Abstract
We have previously described a novel lipoprotein particle consisting of phospholipids, apolipoprotein A-I (apoAI), lipopolysaccharide binding protein (LBP) and Factor H-related proteins (FHRP), and we termed these particles FALP (FHRP-associated lipoprotein particles). Highly purified preparations of FALP contain variable amounts of an unidentified polypeptide triplet of Mr ∼85 000 (tp85). Here we report that tp85 represents fragment D of fibrinogen, as confirmed by N-terminal amino acid sequencing and Western blot analysis with an antifibrinogen antibody. The physical association of fibrinogen with other components of FALP in plasma was further confirmed by sandwich ELISA by using monoclonal antibodies against apoAI, FHRP or LBP to capture the particles and polyclonal antifibrinogen as the detecting antibody. Furthermore, affinity chromatography with anti-FHRP-1–specific IgG showed that fibrinogen is co-immunodepleted with FALP and approximately 17% of total plasma fibrinogen are bound to FALP. LBP is a lipid transfer protein that moves lipopolysaccharide (LPS) to a binding site on CD14 or high-density lipoprotein (HDL). To determine whether fibrinogen affects the lipid transfer activity of LBP on FALP, this activity was measured in FALP prepared with and without fibrinogen. Neither activity of LBP was affected by fibrinogen. The abundance of FALP suggests, instead, an effect of FALP on the function or clearance of fibrinogen or fragment D. (Blood. 2000;95:198-204)
A large body of data suggests functional links between lipoproteins, fibrinogen, and the cardiovascular disease.1-3 Hyperlipoproteinemia (or hypolipoproteinemia) is associated with elevated (or decreased) levels of plasma level of fibrinogen,4-9 and increased plasma levels of both lipid and fibrinogen are risk factors for coronary heart disease. Moreover, treatment of hyperlipidemic patients with fibrates lowers not only lipoprotein levels but also fibrinogen levels10 11 Despite these correlations, a clear mechanistic link between lipoproteins and fibrinogen has yet to be established.
We have recently described a novel lipoprotein particle in normal human plasma (NHP).12 This lipoprotein consists of phospholipids, apolipoprotein A-I (apoAI), lipopolysaccharide binding protein (LBP), and factor H-related proteins (FHRPs). Because FHRP-1 appears to be the numerically dominant component of the particle and because all FHRP-1 in plasma is found in these lipoprotein particles, we named them FALP (FHRP-associated lipoprotein particles). FALP have characteristics of high-density lipoproteins: Mr of ∼200 000, density of 1.219 to 1.264 and the presence of apoAI.12 Electron microscopy revealed a discoid shape with a diameter of ∼11 nm.12 Thus, FALP represents a small subset of high-density lipoprotein (HDL) (∼2% of apoAI containing HDL) with very high density.
FHRP-1, a major component of FALP, is an abundant plasma protein (40 μg/mL) and belongs to a family of protein called FHRPs. FHRPs were discovered as proteins with sequence homology and antigenic cross-reactivity with complement FH.13 So far, 4 (FHRP-1, 2, 3, and 4) have been described and 3 (FHRP-1, 2, and 4) of them are found associated with lipoproteins.12,14 No function has been described for FHRPs. FHRP-1 consists of 5 tandem repeats of a 60-amino acid motif known as the short consensus repeat. Other proteins with short consensus repeats have been reported to be associated with lipoproteins.15 16
We originally isolated FALP while following the activity of LBP, one of the constituents of FALP. LBP is a lipid transfer protein that efficiently moves LPS monomers from LPS aggregates to a binding site on CD14.17,18 CD14 is found on the surface of monocytes and polymorphonuclear leukocytes (PMN) as a glycosylphophatidylinositol-linked protein, as well as in plasma as a soluble form. Both forms of CD14 bind LPS and play a crucial role in mediating cellular responses to LPS.19,20 The transfer activity of LBP (and FALP) dramatically increases the ability of cells to respond to low doses of LPS.11,21,22 LBP is also able to transfer LPS to HDL and neutralize its activity to stimulate inflammatory cells.23 While FALP can modify the efficiency of LPS transfer to HDL by LBP (Park and Wright, in preparation), LBP is able to transfer lipids in the absence of the other FALP components.17,18,23 24 Moreover, although LBP resides on FALP, FALP alone does not have the ability to neutralize LPS and is not thought to be the acceptor for LPS neutralization (Park and Wright, unpublished observation). Thus, the precise functions of FALP remain to be defined.
Our earlier studies revealed the presence of unknown polypeptides of ∼85 kd in FALP. Here we report that these bands are fragments D of fibrinogen and that fibrinogen is a constituent of FALP. The association of fibrinogen with FALP in normal plasma was confirmed by sandwich ELISA (enzyme-linked immunosorbent assay) and affinity immunodepletion using anti-FHRP-1–specific IgG. The physiologic role of fibrinogen on FALP is discussed.
Methods
Reagents
Freshly frozen normal human plasma (citrated) was obtained from the New York Blood Center (New York, NY). Some experiments used normal human plasma prepared from fresh whole blood drawn from healthy human volunteers via venipuncture into EDTA-containing syringes and centrifuged at 1000g for 15 minutes at 4°C. HiPAC-Aldehyde chromatographic resin was obtained from Chromatochem (Missoula, MT). Fibrinogen and human complement factor H (FH) were purchased from Calbiochem (San Diego, CA). Recombinant LBP and recombinant soluble CD14 were prepared as described25 26 and cDNA encoding human FHRP-1 were generous gifts of Dr Rolf Thieringer (Merck Research Laboratory, Rahway, NJ).
Monoclonal antibodies.
3D11 (anti-FH and FHRP), which recognizes C-terminus of FH and FHRP-1,27 was a generous gift from Dr Vesa Koistinen (Helsinki, Finland). Monoclonal anti-LBP, c10, used as a capturing antibody for LBP ELISA or Sandwich ELISA, was a generous gift of Dr Alex Taylor.28 Anti-apoAI was purchased from Calbiochem, antifibrinogen (catalog #311 and #313), and anti-TFPI (tissue factor pathway inhibitor) were purchased from American Diagnostica Inc (Greenwich, CT).
Polyclonal antibodies.
Anti-LBP and anti-FHRP were raised by immunizing rabbits with recombinant LBP and recombinant FHRP-1, respectively. Specificity of these antibodies was confirmed by Western blot using human plasma. Anti-FHRP-1 also recognizes FH as expected, because they share strong homology in the C-terminus.13 Anti-FHRP-1-specific IgG was prepared as described below. Goat anti-FH and goat antifibrinogen antibodies were purchased from Incstar (Stillwater, MN) and rabbit antifibrinogen (catalog #313R) was purchased from American Diagnostica Inc (Greenwich, CT).
Secondary antibodies.
Horseradish peroxidase-conjugated rabbit antigoat IgG and goat antirabbit IgG were purchased from Pierce (Rockford, IL). Alkaline phosphatase-conjugated goat antirabbit IgG was purchased from Bio-Rad (Hercules, CA). Fibrinogen used as a standard for ELISA was purchased from American Diagnostica Inc. ReLPS (S. minnesota, Re595) was purchased from List Biological Laboratories (Campbell, CA). Reconstituted HDL (rHDL) was prepared as previously described.23
Purification of FALP
Chromatography to isolate FALP was performed as previously described,12 with a few minor modifications. Approximately 50 mL of plasma (or serum) was loaded onto a HiPAC-Aldehyde column (packed in an HR 10/30 FPLC column). After washing, adsorbed molecules were eluted into ∼20 mL of 0.5 mol/L ammonium acetate buffer, pH 3.0. Eluates were neutralized by adding 1 mol/L Tris pH = 9.0 at 1/20 volume of the total eluate and immediately dialyzed overnight against 4 L of 20 mmol/L Tris, pH 8.0 with 1 buffer change. Samples were then loaded onto Q Sepharose FF and eluted with a gradient of sodium chloride (NaCl) from 0 to 1 mol/L. Fractions were tested for LBP by ELISA as described below. Fractions containing LBP were pooled, diluted again with 20 mmol/L Tris, pH 8.0 to reduce NaCl concentration lower than 80 mmol/L, loaded onto Mono Q and eluted with a NaCl gradient from 0 to 1 mol/L. Each fraction was tested for LBP by ELISA and the presence of FHRP-1 was confirmed by Western blot. After confirming that peaks of FHRP-1 and LBP overlapped, FALP fractions were pooled and concentrated by ultrafiltration with Ultrafree-15 (Millipore, Bedford, MA), and at the same time, buffer was exchanged into PBS (Dulbecco's Phosphate Buffered Saline, Biowhittaker, Walkersville, MD). FALP was usually recovered at about 140 mmol/L NaCl in Q Sepharose FF and Mono Q chromatography. When indicated, epsilon-aminocaproic acid (EACA) was added to buffers to yield a final concentration of 150 mmol/L (for HiPAC-Aldehyde chromatography) or 50 mmol/L (for Mono Q). FALPs prepared for the purpose of comparison between plasma and serum were made from fresh blood taken from the same donor with purifications performed in parallel.
Polyacrylamide gel electrophoresis
Electrophoresis was performed either with the Phast System (Pharmacia) or the Novex (San Diego, CA) system, according to the manufacturer's recommendations. Western blot analysis was as previously described with minor modifications.12 Briefly, after SDS-PAGE, samples were transferred to nitrocellulose membranes using a Novex transfer system. After transfer, the membranes were blocked with 10% nonfat dry milk, 0.2% Tween 20, and 0.02% sodium azide in PBS for 1 hour in room temperature; washed with PBS containing 0.1% milk, 0.2% Tween 20 (Western buffer); and incubated with primary antibodies in Western buffer either 1 hour at room temperature or 4°C overnight. Bound primary antibodies were detected by the combination of horseradish-conjugated secondary antibodies and chemiluminescent substrates (SuperSignal Substrate System, Pierce), according to the manufacturer's recommendation.
Amino acid sequencing
After SDS-PAGE, polypeptides were transferred to polyvinylidene difluoride (PVDF) membranes, briefly stained with Coomassie Blue, and washed with water. Bands were excised, and proteins were sequenced by the Protein Sequencing Facility at The Rockefeller University.
ELISA
The ELISAs to measure the amounts of LBP and fibrinogen in samples were performed essentially as described previously.12,23Antibodies c10 and polyclonal rabbit anti-LBP were used for capturing and detecting, respectively, in the LBP ELISA. Similarly, antibodies #313 and polyclonal rabbit antifibrinogen (#313R) were used in the fibrinogen ELISA. For the measurement of FHRP, mAb 3D11 and polyclonal rabbit anti-FHRP-1 (or anti-FHRP-1-specific IgG, see below) were used.. A sandwich ELISA to establish the physical association of 2 molecules was performed as described previously,12 23 with slight modifications. Plates were coated with the indicated monoclonal antibodies (5 μg/mL) and blocked with 10% nonfat dry milk in PBS. Samples (normal human plasma) were incubated in the wells for 4 hours to overnight at 4°C, plates were washed, polyclonal antibodies were added to the wells, and plates were further incubated for 4 hours at 4°C. The bound antibody was detected by an alkaline phosphatase-conjugated goat antirabbit IgG or rabbit antigoat IgG, incubated for 2 hours at 4°C. Attophos™ (JBL Scientific, San Luis Obispo, CA), a fluorescence substrate for the alkaline phosphatase, was added after the plates were washed with distilled water. Fluorescence was measured immediately thereafter in a fluorescence plate reader (Cytofluor™, Millipore, Bedford, MA).
Preparation of recombinant FHRP-1
cDNA encoding human FHRP-1 was cloned into an insect cell expression vector with metallothionein promotor, (pRmHa-3, a generous gift from Dr Rolf Thieringer). pRmHa-3 containing the FHRP-1 sequence was transfected with PUChsneo (a co-expression plasmid with hsp70 promoter driving neo expression) into Schneider 2 insect cells. Transfected Schneider 2 cells were grown in serum-free insect cell media (EX-CELL 400, JRH Biosciences, Lenexa, KS) containing G-418 and copper sulfate 1 mmol/L as an inducer for the metallothionein promoter. Purification of expressed recombinant FHRP-1 in culture supernatant was performed using multiple steps of chromatography as follows: Conditioned medium was directly loaded onto a Q sepharose FF (Pharmacia) column, which was pre-equilibrated with 20 mmol/L Bis-Tris-Propane, pH = 6.8 (BTP buffer). Expressed protein appeared in the flow-through fraction, while more than 90% of the protein was adsorbed to the column in this step. The flow-through fraction was directly loaded onto S sepharose FF (Pharmacia) column, also pre-equlibrated with BTP buffer. The column was then washed with BTP buffer containing 250 mmol/L NaCl and eluted with BTP buffer containing 450 mmolL NaCl. This fraction was diluted 5 times with BTP buffer, loaded again onto a uno S (Bio-Rad) column and eluted with a NaCl gradient, 150 to 500 mmol/L in BTP buffer. Recombinant FHRP-1 was recovered in the ∼380 mmol/L NaCl fractions. Samples were immediately dialyzed into 5 mmol/L sodium phosphate, pH 6.8 and loaded onto a hydroxyapatite column (CHT-2, Bio-Rad). Separation was performed by gradient from 5 to 500 mmol/L of sodium phosphate. The presence of recombinant FHRP-1 was monitored by Western blot with goat antihuman FH (Incstar), and the purity of the recombinant FHRP-1 was more than 99% by silver stain and Coomassie blue stain of PAGE gel.
Anti-FHRP antibody affinity chromatography
Rabbit antibody raised against FHRP-1 contains IgG that cross-react with FH. FHRP-1-specific antibodies (anti-FHRPsp) were prepared by removing these cross-reacting antibodies from polyclonal rabbit anti-FHRP-1 IgG (see above) by affinity chromatography using human FH Sepharose beads (NHS-activated HiTrap column, Pharmacia). We were able to remove 99% of the cross-reactivity in 1 step. Then, purified FHRP-1–specific rabbit IgG were coupled to NHS-activated Sepharose beads in parallel with preimmune IgG. Coupling efficiency was determined as 4.75 mg and 4.74 mg per 1 mL column for FHRP-1–specific IgG and preimmune IgG, respectively. For the co-immunodepletion of FHRP-1 and fibrinogen, 5 mL of normal human plasma from freshly drawn heparinized blood was prepared and loaded onto the 1 mL column (anti-FHRPsp and preimmune IgG) equilibrated with PBS 10 U/mL of heparin. The first 1 mL of flow-through was collected and the measurement of FHRP and fibrinogen from the fractions was performed by ELISA as described previously. The columns were regenerated by washing with 0.5 mol/L ammonium acetate and pH = 3.0 and 0.1 mol/L triethylamine, pH = 11.5 sequentially 2 times.
LBP catalyzed transfer of fluorescently labeled LPS (BODIPY-LPS)
LBP catalyzes the transfer of LPS monomers from LPS aggregates to a binding site on CD14 or rHDL.17,23 This activity may be measured in real time using fluorescently labeled LPS (BODIPY-LPS): Fluorescence is quenched in LPS aggregates, and the quenching is relieved on transfer to CD14 or rHDL.18,24 BODIPY-LPS was prepared as described previously,18 and the rate of movement of LPS monomers from aggregates into soluble CD14 (sCD14) or rHDL was measured as described previously18, 24,25 with minor modifications. Briefly, FALP and sCD14 or rHDL were quickly mixed with BODIPY-LPS in buffer (PBS with 1.5 mg/mL bovine serum albumin) in 96-well plates and the increase in fluorescence was measured in real time using a fluorescence plate reader (Cytofluor II, Perspective Biosystems) with the emission and the excitation wavelength of 485 ± 30 and 530 ± 30, respectively. The concentration of LBP in serum- or plasma-derived FALP was measured by LBP ELISA immediately before each experiment to normalize the concentrations of LBP.
PMN adhesion assay
PMN adhesion was measured as described previously.29Briefly, fluorescently labeled PMN were incubated on fibrinogen-coated Terasaki plates for 10 minutes at 37°C in the presence of 10 ng/mL of LPS (Re595) and the FALP preparations. After washing, PMN adherent to the fibrinogen-coated surface were measured by fluorescence plate reader (Cytofluor™, Millipore, Bedford, MA).
Results
Identification of fibrinogen fragments in FALP by amino acid sequencing
FALP was purified from plasma by sequential chromatographic steps on HiPAC-Aldehyde, Q Sepharose FF, and Mono Q columns. Preparations yielded the characteristic bands in SDS-PAGE (Figure1): FHRP-1, LBP and apoAI, all confirmed by Western blot analysis (data not shown). An additional triplet band at 85 kd (previously designated as tp8512) was also prominent. Parallel gels showed that the addition of reducing agent shifted the position of tp85 to the 40-kd region of the gel (Figure 1). N-terminal amino acid sequencing of the 4 bands present under reducing conditions revealed identity with fragments of fibrinogen. Three bands (1, 3, and 4) are from the γ chain and 1 (band 2) is from the β chain of fibrinogen. Bands 1 and 3 share identical N-termini. All the N-terminal sequences fell at predicted sites of plasmin digestion of fragment D (Figure 1).30
FALP preparations contain fibrinogen fragments.
FALP was prepared from normal human plasma as described in “Methods” and subjected to SDS-PAGE under nonreducing conditions (left lane, NR) and reducing conditions (right lane, R). Gels were electrophoretically transferred to a PVDF membrane and stained with Coomassie Blue. Bands in NR appeared identical to those reported previously12 and the previously identified bands are labeled. Four bands from the reduced lane were excised and analyzed for N-terminal amino acid sequence. The sequencing results are shown to the right of the band. Molecular weight markers are shown in kilodaltons.
FALP preparations contain fibrinogen fragments.
FALP was prepared from normal human plasma as described in “Methods” and subjected to SDS-PAGE under nonreducing conditions (left lane, NR) and reducing conditions (right lane, R). Gels were electrophoretically transferred to a PVDF membrane and stained with Coomassie Blue. Bands in NR appeared identical to those reported previously12 and the previously identified bands are labeled. Four bands from the reduced lane were excised and analyzed for N-terminal amino acid sequence. The sequencing results are shown to the right of the band. Molecular weight markers are shown in kilodaltons.
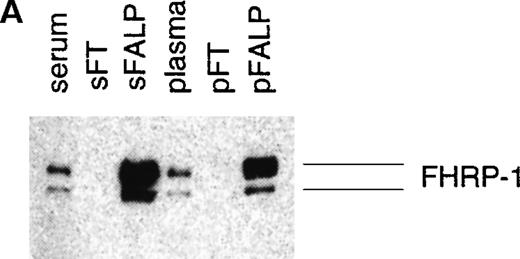
The identification of fibrinogen in FALP was confirmed by Western blot analysis with polyclonal antifibrinogen antibody. A triplet atMr of 85 000 and a band at Mr of 50 000 (previously defined as tp85 and p5012) were stained with antifibrinogen antibody (Figure 2A). From the N-terminal sequence and the size of the bands, it is most likely that these represent fragments D and E of fibrinogen, respectively.30Fragments D and E are products of plasmin digestion of fibrinogen.
Western blot of FALP with antifibrinogen antibody.
(A) FALP purified from normal human plasma was run on SDS-PAGE in nonreducing conditions and subjected to Western blot analysis with polyclonal antifibrinogen antibody (goat antifibrinogen IgG, Incstar). Bands defined previously as tp85 and p50 were stained. (B) HiPAC-Aldehyde column chromatography, the first step purification of FALP, was performed in the presence (right panel) and absence (left panel) of 150 mmol/L EACA. Eluates from these preparations (HE) were run on SDS-PAGE under nonreducing conditions and analyzed by Western blot analysis with goat antifibrinogen IgG. Fragments X, Y, and D are marked based on the molecular weight of the bands. (C) FALP prepared in the presence of EACA during HiPAC-Aldehyde (150 mmol/L) and Mono Q (50 mmol/L) column chromatography, were subjected to Western blot analysis as above with goat antifibrinogen IgG. Presence of LBP and FHRP-1 in FALP was confirmed by ELISA and Western blot analysis, respectively. Molecular weight standards are shown in kilodaltons.
Western blot of FALP with antifibrinogen antibody.
(A) FALP purified from normal human plasma was run on SDS-PAGE in nonreducing conditions and subjected to Western blot analysis with polyclonal antifibrinogen antibody (goat antifibrinogen IgG, Incstar). Bands defined previously as tp85 and p50 were stained. (B) HiPAC-Aldehyde column chromatography, the first step purification of FALP, was performed in the presence (right panel) and absence (left panel) of 150 mmol/L EACA. Eluates from these preparations (HE) were run on SDS-PAGE under nonreducing conditions and analyzed by Western blot analysis with goat antifibrinogen IgG. Fragments X, Y, and D are marked based on the molecular weight of the bands. (C) FALP prepared in the presence of EACA during HiPAC-Aldehyde (150 mmol/L) and Mono Q (50 mmol/L) column chromatography, were subjected to Western blot analysis as above with goat antifibrinogen IgG. Presence of LBP and FHRP-1 in FALP was confirmed by ELISA and Western blot analysis, respectively. Molecular weight standards are shown in kilodaltons.
To determine whether digestion of fibrinogen occurred during purification, fresh plasma was prepared in the presence of epsilon-aminocaproic acid (EACA), a plasmin inhibitor. When the first step of chromatography was performed in the presence of EACA, a band consistent with the molecular weight of uncleaved fibrinogen was observed, which barely entered the main gel out of stacking (Figure 2B, right panel). In contrast, the smaller fragments of fibrinogen consistent with the molecular weight of fragments X, Y, and D were seen in the sample prepared in the absence of EACA (Figure 2B, left panel). On subsequent chromatographic steps, smaller sized bands corresponding to the size of fragment D and E were observed in the sample purified in the absence of EACA (Figure 2A). However, there was no change in the size of the band (corresponding to the uncleaved fibrinogen) in FALP purified in the presence of EACA (Figure 2C). This result suggests that plasmic digestion of fibrinogen occurred during chromatography.
Fibrinogen is associated with FALP in plasma
The ability of fibrinogen to copurify with FALP through 2 discriminating column steps as well as native PAGE12suggests a tight binding interaction.To determine whether this physical association of fibrinogen with FALP occurs in normal human plasma before it is subjected to any chromatographic manipulation, a sandwich ELISA technique (see “Methods”) was used to detect fibrinogen on FALP in whole plasma. Monoclonal antibodies against FHRP-1, LBP, or apoAI were coated on a plate and incubated with NHP. To determine whether they captured not only their target protein but also fibrinogen, polyclonal antifibrinogen antibody was used to detect surface bound protein (Figure 3). We observed that each of these antibodies against components of FALP also captured fibrinogen. In contrast, a control antibody (anti-TFPI) did not capture fibrinogen.
Fibrinogen coprecipitates with components of FALP in sandwich ELISAs.
Monoclonal antibodies against each component of FALP were coated on the plastic surface of Terasaki plates as shown on the X-axis, and sandwich ELISAs were performed as described in Methods. After NHP (diluted 1/200 in PBS) was incubated and washed, polyclonal antifibrinogen antibody was added to detect fibrinogen co-associated with the test antigen. Antitissue factor pathway inhibitor (TFPI) was used as a control antibody. The increase in arbitrary units of fluorescence indicated on the Y-axis is proportional to the amount of bound antifibrinogen antibody. Control experiments were run to demonstrate that each of the monoclonal antibodies bound its own antigen, which could be detected with the corresponding polyclonal antibodies (data not shown). The experiment was repeated twice with similar results. Error bars represent the standard deviations of the triplicate measurements.
Fibrinogen coprecipitates with components of FALP in sandwich ELISAs.
Monoclonal antibodies against each component of FALP were coated on the plastic surface of Terasaki plates as shown on the X-axis, and sandwich ELISAs were performed as described in Methods. After NHP (diluted 1/200 in PBS) was incubated and washed, polyclonal antifibrinogen antibody was added to detect fibrinogen co-associated with the test antigen. Antitissue factor pathway inhibitor (TFPI) was used as a control antibody. The increase in arbitrary units of fluorescence indicated on the Y-axis is proportional to the amount of bound antifibrinogen antibody. Control experiments were run to demonstrate that each of the monoclonal antibodies bound its own antigen, which could be detected with the corresponding polyclonal antibodies (data not shown). The experiment was repeated twice with similar results. Error bars represent the standard deviations of the triplicate measurements.
The small amount of signal from anti-LBP in Figure 3 is expected, since LBP exists in NHP at 2 to 4 μg/mL (Park, unpublished observation), although the concentration of FHRP-1 is reported to be ∼40 μg/mL.31 Thus, the molar ratio of LBP to FHRP-1 in NHP is approximately 1:15-30. FH and FHRP-1 share a highly homologous C-terminal region, and anti-FHRP-1 antibodies also recognize plasma FH, which exists in high concentration.13 To confirm that FHRP-1 was responsible for the association with fibrinogen observed above, we repeated the same experiments using FALP (which does not contain FH) instead of NHP. We observed that monoclonal anti-FHRP3-11 not only captured FHRP-1 but also fibrinogen (data not shown). These observations confirm the proposed association of fibrinogen with FALP.
To quantitate how much fibrinogen is associated with FALP, we performed an immunodepletion of FHRP-1 from normal human plasma by affinity chromatography, then measured the depletion of fibrinogen. Sepharose beads were coupled to FHRP-specific IgG as described in “Methods.” When fresh plasma was passed over this column, we observed that 16.8% of plasma fibrinogen were removed (Table1). From this observation we calculated that fibrinogen is associated with FHRP-1 at a molar ration of ∼1.48:1 (= fibrinogen: FHRP) in normal plasma.
Co-immunodepletion of plasma fibrinogen by anti-FHRP–specific Igg column chromatography
Five ml of fresh normal human plasma was loaded onto a 1 ml NHS-HiTrap column coupled to anti-FHRP–1-specific IgG or preimmune IgG. The first 1 ml of flow-through was collected and the concentration of FHRP and fibrinogen was measured by ELISA as described in “Methods.”
Calculated by (1 − [Imm/Preimm]) × 100, where Imm represents concentration of flow-through fraction from anti-FHRP–1 specific IgG column, and Preimm, that of preimmune IgG column.
Data are the average and the standard errors from 3 independent experiments.
Serum contains the same amount of FALP as plasma but much less fibrinogen
To determine whether fibrinogen is a necessary component of FALP, we compared FALP isolated in parallel from plasma and serum from the same donor. Because fibrinogen is converted to fibrin during clotting, we anticipated an alteration in the available fibrinogen pool. We found that, as expected, serum contained ∼1000 times less fibrinogen than plasma (1.4 ± 0.1 μg/mL vs 2.6 ± 0.3 mg/mL and Figure 4B). During the chromatography with plasma, much of the plasma fibrinogen flows through the first column, but a significant proportion was copurified with FALP as fragment X. However, approximately 10 times less fibrinogen was found in FALP prepared from serum (sFALP) than those found in FALP prepared from plasma (pFALP) (Figure 4B and Table 2). Importantly, the amount of LBP and FHRP-1 present in sFALP and pFALP were comparable (Figure 4A and Table 2). This observation suggests that although fibrinogen is bound to FALP, it may not be a necessary component of FALP.
Serum-derived FALP bears less fibrinogen than plasma-derived FALP but an equal amount of FHRP.
FALP was prepared from serum or plasma from the same donor. Samples derived from an equal amount of serum or plasma (∼30 mL) were diluted equivalently. Western blot analyses were performed with the starting serum or plasma, the flow-through of the first column chromatographic step (sFT and pFT), and the final FALP preparations (sFALP and pFALP). Anti-FHRP was used for the blot shown in panel A, and antifibrinogen was used for the blot shown in panel B. Molecular weight standards are shown in kilodaltons. Bands seen on FALP lanes of antifibrinogen blot (B) correspond to the size of fragment X (X). For this preparation, EACA was used only for the first column chromatography but not for the subsequent chromatographic steps. Therefore, the size of the fibrinogen fragment is bigger than that of the FALP prepared in the absence of EACA shown in Figure 2. The blot was overexposed to visualize the fibrinogen band in sFALP lane.
Serum-derived FALP bears less fibrinogen than plasma-derived FALP but an equal amount of FHRP.
FALP was prepared from serum or plasma from the same donor. Samples derived from an equal amount of serum or plasma (∼30 mL) were diluted equivalently. Western blot analyses were performed with the starting serum or plasma, the flow-through of the first column chromatographic step (sFT and pFT), and the final FALP preparations (sFALP and pFALP). Anti-FHRP was used for the blot shown in panel A, and antifibrinogen was used for the blot shown in panel B. Molecular weight standards are shown in kilodaltons. Bands seen on FALP lanes of antifibrinogen blot (B) correspond to the size of fragment X (X). For this preparation, EACA was used only for the first column chromatography but not for the subsequent chromatographic steps. Therefore, the size of the fibrinogen fragment is bigger than that of the FALP prepared in the absence of EACA shown in Figure 2. The blot was overexposed to visualize the fibrinogen band in sFALP lane.
LBP and fibrinogen concentration of serum-derived and plasma-derived FALP
FALP was purified in parallel from approximately 32 mL of fresh serum (sFALP) or plasma (pFALP), as described in “Methods” and reconstituted in approximately 0.2 mL of PBS. The LBP and fibrinogen concentrations of the FALP preparations were measured by ELISA as described in Materials and Methods.
Data are the average and the standard errors from three measurements.
Expressed as the concentration of full-length fibrinogen. The experiment was performed twice with similar results.
Fibrinogen in FALP does not affect the ability of LBP to transfer LPS
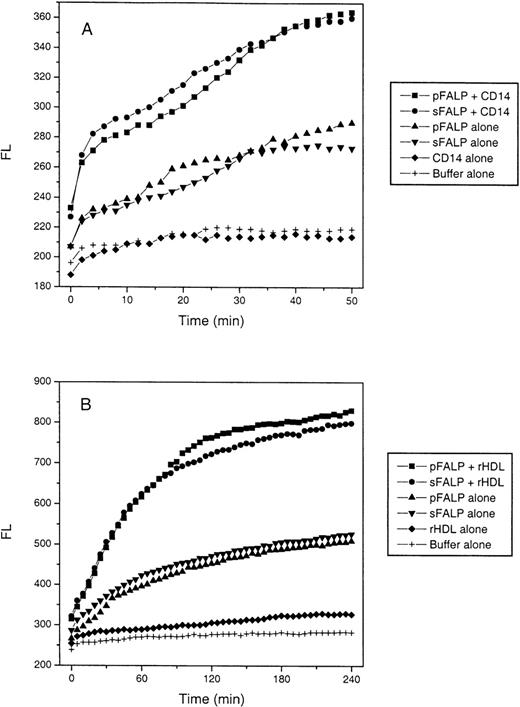
To test whether fibrinogen affects LBP-mediated LPS transfer to CD14, we compared the LPS transfer activity of fibrinogen-sufficient FALP from plasma with that of fibrinogen-deficient FALP from serum. Transfer of BODIPY-LPS from micelles to sCD14 was measured in real time. We observed no significant difference between the preparations (Figure 5A). LBP transfers LPS not only to CD14 but also to HDL.23 To test whether fibrinogen has a role in LBP-mediated LPS transfer to HDL, we performed the same assay, except that sCD14 was replaced by reconstituted HDL (rHDL). Because transfer to HDL is slower than to sCD14,24 fluorescence was observed over a longer period. As seen in Figure 5B, fibrinogen-depleted FALP (sFALP) did not exhibit a significant difference from fibrinogen-rich FALP (pFALP) in the transfer rate. These experiments were repeated using whole serum or whole plasma instead of purified FALPs, and identical results were obtained (Figure6A and 6B).
LBP lipid transfer activity is equal in plasma- and serum-derived FALP.
(A) LBP-catalyzed transfer of LPS to CD14 FALPs were mixed quickly with BODIPY-LPS and sCD14 in a 96-well plate at room temperature, and the increase in fluorescence was measured every 2 minutes for 50 minutes. The final concentration of LBP in pFALP and sFALP was 0.56 and 0.43 μg/mL, respectively. Final concentrations of sCD14 and BODIPY-LPS were 2 and 0.8 μg/mL, respectively. Controls included BODIPY-LPS incubated with pFALP or sFALP alone (pFALP alone, sFALP alone), incubated with sCD14 alone (CD14 alone), or buffer alone (Buffer alone). The Y-axis represents arbitrary fluorescence units. (B) LBP-catalyzed transfer of LPS to HDL FALPs were mixed with BODIPY-LPS and rHDL at 37°C, and the increase in fluorescence was measured every 5 minutes for 4 hours. The final concentrations of the each component are: rHDL 100 μg/mL (expressed as apoAI content), BODIPY-LPS 0.6 μg/mL, and pFALP and sFALP 0.5 μg/mL of LBP. Further dilution of pFALP and sFALP to 0.25, 0.125, 0.06, and 0.03 μg/mL of LBP did not show any difference between pFALP and sFALP as well (data not shown). Controls were included as above, except that rHDL was used instead of sCD14. These experiments were repeated twice with similar results.
LBP lipid transfer activity is equal in plasma- and serum-derived FALP.
(A) LBP-catalyzed transfer of LPS to CD14 FALPs were mixed quickly with BODIPY-LPS and sCD14 in a 96-well plate at room temperature, and the increase in fluorescence was measured every 2 minutes for 50 minutes. The final concentration of LBP in pFALP and sFALP was 0.56 and 0.43 μg/mL, respectively. Final concentrations of sCD14 and BODIPY-LPS were 2 and 0.8 μg/mL, respectively. Controls included BODIPY-LPS incubated with pFALP or sFALP alone (pFALP alone, sFALP alone), incubated with sCD14 alone (CD14 alone), or buffer alone (Buffer alone). The Y-axis represents arbitrary fluorescence units. (B) LBP-catalyzed transfer of LPS to HDL FALPs were mixed with BODIPY-LPS and rHDL at 37°C, and the increase in fluorescence was measured every 5 minutes for 4 hours. The final concentrations of the each component are: rHDL 100 μg/mL (expressed as apoAI content), BODIPY-LPS 0.6 μg/mL, and pFALP and sFALP 0.5 μg/mL of LBP. Further dilution of pFALP and sFALP to 0.25, 0.125, 0.06, and 0.03 μg/mL of LBP did not show any difference between pFALP and sFALP as well (data not shown). Controls were included as above, except that rHDL was used instead of sCD14. These experiments were repeated twice with similar results.
Plasma and serum have identical LBP lipid transfer activity.
The BODIPY-LPS transfer assays were performed as Figure 5, except that whole plasma and whole serum from a single blood sample were used instead of purified FALPs. (A) For the LPS transfer to sCD14, sCD14 3 μg/mL, BODIPY-LPS 0.5 μg/mL, and a 1/100 dilution of whole plasma or serum were used. (B) For the LPS transfer to rHDL, rHDL 100 μg/mL, BODIPY-LPS 0.5 μg/mL and a 1/20 dilution of whole plasma or serum were used.
Plasma and serum have identical LBP lipid transfer activity.
The BODIPY-LPS transfer assays were performed as Figure 5, except that whole plasma and whole serum from a single blood sample were used instead of purified FALPs. (A) For the LPS transfer to sCD14, sCD14 3 μg/mL, BODIPY-LPS 0.5 μg/mL, and a 1/100 dilution of whole plasma or serum were used. (B) For the LPS transfer to rHDL, rHDL 100 μg/mL, BODIPY-LPS 0.5 μg/mL and a 1/20 dilution of whole plasma or serum were used.
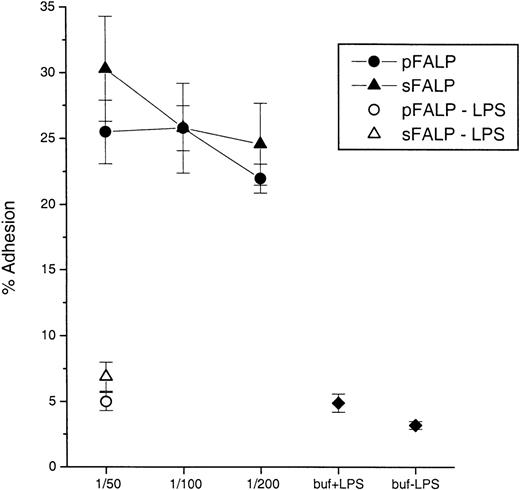
The ability of LBP to transfer BODIPY-LPS from micelles to sCD14 correlates well with the ability of LBP to stimulate PMN in the presence of low concentrations of LPS.18,25 28 We tested whether sFALP or pFALP showed differences in their ability to stimulate PMN adhesion in the presence of LPS. PMN were incubated with LPS and sFALP or pFALP, and assayed for integrin-mediated adhesion. Figure7 shows that there was no difference between sFALP and pFALP in the ability to enable stimulation of PMN by LPS. These results strongly suggest that fibrinogen in FALP does not have a significant role in modulating the lipid transfer and cell-stimulating activity of FALP-associated LBP.
pFALP and sFALP both enable responses of PMN to LPS.
PMN adhesion was measured as described in “Methods” using plasma- or serum-derived FALP preparations incubated with LPS (10 ng/mL). The LBP concentrations of pFALP and sFALP were 14.4 and 16.9 μg/mL before dilution, respectively. 3 different dilutions (1/50, 1/100, and 1/200 that correspond to ∼0.3, 0.15, and 0.07 μg/mL of LBP, respectively) of FALPs were tested. Each data point and error bar represents the average and the standard deviation from triplicate sample preparations. Controls included pFALP or sFALP incubated with PMN without LPS (pFALP-LPS or sFALP-LPS), and PMN incubated without pFALP or sFALP (buf+LPS) or with buffer alone (buf-LPS). The experiments were repeated twice with similar results.
pFALP and sFALP both enable responses of PMN to LPS.
PMN adhesion was measured as described in “Methods” using plasma- or serum-derived FALP preparations incubated with LPS (10 ng/mL). The LBP concentrations of pFALP and sFALP were 14.4 and 16.9 μg/mL before dilution, respectively. 3 different dilutions (1/50, 1/100, and 1/200 that correspond to ∼0.3, 0.15, and 0.07 μg/mL of LBP, respectively) of FALPs were tested. Each data point and error bar represents the average and the standard deviation from triplicate sample preparations. Controls included pFALP or sFALP incubated with PMN without LPS (pFALP-LPS or sFALP-LPS), and PMN incubated without pFALP or sFALP (buf+LPS) or with buffer alone (buf-LPS). The experiments were repeated twice with similar results.
Discussion
Here we show evidence that fibrinogen, an abundant plasma protein with a well-established function, is part of a novel HDL particle containing LBP and FHRP-1. In fact, we are not the first to report that fibrinogen may be associated with HDL. Kunitake et al32showed that fibrinogen copurified with HDL when a mild affinity chromatography procedure using an anti-apoAI antibody was used. Mild affinity chromatographic conditions were used to isolate HDL in both cases. Affinity purification is thought to be preferable to ultracentrifugation for the isolation of minor components of HDL because they tend to be dissociated from HDL during ultracentrifugation.33
It appears that fragment D on FALP is the result of plasmin activation during chromatography. When plasmin was inhibited by EACA in the chromatographic buffers, we observed longer fragments or whole fibrinogen associated with FALP (Figure 2). It is therefore more likely that the whole fibrinogen molecule is present on FALP in normal plasma. Indeed, on native PAGE of FALP prepared in the presence of EACA, the apparent Mr of FALP was >400 000 (data not shown), in contrast to Mr of ∼200 000 when FALP prepared without the inhibitor of plasmin was analyzed.12 It is interesting to point out that it was also the N-terminal sequence of fragment D that Kunitake et al32 reported associated with HDL. Although FALP binds both fibrinogen and fragment D, it appears that the majority of FALP does not bind fibrin because serum and plasma yield equal quantities of FALP (Table 2 and Figure 4). It seems most likely that conformational changes that occur during fibrin formation destroy the binding epitopes on fibrinogen for FALP.
The abundance of FALP (∼40 μg/mL of FHRP-1 in plasma,31and Park unpublished observation) suggests that the interaction of fibrinogen with FALP may be significant. About 16% of plasma fibrinogen is associated with FHRP-1 (or FALP) and can be adsorbed with an anti-FHRP affinity column (Table 1). If we assume that the plasma fibrinogen level is 3 mg/mL and the FHRP-1 level is ∼40 μg/mL, it can be calculated that 1.5 molecule of fibrinogen is associated with 1 FHRP-1 particle. At this time, we do not know whether the fibrinogen on FALP is different from free fibrinogen in terms of structure or function. The answer will become clear when fibrinogen is purified from FALP and compared with plasma fibrinogen. At a minimum, fibrinogen on FALP is able to participate in polymerization to form fibrin, since we observe that we can isolate FALP deficient in fibrinogen from serum (Table 2 and Figure 4).
The physiologic role of fibrinogen on FALP is not clear at this time. We tested whether it affects the function of LBP, because this lipid transfer protein is associated with FALP. Preparations of fibrinogen-sufficient and fibrinogen-deficient FALP exhibited equal LBP function in the following assays: LPS transfer to sCD14, LPS transfer to HDL, and enhancement of PMN adhesion in response to LPS. Although our fibrinogen-deficient preparation was not completely devoid of fibrinogen, the strong depletion of fibrinogen (Table 2) and the quantitative similarity of the LBP-activity between preparations suggest that fibrinogen does not affect LBP function. Because FALP also bind fragment D, and because FALP is far more abundant than fragment D in plasma, it is possible that FALP may contribute to the binding and clearance of fragment D. The relative affinities of fibrinogen and fragment D for FALP is a subject of current investigation.
Although the functional significance of fibrinogen binding to FALP remains obscure, the expression pattern of the constituents of FALP suggests linked functions. Both LBP and fibrinogen are acute phase reactants and both are involved in localization of infection. Moreover, fibrinogen and HDL levels are inversely correlated in humans.4,6,7,34 Increased fibrinogen level may be directly involved in lowering the HDL levels by binding to HDL and changing the structure and the physical characteristics of HDL, as suggested for serum amyloid A.35 36
It will also be of interest to test whether FALP levels correlate with fibrinogen levels and whether FALP rises in the acute phase.
Acknowledgments
We thank Dr Rolf Thieringer at Merck Research Laboratory for kindly providing recombinant sCD14, the cDNA encoding human FHRP-1, and BODIPY-LPS. Protein sequence analysis was provided by The Rockefeller University Protein Sequencing Facility.
Supported by National Institutes of Health Grants AI-01333-01 to C. Thomas Park.
Reprints:Samuel D. Wright, Merck Research Laboratory, Department of Lipid Biochemistry, PO Box 2000, R80W-250, Rahway, NJ 07065.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal