To the Editor:
In a recent issue of Blood, Guenechea et al1reported that ex vivo cytokine-expanded CD34+ cord blood cells retained nonobese diabetic/severe combined immunodeficient (NOD/SCID) repopulating activity of fresh cells, although there was a significant delay in the time to achieve similar levels of engraftment. They also found that there was little change in the quality of engraftment in terms of represented lineages 120 days after inoculation, although they suggest a trend toward a reduction of the myeloid (CD33+) compartment.
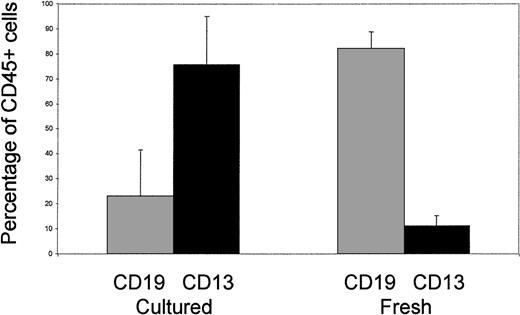
To develop a clinically applicable protocol, we have recently examined the potential for expansion of cord blood progenitors under serum-free conditions. As part of this study, we analyzed the characteristics of NOD/SCID engraftment of cells cultured ex vivo for 14 days (Fig1). In contrast to the findings reported above, we found that culture with interleukin-6 (IL-6) (10 ng/mL), IL-11 (10 ng/mL), Flt3-ligand (FL) (50 ng/mL), and thrombopoietin (TPO) (10 ng/mL) was associated with a switch from dominant B lymphopoiesis (CD19+) toward myelopoiesis (CD13+) in animals analyzed after 40 days in the absence of in vivo cytokine support. Furthermore, in other studies using alternative combinations of cytokines (IL-3 [20 ng/mL], IL-6 [20 ng/mL], stem cell factor [SCF] [100 ng/mL], and FL [100 ng/mL]), we found that the same switch in engraftment quality occurred after 48 hours of ex vivo culture (Demaison et al, in press). These findings can be interpreted in two ways. Firstly, this may reflect a forced commitment of the mutipotent SCID-repopulating cell (SRC) to myelopoiesis, and/or selective inhibition of B-lymphoid commitment, and is similar phenotypically to the patterns of engraftment observed in NOD/SCID mice supplemented in vivo with FL either alone or in combination with IL-7 and SCF, or a combination of SCF, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-3.2 If this is the case, our findings suggest that these changes in commitment are initiated by exposure to cytokines in vitro and, more importantly, do not revert spontaneously in vivo. Secondly, there may be a selective expansion or retention of SRCs intrinsically restricted to the myeloid lineage. This possibility is supported by previous studies that used retroviral marking or radiation-induced chromosomal aberrations to track distinct cell lineages in mice, and our own recent experiments that indicate that murine retroviral vectors can selectively mark ex vivo–cultured human CD34+ cells that repopulate NOD/SCID mice in a myeloid-restricted pattern3,4 (Demaison et al, in press). Furthermore, good evidence has emerged for a murine clonogenic common lymphoid precursor (CLP) population that possesses lymphoid-restricted (T, B, and natural killer) repopulating activity.5Heterogeneity within the SRC compartment is not surprising. For example, a small population of CD34−lin−cells has the capacity to repopulate in a pattern similar to that of cord blood and adult CD34+ populations.6Conversely, second-gestation human fetal blood samples engraft in the absence of cytokine support with a pronounced erythroid bias when compared with cord blood or adult samples (Pahal G and Thrasher AJ, unpublished observations, 1999).
Lineage distribution in NOD/SCID mice engrafted with fresh cord blood CD34+ cells or cord blood CD34+ cells cultured for 14 days in serum-free medium supplemented with IL-6 (10 ng/mL), IL-11 (10 ng/mL), FL (50 ng/mL), and TPO (10 ng/mL). Nine mice engrafted with 2 × 105 to 106 CD34+ cells from 2 cord samples were analyzed in each group.
Lineage distribution in NOD/SCID mice engrafted with fresh cord blood CD34+ cells or cord blood CD34+ cells cultured for 14 days in serum-free medium supplemented with IL-6 (10 ng/mL), IL-11 (10 ng/mL), FL (50 ng/mL), and TPO (10 ng/mL). Nine mice engrafted with 2 × 105 to 106 CD34+ cells from 2 cord samples were analyzed in each group.
As clearly shown in another recent publication, the time for reconstitution after cord blood transplantation, which is directly related to the cell dose, may be a significant limiting factor.7 Selective expansion of alternative repopulating cell populations, such as those restricted to the myeloid lineage, may therefore be of significant benefit for hematopoietic support in the early time period after cord transplantation. At the same time, potential changes in the commitment of repopulating cells initiated by ex vivo culture need to be clarified. The reasons for the differences in engraftment quality noted in Guenechea’s study and our own are not entirely clear but could relate to the timing of analysis (120v 40 days), or to differences in ex vivo culture conditions. Further studies on the quantitation, kinetics, and quality of repopulating cell engraftment after ex vivo culture of cord blood cells are therefore warranted if stem cell expansion protocols are to be effectively optimized for clinical use.
ACKNOWLEDGMENT
A.J.T. is a Wellcome Clinical Scientist.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal