Abstract
Human erythroid precursors grown in culture possess membrane receptors that bind and internalize acid isoferritin. These receptors are regulated by the iron status of the cell, implying that ferritin iron uptake may represent a normal physiologic pathway. The present studies describe the fate of internalized ferritin, the mechanisms involved in the release of its iron, and the recognition of this iron by the cell. Normal human erythroid precursors were grown in a 2-phase liquid culture that supports the proliferation, differentiation, and maturation of erythroid precursors. At the stage of polychromatic normoblasts, cells were briefly incubated with 59Fe- and/or125I-labeled acid isoferritin and chased. The125I-labeled ferritin protein was rapidly degraded and only 50% of the label remained in intact ferritin protein after 3 to 4 hours. In parallel, 59Fe decreased in ferritin and increased in hemoglobin. Extracellular holoferritin uptake elevated the cellular labile iron pool (LIP) and reduced iron regulatory protein (IRP) activity; this was inhibited by leupeptin or chloroquine. Extracellular apoferritin taken up by the cell functioned as an iron scavenger: it decreased the level of cellular LIP and increased IRP activity. We suggest that the iron from extracellular is metabolized in a similar fashion by developing erythroid cells as is intracellular ferritin. Following its uptake, extracellular ferritin iron is released by proteolytic degradation of the protein shell in an acid compartment. The released iron induces an increase in the cellular LIP and participates in heme synthesis and in intracellular iron regulatory pathways.
DEVELOPING ERYTHROID CELLS (DEC) take up substantial amounts of iron, mainly for heme synthesis. Iron in excess of immediate cellular requirements is stored in ferritin. Ferritin functions as an iron-storage protein and is essential for cellular iron homeostasis. The amount of iron in cells exceeds its solubility, and it is necessary to concentrate excess iron and maintain its solubility. Excess cellular iron is stored in a soluble and nontoxic form in ferritin. Isoferritins are heteropolymers, consisting of 24 subunits of two types: H (heavy) and L (light), with a molecular mass of approximately 21 kD and 19 kD, respectively. The relative ratio of the two subunits is characteristic of tissue and cell type.1Isoferritins rich in H-type subunits have a lower isoelectric point (pI) than isoferritins composed predominantly of L-type subunits (basic iso-Fts). The ferritin protein shell surrounds a central iron core that contains up to 4,500 iron atoms.1-4 Recent evidence suggests that ferritin is not simply an “iron sink,” but plays a dynamic role in cellular iron metabolism. In addition to its iron-scavenging capacity, ferritin serves also as a potential source of iron for the synthesis of heme5 and iron-containing enzymes6 and for replenishing the labile iron pool (LIP)7 believed to be identical to the chelatable iron pool.8 The presumed mechanism of ferritin iron mobilization is protein degradation by acid proteases in an acidic compartment of the cell.5
The critical need for iron in all living cells on the one hand, and its toxicity on the other is underlined by a subtle coordinated mechanism of regulation of iron sensing, acquisition, storage, and utilization, which is particularly important when iron is in great demand, such as in hemoglobin-synthesizing cells. Transferrin receptor (TfR) expression, which is the major route of iron uptake, is postranscriptionally regulated through the stability of its mRNA. The biosynthesis of ferritin subunits and that of erythroid δ-aminolevulonic acid synthase are also regulated by cellular iron status. mRNAs for TfR carry at the 3′, and for ferritin at the 5′ end, specific stem-loop structures, or iron-responsive elements (IRE), which reversibly bind iron-regulatory proteins (IRP-1 and IRP-2).9,10 Low cellular iron levels activate IRP-1 by removing an attached iron-sulfur cluster10 and the half-life of IRP-2 is prolonged under these conditions,11causing them to bind to the ferritin-IRE and to inhibit translation. When cellular iron levels rise, IRP-1 is inactivated and IRP-2 is degraded following oxidation and ubiquitination.12,13 The absence of IRP binding to IRE allows continued apoferritin translation. It is suggested that the regulation of intracellular iron metabolism by the above mechanisms is through cytosolic LIP. The level of the LIP is thus both sensed and homeostatically controlled by IRPs.7Iron newly acquired by the cell emerges initially in the LIP and excess iron is subsequently sequestered within the ferritin molecule.
Iron delivery to mammalian cells in general and to erythroid cells in particular is largely attributed to diferric transferrin. In a previous report, we have shown that developing human erythroid cells possess on their surface, in addition to TfR, receptors that bind specifically and internalize acid isoferritin.14 This was performed by a specific, saturable process, distinct from the uptake of iron associated with albumin. It was highly regulated by the iron status of the cell and by its degree of maturation. Internalized ferritin-iron is utilized for heme synthesis and, thus, this process could represent a physiologic pathway for iron assimilation.15 Ferritin receptors that internalize ferritin are also found on cells of the human T-cell line MOLT4.16
In another study, we have shown that intracellularly synthesized ferritin released its iron by a proteolytic process in a lysosome-like compartment.5 We hypothesized that extracellular ferritin, once internalized by the cell, is indistinguishable from intracellular ferritin in its iron release pathway and its effect on cellular iron metabolism. To test this hypothesis, we monitored the course of the internalized ferritin-iron and ferritin-protein in human DEC grown in liquid culture and studied the effects of leupeptin, a reversible inhibitor of trypsin-like and cysteine proteases, and chloroquine, a weak, acidophylic base known to inhibit lysosomal and siderosomal function by rising their pH.17 We found that both extracellular holoferritin and apoferritin were rapidly internalized by the cells and were degraded by proteolysis in an acidic cellular compartment. This was followed by iron transfer from the internalized ferritin into hemoglobin. Treatment with the above inhibitors prevented ferritin degradation, as well as transfer of its iron. The effect of internalized ferritin on intracellular iron metabolism was studied by measuring the levels of LIP and the activity of IRP. The results indicated that internalized holoferritin increased cellular LIP and decreased IRP, whereas apoferritin had an opposite effect, which suggests that internalized holoferritin is an iron donor, while apoferritin behaves like an iron chelator.
The results of the present study show that extracellular ferritin and apoferritin taken up by developing human erythroid cells grown in liquid culture modify intracellular iron metabolism. Iron from extracellular holoferritin is released by a proteolytic mechanism and represses IRP activity, while apoferritin chelates cellular iron, decreases cellular LIP, and activates IRP. Extracellular ferritin-iron once taken up is processed by the cell in the same way as intracellular ferritin-iron.
MATERIALS AND METHODS
Erythroid cell cultures.
The 2-phase liquid culture was used as previously described.18 19 Briefly, mononuclear cells were isolated from peripheral blood samples of normal donors by Ficoll-Hypaque density gradient centrifugation and seeded in alpha-minimal essential medium (α-MEM) supplemented with 10% fetal calf serum (FCS) (both from Biological Industries, Beit-Haemek, Israel), 1 μg/mL cyclosporine A (Sandoz, Basel, Switzerland), and 10% conditioned medium from the 5637 bladder carcinoma cell line. The cultures were incubated at 37°C, under an atmosphere of 5% CO2 in air, with extra humidity. After 7-day incubation in this phase I culture, the nonadherent cells were harvested, washed, and recultured in fresh medium composed of α-MEM, 30% FCS, 1% deionized bovine serum albumin (BSA), 10−5 mol/L β-mercaptoethanol, 15 mmol/L glutamine, 10−6 mol/L dexamethasone, and 1 U/mL human recombinant erythropoietin (Ortho Pharmaceutical, Raritan, NJ). This part of the culture is referred to as phase II. After 5 or 6 days of incubation, erythroblasts were purified by centrifugation on 45% Percol (density, 1.0585 g/mL; Pharmacia, Uppsala, Sweden). The upper layer, which contained mainly proerythroblasts and basophilic normoblasts, was collected, washed, and resuspended in the original medium for future incubation. Cell samples were analyzed between the sixth and twelfth days of phase II. Viability of the cells was determined by trypan blue exclusion and was higher than 95%. The majority of erythroid cells were basophilic normoblasts on day 6 of phase II, polychromatophilic normoblasts on days 8 to 10, and orthochromatic normoblasts on day 12.
Ferritin.
Ferritin was isolated from human term placenta and fractionated into isoferritins as previously described.20 The isoferritin used in the present experiments was the “acid I” fraction, which has the highest H to L subunit ratio of the placental isoferritins and as such the most acidic pI, designated in this report as “ferritin.”
Preparation of apoferritin.
Apoferritin was prepared as described previously,15 21 by the reduction and the subsequent chelation of the iron core of the ferritin. In brief, human acid I isoferritin was dialyzed overnight against a 500-fold excess (vol/vol) of 0.2 mol/L acetate buffer, pH 5.5, containing 1% thioglycolic acid and 10 mmol/L 2,2’-bipyridyl, at 4°C. It was subsequently dialyzed 4 times against 0.2 mol/L Na-acetate buffer, pH 5.5, and twice against 10 mmol/L 3-(N-morpholino) propanesulfonic acid (MOPS) buffer, pH 7.0.
Preparation of 59Fe-labeled ferritin.
Apoferritin was labeled with 59Fe in 0.2 mol/L MOPS buffer, pH 7.0, with no additional Fe-binding ligands, using a mixture of 5%59Fe(III), as 59FeCl3 (Dupont-NEN, Boston, MA) and 95% 56Fe(II) as ferrous sulfate. Equilibration between Fe(II) and Fe(III) was achieved in 0.1N HCl. The iron loading was performed essentially as described by Levi et al.22 MOPS buffer was chosen over 2-[N-Morpholino] ethanesulfonic acid (MES) buffer, since the auto-oxidation rate of iron in MOPS was slower than in any of several other “Good” buffers tested, thereby decreasing the tendency for the formation of non–ferritin-bound ferric-hydroxypolymers. Labeling was performed in the presence of 100 U/mL catalase to reduce harmful free radical reactions. Aliquots of the 59Fe solution, each providing a final concentration of 0.1 mmol/L iron, were added to a solution that contained 0.25 mg/mL apoferritin, to give a total of 800 to 1,000 iron atoms per ferritin molecule. Ferritin labeling was followed spectroscopically at 310 nm. Aliquots of Fe(II) were added only after the previous Fe(II) was completely oxidized. Small amounts of iron polymers and aggregated ferritin were removed by filtration before the use of the ferritin preparations.15Incorporation of the ferritin-associated iron into the ferritin iron core under the conditions employed has been firmly established.23
Preparation of 125I-labeled ferritin.
Ferritin was iodinated by solid-phase enzymatic radioiodination with Enzymobead (Bio-Rad Laboratories, Richmond, CA) according to the procedure supplied by the manufacturer as described previously.14 125I-ferritin was separated from free iodine on a Bio-Spin 30 column (Bio-Rad).
Ferritin uptake and degradation by erythroid precursor cells.
Cells were washed 3 times with phosphate-buffered saline (PBS) and then incubated in serum-free phase II medium supplemented with 3% BSA, named “incubation medium.” Cells were incubated for 30 minutes with 4 nmol/L ferritin labeled with either 59Fe or125I, or with 4 nmol/L apoferritin labeled with125I (pulse labeling). The cells were then washed once with incubation medium and incubated again with incubation medium that contained 400 nmol/L unlabeled ferritin or apoferritin. Incubation was continued for indicated times and cell lysates were prepared for sodium-lauryl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Where indicated, cells were preincubated for 60 minutes, before pulse labeling, in serum-free medium with 15 μmol/L chloroquine or 20 μg/mL leupeptin. Inhibitors were added again after each change of medium and fresh leupeptin, which has a short half-life at 37°C, was added every 12 hours. Cells were harvested at indicated times, washed 4 times with PBS, and lysed on ice in solubilization buffer containing 1% Triton X-100 (Pierce, Rockford, IL), 10 μg/mL aprotinin, 10 μg/mL leupeptin (both from Boehringer Mannheim, Mannheim, Germany), 10 μg/mL benzamidine, 3.7μg/mL N-tosyl-L-phenyl-alanine-chloromethyl-ketone (TPCK), 1 μmol/L N-tosyl-L-lysine-chloromethyl-ketone (TLCK), 0.25 mmol/L phenylmethylsulfonylfluoride (PMSF), and 0.02% sodium azide (all from Sigma-Israel, Rehovoth, Israel) in 10 mmol/L Tris-HCl, pH 7.4. The lysates were centrifuged at 10,000g for 15 minutes. The supernates were collected and stored at −80°C until use. Lysates were analyzed for the whole ferritin molecule by SDS-PAGE on 6% gels without heating the samples under nonreducing conditions. For analysis of subunits, samples were heated for 3 minutes at 96°C under reducing conditions, and analyzed on 10% to 15% SDS-PAGE gradient gels. Gels were dried and exposed to phosphor-imaging (Fujix Bas 1000 Fuji, Japan) and/or autoradiography. Quantitation of the radioactivity in the gels was done by scanning with the phosphor imager.
Electromobility RNA gel retardation assay.24
Cells were washed twice with ice-cold PBS and then lysed by incubation on ice for 30 minutes with solubilization buffer that contained 25 mmol/L Tris-HCl, pH 8, 40 mmol/L KCl, and the previously specified protease inhibitors. Cell nuclei were precipitated at 10,000gfor 15 minutes. Protein content of the supernatant was measured with the “BCA Protein Assay Reagent” (Pierce).
Freshly prepared lysates containing 10 μg protein were incubated for 10 minutes at room temperature with 15 μL of an32P-labeled IRE Probe cocktail (containing 30,000 cpm/lane IRE Probe 24, 2 μg/lane tRNA, 3.3% glycerol, 1 μL/lane ACE-RNAse inhibitor [5Prime→3Prime Inc, West Chester, PA]). Incubation was performed for 5 minutes at room temperature without (active IRP) or with 2% β-mercaptoethanol (total IRP). The reaction mixture was applied to an 8% native polyacrylamide gel and electrophoresis was allowed to proceed for 2 hours at 180 V. As a marker for the32P-IRE-IRP migration, recombinant IRP was used. Gels were fixed by immersing them in a mixture containing 40% methanol and 10% acetic acid in water and subsequently dried. Gels were exposed to phosphor imaging and/or autoradiography.
Measurement of the cellular LIP.
Cellular LIP was measured with the fluorescent metal sensor calcein-aceto-methory (AM) as previously described.25 Cells were incubated without (control) or with either 40 nmol/L H-rich holoferritin or 40 nmol/L apoferritin for 64 hours. Cells were then incubated for 5 minutes at 37°C in MEM-BSA containing 250 nmol/L calcein-AM. After calcein loading, the cells were washed 3 times and resuspended in 20 mmol/L Na-HEPES, 145 mmol/L NaCl, pH 7.2, 37°C. Fluorescence (488 nm excitation, 517 nm emission) was measured in a continuous mode using a PTI fluorescence station (PTI, New Brunswick, NJ) with the cells constantly stirred and kept at 37°C. After attaining a stable baseline, anticalcein antibodies were added (for quenching extracellular probe fluorescence) and the amount of intracellular metal, bound to calcein (CA-Fe), was assessed by addition of 100 μmol/L of the fast permeating chelator isonicotinoyl-salicyl-aldehyde-hydrazone (SIH, kindly donated by Dr Prem Ponka, Montreal, Canada).
RESULTS
Iron release from internalized ferritin.
We have previously shown that H-subunit–rich extracellular ferritin is taken up by human DEC in culture by a receptor-mediated process and its iron is incorporated into heme.15 Likewise, iron from intracellular ferritin is released in a lysosome-like compartment by a proteolytic process.5 Proteolysis in acid compartments could correspondingly be involved in the release of iron from ferritin internalized by DEC. To test this hypothesis, cells were treated with leupeptin, a reversible inhibitor of trypsin-like and cysteine proteases, or with chloroquine, a weak, acidophilic base known to inhibit lysosomal and siderosomal function by raising their pH.17
59Fe-labeled ferritin (“acid I” placental isoferritin,20 see Materials and Methods) was used to follow the fate of ferritin iron internalized by DEC. Cells on day 8 of phase II were pulse-labeled for 30 minutes with 4 nmol/L59Fe-ferritin and chased with unlabeled ferritin as described in Materials and Methods. Incubation was continued for times as indicated. The distribution of radioiron between ferritin and hemoglobin in the lysates was followed by SDS-PAGE under nonreducing conditions, followed by phosphor imaging and scanning in the phosphor imager. Figure 1 shows that already after the 30-minute pulse, some radioiron appeared in hemoglobin. Internalized 59Fe-ferritin released its iron and a decrease in 59Fe in ferritin was observed concomitant with an increase of 59Fe in hemoglobin. Thus, in control cells at time 0 (after a 30-minute pulse and chase), 28% of the radioiron was in hemoglobin, and, at 48 hours, 60% was in hemoglobin. The effect of leupeptin or chloroquine was measured by preincubating the cells for 60 minutes with either 20 μg/mL leupeptin or 15 μmol/L chloroquine followed by labeling with a 30-minute pulse with 4 nmol/L59Fe-ferritin and chasing with unlabeled ferritin as described earlier. The inhibitors were present during the entire experiment; leupeptin was renewed every 12 hours.
Distribution of radioiron between internalized ferritin and hemoglobin (Hb) following inhibition of protein degradation. DEC on day 8 of phase II were pulse labeled for 30 minutes with 4 nmol/L59Fe-ferritin, then washed and chased with 400 nmol/L unlabeled ferritin. Inhibition of protein degradation was initiated 60 minutes before the 59Fe-ferritin pulse by 20 μg/mL leupeptin or 15 μmol/L chloroquine, and inhibitors were present throughout the experiment. Leupeptin, which has a short half-life at 37°C, was added every 12 hours. Equal amounts of protein from cell lysates were analyzed on SDS-PAGE under nonreducing conditions followed by phosphor imaging and scanning with the phosphor imager.59Fe-Hb was expressed as percentage of the label in Hb and ferritin. [59Fe-Hb/(59Fe-Hb +59Fe-ferritin)] × 100. Unlabeled Hb was used as a marker (not shown).
Distribution of radioiron between internalized ferritin and hemoglobin (Hb) following inhibition of protein degradation. DEC on day 8 of phase II were pulse labeled for 30 minutes with 4 nmol/L59Fe-ferritin, then washed and chased with 400 nmol/L unlabeled ferritin. Inhibition of protein degradation was initiated 60 minutes before the 59Fe-ferritin pulse by 20 μg/mL leupeptin or 15 μmol/L chloroquine, and inhibitors were present throughout the experiment. Leupeptin, which has a short half-life at 37°C, was added every 12 hours. Equal amounts of protein from cell lysates were analyzed on SDS-PAGE under nonreducing conditions followed by phosphor imaging and scanning with the phosphor imager.59Fe-Hb was expressed as percentage of the label in Hb and ferritin. [59Fe-Hb/(59Fe-Hb +59Fe-ferritin)] × 100. Unlabeled Hb was used as a marker (not shown).
Compared with controls, considerably less radioiron was observed in hemoglobin following incubation with either leupeptin or chloroquine. Thus, only 16% to 19% and 25% to 26% of the radioiron was in hemoglobin 30 minutes and 48 hours after the chase, respectively. Thus, inhibition of proteolysis by leupeptin as well as inhibition of lysosome-like activity by chloroquine caused radioiron to remain in the internalized ferritin and inhibited its transfer to hemoglobin.
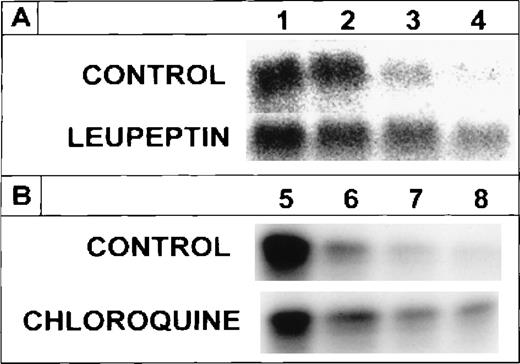
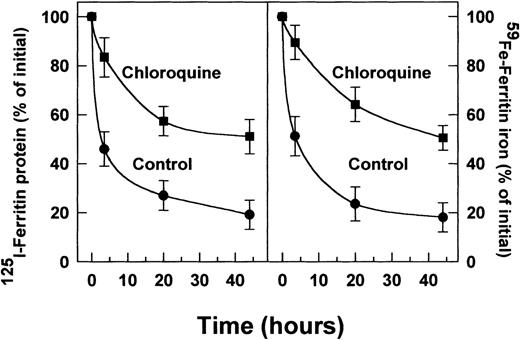
The suppression of radioiron release from internalized ferritin by leupeptin and by chloroquine implies that ferritin protein degradation is necessary for its iron release. To clarify this point, cells on day 8 of phase II were treated with leupeptin or chloroquine and pulse-labeled with 4 nmol/L ferritin whose protein moiety was labeled with 125I, followed by a chase with unlabeled ferritin, as described earlier. Incubation was continued for indicated periods. The cellular content of the 125I-ferritin was followed by SDS-PAGE under reducing conditions. Following its uptake,125I-ferritin underwent proteolysis in untreated, control cells, while in cells treated with leupeptin or chloroquine,125I-ferritin protein degradation was inhibited (Fig2). The half-life of the radioiodinated ferritin-protein was calculated to be 3.5 hours. The radioiron content of ferritin in the presence or absence of chloroquine correlated with the decay of the 125I-label of the ferritin protein (Fig3). Thus, release of ferritin iron is dependent on proteolysis.
Effect of leupeptin or chloroquine on the degradation of internalized 125I-ferritin protein. Human DEC were incubated on day 8 of phase II for 60 minutes with either 20 μg/mL leupeptin or 15 μmol/L chloroquine. Cells were pulse labeled for 30 minutes with 4 nmol/L 125I-ferritin then washed and chased with 400 nmol/L unlabeled ferritin. Incubation was continued for 0, 3, 8, and 24 hours (A, lanes 1, 2, 3, and 4, respectively) or for 0, 3, 24, and 48 hours (B, lanes 5, 6, 7, and 8, respectively). Inhibitors were refreshed after each wash and fresh leupeptin was added every 12 hours. Equal amounts of protein from cell lysates were analyzed on SDS-PAGE under reducing conditions.
Effect of leupeptin or chloroquine on the degradation of internalized 125I-ferritin protein. Human DEC were incubated on day 8 of phase II for 60 minutes with either 20 μg/mL leupeptin or 15 μmol/L chloroquine. Cells were pulse labeled for 30 minutes with 4 nmol/L 125I-ferritin then washed and chased with 400 nmol/L unlabeled ferritin. Incubation was continued for 0, 3, 8, and 24 hours (A, lanes 1, 2, 3, and 4, respectively) or for 0, 3, 24, and 48 hours (B, lanes 5, 6, 7, and 8, respectively). Inhibitors were refreshed after each wash and fresh leupeptin was added every 12 hours. Equal amounts of protein from cell lysates were analyzed on SDS-PAGE under reducing conditions.
Effect of chloroquine on radiolabeled (125 I) ferritin-protein degradation (A) and radioiron loss (B) from extracellular ferritin taken up by DEC. Incubations were performed for 0, 5, 20, and 44 hours. Other experimental conditions were as described in Figs 1 and 2. The intensity of the ferritin-label was measured by scanning with the phosphor imager. Means ± SD of 3 independent experiments.
Effect of chloroquine on radiolabeled (125 I) ferritin-protein degradation (A) and radioiron loss (B) from extracellular ferritin taken up by DEC. Incubations were performed for 0, 5, 20, and 44 hours. Other experimental conditions were as described in Figs 1 and 2. The intensity of the ferritin-label was measured by scanning with the phosphor imager. Means ± SD of 3 independent experiments.
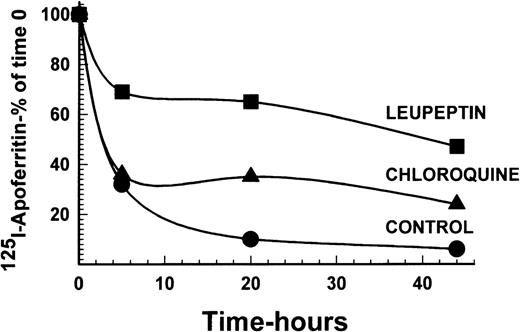
To examine the necessity for iron to be present in ferritin for protein degradation, DEC were incubated with 125I-apoferritin, in the presence or absence of 20 μg/mL leupeptin or 15 μmol/L chloroquine. Following a chase with apoferritin as above, analysis by SDS-PAGE under reducing conditions showed that apoferritin was degraded in DEC, with a half-life of approximately 2.5 hours. Leupeptin, as well as chloroquine, inhibited apoferritin degradation, leupeptin being more effective than chloroquine at the concentrations used (Fig4). Hence, iron in ferritin is not essential for degradation of the protein.
Radiolabeled apoferritin protein degradation following inhibition by leupeptin or chloroquine. Cells were labeled with 4 nmol/L 125I-apoferritin and chased with 400 nmol/L unlabeled apoferritin. Incubations were performed for 0, 5, 20, and 44 hours. Other experimental conditions were as described in Figs 1 and 2. The intensity of the apoferritin-label was measured by scanning with the phosphor imager of SDS-PAGE performed under reducing conditions. One of 2 experiments with comparable results is shown.
Radiolabeled apoferritin protein degradation following inhibition by leupeptin or chloroquine. Cells were labeled with 4 nmol/L 125I-apoferritin and chased with 400 nmol/L unlabeled apoferritin. Incubations were performed for 0, 5, 20, and 44 hours. Other experimental conditions were as described in Figs 1 and 2. The intensity of the apoferritin-label was measured by scanning with the phosphor imager of SDS-PAGE performed under reducing conditions. One of 2 experiments with comparable results is shown.
The effect of extracellular ferritin on the LIP.
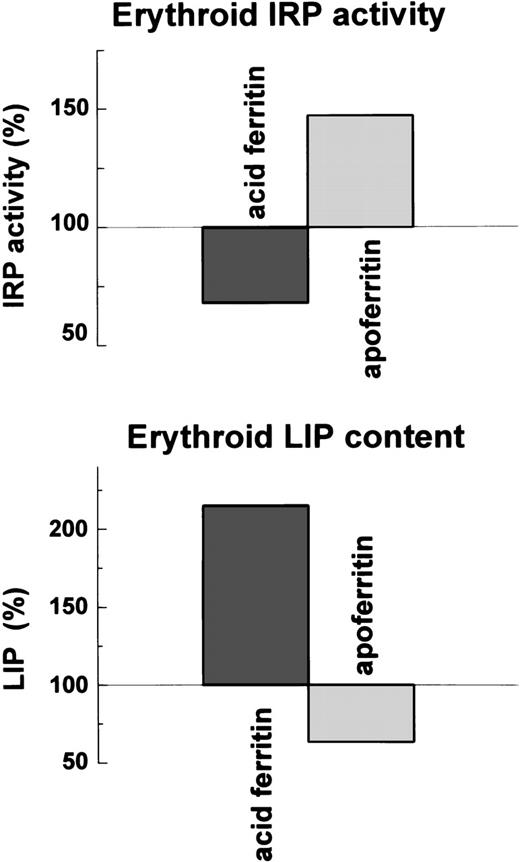
The cellular compartment representing the metabolically active form of iron is thought to be a weakly bound low molecular fraction that possibly connects between cytosolic ferritin and hemoglobin. This compartment is referred to as the chelatable iron or LIP.6If iron is released from internalized extracellular ferritin and is transferred from there to hemoglobin, it would probably be channeled through the LIP. Therefore, we measured the LIP in DEC after incubation with either 40 nmol/L ferritin or 40 nmol/L apoferritin for 64 hours beginning on day 6 of phase II. Ferritin uptake by DEC led to a 200% increase in intracellular LIP compared with control cells, whereas apoferritin caused a decrease in LIP to 50% of controls (Fig5).
Effect of extracellular ferritin on the LIP. Cellular LIP was measured with the fluorescent tracer calcein. DEC were incubated with 40 nmol/L H-rich holoferritin or apoferritin for 64 hours, beginning at day 6 of phase II or with medium only (control). Cells were loaded with 250 nmol/L calcein AM for 5 minutes at 37°C. Fluorescence was measured before and after chelation with 100 μmol/L SIH. Relative cellular LIP values are demonstrated.
Effect of extracellular ferritin on the LIP. Cellular LIP was measured with the fluorescent tracer calcein. DEC were incubated with 40 nmol/L H-rich holoferritin or apoferritin for 64 hours, beginning at day 6 of phase II or with medium only (control). Cells were loaded with 250 nmol/L calcein AM for 5 minutes at 37°C. Fluorescence was measured before and after chelation with 100 μmol/L SIH. Relative cellular LIP values are demonstrated.
Effect of extracellular ferritin on IRP activity.
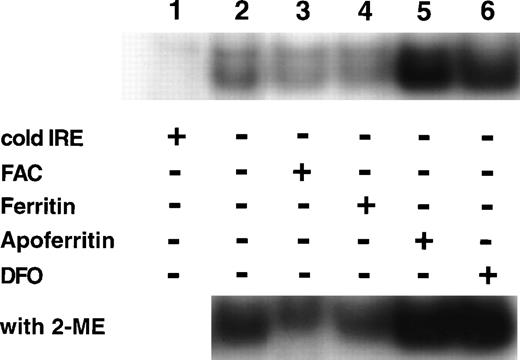
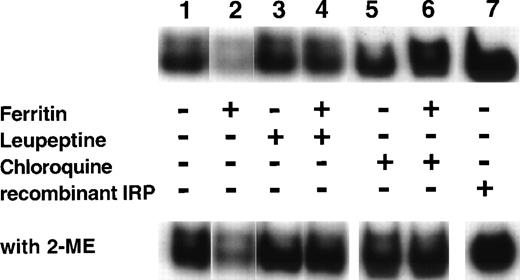
If IRP activity is regulated by the magnitude of intracellular LIP, the addition of extracellular ferritin or apoferritin, modifying cellular LIP levels (as shown in Fig 5), should affect IRP activity. Cells were incubated on day 6 of phase II for 64 hours with either 40 nmol/L ferritin, 40 nmol/L apoferritin, 100 μmol/L ferric ammonium citrate (FAC) or 50 μmol/L deferoxamine (DFO). The relative activities of IRP are shown in Fig 6. Ferritin caused a reduction in IRP activity similar to the decline in activity observed by incubating the cells for the same time period with FAC. Incubation with apoferritin led to increased IRP activity similar to that observed after incubation with DFO. Because identical amounts of protein were applied from cellular lysates, it seems that total IRP levels were affected as well, as shown by IRP activity in the presence of β-mercaptoethanol.
Regulation of IRP activity by extracellular ferritin. Cells were incubated with medium only (control, lane 2), 100 μmol/L ferric ammonium citrate (FAC, lane 3), 40 nmol/L H-rich holoferritin or apoferritin (lanes 4 and 5, respectively), or with 50 μmol/L DFO (lane 6) for 64 hours, beginning at day 6 of phase II. Fresh cytoplasm extracts (10 μg protein) were analyzed for IRP activity by electromobility-RNA gel retardation. In lane 1, a 100-fold excess of unlabeled IRE was added as a specific competitor to the labeled IRE. Cell lysates were analyzed without β-mercaptoethanol (2-ME) (top panel) or after incubation with 2% 2-ME for 5 minutes at room temperature (bottom panel).
Regulation of IRP activity by extracellular ferritin. Cells were incubated with medium only (control, lane 2), 100 μmol/L ferric ammonium citrate (FAC, lane 3), 40 nmol/L H-rich holoferritin or apoferritin (lanes 4 and 5, respectively), or with 50 μmol/L DFO (lane 6) for 64 hours, beginning at day 6 of phase II. Fresh cytoplasm extracts (10 μg protein) were analyzed for IRP activity by electromobility-RNA gel retardation. In lane 1, a 100-fold excess of unlabeled IRE was added as a specific competitor to the labeled IRE. Cell lysates were analyzed without β-mercaptoethanol (2-ME) (top panel) or after incubation with 2% 2-ME for 5 minutes at room temperature (bottom panel).
Effect of protease inhibitors on the regulation of IRP activity by ferritin.
Ferritin downregulates IRP activity (Fig 6), presumably by release of ferritin iron causing an increase in the cellular LIP. As apoferritin enhanced IRP activity (Fig 6), inhibiting iron release from ferritin might be expected to abolish the decrease in IRP activity. Cells, beginning at day 6 of phase II, were incubated with 40 nmol/L ferritin for 64 hours and, where indicated, leupeptin or chloroquine was added. Both leupeptin and chloroquine inhibited ferritin protein degradation and suppressed iron release from ferritin (Fig 2). Figure7 shows that, indeed, both leupeptin and chloroquine prevented the downregulation of IRP activity by ferritin.
Effect of leupeptin and chloroquine on the influence of ferritin on IRP activity. DEC were incubated for 64 hours, beginning on day 6 of phase II, with the following: medium only (lane 1), 40 nmol/L ferritin (lane 2), 20 μg/mL leupeptin (lane 3), 40 nmol/L ferritin together with 20 μg/mL leupeptin (lane 4), 15 μmol/L chloroquine (lane 5), or 40 nmol/L ferritin together with 15 μmol/L chloroquine (lane 6). Fresh cytoplasmic extracts (10 μg protein) were analyzed for IRP activity by electromobility-RNA gel retardation. A control containing 120 ng of recombinant IRP-1 was included (lane 7). Cell lysates were analyzed without 2-ME (top panel) or after incubation with 2% 2-ME for 5 minutes at room temperature (lower panel).
Effect of leupeptin and chloroquine on the influence of ferritin on IRP activity. DEC were incubated for 64 hours, beginning on day 6 of phase II, with the following: medium only (lane 1), 40 nmol/L ferritin (lane 2), 20 μg/mL leupeptin (lane 3), 40 nmol/L ferritin together with 20 μg/mL leupeptin (lane 4), 15 μmol/L chloroquine (lane 5), or 40 nmol/L ferritin together with 15 μmol/L chloroquine (lane 6). Fresh cytoplasmic extracts (10 μg protein) were analyzed for IRP activity by electromobility-RNA gel retardation. A control containing 120 ng of recombinant IRP-1 was included (lane 7). Cell lysates were analyzed without 2-ME (top panel) or after incubation with 2% 2-ME for 5 minutes at room temperature (lower panel).
Relationship of LIP concentration to IRP activity.
If IRP activity is affected by LIP concentration, LIP concentration and the IRP activity should be inversely correlated. Indeed, ferritin by releasing its iron, and apoferritin, presumably by binding iron, altered the magnitude of LIP and the activity of IRP in an inversely correlated manner (Fig 8).
Effect of ferritin and apoferritin on LIP levels and IRP activity in erythroid cells . Cellular LIP was measured with a fluorescent tracer and cellular IRP activity by electromobility-RNA gel retardation.
Effect of ferritin and apoferritin on LIP levels and IRP activity in erythroid cells . Cellular LIP was measured with a fluorescent tracer and cellular IRP activity by electromobility-RNA gel retardation.
DISCUSSION
Cellular ferritin has been long thought to serve as an iron storage protein that protects cells against the toxic effects of free iron. We have previously shown that in DEC, ferritin may also function as a metabolically active reservoir and donate its iron for heme synthesis.5 Cellular ferritin in DEC is derived from 2 sources: (1) de novo, intracellularly synthesized ferritin, which is regulated by the iron status of the cell through the activity of IRP10-13; and (2) extracellular ferritin, the uptake of which is mediated through specific surface receptors.14,15By studying the turnover of the de novo synthesized ferritin in DEC, we have shown that in order to release its iron to LIP, representing the metabolically active form of cellular iron, and subsequently to heme, this ferritin should be proteolytically degraded in an acid compartment (eg, lysozomes) of the cell.5 7
In the present study, we have monitored the course of extracellular ferritin internalized by DEC. We hypothesized that if extracellular-derived ferritin internalized by the cells behaves like intracellularly synthesized ferritin, it should have a similar effect on cellular iron metabolism. To test this hypothesis, we incubated DEC, at their basophilic normoblast stage, with acid ferritin containing59Fe or ferritin whose protein was labeled with125I. Our results showed that short (30-minute) incubation was enough for internalization of discernable amounts of ferritin into the cells. 59Fe was observed in hemoglobin immediately following the pulse (Fig 1, Time 0); with time, 59Fe decreased in ferritin and increased in hemoglobin. This was associated with a decrease in 125I in ferritin. Leupeptin, a reversible inhibitor of trypsin-like and cysteine proteases, and chloroquine, a weak acidophylic base known to inhibit lysosomal and siderosomal functions by increasing their pH 17, inhibited the rate of release of ferritin iron and the decay of ferritin protein. These results indicate that extracellular ferritin can be taken up by the cells, and once internalized its iron is transferred into heme by a process that involves proteolytic degradation in an acid compartment of the cell. These results showed a pattern previously established for endogenous, intracellular ferritin.5
When these experiments were repeated with apoferritin, the results showed that apoferritin too is internalized and proteolytically degraded, with a half-life of 2.5 hours. Thus, in contrast to transferrin uptake, which is specific to iron-loaded transferrin, both iron-loaded holoferritin and iron-free apoferritin can be taken up and proteolytically degraded by DEC.
We further studied the effect of internalized ferritin and apoferritin on cellular iron metabolism by measuring LIP levels and IRP activity. We found that ferritin increased, whereas apoferritin depressed cellular LIP. The latter results are in agreement with previous studies in which mouse erythroleukemic cells that were transfected with and overexpressed the H-ferritin subunit showed a significant lowering of cellular LIP26,27 in association with decreased hemoglobin production.27 IRP activity was measured by an RNA gel retardation assay. Ferritin was found to decrease, and apoferritin to increase IRP activity (Figs 6 through 8). These results demonstrated that ferritin behaves as an iron donor (eg, ferric ammonium citrate). Leupeptin, a reversible inhibitor of trypsin-like and cysteine proteases, and chloroquine, a weak, acidophylic base known to inhibit lysosomal and siderosomal functions by increasing their pH,17 with or without added ferritin, caused an increase in IRP activity apparently by inhibiting ferritin proteolysis and, as such, decreasing LIP level.5 7 Apoferritin behaved in a manner similar to an iron chelator (eg, deferoxamine).
The mechanism by which extracellular apoferritin may decrease cellular LIP is an enigma, since it is difficult to conceive how could apoferritin migrate from the endosome (if, indeed it is taken up into an endosome) to the cytoplasm. However, apoferritin potentially could prevent endosomal iron from being released into the cytoplasm.
Our results suggest an inverse relation between cellular LIP and IRP activity. Moreover, leupeptin or chloroquine, by inhibiting proteolysis, causes an increase in IRP activity, presumably via a decrease in the LIP levels.5,7 The concept of LIP levels regulating IRP activity and vice versa has been dealt with extensively,7,28,29 but it has not been easy to substantiate because of difficulties in measuring LIP.30Recently, we have implicated such an association for K562 cells using the calcein fluorescent method for LIP measurements.7 The present studies, employing a novel fluorescence-based method for the determination of LIP in living cells,25 strongly favor this concept.
Extracellular acid isoferritin may have a physiologic role in iron acquisition by erythroid cells as its uptake is regulated by the iron status of the cell.15 However, it is unlikely that serum ferritin serves such a role, since serum ferritin is iron poor (behaving rather like apoferritin31) and is of a basic nature, whereas DEC have a higher affinity for acid ferritin.14,15 In the local microenvironment of the bone marrow, erythroid precursors are associated with a central reticuloendothelial cell in erythropoietic islands. Electron microscopic studies have long suggested that ferritin may be transferred from macrophages to erythroid cells.32Recently, it was found that some mouse embryos lacking transferrin receptors might still produce near-normal amounts of erythrocytes early in gestation, which suggests ferritin uptake directly from the erythropoietic milieu or from macrophages.33 Preliminary studies in our laboratory using the 2-phase erythroid culture system (unpublished data, May 1997) have shown the transfer of radioiron from macrophage ferritin to erythroid cells and its incorporation into hemoglobin.
Our work shows that extracellular ferritin or apoferritin internalized by DEC modify intercellular iron metabolism by donating iron to or depriving iron from the LIP, resulting in modified IRP activity. Iron is released from ferritin by a proteolytic event, most probably in an acidic compartment, as indicated by the prevention of iron release and suppression of IRP activity by added ferritin, following the use of protease inhibitors and chloroquine. Internalized extracellular ferritin is apparently metabolized in a manner identical with endogenous, intracellular ferritin.5 7
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A.M. Konijn, PhD, Department of Human Nutrition and Metabolism, The Hebrew University, Faculty of Medicine, PO Box 12272, Jerusalem 91120, Israel; e-mail:konijn@md2.huji.ac.il.

![Fig. 1. Distribution of radioiron between internalized ferritin and hemoglobin (Hb) following inhibition of protein degradation. DEC on day 8 of phase II were pulse labeled for 30 minutes with 4 nmol/L59Fe-ferritin, then washed and chased with 400 nmol/L unlabeled ferritin. Inhibition of protein degradation was initiated 60 minutes before the 59Fe-ferritin pulse by 20 μg/mL leupeptin or 15 μmol/L chloroquine, and inhibitors were present throughout the experiment. Leupeptin, which has a short half-life at 37°C, was added every 12 hours. Equal amounts of protein from cell lysates were analyzed on SDS-PAGE under nonreducing conditions followed by phosphor imaging and scanning with the phosphor imager.59Fe-Hb was expressed as percentage of the label in Hb and ferritin. [59Fe-Hb/(59Fe-Hb +59Fe-ferritin)] × 100. Unlabeled Hb was used as a marker (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/9/10.1182_blood.v94.9.3205/4/m_blod42125001w.jpeg?Expires=1765939084&Signature=ufrt9udxZqft~eee014yuL4gZ9-pJXs7V3h1YAQp3nBjJmrMhiXepIKhHBiZkI5~E4w0Tn~Dmze3oS1UR6mCUgj1-qZeveJkpsfjDBAC6BPYYJANSJMnbw4mcMSqhcS-vgxqK1ocx7983GTOnB7WnCYYUMy69ZTLzQR6B0LOjEnVO~LH-dnLtfmEJMi1NK8ES8p2RiumgqBklgQKtKh5QMO1vLUeR6Vs8VKN3Wf-o0Lq94Qr2RojhyJW4hJ6mtEc3yO98EBiSdm9Hee9r8y5ltv0FmOm~owD5AI0hwaK~b6-U-p~V2GiRlEr4k1J6vO9H69AhYPekqnDu64J2q6MAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal