected apoptotic cells by dendritic cells.42,43 The CD36 gene is known to be an Oct-2–specific target gene in murine B cells and some Mph cell lines.40 Our studies suggest that in human dendritic cells the expression of the CD36 gene might also be controlled by Oct-2. However, this assumption is based solely on the observation that Oct-2 expression declines with a comparable kinetics as the expression of CD36 during the development and maturation of dendritic cells. In contrast, no such correlation was notable in macrophages. Therefore, direct experimental evidence is needed to determine whether in human dendritic cells the cd36 gene is indeed a candidate for an Oct-2–dependent regulation.
The specific expression patterns of Rel and octamer proteins during dendritic cell differentation might be a useful molecular marker to determine the maturation state of dendritic cells generated in vitro. In clinical trials, monocyte-conditioned medium is frequently used to induce dendritic cell maturation.29 However, in addition to several cytokines, this supernatant contains other unknown and possibly inhibitory components. Thus, variations in its maturation-inducing potency are commonly observed. Alternatively, a cocktail consisting of TNF-α, IL-1β, IL-6, and PGE2 has been proposed as a substitute for monocyte-conditioned medium.30 Others described TNF-α to be sufficient to induce dendritic cell maturation,44 but this requires the presence of fetal calf serum.29 30 Identical expression patterns of Rel and octamer factors were observed in dendritic cells grown in media containing either monocyte-conditioned medium or a defined cytokine cocktail, indicating that this cocktail can replace monocyte-conditioned medium. In contrast, dendritic cells treated with TNF-α alone displayed a factor pattern typical for an intermediate state of dendritic cell maturation characterized by a lower CD83 and CD25 expression, when compared with monocyte-conditioned medium–treated dendritic cells (data not shown). Thus, the expression patterns of Rel and octamer factors are useful additional markers for determining the maturation state of dendritic cells.
Acknowledgments
The authors thank C. Staskewitz and C. Kurzmann for excellent technical assistance and Drs N. R. Rice and T. Wirth for gifts of reagents. We are indebted to Drs A. McLellan, A. Schimpl, F. Weih, A. Wilisch-Neumann, and T. Wirth for critical reading of the manuscript.
Supported by a grant from the German Ministry of Education and Research (01GE9602/4 and IZKF Würzburg to E.K.) and grants from the Deutsche Forschungsgemeinschaft, SFB 465 (to E.K., H.W.F. and E.S.), and NE 608/1-2 (to M.N.). M.N. is also supported by a fellowship from the habilitation program of the Deutsche Forschungsgemeinschaft (NE 608/2-1).
The first 2 authors contributed equally to this work.
Reprints:M. Neumann, Institute of Pathology, Department of Molecular Pathology, University of Würzburg, Josef-Schneider-Strasse 2, D-97080 Würzburg, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal